Abstract
Objective
To wholly evaluate the prognostic value of CHT for pT1-3N0-1 breast cancer patients with HR+, HER2− subtype using the Surveillance, Epidemiology, and End Results (SEER) database.
Method
A total of 126,102 eligible cases diagnosed between January 2010 and December 2018 were included in the SEER database. A propensity-score matched (PSM) study with competing risk analysis was conducted. The Kaplan–Meier method was used to visualize the survival disparities between chemotherapy (CHT) and no CHT groups. The cumulative incidences of different subgroups were compared by Fine-Gray’s test.
Results
Before PSM, patients in the CHT group had worse OS and CSS (both P < 0.001). After PSM, we were surprised that patients in the CHT group had a better OS than those in the no CHT group (HR 0.74, 95% CI 0.68–0.80, P < 0.001), while no significant survival disparities were observed for CSS (HR 1.00, 95% CI 0.89–1.12, P = 0.952). In the competing risk analysis, the OS disparities between the CHT and no CHT groups were mainly attributed to deaths of other causes (subdistribution HR [95% CI] 0.50 [0.44–0.57]). After adjusting for other competitive risk events, there was no significant difference in cumulative death risk of breast cancer between the CHT and no CHT groups (subdistribution HR [95% CI] 1.01 [0.90–0.1.13]).
Conclusion
The present study is the first, to our knowledge, to wholly evaluate the prognostic value of CHT for pT1-3N0-1 breast cancer patients with HR+, HER2− subtype using a propensity-score matched study with competing risk analysis. All pT1-3N0-1 breast cancer patients with HR+, HER2− subtype do not benefit from CHT. Genetic testing may be the only effective tool to determine the need for CHT at the present.
Keywords: Breast cancer, Overall survival, Cancer-specific survival, Competing risk analysis, Propensity score matching, Chemotherapy
Introduction
Breast cancer has become the largest threat to women’s health worldwide. The latest cancer statistics in 2020 show that there are 276,480 new cases of breast cancer in women, accounting for 30% of female cancers, with a mortality rate of 22% (Siegel et al. 2020). Among them, breast cancer with hormone receptor (HR)+ and human epidermal growth factor receptor 2 (HER2)− subtype account for the vast majority of the total population. The prognosis of patients, as well as treatment strategy making, is closely related to different molecular types (Colleoni et al. 2016; Prat et al. 2014; Zhou et al. 2020). Chemotherapy (CHT) is regarded as an important role in the treatment of breast cancer. Previous studies showed that in some selected breast cancer patients, such as triple negative, larger tumor, and more positive lymph nodes, CHT can significantly improve overall survival (OS) and cancer-specific survival (CSS). However, to date, the effect of CHT remains still unclear for HR+, HER2−, early-stage breast cancer. Additionally, inevitable side effects along with CHT often brings trouble to breast cancer patients.
Currently, most guidelines recommend that patients with HR+, HER2−, early-stage breast cancer undergo genetic testing to determine the need for CHT (Gradishar et al. 2020; Krop et al. 2017), such as Oncotype DX and MammaPrint testing. However, the high cost of genetic testing and copyright issues limit its accessibility in both developing and developed countries (Turner et al. 2015). Moreover, the results of oncotype DX were divided into three groups: high, medium, and low risk. For high- and low-risk patients, endocrine therapy plus CHT or endocrine therapy alone can be chosen according to guidelines, but the systemic therapy for intermediate-risk patients (26–30 points), which accounts for about 22–36% (McVeigh and Kerin 2017; Chin-Lenn et al. 2018), is not clear (Gradishar et al. 2020). The results of TAILORx trial suggest that the 21-gene assay may identify up to 85% of women with early breast cancer who can be spared adjuvant CHT, especially those who are older than 50 years of age and have a recurrence score of 25 or lower, as well as women 50 years of age or younger with a recurrence score of 15 or lower (Sparano et al. 2018).
Thus, using a large population-based cancer database, a propensity-score matched study with competing risk analysis was conducted to wholly evaluate the prognostic value of CHT for pT1-3N0-1 breast cancer patients with HR+, HER2− subtype. Second, we also constructed a dynamic nomogram based on the competing risk model to predict the CSS of these patients.
Materials and methods
Data source and patient selection
The Surveillance, Epidemiology, and End Results (SEER) database (Incidence—SEER Research Plus Data, 18 Registries, Nov 2020 Sub [2000–2018]), a population-based cancer database covering nearly 30% of the US population, was acquired by SEER*Stat software (version 8.3.9) to identify patients diagnosed between January 2010 and December 2018, according to the 3rd edition of International Classification of Diseases for Oncology (ICD-O-3).
Female patients diagnosed with HR+/HER2−, T1-3N0-1M0 invasive ductal carcinoma as their primary and only cancer were considered eligible for our study. We selected these patients as per the 8th edition of the American Joint Committee on Cancer (AJCC) staging system to generate a uniform dataset. Cases diagnosed without pathological results support lacked complete demographic, clinicopathological and treatment information and those identified by autopsy or death certificate only, follow-up < 3 months were excluded. Finally, 126,102 patients fulfilled the aforementioned criteria were included in this study. The flowchart showing detailed patients’ derivation is illustrated in Fig. 1. Not any informed consent or ethical permission is required since the identifiable information of patients is not publicly available in the SEER database.
Fig. 1.
Flowchart showing derivation of patient selection
In this study, T stages were redefined into T1, T2 and T3. Based on the code information in SEER, marital status was reclassified as single/unmarried, married and divorced /separated/widowed; race was reclassified as white, black and others (Asian or Pacific Islander and American Indian/Alaska Native). Similarly, tumor location was reclassified into three categories: outer quadrant, inner quadrant, and others. OS and CSS were employed as the outcomes of interest. OS was measured from the date of diagnosis to that of the mortality of all causes or the last follow-up. CSS was defined as a period from the diagnosis to the death attributed to breast cancer.
Statistical analysis
Baseline characteristics were presented as frequencies and descriptive statistics, and the distribution differences between the CHT and no CHT groups were measured by the Chi-square test or Wilcoxon–Mann–Whitney test, where appropriate. Propensity score matching (PSM) was adopted to reduce imbalanced distributions of the potential confounding factors. In this study, patients not receiving chemotherapy were matched to those receiving chemotherapy in random order, using the nearest-neighbor matching approach with the ratio of 1:1 (maximum caliper distance, 0.03). The balance of covariates after PSM was evaluated by standardized mean differences (SMDs), with a value of less than 10 percent considered as sufficiently balanced. The Kaplan–Meier method and the log-rank test were used to visualize and compare the survival disparities between CHT and no CHT groups, respectively.
Moreover, although the Cox proportional hazards regression model is the most common method to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for analyzing time-to-event data, it tends to produce inaccurate estimates when fitting the competing nature of multiple causes of the same event. Given breast cancer only accounts for 41.5% of all deaths, competing risks are especially relevant in our study. To estimate the unbiased death risk of breast cancer, we applied a proportional subdistribution hazard regression, which connects regression coefficients to a cumulative incidence function in the presence of competing risks. The cumulative incidences of different subgroups were compared by Fine-Gray’s test. Then, a dynamic nomogram based on the proportional subdistribution hazard model was constructed to predict 3- and 5-year CSS, in which predictors were selected by stepwise backward elimination method. Harrell’s Concordance index (C-index) and calibration curves (1000 bootstrap resamples) were used to assess the discriminative performance and calibrate the evaluation, respectively. All statistical analyses were performed with R 4.0.2 software (R Foundation, Vienna, Austria); the statistical significance was considered as a two-sided P value < 0.05.
Results
Patient demographics and characteristics
Totally, 126,102 female patients diagnosed with HR+/HER2−, T1-3N0-1M0 invasive ductal carcinoma were included in our study (median [interquartile range IQR] age, 60 [51–69] years; 101,787 [80.7%] white), of which 33,773 (26.8%) were in the CHT group while the rest 92,329 (73.2%) were in the no CHT group. Median follow-up of the whole cohort was 51 months (IQR 29–76 months). There were 6650 cases that died during the follow-up, of which 2761 (41.5%) were deaths attributed to breast cancer. Baseline characteristics of patients are summarized in Table 1. Except for laterality, the distributions of other characteristics, all showed significant differences between the CHT and no CHT groups (all P < 0.001). Patients who received chemotherapy seemed to be younger, diagnosed with larger tumor burden, more positive lymph nodes, etc.
Table 1.
Patients demographic and clinicopathological characteristics
| Characteristic | All patients (n = 126,102) | No. of patients (%) | P value | |
|---|---|---|---|---|
| No chemotherapy (n = 92,329) | Chemotherapy (n = 33,773) | |||
| Age, median (IQR), years | 60 (51–69) | 63 (54–71) | 53 (45–62) | < 0.001 |
| Race | < 0.001 | |||
| White | 101,787 (80.7) | 75,790 (82.1) | 25,997 (77.0) | |
| Black | 10,864 (8.6) | 6920 (7.5) | 3944 (11.7) | |
| Othersa | 13,451 (10.7) | 9619 (10.4) | 3832 (11.3) | |
| Marital status | < 0.001 | |||
| Single/unmarried | 18,793 (14.9) | 12,992 (14.1) | 5801 (17.2) | |
| Married | 78,215 (62.0) | 56,330 (61.0) | 21,885 (64.8) | |
| Divorced/separated/widowed | 29,094 (23.1) | 23,007 (24.9) | 6087 (18.0) | |
| Site | < 0.001 | |||
| Outer | 55,401 (43.9) | 40,433 (43.8) | 14,968 (44.3) | |
| Inner | 25,778 (20.4) | 19,449 (21.1) | 6329 (18.7) | |
| Othersb | 44,923 (35.6) | 32,447 (35.1) | 12,476 (36.9) | |
| T stage | < 0.001 | |||
| T1 | 93,082 (73.8) | 76,693 (83.1) | 16,389 (48.5) | |
| T2 | 30,356 (24.1) | 14,918 (16.2) | 15,438 (45.7) | |
| T3 | 2664 (2.1) | 718 (0.8) | 1946 (5.8) | |
| N stage | < 0.001 | |||
| N0 | 97,790 (77.5) | 81,138 (87.9) | 16,652 (49.3) | |
| N1 | 28,312 (22.5) | 11,191 (12.1) | 17,121 (50.7) | |
| Tumor size, median (IQR), mm | 14 (9–21) | 12 (8–18) | 21 (15–30) | < 0.001 |
| No. of positive lymph nodes | < 0.001 | |||
| 0 | 98,187 (77.9) | 81,134 (87.9) | 17,053 (50.5) | |
| 1 | 18,252 (14.5) | 8548 (9.3) | 9704 (28.7) | |
| 2 | 6551 (5.2) | 1981 (2.1) | 4570 (13.5) | |
| 3 | 3112 (2.5) | 666 (0.7) | 2446 (7.2) | |
| Grade | < 0.001 | |||
| Well | 39,409 (31.3) | 35,646 (38.6) | 3763 (11.1) | |
| Moderate | 60,883 (48.3) | 46,050 (49.9) | 14,833 (43.9) | |
| Poor/unknown | 25,810 (20.5) | 10,633 (11.5) | 15,177 (44.9) | |
| Laterality | 0.830 | |||
| Left | 63,367 (50.3) | 46,379 (50.2) | 16,988 (50.3) | |
| Right | 62,735 (49.7) | 45,950 (49.8) | 16,785 (49.7) | |
| ER | < 0.001 | |||
| Negative | 1350 (1.1) | 329 (0.4) | 1021 (3.0) | |
| Positive | 124,752 (98.9) | 92,000 (99.6) | 32,752 (97.0) | |
| PR | < 0.001 | |||
| Negative | 13,178 (10.5) | 6971 (7.6) | 6207 (18.4) | |
| Positive | 112,924 (89.5) | 85,358 (92.4) | 27,566 (81.6) | |
| Surgery | < 0.001 | |||
| Lumpectomy | 86,730 (68.8) | 68,003 (73.7) | 18,727 (55.4) | |
| Mastectomy | 39,372 (31.2) | 24,326 (26.3) | 15,046 (44.6) | |
| Radiation | < 0.001 | |||
| None/unknown | 45,361 (36.0) | 33,592 (36.4) | 11,769 (34.8) | |
| Yes | 80,741 (64.0) | 58,737 (63.6) | 22,004 (65.2) | |
IQR interquartile range, ER estrogen receptor, PR progesterone receptor
aOthers consisting of Asian or Pacific Islander and American Indian/Alaska Native
bOthers including central/nipple, overlapping, NOS
The prognostic role of CHT via the PSM method
The Kaplan–Meier curves showed patients in the CHT group had a worse OS and CSS (both P < 0.001, Fig. 2A, B). After PSM, there were 20,347 patients in the CHT group matched to those in the no CHT group with a ratio of 1:1; all the baseline characteristics were well between-group balanced (all SMD value < 10%, Fig. 3). Among matched patients, we were surprised that patients in the CHT group had a better OS than those in the no CHT group (HR 0.74, 95% CI 0.68–0.80, P < 0.001), while no significant survival disparities were observed for CSS (HR 1.00, 95% CI 0.89–1.12, P = 0.952, Fig. 2C, D).
Fig. 2.
A Overall survival (OS) and B cancer-specific survival (CSS) of patients with or without chemotherapy before propensity score matching (PSM); C OS and D CSS of patients with or without chemotherapy after PSM
Fig. 3.

Distribution of standardized mean differences (SMDs) between the CHT and no CHT groups before and after propensity score matching (PSM)
The prognostic role of CHT in a competing risk analysis
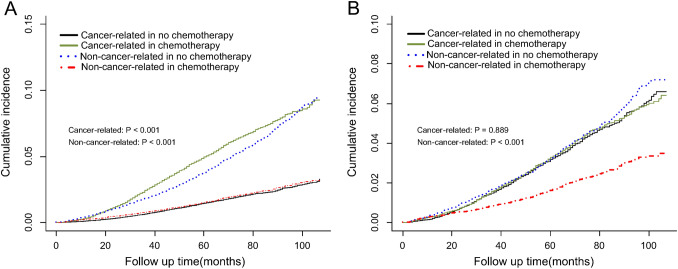
As mentioned above, there were only 2761 (41.5%) deaths attributed to breast cancer. To obtain unbiased estimates of the death risk of breast cancer, thus we also conducted a competing risk analysis. As is shown in Fig. 4, the OS disparities between the CHT and no CHT groups were mainly attributed to deaths of other causes (subdistribution HR [95% CI] 0.50 [0.44–0.57]). After adjusting for other competitive risk events, there was no significant difference in cumulative death risk of breast cancer between the CHT and no CHT groups (subdistribution HR [95% CI] 1.01 [0.90–0.1.13]).
Fig. 4.
The competing risk analysis for pT1-3N0-1 breast cancer patients with HR+, HER2− subtype A before and B after propensity score matching (PSM)
A dynamic nomogram based on the proportional subdistribution hazard model
Then, based on the proportional subdistribution hazard model, a dynamic nomogram was developed and validated to predict 3- and 5-year CSS (https://web-calculator.shinyapps.io/Dynamic_nomogram_for_CSS_of_breast_cancer/) (Fig. 5). The selected predictors in the final model were as follows: age, tumor size, site, race, marriage, T stage, N stage, grade, number of positive lymph nodes, ER status, progesterone receptor status and radiation. The C-indices of the dynamic nomogram in the development and the validation cohort were 0.814 (95% CI 0.802–0.826) and 0.832 (0.820–0.844), respectively. Calibration curves showed the dynamic nomogram was well calibrated, with superb agreement achieved between the actual and predicted probabilities of 3- and 5-year CSS (Fig. 6).
Fig. 5.
A dynamic nomogram to predict cancer-specific survival (CSS) at any follow-up time point for pT1-3N0-1 breast cancer patients with HR+, HER2− subtype
Fig. 6.
Calibration curves of cancer-specific survival (CSS) for the dynamic nomogram. A At 3-year and C 5-year in the development cohort; B at 3-year and D 5-year in the validation cohort
Discussion
During the past few years, breast cancer has become the most commonly diagnosed cancer worldwide, which certainly imposes healthy burdens for women. As one of the most common subtypes, breast cancer with HR+ and HER2− subtype account for the majority of all patients. Recently, a cohort study based on the SEER database conducted by Wen et al. found that for pT1-3N0-1 breast cancer with HR+ , HER2− subtype, patients at high-risk judged by their nomogram should receive CHT, while those in the low-risk group may be exempt from CHT (Wen et al. 2021). However, after screening the clinical data of patients from the SEER database, we found that there were much more pT1-3N0-1 breast cancer with HR+, HER2− subtype died of other causes rather than breast cancer. In this situation, we have to conduct a competing risk analysis to wholly evaluate the prognostic value of CHT for pT1-3N0-1 breast cancer patients with HR+, HER2− subtype, although the Cox proportional hazards regression model is the most common method for analyzing time-to-event data.
In this study, before PSM, the OS and CSS of the CHT group were both inferior to those in the no CHT group, with the more obvious trend in term of CSS. There may be some possible reasons for this phenomenon. On one hand, patients in the CHT group had larger tumors, higher stages, and more positive lymph nodes. On the other, CHT often brings inevitable adverse reactions and side effects to patients. And as summarized in Table 1, obvious imbalanced distributions of the potential confounding factors existed between the CHT and no CHT groups, which need PSM to further generate sufficiently balanced groups for comparison. Moreover, unlike the study of Wen et al. (2021), we extended the follow-up time of the included cases to more than 100 months to reflect the recurrence risk of breast cancer more comprehensively. Several studies have shown that the recurrence time of HR + breast cancer can be as long as 20 years after initial diagnosis (Blows et al. 2010; Davies et al. 2013; Sestak et al. 2013; Sgroi et al. 2013; Pan et al. 2017; Dowsett et al. 2018). According to the NCIC CTG MA. 17 trial (Jin et al. 2012), for some high-risk HR+ breast cancer, 10-year adjuvant endocrine therapy showed better disease-free survival and OS than 5-year therapy. Therefore, it is necessary to extend the follow-up time of the included cases to assess the risk more completely.
After PSM, when the baseline information (demographic and clinical characteristics) of the two groups were balanced, we were surprised to find that patients in the CHT group had better OS than those in the no CHT group, but there was no significant difference in CSS between the two groups. Given breast cancer only accounts for 41.5% of all deaths, competing risks are especially relevant in our study. To estimate the unbiased death risk of breast cancer, we applied a propensity-score matched study with competing risk analysis.
As expected, in the competing risk analysis, the difference in OS between the two groups was mainly due to non-cancer-related deaths. After adjusting for the non-cancer-related deaths, there was no statistically significant difference found between the two groups. In recent years, there have been a number of single-center and multicenter retrospective analyses discussing the need for CHT in early-stage patients with HR+, HER2− breast cancer based on clinicopathologic factors (Ravdin et al. 2001; Goldhirsch et al. 2007; Chia et al. 2004). It has been shown that the majority of early-stage patients with breast cancer who did not receive CHT also had a relatively low rate of locoregional or distant recurrence within 10 years after diagnosis (Chia et al. 2004). According to the MINDACT trail (Cardoso et al. 2016), Similar rates of survival without distant metastasis were reported in the subgroup of patients who had HR+/HER2−, and either node-negative or node-positive disease. The study suggests that the biologic characteristics of the tumor are as important as tumor burden with respect to treatment decisions and patients’ outcomes, even among patients with one to three positive nodes. Unlikely to the findings from Wen et al., we found that all the pT1-3N0-1 breast cancer patients with HR+, HER2− subtype do not benefit from CHT, both in the PSM analysis and in the PSM-based competing risk analysis. For HR+, HER2− early breast cancer, it is difficult to reliably identify high-risk patients who may benefit from adjuvant chemotherapy based solely on traditional clinicopathological features. About 2/3 of patients with node-negative breast cancer can be cured by locoregional therapy, accounting for more than 50% of patients with early breast cancer (Harbeck and Thomssen 2011). In addition, 25–30% of patients with 1–3 positive lymph nodes remain free of distant metastases without adjuvant chemotherapy (Mook et al. 2009). These patients can safely avoid the toxic effects of chemotherapy. However, the results of the NSABP-B20, TAILORx, and SWOG 8814 trials demonstrated a clear correlation between chemotherapy benefit and Oncotype DX outcomes (Sparano et al. 2018; Albain et al. 2010; Paik et al. 2006). Thus, according to the findings from our and previous studies, we thought genetic testing may be the only effective tool to determine the need for CHT at present.
The study also has several limitations need consideration. First, we were unable to obtain treatment details from the SEER database. Therefore, our analysis may be one-sided. Second, underlying disease information was considered as a potential confounding factor affecting treatment options, however, it was also not available to public applicants. Last but not least, although PSM could well balance the potential confounding between the CHT and no CHT groups, we failed to take gene-related information into consideration from the SEER database. Further creditable studies with abundant gene characteristics are warranted to confirm our findings.
Conclusion
To our knowledge, our study is the first to wholly evaluate the prognostic value of CHT for pT1-3N0-1 breast cancer patients with HR+, HER2− subtype using a large population-based propensity-score matched study with competing risk analysis. And we found all the pT1-3N0-1 breast cancer patients with HR+, HER2− subtype do not benefit from CHT, that is, genetic testing may be the only effective tool to determine the need for CHT at the present.
Abbreviations
- SEER
Surveillance, epidemiology, and end results
- PSM
Propensity-score matched
- CHT
Chemotherapy
- HR
Hormone receptor
- HER2
Human epidermal growth factor receptor 2
- OS
Overall survival
- CSS
Cancer specific survival
- ICD-O-3
The 3rd edition of International Classification of Diseases for Oncology
- AJCC
American Joint Committee on Cancer
- SMDs
Standardized mean differences
- HRs
Hazard ratios
- Cis
Confidence intervals
- C-index
Concordance index
- IQR
Interquartile range
Author contributions
Dr HD had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: HD, LT. Acquisition, analysis, or interpretation of data: HD, XS. Statistical analysis: HD, XL, PF. Drafting of the manuscript: HD, LT. Supervision: LT.
Funding/support
Wuhan Municipal Health Commission, Grant/Award Number: WX19Q49; Health Commission of Hubei province, Grant/Award Number: WJ2019H396; Wuhan Medical Scientific Research Project: WX21D69.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Declarations
Conflict of interest
None declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hong Dong and Xinyu Su contributed equally.
References
- Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE et al (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11(1):55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C et al (2010) Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 7(5):e1000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga JY, Brain E, Causeret S, DeLorenzi M et al (2016) Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375(8):717–729 [DOI] [PubMed] [Google Scholar]
- Chia SK, Speers CH, Bryce CJ, Hayes MM, Olivotto IA (2004) Ten-year outcomes in a population-based cohort of node-negative, lymphatic, and vascular invasion-negative early breast cancers without adjuvant systemic therapies. J Clin Oncol 22(9):1630–1637 [DOI] [PubMed] [Google Scholar]
- Chin-Lenn L, De Boer RH, Segelov E, Marx GM, Hughes TM, McCarthy NJ, White SC, Foo SS, Rutovitz JJ, Della-Fiorentina S et al (2018) The impact and indications for Oncotype DX on adjuvant treatment recommendations when third-party funding is unavailable. Asia Pac J Clin Oncol 14(6):410–416 [DOI] [PubMed] [Google Scholar]
- Colleoni M, Sun Z, Price KN, Karlsson P, Forbes JF, Thurlimann B, Gianni L, Castiglione M, Gelber RD, Coates AS et al (2016) Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the international breast cancer study group trials I to V. J Clin Oncol 34(9):927–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A, Bonfill X et al (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381(9869):805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett M, Sestak I, Regan MM, Dodson A, Viale G, Thurlimann B, Colleoni M, Cuzick J (2018) Integration of clinical variables for the prediction of late distant recurrence in patients with estrogen receptor-positive breast cancer treated with 5 years of endocrine therapy: CTS5. J Clin Oncol 36(19):1941–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2007) Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 18(7):1133–1144 [DOI] [PubMed] [Google Scholar]
- Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD et al (2020) Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw 18(4):452–478 [DOI] [PubMed] [Google Scholar]
- Harbeck N, Thomssen C (2011) A new look at node-negative breast cancer. Oncologist 16(Suppl 1):51–60 [DOI] [PubMed] [Google Scholar]
- Jin H, Tu D, Zhao N, Shepherd LE, Goss PE (2012) Longer-term outcomes of letrozole versus placebo after 5 years of tamoxifen in the NCIC CTG MA.17 trial: analyses adjusting for treatment crossover. J Clin Oncol 30(7):718–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krop I, Ismaila N, Stearns V (2017) Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American society of clinical oncology clinical practice focused update guideline summary. J Oncol Pract 13(11):763–766 [DOI] [PubMed] [Google Scholar]
- McVeigh TP, Kerin MJ (2017) Clinical use of the Oncotype DX genomic test to guide treatment decisions for patients with invasive breast cancer. Breast Cancer (dove Med Press) 9:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mook S, Schmidt MK, Viale G, Pruneri G, Eekhout I, Floore A, Glas AM, Bogaerts J, Cardoso F, Piccart-Gebhart MJ et al (2009) The 70-gene prognosis-signature predicts disease outcome in breast cancer patients with 1–3 positive lymph nodes in an independent validation study. Breast Cancer Res Treat 116(2):295–302 [DOI] [PubMed] [Google Scholar]
- Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24(23):3726–3734 [DOI] [PubMed] [Google Scholar]
- Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M et al (2017) 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 377(19):1836–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat A, Carey LA, Adamo B, Vidal M, Tabernero J, Cortes J, Parker JS, Perou CM, Baselga J (2014) Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J Natl Cancer Inst. 10.1093/jnci/dju152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, Parker HL (2001) Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol 19(4):980–991 [DOI] [PubMed] [Google Scholar]
- Sestak I, Dowsett M, Zabaglo L, Lopez-Knowles E, Ferree S, Cowens JW, Cuzick J (2013) Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst 105(19):1504–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgroi DC, Sestak I, Cuzick J, Zhang Y, Schnabel CA, Schroeder B, Erlander MG, Dunbier A, Sidhu K, Lopez-Knowles E et al (2013) Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 14(11):1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70(1):7–30 [DOI] [PubMed] [Google Scholar]
- Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr et al (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379(2):111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BM, Skinner KA, Tang P, Jackson MC, Soukiazian N, Shayne M, Huston A, Ling M, Hicks DG (2015) Use of modified Magee equations and histologic criteria to predict the Oncotype DX recurrence score. Mod Pathol 28(7):921–931 [DOI] [PubMed] [Google Scholar]
- Wen N, Qiu J, Xu L, Wang Y, Zhang J, Xie Y, Lv Q, Du Z (2021) Adjuvant chemotherapy guidance for pT1-3N0-1 breast cancer patients with HR(+), HER2(-) subtype: a cohort study based on the SEER database. Ann Transl Med 9(24):1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wang SP, Zeng W, Chen SC, Huang YH, Zhou L, Wang M, Wei W, Zhang C, Liu ZM et al (2020) The effect of metastasis patterns on survival in male patients with different breast cancer subtypes: results from the surveillance, epidemiology, and end results (SEER) database. Transl Cancer Res 9(4):2267–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.