Abstract
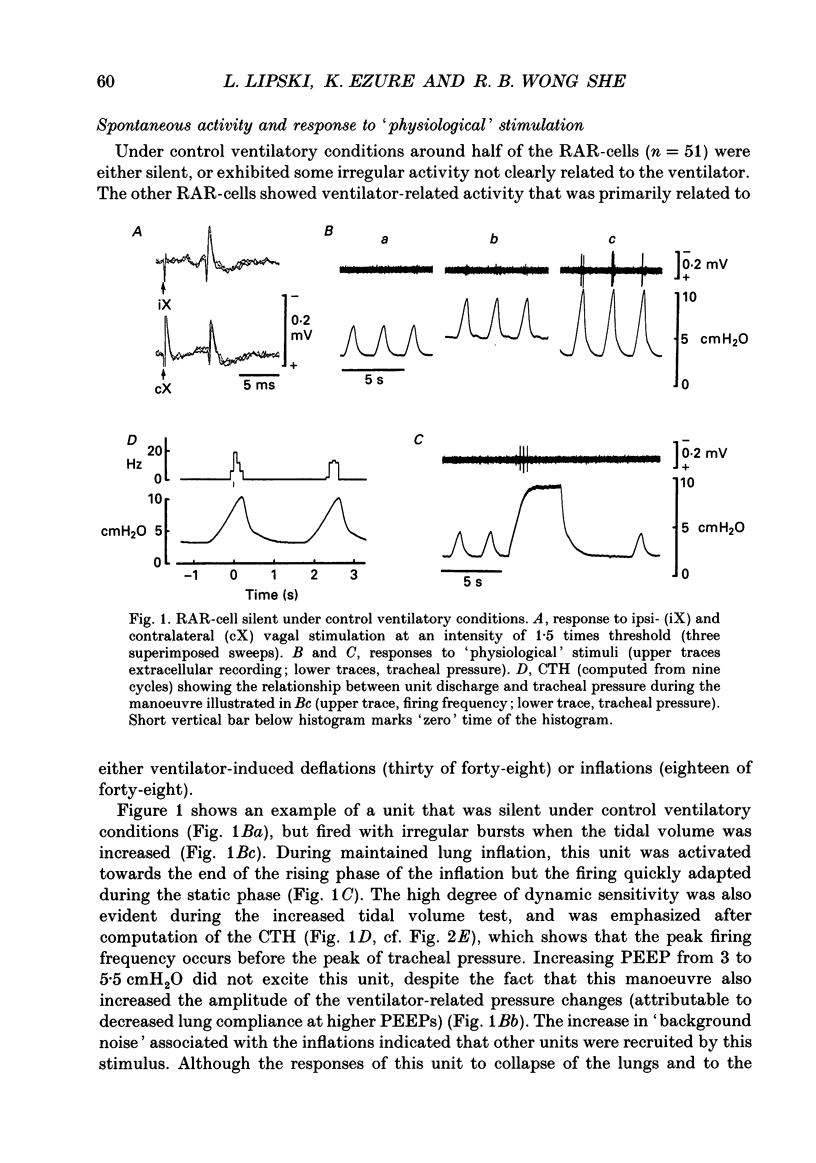
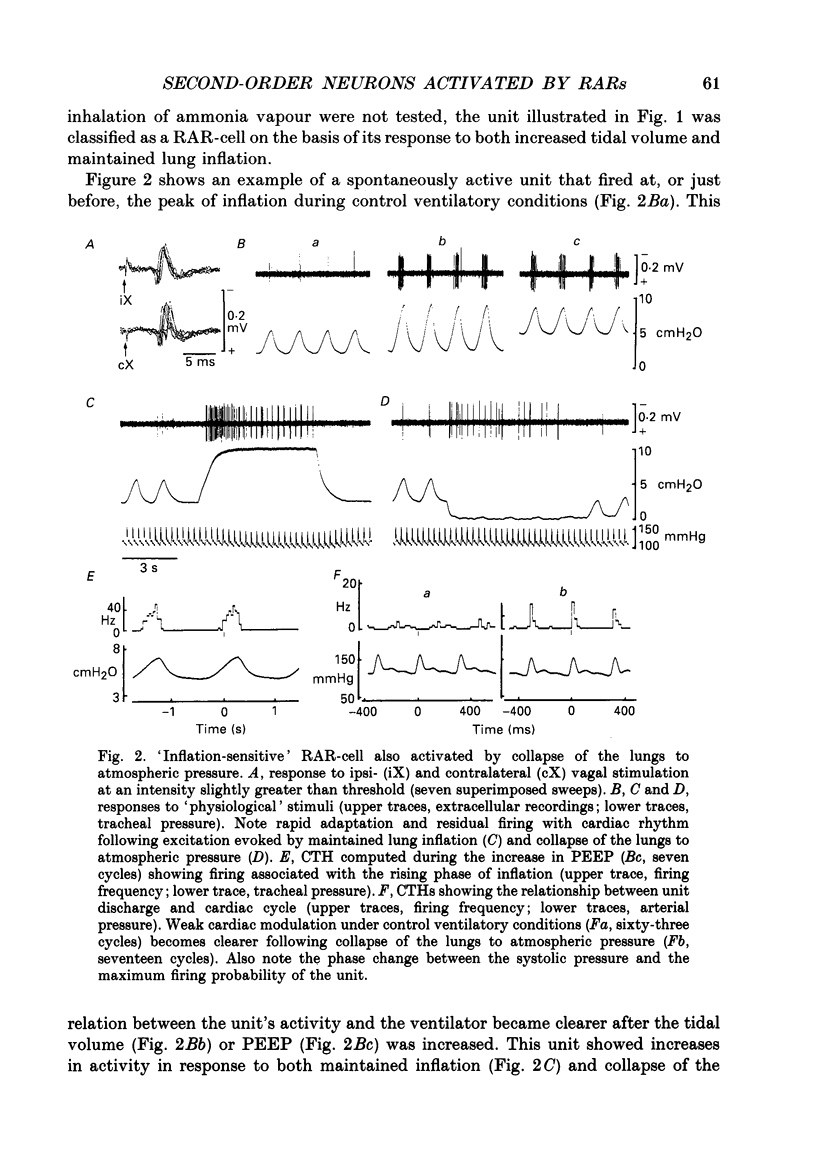
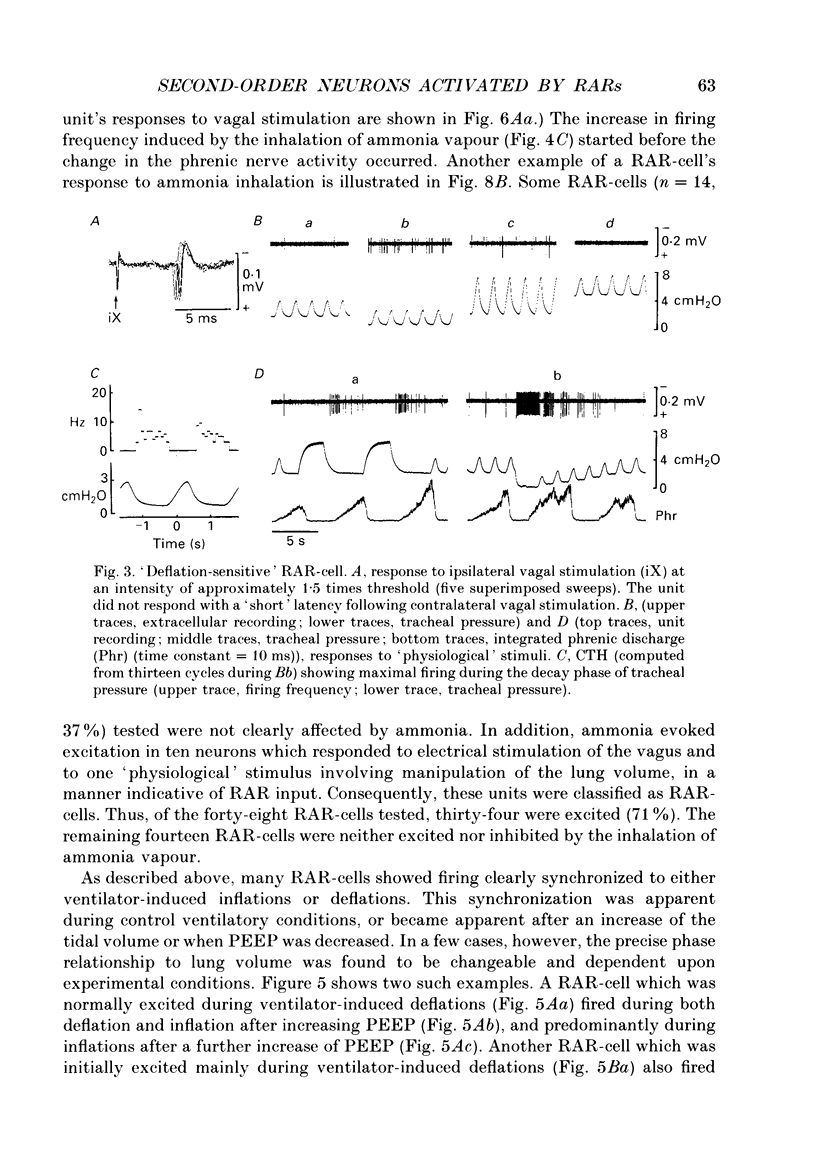
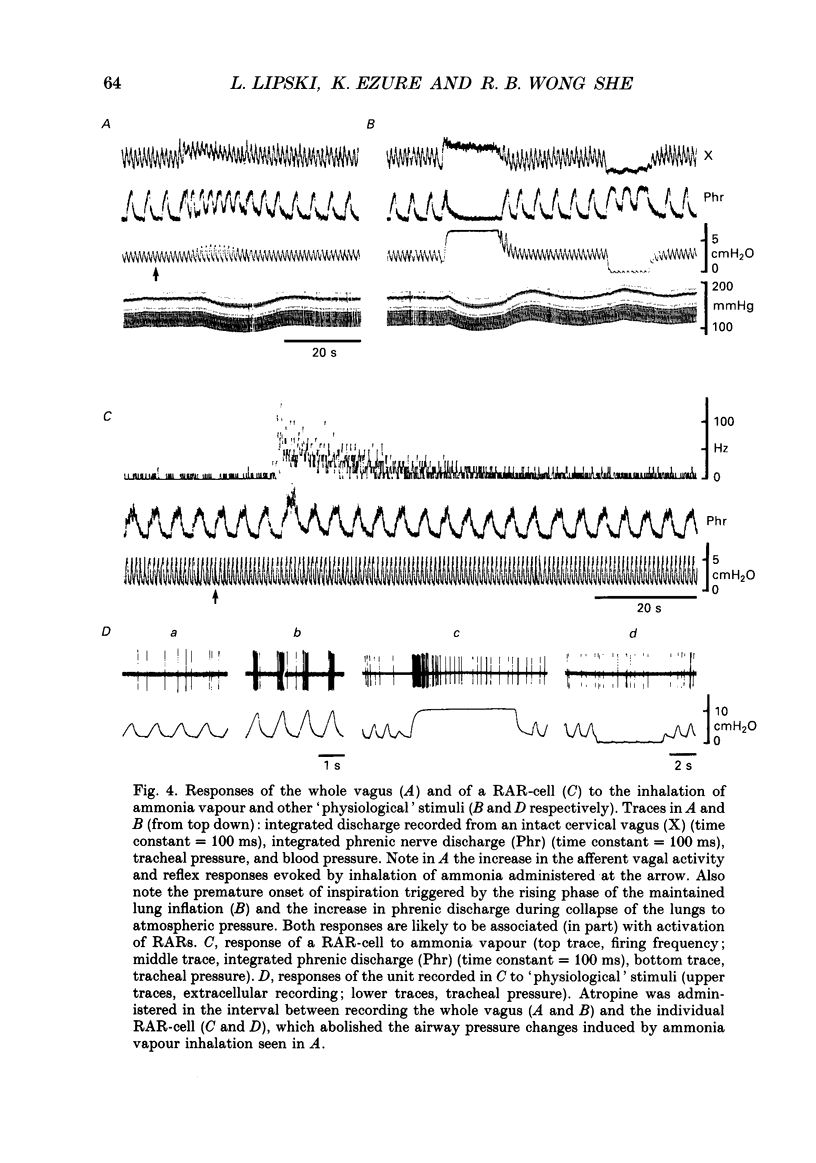
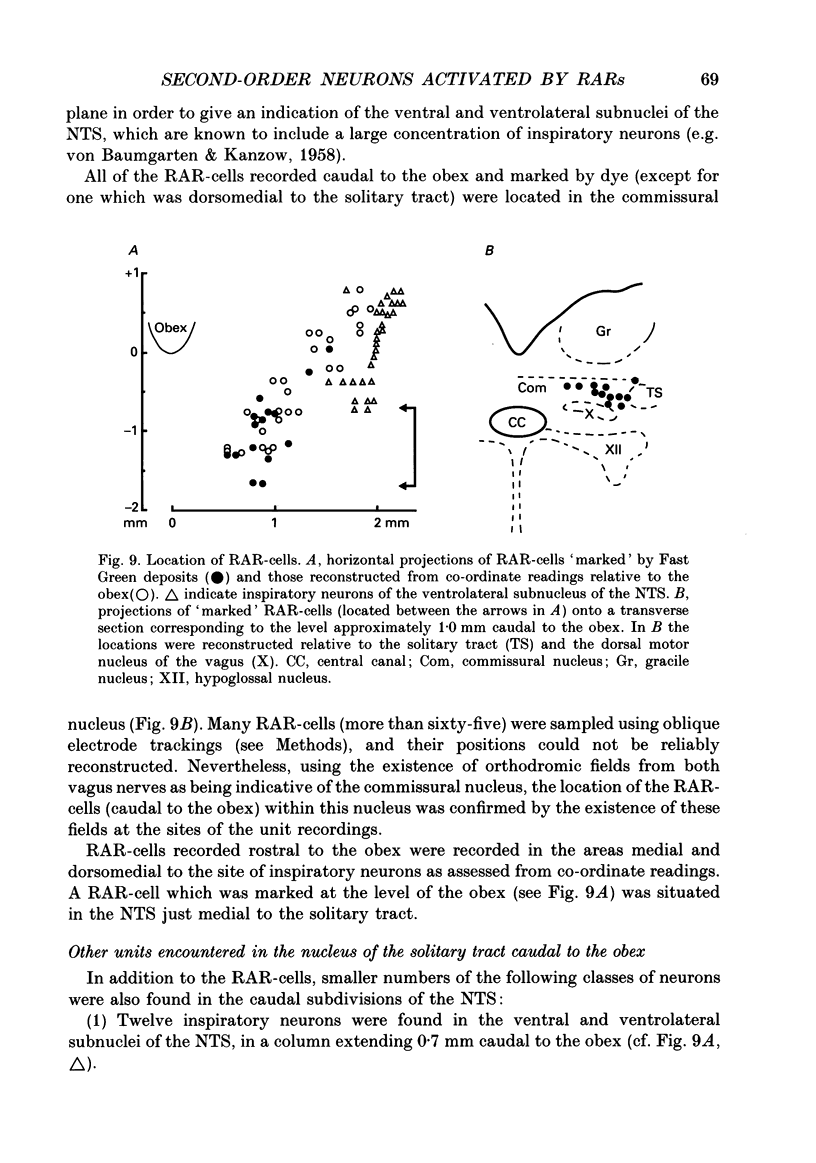
1. Extracellular and intracellular recordings were made in the caudal subdivisions of the nucleus tractus solitarii (NTS) to locate and characterize neurons excited by afferents from pulmonary rapidly adapting receptors (RARs) in Nembutal-anaesthetized cats. 2. Neurons identified as second-order cells activated by RARs (RAR-cells) were activated by electrical stimulation of myelinated afferents in the cervical portion of the vagus nerve(s) and by at least two of the following 'physiological' stimuli: (a) collapse of the lungs to atmospheric pressure; (b) hyperinflation of the lungs by either increasing tidal volume or maintained lung inflations; and (c) brief inhalation of ammonia vapour. 3. Of the ninety-nine RAR-cells identified and studied extracellularly, eighty-four were localized within the commissural nucleus of the NTS. Seventy-four cells responded monosynaptically to electrical stimulation of both ipsi- and contralateral vagal stimulation. The remaining ten RAR-cells located in the commissural nucleus and the fifteen located in the caudal portion of the medial subnucleus of the NTS rostral to the obex, responded to the ipsilateral vagus only. 4. Under control ventilatory conditions (bilateral pneumothorax, positive end-expiratory pressure of approx. 2 cmH2O), forty-eight of the ninety-nine RAR-cells showed spontaneous ventilator-related activity, occurring primarily during ventilator-induced deflations (thirty of forty-eight). 'Reversal' of this ventilator-related modulation, from firing predominantly during lung deflations to lung inflations, and vice versa, could be induced in eleven of the RAR-cells by changing the lung volume or by the inhalation of ammonia vapour. 5. Modulation of firing in synchrony with the central respiratory rhythm was observed in the activity of fourteen of the ninety-nine RAR-cells. 6. Intracellular recordings were made from twenty-two NTS neurons caudal to the obex that received monosynaptic excitatory postsynaptic potentials (EPSPs) from both ipsi- and contralateral vagus nerves. Two were positively identified as RAR-cells. 7. It is concluded that the commissural nucleus of the NTS is the major location of RAR-cells, and that the response characteristics of these neurons largely, but not entirely, correspond to the known properties of RARs.
Full text
PDF





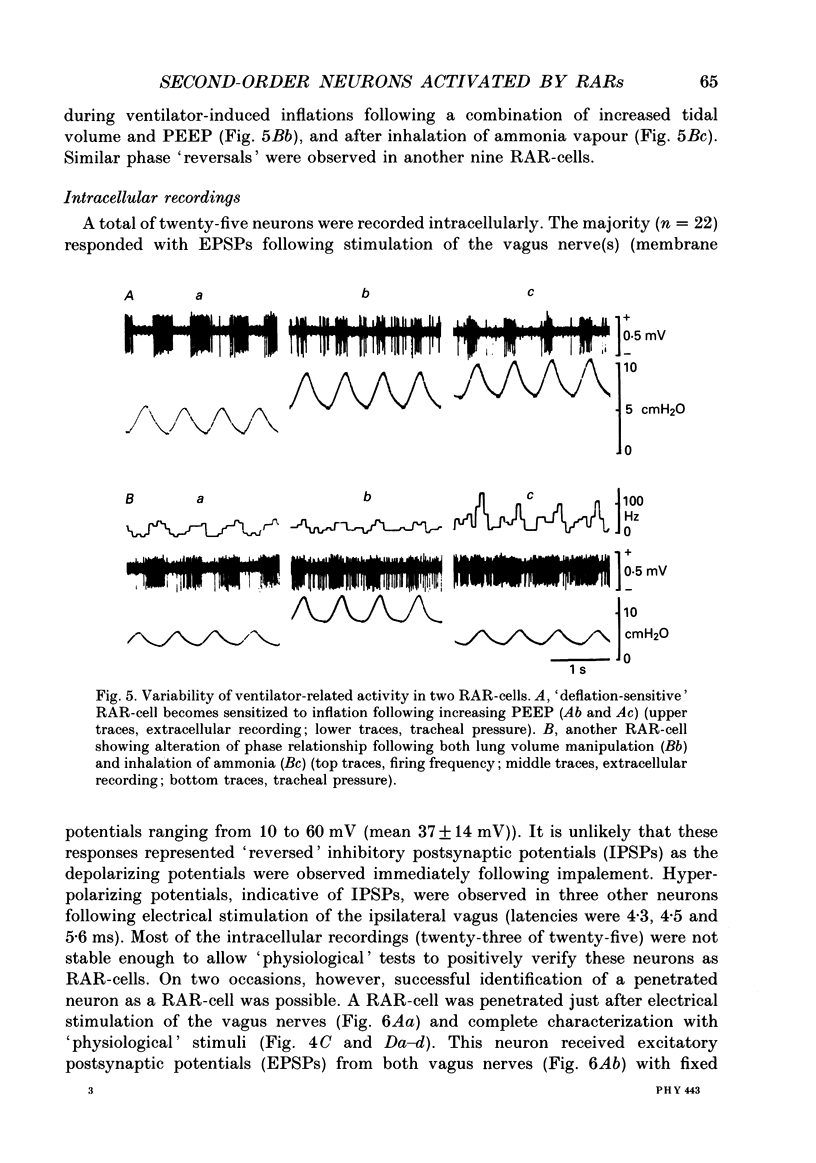
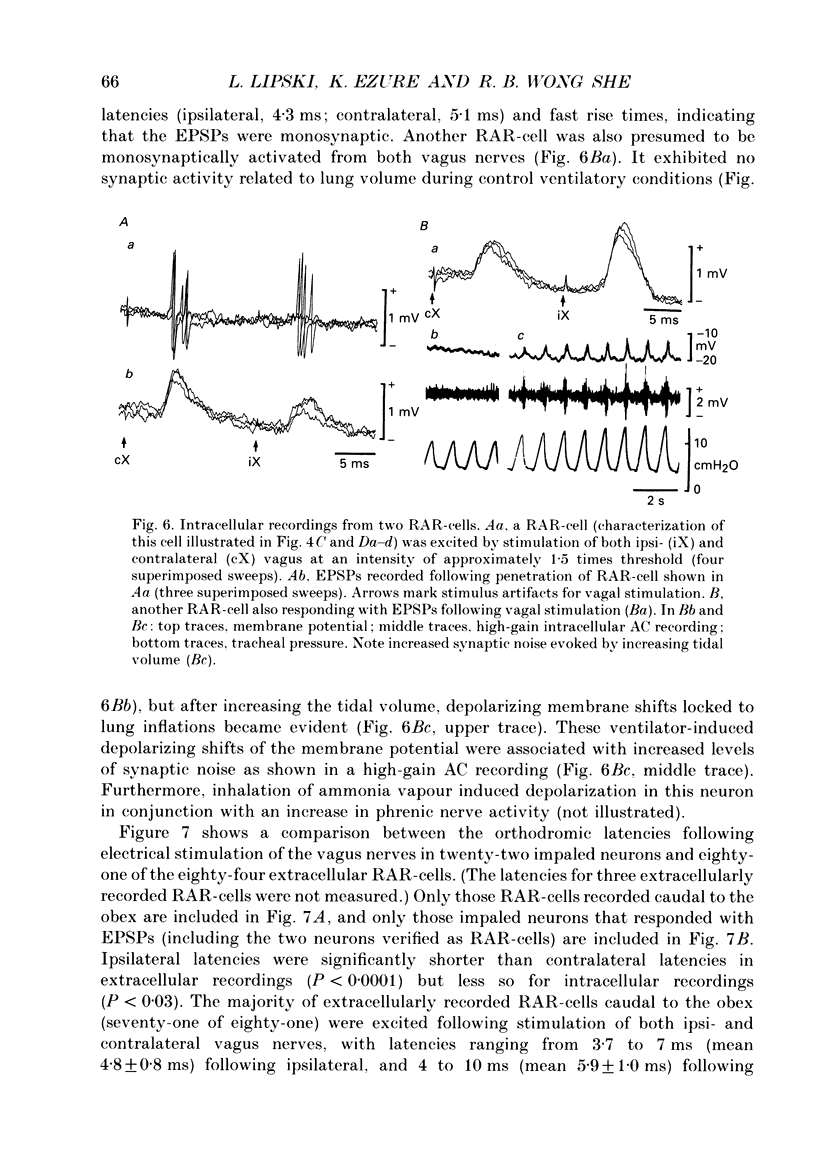
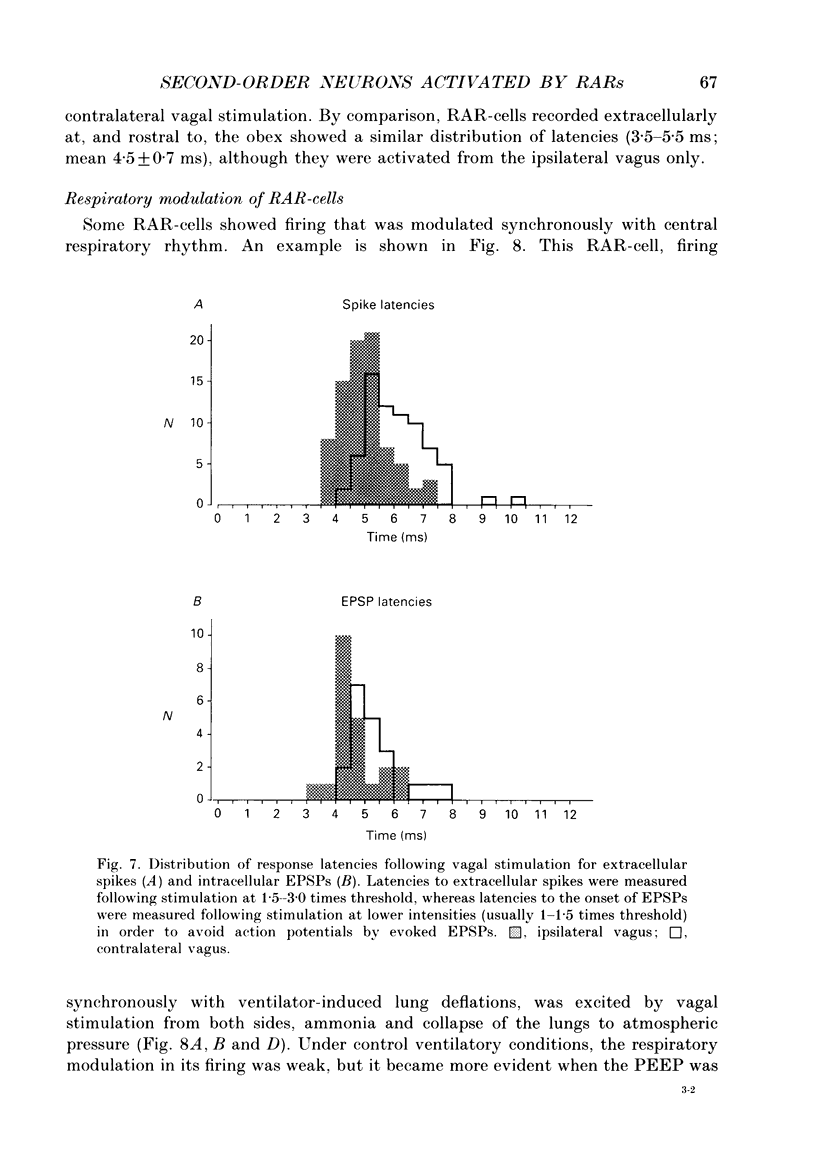
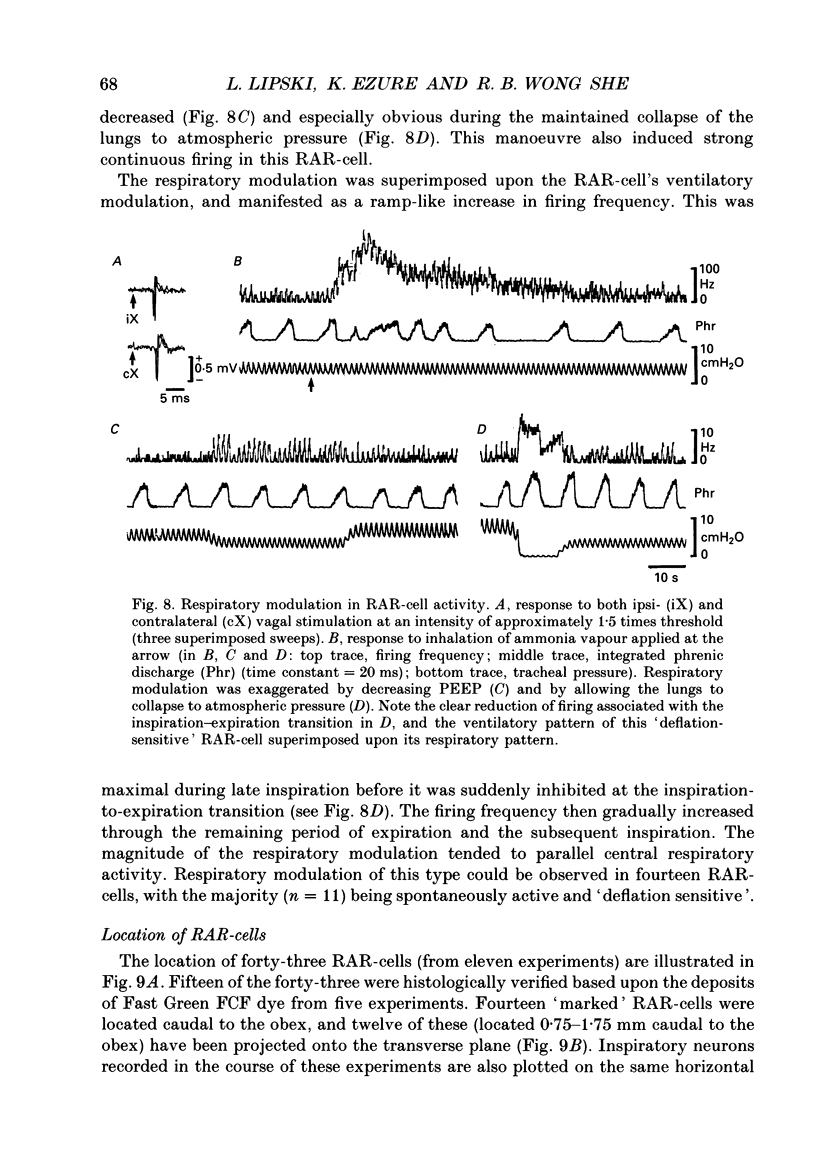








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. J., Luck J. C. A comparative study of irritant and type J receptors in the cat. Respir Physiol. 1974 Jul;21(1):47–60. doi: 10.1016/0034-5687(74)90006-1. [DOI] [PubMed] [Google Scholar]
- Bennett J. A., Goodchild C. S., Kidd C., McWilliam P. N. Neurones in the brain stem of the cat excited by vagal afferent fibres from the heart and lungs. J Physiol. 1985 Dec;369:1–15. doi: 10.1113/jphysiol.1985.sp015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A. J. Dorsal respiratory group neurons in the medulla of cat: spinal projections, responses to lung inflation and superior laryngeal nerve stimulation. Brain Res. 1977 Oct 28;135(2):231–254. doi: 10.1016/0006-8993(77)91028-9. [DOI] [PubMed] [Google Scholar]
- Bianchi A. L., Barillot J. C. Activity of medullary respiratory neurones during reflexes from the lungs in cats. Respir Physiol. 1975 Dec;25(3):335–352. doi: 10.1016/0034-5687(75)90008-0. [DOI] [PubMed] [Google Scholar]
- Bonham A. C., McCrimmon D. R. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. J Physiol. 1990 Aug;427:261–280. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniack N. S., von Euler C., Głogowska M., Homma I. Characteristics and rate of occurrence of spontaneous and provoked augmented breaths. Acta Physiol Scand. 1981 Mar;111(3):349–360. doi: 10.1111/j.1748-1716.1981.tb06747.x. [DOI] [PubMed] [Google Scholar]
- Davies A., Roumy M. The effect of transient stimulation of lung irritant receptors on the pattern of breathing in rabbits. J Physiol. 1982 Mar;324:389–401. doi: 10.1113/jphysiol.1982.sp014119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A., Sant'Ambrogio F., Sant'Ambrogio G. Onset of inspiration in rabbit during artificial ventilation. J Physiol. 1981 Sep;318:17–23. doi: 10.1113/jphysiol.1981.sp013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. O., Kubin L., Pack A. I. Pulmonary stretch receptor relay neurones of the cat: location and contralateral medullary projections. J Physiol. 1987 Feb;383:571–585. doi: 10.1113/jphysiol.1987.sp016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. O., Kubin L. Projection of pulmonary rapidly adapting receptors to the medulla of the cat: an antidromic mapping study. J Physiol. 1986 Apr;373:63–86. doi: 10.1113/jphysiol.1986.sp016035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. O., Metzler J., Silage D. A., Pack A. I. Effects of lung inflation on the excitability of dorsal respiratory group neurons. Brain Res. 1986 Feb 26;366(1-2):22–36. doi: 10.1016/0006-8993(86)91278-3. [DOI] [PubMed] [Google Scholar]
- Donoghue S., Garcia M., Jordan D., Spyer K. M. The brain-stem projections of pulmonary stretch afferent neurones in cats and rabbits. J Physiol. 1982 Jan;322:353–363. doi: 10.1113/jphysiol.1982.sp014041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B. Afterpotentials and transduction properties in different types of central neurones. Arch Ital Biol. 1984 Mar;122(1):17–30. [PubMed] [Google Scholar]
- Jonzon A., Pisarri T. E., Coleridge J. C., Coleridge H. M. Rapidly adapting receptor activity in dogs is inversely related to lung compliance. J Appl Physiol (1985) 1986 Nov;61(5):1980–1987. doi: 10.1152/jappl.1986.61.5.1980. [DOI] [PubMed] [Google Scholar]
- Jordan D., Spyer K. M. Brainstem integration of cardiovascular and pulmonary afferent activity. Prog Brain Res. 1986;67:295–314. doi: 10.1016/s0079-6123(08)62769-7. [DOI] [PubMed] [Google Scholar]
- Kalia M., Mesulam M. M. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol. 1980 Sep 15;193(2):435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- Kalia M. Neuroanatomical organization of the respiratory centers. Fed Proc. 1977 Sep;36(10):2405–2411. [PubMed] [Google Scholar]
- Kalia M., Richter D. Morphology of physiologically identified slowly adapting lung stretch receptor afferents stained with intra-axonal horseradish peroxidase in the nucleus of the tractus solitarius of the cat. I. A light microscopic analysis. J Comp Neurol. 1985 Nov 22;241(4):503–520. doi: 10.1002/cne.902410409. [DOI] [PubMed] [Google Scholar]
- Kalia M., Richter D. Rapidly adapting pulmonary receptor afferents: I. Arborization in the nucleus of the tractus solitarius. J Comp Neurol. 1988 Aug 22;274(4):560–573. doi: 10.1002/cne.902740406. [DOI] [PubMed] [Google Scholar]
- Kalia M., Richter D. Rapidly adapting pulmonary receptor afferents: II. Fine structure and synaptic organization of central terminal processes in the nucleus of the tractus solitarius. J Comp Neurol. 1988 Aug 22;274(4):574–594. doi: 10.1002/cne.902740407. [DOI] [PubMed] [Google Scholar]
- King G. W. Topology of ascending brainstem projections to nucleus parabrachialis in the cat. J Comp Neurol. 1980 Jun 15;191(4):615–638. doi: 10.1002/cne.901910408. [DOI] [PubMed] [Google Scholar]
- Kubin L., Davies R. O. Sites of termination and relay of pulmonary rapidly adapting receptors as studied by spike-triggered averaging. Brain Res. 1988 Mar 8;443(1-2):215–221. doi: 10.1016/0006-8993(88)91615-0. [DOI] [PubMed] [Google Scholar]
- Loewy A. D., Burton H. Nuclei of the solitary tract: efferent projections to the lower brain stem and spinal cord of the cat. J Comp Neurol. 1978 Sep 15;181(2):421–449. doi: 10.1002/cne.901810211. [DOI] [PubMed] [Google Scholar]
- Maley B. E., Panneton W. M. Enkephalin-immunoreactive neurons in the nucleus tractus solitarius project to the parabrachial nucleus of the cat. Brain Res. 1988 Mar 1;442(2):340–344. doi: 10.1016/0006-8993(88)91521-1. [DOI] [PubMed] [Google Scholar]
- Marino P. L., Davies R. O., Pack A. I. The responses of I beta cells to increases in the rate of lung inflation. Brain Res. 1981 Aug 31;219(2):289–305. doi: 10.1016/0006-8993(81)90292-4. [DOI] [PubMed] [Google Scholar]
- Mills J. E., Sellick H., Widdicombe J. G. Activity of lung irritant receptors in pulmonary microembolism, anaphylaxis and drug-induced bronchoconstrictions. J Physiol. 1969 Aug;203(2):337–357. doi: 10.1113/jphysiol.1969.sp008867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack A. I., DeLaney R. G. Response of pulmonary rapidly adapting receptors during lung inflation. J Appl Physiol Respir Environ Exerc Physiol. 1983 Sep;55(3):955–963. doi: 10.1152/jappl.1983.55.3.955. [DOI] [PubMed] [Google Scholar]
- Pack A. I. Sensory inputs to the medulla. Annu Rev Physiol. 1981;43:73–90. doi: 10.1146/annurev.ph.43.030181.000445. [DOI] [PubMed] [Google Scholar]
- Paintal A. S. Vagal sensory receptors and their reflex effects. Physiol Rev. 1973 Jan;53(1):159–227. doi: 10.1152/physrev.1973.53.1.159. [DOI] [PubMed] [Google Scholar]
- Pantaleo T., Corda M. Respiration-related neurons in the medial nuclear complex of the solitary tract of the cat. Respir Physiol. 1986 May;64(2):135–148. doi: 10.1016/0034-5687(86)90037-x. [DOI] [PubMed] [Google Scholar]
- Sampson S. R., Vidruk E. H. Properties of 'irritant' receptors in canine lung. Respir Physiol. 1975 Oct;25(1):9–22. doi: 10.1016/0034-5687(75)90047-x. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio G. Nervous receptors of the tracheobronchial tree. Annu Rev Physiol. 1987;49:611–627. doi: 10.1146/annurev.ph.49.030187.003143. [DOI] [PubMed] [Google Scholar]
- Sellick H., Widdicombe J. G. The activity of lung irritant receptors during pneumothorax, hyperpnoea and pulmonary vascular congestion. J Physiol. 1969 Aug;203(2):359–381. doi: 10.1113/jphysiol.1969.sp008868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon R. Intercostal and abdominal muscle afferent influence on medullary dorsal respiratory group neurons. Respir Physiol. 1980 Jan;39(1):73–94. doi: 10.1016/0034-5687(80)90015-8. [DOI] [PubMed] [Google Scholar]
- WIDDICOMBE J. G. Receptors in the trachea and bronchi of the cat. J Physiol. 1954 Jan;123(1):71–104. doi: 10.1113/jphysiol.1954.sp005034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe J. G., Sellick H. Vagal deflation and inflation reflexes mediated by lung irritant receptors. Q J Exp Physiol Cogn Med Sci. 1970 Apr;55(2):153–163. doi: 10.1113/expphysiol.1970.sp002060. [DOI] [PubMed] [Google Scholar]
- Yu J., Coleridge J. C., Coleridge H. M. Influence of lung stiffness on rapidly adapting receptors in rabbits and cats. Respir Physiol. 1987 May;68(2):161–176. doi: 10.1016/s0034-5687(87)80003-8. [DOI] [PubMed] [Google Scholar]
- Yu J., Roberts A. M. Indirect effects of histamine on pulmonary rapidly adapting receptors in cats. Respir Physiol. 1990 Feb;79(2):101–110. doi: 10.1016/0034-5687(90)90010-v. [DOI] [PubMed] [Google Scholar]


