Abstract
Two independent pathways operate in plants for the synthesis of isopentenyl diphosphate and dimethylallyl diphosphate, the central intermediates in the biosynthesis of all isoprenoids. The mevalonate pathway is present in the cytosol, whereas the recently discovered mevalonate-independent pathway is localized to plastids. We have used isolated peppermint (Mentha piperita) oil gland secretory cells as an experimental model system to study the effects of the herbicides fosmidomycin, phosphonothrixin, methyl viologen, benzyl viologen, clomazone, 2-(dimethylamino)ethyl diphosphate, alendronate, and pamidronate on the pools of metabolites related to monoterpene biosynthesis via the mevalonate-independent pathway. A newly developed isolation protocol for polar metabolites together with an improved separation and detection method based on liquid chromatography-mass spectrometry have allowed assessment of the enzyme targets for a number of these herbicides.
The growing body of gene sequence information from a variety of plants, in combination with experimental genomics, is beginning to revolutionize the understanding of plant metabolism. These new technologies based on sequence information can be readily integrated with traditional biochemical and genetic approaches to add new dimensions to the study of complex metabolic pathways in plants. T-DNA and transposon insertion mutagenesis (Feldmann, 1991; Tissier et al., 1999), the analysis of synteny between different plant species (Devos and Gale, 2000), the use of chimeraplasty to create specific mutations in plant genes (Beetham et al., 1999), and the introduction of activation tagging as a gain-of-function screen (Weigel et al., 2000) have already revealed new insights into the function of specific genes. Microarray tracking of global gene expression in a plant cell or tissue (Van Hal et al., 2000) and proteomic analysis of protein expression patterns (Thiellement et al., 1999) have evolved as additional powerful tools to draw a broader picture of how plants alter biochemical processes over time or in response to environmental stimuli. Furthermore, metabolomics provides complementary information from profiles of metabolites produced by various plants and plant tissues in response to change. For example, this approach has been used successfully to study metabolite flux perturbations by gas chromatography-mass spectrometry in transgenic potato tubers modified in Suc metabolism (Roessner et al., 2000).
A central focus of this laboratory is isoprenoid metabolism in plants. Using peppermint (Mentha × piperita) as a model system, we have used traditional reverse genetics as well as functional genomics approaches to define the biosynthetic steps involved in the formation of peppermint isoprenoids that are synthesized in specialized anatomical structures termed oil glands (Lange et al., 2000a). The secretory cells of the oil glands are highly specialized for the synthesis of monoterpenes via the plastidial mevalonate-independent pathway (Fig. 1), and these fully functional, isolated cells are nonspecifically permeable to low-Mr (≤1,000), water-soluble compounds (McCaskill et al., 1992). As a consequence of their anatomy and the method of isolation (Gershenzon et al., 1992), the cytoplasm of the isolated secretory cells is depleted of endogenous metabolites. However, the intracellular composition of cofactors and substrates can be adjusted by the buffer in which the cells are suspended (McCaskill and Croteau, 1995). We have recently developed a method that allows the simultaneous separation and detection of polar intracellular metabolites of plant isoprenoid biosynthesis by a combination of HPLC and mass spectrometry (LC-MS; R.E.B. Ketchum, B.M. Lange, and R. Croteau, unpublished data). Here, we describe the use of this analytical technique to identify the mode-of-action of herbicides that target monoterpene biosynthesis in peppermint. This procedure involves the feeding of isolated peppermint oil gland secretory cells with labeled precursors of isoprenoid biosynthesis in the presence of putative inhibitors of this pathway (Fig. 2), the subsequent isolation of polar intracellular metabolites, and the separation and characterization of these metabolites by LC-MS.
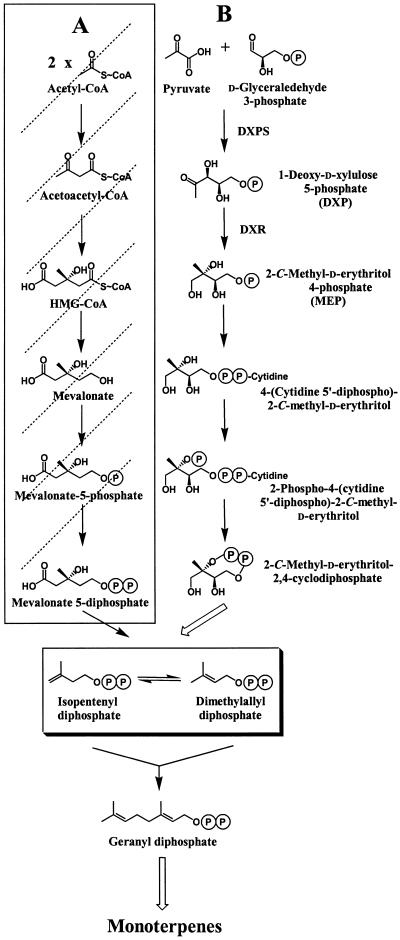
Figure 1.
Monoterpene biosynthesis in peppermint oil gland secretory cells. In plants, two independent pathways are used in the formation of the central precursors of isoprenoids, IPP, and DMAPP: the cytosolic mevalonate pathway (A) and the plastidial mevalonate-independent pathway (B; Lange et al., 2000b). Further reactions include the conversion of IPP to DMAPP, catalyzed by IPP isomerase (Ogura, 1999), followed by sequential condensation reactions catalyzed by prenyltransferases (Ogura, 1999). These prenyl diphosphates (e.g. geranyl diphosphates) undergo cyclizations and subsequent secondary transformation (largely redox) reactions leading to diverse isoprenoid end products (McGarvey and Croteau, 1995). In peppermint secretory cells, the mevalonate pathway is blocked as indicated by dotted lines (McCaskill and Croteau, 1995), making these cells an excellent in vivo system for studying herbicide effects on the mevalonate-independent pathway.
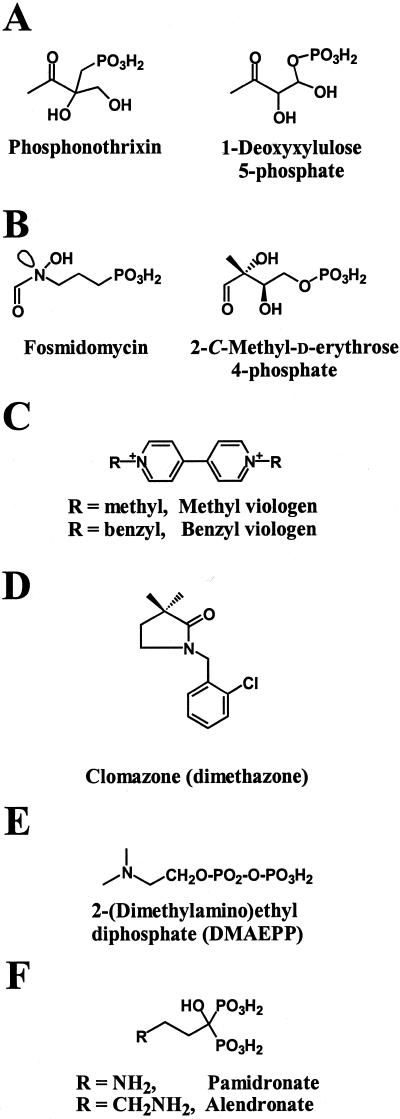
Figure 2.
A through F, Structures of herbicides used in this study. For comparison, the structures of 1-deoxy-d-xylulose 5-phosphate and 2-C-methyl-d-erythrose 4-phosphate (presumed intermediate of the DXR reaction) are also illustrated.
RESULTS AND DISCUSSION
Inhibitors of Isoprenoid Biosynthesis
This study focuses on putative inhibitors of the early steps of isoprenoid biosynthesis leading to the production of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) and their assembly to prenyl diphosphates (Fig. 1). Foliar application of phosphonothrixin (Fig. 2A), a compound isolated from the fermentation broth of Saccharothrix sp. ST-888, induces non-selective leaf chlorosis in several plant species (Kimura et al., 1995; Takahashi et al., 1995). However, no antibiotic effects of this compound have been observed in paper-disc assays against certain eubacteria, ascomycetes, and yeasts (Takahashi et al., 1995). Phosphonothrixin was included in this screening program because of its notable structural similarity to deoxyxylulose phosphate (DXP), the first intermediate of the mevalonate-independent pathway of isoprenoid biosynthesis (Fig. 2A).
Fosmidomycin, a nitrogen-containing phosphonate antibiotic (Fig. 2B) produced by Streptomyces lavendulae (Okuhara et al., 1980), has been shown previously to affect isoprenoid biosynthesis in several bacteria (Shigi, 1989) and in the malaria-causing parasite Plasmodium falciparum (Jomaa et al., 1999), and has been demonstrated to possess herbicidal activity (Patterson, 1987; Kamuro et al., 1991). The mode-of-action of this compound has recently been reported to involve the inhibition of 1-deoxyxylulose 5-phosphate reductoisomerase (DXR) of the mevalonate-independent pathway (Kuzuyama et al., 1998; Zeidler et al., 1998; Fellermeier et al., 1999; Jomaa et al., 1999).
Methyl viologen (paraquat) and its structural analog benzyl viologen (Fig. 2C) cause oxidative stress by generating reactive oxygen species (Hassan and Fridovich, 1979; Bus and Gibson, 1984). In a variety of bacteria, benzyl viologen treatment leads to the accumulation of 2-C-methyl-d-erythritol 2,4-cyclodiphosphate (Turner et al., 1992; Ogrel et al., 1996; Ostrovsky et al., 1998), an intermediate of the mevalonate-independent pathway (Herz et al., 2000; Takagi et al., 2000).
The herbicide clomazone (dimethazone; Fig. 2D) produces leaf bleaching by significantly reducing the levels of plastidial pigments such as carotenoids and chlorophylls (Sandmann and Böger, 1986; Duke and Kenyon, 1988). The enzyme target of this herbicide is not yet defined (Lutzow et al., 1990; Croteau, 1992).
2-(Dimethylamino) ethyl diphosphate (DMAEPP; Fig. 2E) is a presumptive transition-state analog of the intermediate of the IPP isomerase reaction (Mühlbacher and Poulter, 1988), and this compound has been shown recently to inhibit isoprenoid biosynthesis in plants (McCaskill and Croteau, 1999).
Nitrogen-containing bisphosphonates have been used as therapeutic agents in treating disorders such as osteoporosis, metastatic bone disease, and Paget's disease (Fleisch, 1998). In recent work, these bisphosphonates, including alendronate and pamidronate (Fig. 2F), have been shown to inhibit mammalian IPP isomerase and farnesyl diphosphate synthase activity in vitro (Van Beek et al., 1999) and farnesyl diphosphate synthase and geranylgeranyl diphosphate synthase activity in plants in vivo (Oberhauser et al., 1998; Cromartie et al., 1999).
Effects of Inhibitor Treatments on Monoterpene and Sesquiterpene Accumulation
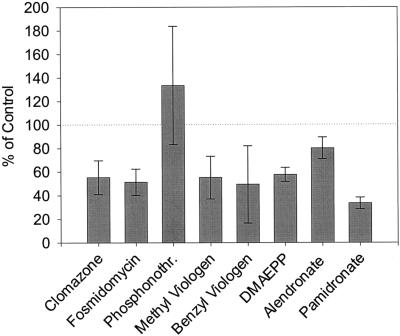
Secretory cells were isolated from peppermint leaves and incubated with a cofactor mix to reconstitute functional isoprenoid biosynthesis. Under these conditions, [2-14C]pyruvate, a precursor of isoprenoid biosynthesis via the mevalonate-independent pathway, was efficiently incorporated into monoterpenes and sesquiterpenes (65–70 pmol/h × 105 secretory cell clusters) which were trapped in n-pentane overlaying the suspension. Compared with these controls, the herbicide-treated cells showed significant inhibition of monoterpene and sesquiterpene biosynthesis at the 2-mm level: pamidronate (incorporation of label from [2-14C]pyruvate decreased to 33% of control), benzyl viologen (to 49%), fosmidomycin (to 51%), methyl viologen (to 55%), clomazone (to 55%), DMAEPP (to 58%), and alendronate (to 80%) (Fig. 3). In contrast, phosphonothrixin (at 2 mm) did not appear to inhibit isoprenoid biosynthesis in peppermint secretory cells and was, thus, excluded from further investigations.
Figure 3.
Incorporation of [2-14C]pyruvate into monoterpenes and sesquiterpenes of isolated peppermint oil gland secretory cells in the presence of putative inhibitors of isoprenoid biosynthesis. The sd of four independent experiments is indicated.
Effects of Inhibitors on Prenyl Diphosphate Accumulation
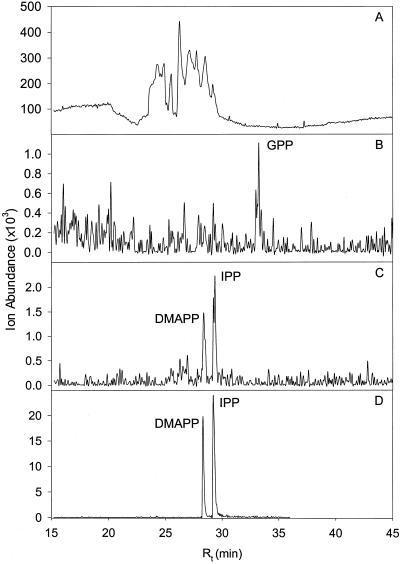
Suspensions of isolated secretory cells were incubated with [2,3-13C2]pyruvate in the presence of the cofactor mix, as before, and the cells were then harvested by centrifugation without disruption. The supernatant was discarded and the pelleted secretory cells were disrupted by sonication to release intracellular metabolites. (Note that because of the permeable nature of these cells, the incubation buffer and cytosol are in equilibrium; plastidial metabolites, however, are likely sequestered at this locale.) Contaminating proteins in the cell extract were denatured by addition of CHCl3, and soluble metabolites were adsorbed to an anion-exchange resin that was subsequently eluted using a volatile buffer. After concentration under vacuum, metabolites were analyzed by an LC-MS method optimized for the separation and detection of prenyl diphosphates {IPP, m/z 245 [(M − H)−], retention time (Rt) 29.4 min; DMAPP, m/z 245 [(M − H)−], Rt 28.4 min; geranyl diphosphate (GPP), m/z 313 [(M − H)−], Rt 33.2 min}. Both IPP and DMAPP were detectable in extracts obtained from (untreated control) oil gland secretory cells (ratio ≈ 1:1; Fig. 4). No de novo incorporation of [2,3-13C2]pyruvate into IPP and DMAPP was detected, as indicated by the absence of M+2 signals. However, a considerable de novo incorporation of label into GPP was observed (M+2 signal corresponded to 34% of the signal at Rt 33.2 min; the M+4 signal, corresponding to the incorporation of one molecule of 13C2-IPP and one molecule of 13C2-DMAPP, was too low to allow accurate quantification; Table I). The incorporation of [2,3-13C2]pyruvate into GPP without a concomitant detectable incorporation into IPP or DMAPP was unexpected. It is likely that the detected pools of DMAPP and IPP pools are compartmentalized in the cytoplasm and separated from the plastidial biosynthetic pathway that is responsible for the de novo incorporation of [2,3-13C2]pyruvate into GPP (Soler et al., 1992; Bouvier et al., 2000).
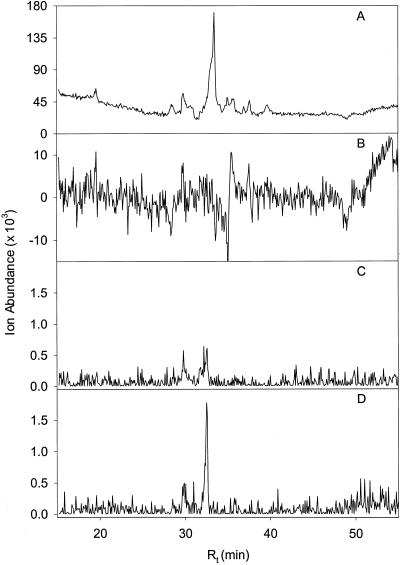
Figure 4.
LC-MS analysis of endogenous pools of prenyl diphosphates in isolated peppermint oil gland secretory cells. A, Total ion chromatogram (TIC; m/z 50–350); B, detection of endogenous GPP in the m/z 313 [(M − H)−] extracted ion chromatogram (EIC); C, detection of endogenous DMAPP and IPP in the m/z 245 [(M − H)−] EIC; D, EIC of a mixture of authentic DMAPP and IPP standards at m/z 245 [(M − H)−].
Table I.
Herbicide-mediated accumulation of prenyl diphosphates in isolated peppermint oil gland secretory cells
| Treatment | IPP
|
DMAPP
|
GPP
|
|||
|---|---|---|---|---|---|---|
| % of Control | 12C:13C | % of Control | 12C:13C | % of Control | 12C:13C | |
| Control | 100 | 100:0 | 100 | 100:0 | 100 | 66:34 |
| Clomazone | 0 | n.a.a | 0 | n.a. | 0 | n.a. |
| Fosmidomycin | 392 | 93:7 | 275 | 84:16 | 128 | 100:0 |
| Methyl viologen | 0 | n.a. | 0 | n.a. | 0 | n.a. |
| Benzyl viologen | 0 | n.a. | 0 | n.a. | 0 | n.a. |
| DMAEPP | 225 | 70:30 | 118 | 68:32 | 61 | 70:30 |
| Alendronate | 110 | 42:58 | 109 | 47:53 | 0 | n.a. |
| Pamidronate | 116 | 42:58 | 116 | 57:43 | 0 | n.a. |
Each treatment was at 2 mm of the indicated herbicide in the presence of 2 mm [2,3-13C2]pyruvate.
n.a., Not applicable.
According to recent results (Arigoni et al., 1999; McCaskill and Croteau, 1999), the inhibition of plant IPP isomerase should lead to the accumulation of IPP as the end product of the mevalonate-independent pathway. However, in the present case, treatment of cells with DMAEPP, an inhibitor of IPP isomerase, resulted not only in the anticipated increased accumulation of IPP (225% of control) but also in detectably increased accumulation of DMAPP (118% of control), suggesting that, as recently proposed for Escherichia coli (Hahn et al., 1999; Rodriguez-Concepcion et al., 2000), the mevalonate-independent pathway may diverge at some point to yield IPP and DMAPP independently. Compared with untreated controls, the DMAEPP-treated samples also showed substantially decreased GPP levels (reduced to 61% of control), indicating that DMAEPP, in addition to influencing IPP isomerase activity, may act as an inhibitor of GPP synthase. Preliminary experiments with purified GPP synthase (isolated from peppermint oil gland secretory cells) confirmed the inhibitory effect of DMAEPP on this enzyme (C.C. Burke, B.M. Lange, and R. Croteau, unpublished data). In the presence of DMAEPP, a considerable proportion of the observed IPP, DMAPP, and GPP pools was produced by de novo incorporation of label from [2,3-13C2]pyruvate (approximately 30% enrichment), indicating that DMAEPP did not completely inhibit the activities of IPP isomerase and GPP synthase. Because the ratios of IPP to DMAPP, IPP to GPP, and DMAPP to GPP did not change in the presence of this IPP isomerase inhibitor, we now suggest that IPP isomerase of peppermint, similar to IPP isomerase of E. coli (Hahn et al., 1999), is not an essential component of isoprenoid biosynthesis via the mevalonate-independent pathway. However, this enzyme could play a regulatory role in adjusting IPP:DMAPP ratios under some physiological conditions.
Treatment with fosmidomycin led to a significant increase in the intracellular levels of IPP, DMAPP, and GPP but with only marginally increased contribution from de novo synthesis as observed by isotope incorporation. Thus, isoprenoid biosynthesis was effectively inhibited by fosmidomycin at a step prior to the formation of IPP and DMAPP. The accumulation of IPP, DMAPP, and GPP may result from a regulatory feedback loop that allows existing pools of intermediates to proceed to these prenyl diphosphates, whereas labeled pyruvate is not incorporated. An alternative explanation is that, in addition to the inhibition of the reductoisomerase, these compounds accumulate as a result of inhibition of enzymes downstream of GPP synthase.
Treatment with alendronate and pamidronate led to the disappearance of GPP, whereas IPP and DMAPP signals increased in intensity (IPP and DMAPP ≈ 116% of control for alendronate treatment; IPP and DMAPP ≈ 110% of control for pamidronate treatment). A considerable proportion of each of these metabolites originated from de novo incorporation of [2,3-13C2]pyruvate (for IPP, the M+2 signal indicated 58% enrichment for both inhibitor treatments; for DMAPP, the M+2 signal indicated 53% enrichment with alendronate treatment and 43% enrichment with pamidronate treatment). These results provide the first evidence that both compounds exert a strong inhibitory activity on GPP synthase, which is consistent with the known inhibitory effect on other plant prenyltransferases (Oberhauser et al., 1998; Cromartie et al., 1999). This inhibition was confirmed by direct observation using GPP synthase from peppermint (C.C. Burke, B.M. Lange, and R. Croteau, unpublished data).
Parallel incubations of [2,3-13C2]pyruvate in the presence of 2 mm methyl viologen, benzyl viologen, or clomazone resulted in the disappearance of all of the prenyl diphosphates, indicating that the previously observed reduction in monoterpene production was caused by an inhibition of one or more enzymatic steps upstream of the formation of IPP and DMAPP.
Effects of Inhibitors on the Accumulation of Other Metabolites
To elucidate the enzyme targets of inhibitors of monoterpene biosynthesis, intracellular metabolites were separated and detected using an LC-MS method optimized for the analysis of a broad range of organic mono-, di-, and triphosphate esters. The total ion chromatograms (TICs) resulting from each treatment were normalized to the intensity of the signals for adenosine 5′-monophosphate, the level of which did not change appreciably over the course of the experiment (data not shown). The TIC from extracts of control incubations (no treatment) samples was then subtracted from the TIC of inhibitor-treated samples, thereby yielding a subtraction TIC showing only differences between samples with positive signals indicating a herbicide-mediated increase in the level of the corresponding metabolites and negative signals indicating the opposite effect.
The most pronounced feature of the subtraction TIC resulting from the fosmidomycin-treated cells was the appearance of a signal at m/z 213 [(M − H)−] (32.4 min) that was 4-fold higher in abundance than in control samples (Fig. 5). The signal for DXP {m/z 213 [(M − H)−]; 29.8 min} was unchanged compared with controls. The same new compound accumulated when purified recombinant DXP reductoisomerase from E. coli was co-incubated with DXP and fosmidomycin (according to Kuzuyama et al., 1998; data not shown). These results suggest the direct observation of 2-C-methyl-d-erythrose-4-phosphate (Fig. 2B) as an intermediate of the reaction catalyzed by DXP reductoisomerase in the presence of fosmidomycin (see Fig. 1), but the identity of this aldose phosphate has not yet been confirmed.
Figure 5.
LC-MS analysis of fosmidomycin-mediated perturbation of metabolite pools in isolated peppermint oil gland secretory cells. A, TIC of control cell extracts subtracted from TIC of extracts obtained from fosmidomicin-treated cells; B, EIC of control cells at m/z 213 [(M − H)−] for detection of DXP; C, EIC of fosmidomycin-treated cells {m/z 213 [(M − H)−] for DXP}. D, EIC of control cell extracts subtracted from EIC of fosmidomycin-treated cells to emphasize increase in DXP in fosmidomycin-treated cells.
Treatment of secretory cells with clomazone resulted in the appearance of new signals at m/z 441 [(M − H)−] (Rt 32.6 min) and m/z 259 [(M − H)−] (Rt 35.2 min) as indicated by the difference in TIC (clomazone treatment − untreated control). However, only the signal at 32.6 min afforded a ratio of molecular ions indicating incorporation of label from [2,3-13C2]pyruvate (the M+2 signal indicated 31% enrichment, and the M+4 signal indicated 20% enrichment; Fig. 6). These molecular ions do not correspond to any known intermediate of the mevalonate-independent pathway (or any metabolite of clomazone itself [Weimer et al., 1992]), and further structural analyses will be required for identification.
Figure 6.
LC-MS analysis of clomazone-mediated perturbation of metabolite pools in isolated peppermint oil gland secretory cells. The large panel illustrates the TIC of control cell extracts subtracted from the TIC of extracts obtained from clomazone-treated cells. The two insets show the mass spectra of the products eluting at 32.6 and 35.2 min. The small inset shows details of the unusual molecular ion distribution (m/z 441, 443, 445) of the compound eluting at 32.6 min.
As indicated above, treatment with DMAEPP, alendronate, or pamidronate influenced the metabolism of prenyl diphosphates but did not result in the accumulation of novel metabolites. Treatment with methyl viologen or benzyl viologen led to a multitude of changes involving increased as well as decreased levels of several unidentified metabolites; these results suggest that neither compound acts on a specific target enzyme of terpenoid metabolism. These inhibitors almost certainly disrupt the general redox chemistry of peppermint oil gland secretory cells and may influence the activity of a number of enzymes only indirectly related to isoprenoid biosynthesis. In contrast to experiments with bacteria (Ogrel et al., 1996; Ostrovsky et al., 1998), no detectable 2-C-methyl-d-erythritol-2,4-cyclodiphosphate {m/z 277 [(M − H)−]} was accumulated as a consequence of treating peppermint secretory cells with benzyl viologen (data not shown).
CONCLUSIONS
The LC-MS separation and identification methods used here allowed the detection of endogenous pools of intermediates of isoprenoid biosynthesis formed via the mevalonate-independent pathway (i.e. DXP, IPP, DMAPP, and GPP) in peppermint oil gland secretory cells. The results obtained with untreated (control) cells provide evidence that the enzymes that use these substrates (e.g. DXR, GPPS, and limonene synthase) may catalyze slow steps of monoterpene biosynthesis in peppermint. Other established intermediates of the mevalonate-independent pathway in plants, including 2-C-methyl-d-erythritol 4-phosphate (Fellermeier et al., 1999; Lange and Croteau, 1999), 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol (Kuzuyama et al., 2000a; Rohdich et al., 2000a), 2-phospho-4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol (Kuzuyama et al., 2000b; Rohdich et al., 2000b), and 2-C-methyl-d-erythritol 2,4-cyclodiphosphate (a confirmed intermediate of the mevalonate-independent pathway in E. coli; Herz et al., 2000; Takagi et al., 2000), were not accumulated at detectable levels under the experimental conditions used. We were able to confirm the enzyme target of fosmidomycin as DXR, and we found, for the first time, to our knowledge, GPP synthase to be a target for DMAEPP (an IPP isomerase inhibitor), alendronate, and pamidronate (both are prenyltransferase inhibitors). Clomazone treatment of peppermint secretory cells resulted in the accumulation of an unknown metabolite, the structure of which may provide insight into the as-yet-undefined steps of the mevalonate-independent pathway.
MATERIALS AND METHODS
Plant Material and Chemicals
Peppermint (Mentha × piperita L. cv Black Mitcham) plants were propagated and grown as previously described (Gershenzon et al., 1992). [2-14C]Pyruvate (0.59 GBq mmol−1) was obtained from DuPont/NEN (Wilmington, DE); [2,3-13C2]pyruvate was purchased from Cambridge Isotope Laboratories (Andover, MA); phosphonothrixin and fosmidomycin were gifts from Dr. Phil Proteau (Oregon State University, Corvallis); clomazone was generously provided by the FMC Corporation (Philadelphia; courtesy of Dr. D.A. Baver); alendronate and pamidronate were gifts from Dr. Eric Oldfield (University of Illinois, Urbana-Champaign); DMAEPP was prepared synthetically (Mühlbacher and Poulter, 1988); and methyl viologen and benzyl viologen were purchased from Sigma (St. Louis). Except where noted, all other chemicals were obtained from Sigma. The inhibitors were stored as stock solutions (phosphonothrixin, 62 mm in water; fosmidomycin, 100 mm in 1 mm Tris/HCl, pH 6; clomazone, 100 mm in ethanol; alendronate and pamidronate, both 100 mm in water; methyl viologen and benzyl viologen, both 100 mm in methanol).
Isolation of Oil Gland Secretory Cells
Apical leaves (15–20 g, <10 cm in length) were harvested from vegetative stems of peppermint. Following isolation according to Gershenzon et al. (1992), the secretory cells were washed with 25 mm Tris/HCl buffer (pH 7.3) containing 200 mm sorbitol, 10 mm Suc, 5 mm MgCl2, 10 mm KCl, 1 mm ethyleneglycol bis(β-aminoethyl ether), 8.5 mm Na2HPO4, and 0.1 mm Na4P2O7 and were then suspended in the same buffer supplemented with 2 mm ATP, 0.1 mm NADPH, 0.1 mm NAD+, 5 mm phosphoenolpyruvate, and 5 mm Glc-6-P. Cell density was determined using a hemacytometer and was adjusted to 1 to 2 × 106 cellular discs (each containing eight secretory cells) per milliliter suspension.
Incubation with [2-14C]Pyruvate and Quantification of Incorporation into Monoterpenes and Sesquiterpenes
The appropriate amounts of inhibitors from stock solutions (2 mm final concentration in secretory cell feeding assay) were transferred to 15-mL screw-cap glass vials, and the solvents were allowed to evaporate for 15 min at room temperature with occasional swirling of the tube. The isolated oil gland secretory cell suspension (1 mL, corresponding to 1.2–1.4 × 106 secretory cell clusters) and [2-14C]pyruvate (1.1 × 107 dpm, final concentration 0.3 mm) were added, the suspension was overlaid with 3 mL of n-pentane, and the suspended cells were aerated and incubated at 23°C for 1.5 h. At the end of the incubation period, the n-pentane layer was recovered, and the suspension was extracted three times with 1 mL of diethyl ether. The combined organic extracts were adjusted to a volume of 5 mL with diethyl ether, were washed with 1 mL of 1 m Na2CO3, and were dried over Na2SO4. A 5-μL aliquot of the mixture was removed for liquid scintillation counting to quantify the accumulated monoterpenes and sesquiterpenes. The identities of these organic-soluble isoprenoid end products (comprised largely of p-menthane monoterpenoids with lesser amounts of several sesquiterpene olefins [Gershenzon et al., 1992]) were verified in selected samples using radio-gas chromatography as previously described (McCaskill and Croteau, 1995).
Incubations with [2,3-13C2]Pyruvate, Extraction of Polar Metabolites, and Sample Preparation for LC-MS
Incubations were carried out for 1 h as described above, but without the n-pentane overlay and with [2,3-13C2]pyruvate (2 mm) as a substrate instead of [2-14C]pyruvate. At the end of the incubation period, the isolated secretory cells were gently pelleted by centrifugation (300g, 5 min), the supernatant was discarded, and the loose pellet was transferred to a 5-mL glass vial. The suspended cells were disrupted (verified by light microscopy) by sonication for 1 min at 95 W in a Virsonic 475 Ultrasonic Cell Disrupter (Virtis, Gardiner, NY) equipped with a 3.2-mm microprobe tip. The resulting homogenate was extracted with 2 × 1 mL CHCl3, and the aqueous layer was transferred to a 1.5-mL Eppendorf vial and centrifuged (10,000g, 10 min) to pellet cellular debris. The supernatant was then adsorbed to an anion-exchange cartridge (IC-OH, Alltech, Deerfield, IL; the cartridge was conditioned with water according to the manufacturer's instructions), the resin was washed with water to remove uncharged metabolites, and polar, negatively charged metabolites were eluted with 2 m ammonium acetate, pH 6. The eluted samples were then concentrated to dryness under vacuum and were dissolved in 100 μL of 0.1 mm ammonium acetate, pH 6, and stored at −20°C until further analysis.
Analysis of Polar Metabolites by LC-MS
The LC-MS instrumentation consisted of a Hewlett-Packard (Agilent) Series 1100 HPLC (Agilent Technologies, Palo Alto, CA) with a model 1946A mass detector. Polar metabolites were separated on a 200- × 4-mm Nucleodex β-OH Cyclodextrin column (Macherey-Nagel, Düren, Germany). Two different methods were used for gradient elution, depending on the polarity of the analytes of interest. The aqueous phase was 10 mm ammonium acetate, pH 6.5; flow rate was 1 mL min−1 (Table II).
Table II.
HPLC methods for gradient elution of polar metabolites
| Method
A
|
Method B
|
||
|---|---|---|---|
| Time | % Acetonitrile | Time | % Acetonitrile |
| 0 | 90 | 0 | 90 |
| 15 | 90 | 15 | 90 |
| 35 | 65 | 30 | 50 |
| 45 | 65 | 35 | 50 |
| 45.1 | 90 | 35.1 | 90 |
| 55 | 90 | 45 | 90 |
Valve switching on the mass detector allowed the solvent stream to be diverted to waste for the first 15 min of the chromatographic run. This procedure allowed adsorption of the polar metabolites of interest while washing through the high concentration of salts and other compounds present in the secretory cell extracts. Detection of polar metabolites was achieved by atmospheric pressure ionization-electrospray mass detection in the negative ion mode. For prenyl diphosphates (method A), the drying/carrier gas was nitrogen heated to 350°C with a flow of 12 L min−1 at a pressure of 20 psi. The capillary was set to 4,000 V with a fragmentor voltage of 75 V and a gain setting of 1. Ions in the range m/z 50 to 350 were scanned from 15 min after injection until the end of the run. For profiling other polar metabolites (method B), the drying/carrier gas was nitrogen-heated to 350°C, with flow of 10 L min−1 at a pressure of 30 psi. The capillary was set to 5,000 V with a fragmentor voltage of 100 V and a gain setting of 1. Ions in the range m/z 50 to 800 were scanned starting 15 min after injection until the end of the run. Chromatographic separations were simultaneously monitored at 212 to 228 nm using diode array detection. Sample injection volume varied from 10 to 40 μL.
ACKNOWLEDGMENTS
We thank Dr. Phil Proteau (Oregon State University) for the gifts of fosmidomycin and phosphonothrixin, Dr. Eric Oldfield (University of Illinois) for the gifts of alendronate and pamidronate, Dr. D.A. Baver (FMC Corporation) for the gift of clomazone, and Sue Vogtman (Washington State University) for growing the plants.
Footnotes
This work was supported by a grant from the U.S. Department of Energy.
LITERATURE CITED
- Arigoni D, Eisenreich W, Latzel C, Sagner S, Radykewicz T, Zenk MH, Bacher A. Dimethylallyl pyrophosphate is not the committed precursor of isopentenyl pyrophosphate during terpenoid biosynthesis from 1-deoxyxylulose in higher plants. Proc Natl Acad Sci USA. 1999;96:1309–1314. doi: 10.1073/pnas.96.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beetham RB, Kipp PB, Sawycky XL, Arntzen CJ, May GD. A tool for functional plant genomics: chimeric RNA/DNA oligonucleotides cause in vivo gene-specific mutations. Proc Natl Acad Sci USA. 1999;96:8774–8778. doi: 10.1073/pnas.96.15.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier R, Suire C, d'Harlingue A, Backhaus RA, Camara B. Molecular cloning of geranyl diphosphate synthase and compartmentation of monoterpene synthesis in plant cells. Plant J. 2000;24:241–252. doi: 10.1046/j.1365-313x.2000.00875.x. [DOI] [PubMed] [Google Scholar]
- Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromartie TH, Fisher KJ, Grossman JN. The discovery of a novel site of action for herbicidal bisphosphonates. Pestic Biochem Physiol. 1999;63:114–126. [Google Scholar]
- Croteau R. Clomazone does not inhibit the conversion of isopentenyl pyrophosphate to geranyl, farnesyl, or geranylgeranyl pyrophosphate in vitro. Plant Physiol. 1992;98:1515–1517. doi: 10.1104/pp.98.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos KM, Gale MD. Genome relationships: the grass model in current research. Plant Cell. 2000;12:637–646. doi: 10.1105/tpc.12.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke SO, Kenyon WH. Effects of dimethazone (FMC 57020) on chloroplast development: II. Pigment synthesis and photosynthetic function in cow pea (Vigna unguiculata L.) primary leaves. Pestic Biochem Physiol. 1988;25:11–18. [Google Scholar]
- Feldmann KA. T-DNA insertion mutagenesis in Arabidopsis: mutational spectrum. Plant J. 1991;1:71–82. [Google Scholar]
- Fellermeier M, Kis K, Sagner S, Maier U, Bacher A, Zenk MH. Cell-free conversion of 1-deoxy-d-xylulose 5-phosphate and 2-C-methyl-d-erythritol 4-phosphate into β-carotene in higher plants and its inhibition by fosmidomycin. Tetrahedron Lett. 1999;40:2743–2746. [Google Scholar]
- Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- Gershenzon J, McCaskill D, Rajaonarivony JIM, Mihaliak C, Karp F, Croteau R. Isolation of secretory cells from plant glandular trichomes and their use in biosynthetic studies of monoterpenes and other gland products. Anal Biochem. 1992;200:130–138. doi: 10.1016/0003-2697(92)90288-i. [DOI] [PubMed] [Google Scholar]
- Hahn FM, Hurlburt AP, Poulter CD. Escherichia coli open reading frame 696 is idi, a nonessential gene encoding isopentenyl diphosphate isomerase. J Bacterol. 1999;181:4499–4504. doi: 10.1128/jb.181.15.4499-4504.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan HM, Fridovich I. Paraquat and Escherichia coli: mechanism of production of extracellular superoxide radical. J Biol Chem. 1979;254:10846–10852. [PubMed] [Google Scholar]
- Herz S, Wungsintaweekul J, Schuhr CA, Hecht S, Lüttgen H, Sagner S, Fellermeier M, Eisenreich W, Zenk MH, Bacher A. Biosynthesis of terpenoids: ygbB protein converts 2-phospho-4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol to 2-C-methyl-d-erythritol 2,4-cyclodiphosphate. Proc Natl Acad Sci USA. 2000;97:2486–2490. doi: 10.1073/pnas.040554697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomaa H, Wiesner J, Sanderbrand S, Altinciek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler HK. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- Kamuro Y, Kawai T, Kakiuchi T, inventors. March 26, 1991. Herbicidal methods and compositions comprising fosmidomycin. Fujisawa Pharmaceutical Co. Ltd., U.S. Patent Application No. 5,002,602
- Kimura T, Nakamura K, Takahshi E. Phosphonothrixin, a novel herbicidal antibiotic produced by Saccharothrix sp. ST-888: II. Structure determination. J Antibiot. 1995;48:1130–1333. doi: 10.7164/antibiotics.48.1130. [DOI] [PubMed] [Google Scholar]
- Kuzuyama T, Shimizu T, Takahashi S, Seto H. Fosmidomycin, a specific inhibitor of 1-deoxy-d-xylulose 5-phosphate reductoisomerase in the nonmevalonate pathway for terpenoid biosynthesis. Tetrahedron Lett. 1998;39:7913–7916. [Google Scholar]
- Kuzuyama T, Takagi M, Kaneda K, Watanabe H, Dairi T, Seto H. Formation of 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol from 2-C-methyl-d-erythritol 4-phosphate by 2-C-methyl-d-erythritol 4-phosphate cytidyltransferase, a new enzyme in the nonmevalonate pathway. Tetrahedron Lett. 2000a;41:703–706. [Google Scholar]
- Kuzuyama T, Takagi M, Kaneda K, Watanabe H, Dairi T, Seto H. Studies on the nonmevalonate pathway: conversion of 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol to its 2-phospho derivative by 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol kinase. Tetrahedron Lett. 2000b;41:2925–2928. [Google Scholar]
- Lange BM, Croteau R. Isoprenoid biosynthesis via a mevalonate-independent pathway in plants: cloning and heterologous expression of 1-deoxy-d-xylulose 5-phosphatereductoisomerase from peppermint. Arch Biochem Biophys. 1999;365:170–174. doi: 10.1006/abbi.1999.1168. [DOI] [PubMed] [Google Scholar]
- Lange BM, Rujan T, Martin W, Croteau R. Isoprenoid biosynthesis: the evolution of two ancient and distinct pathways across genomes. Proc Natl Acad Sci USA. 2000b;97:13172–13177. doi: 10.1073/pnas.240454797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange BM, Wildung MR, Stauber EJ, Sanchez C, Pouchnik D, Croteau R. Probing essential oil biosynthesis by functional evaluation of expressed sequence tags from mint glandular trichomes. Proc Natl Acad Sci USA. 2000a;97:2934–2939. doi: 10.1073/pnas.97.6.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzow M, Beyer P, Kleinig H. The herbicide clomazone does not inhibit the prenyl diphosphate-forming enzymes in plastids. Z Naturforsch. 1990;45c:856–858. [Google Scholar]
- McCaskill D, Croteau R. Monoterpene and sesquiterpene biosynthesis in glandular trichomes of peppermint (Mentha × piperita) rely exclusively on plastid-derived isopentenyl diphosphate. Planta. 1995;197:49–56. [Google Scholar]
- McCaskill D, Croteau R. Isopentenyl diphosphate is the terminal product of the deoxyxylulose 5-phosphate pathway for terpenoid biosynthesis in plants. Tetrahedron Lett. 1999;40:653–656. [Google Scholar]
- McCaskill D, Gershenzon J, Croteau R. Morphology and monoterpene biosynthetic capabilities of secretory cell clusters isolated from glandular trichomes of peppermint (Mentha × piperita L.) Planta. 1992;187:445–454. doi: 10.1007/BF00199962. [DOI] [PubMed] [Google Scholar]
- McGarvey DJ, Croteau R. Terpenoid metabolism. Plant Cell. 1995;7:1015–1026. doi: 10.1105/tpc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlbacher M, Poulter CD. Isopentenyl diphosphate isomerase: inactivation of the enzyme with active-site-directed irreversible inhibitors and transition-state analogues. Biochemistry. 1988;20:7315–7328. doi: 10.1021/bi00419a021. [DOI] [PubMed] [Google Scholar]
- Oberhauser V, Gaudin J, Fonné-Pfister, Schär P. New target enzyme(s) for bisphosphonates: inhibition of geranylgeranyl diphosphate synthase. Pestic Biochem Physiol. 1998;60:111–117. [Google Scholar]
- Ogrel OD, Fegeding KV, Kapreliants AS, Lysak EI, Ngo MS, Sudarikov AB, Kharatian EF, Ostrovsky DN. 2-C-Methyl-d-erythritol-2,4-cyclopyrophosphate participates in bacterial oxidative stress reactions and their persistence in macrophages. Biochemistry (Moscow) 1996;61:921–927. [Google Scholar]
- Ogura K. Isomerase and prenyl transferases. In: Cane DE, editor. Comprehensive Natural Products Chemistry. 2: Isoprenoids Including Carotenoids and Steroids. Oxford: Pergamon Press; 1999. pp. 69–96. [Google Scholar]
- Okuhara M, Kuroda Y, Goto T, Okamoto M, Terano H, Kohsaka M, Aoki H, Imanaka H. Studies on new phosphonic acid antibiotics: III. Isolation and characterization of FR-31564, FR-32863 and FR-33289. J Antibiot. 1980;33:24–28. doi: 10.7164/antibiotics.33.24. [DOI] [PubMed] [Google Scholar]
- Ostrovsky D, Diomin G, Lysak E, Mateeva E, Ogrel O, Trutko S. Effect of oxidative stress on the biosynthesis of 2-C-methyl-d-erythritol-2,4-cyclopyrophosphate and isoprenoids by several bacterial strains. Arch Microbiol. 1998;171:69–72. doi: 10.1007/s002030050680. [DOI] [PubMed] [Google Scholar]
- Patterson DR, inventor. September 15, 1987. Herbicidal hydroxyamino phosphonic acids and derivatives. Rohm and Haas Corp., U.S. Patent Application No. 4,693,742
- Rodriguez-Concepcion M, Campos N, Lois ML, Maldonado C, Hoeffler JF, Grosdemange-Billiard C, Rohmer M, Boronat A. Genetic evidence for branching in the isoprenoid pathway for the production of isopentenyl diphosphate and dimethylallyl diphosphate in Escherichia coli. FEBS Lett. 2000;473:328–332. doi: 10.1016/s0014-5793(00)01552-0. [DOI] [PubMed] [Google Scholar]
- Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L. Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J. 2000;23:131–142. doi: 10.1046/j.1365-313x.2000.00774.x. [DOI] [PubMed] [Google Scholar]
- Rohdich F, Wungsintaweekul J, Eisenreich W, Richter G, Schuhr CA, Zenk MH, Bacher A. Biosynthesis of terpenoids: 4-diphosphocytidyl-2-C-methyl-d-erythritol synthase of Arabidopsis thaliana. Proc Natl Acad Sci USA. 2000a;97:6451–6456. doi: 10.1073/pnas.97.12.6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohdich F, Wungsintaweekul J, Lüttgen H, Fischer M, Eisenreich W, Schuhr CA, Fellermeier M, Schramek N, Zenk MH, Bacher A. Biosynthesis of terpenoids: 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase from tomato. Proc Natl Acad Sci USA. 2000b;97:8251–8256. doi: 10.1073/pnas.140209197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann G, Böger P. Interference of dimethazone with formation of terpenoid compounds. Z Naturforsch. 1986;41c:729–732. [Google Scholar]
- Shigi Y. Inhibition of bacterial isoprenoid synthesis by fosmidomycin, a phosphonic acid-containing antibiotic. J Antimicrob Chemother. 1989;24:131–145. doi: 10.1093/jac/24.2.131. [DOI] [PubMed] [Google Scholar]
- Soler E, Feron G, Clastre M, Dargent R, Gleizes M, Ambid C. Evidence for a geranyl-diphosphate synthase located within the plastids of Vitis vinifera L. cultivated in vitro. Planta. 1992;187:171–175. doi: 10.1007/BF00201934. [DOI] [PubMed] [Google Scholar]
- Takagi M, Kuzuyama T, Kaneda K, Watanabe H, Dairi T, Seto H. Tetrahedron Lett 41: 3395–3398. 2000. Studies on the nonmevalonate pathway: formation of 2-C-methyl-d-erythritol 2,4-cyclodiphosphate from 2-phospho-4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol. [Google Scholar]
- Takahashi E, Kimura T, Nakamura K, Iida M. Phosphonothrixin, a novel herbicidal antibiotic produced by Saccharothrix sp. ST-888: I. Taxonomy, fermentation, isolation and biological properties. J Antibiot. 1995;48:1124–1129. doi: 10.7164/antibiotics.48.1124. [DOI] [PubMed] [Google Scholar]
- Thiellement H, Bahrmann N, Damerval C, Plomion C, Rossignol M, Santoni V, Vienne D, Zivy M. Proteomics for genetic engineering studies in plants. Electrophoresis. 1999;20:2013–2026. doi: 10.1002/(SICI)1522-2683(19990701)20:10<2013::AID-ELPS2013>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Tissier AF, Marillonnet S, Klimyuk V, Patel K, Torres MA, Murphy G, Jones JDG. Multiple independent defective suppressor-mutator transposon insertions in Arabidopsis: a tool for functional genomics. Plant Cell. 1999;11:1841–1852. doi: 10.1105/tpc.11.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Santos H, Fareleira P, Pacheco I, LeGall J, Xavier AV. Structure determination of a novel cyclic phosphocompound isolated from Desulfovirio desulfuricans. Biochem J. 1992;15:387–390. doi: 10.1042/bj2850387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beek E, Pietermann E, Cohen L, Löwik C, Papapoulos S. Nitrogen-containing bisphosphonates inhibit isopentenyl pyrophosphate isomerase/farnesyl pyrophosphate synthase activity with relative potencies corresponding to their antiresorptive potencies in vitro and in vivo. Biochem Biophys Res Commun. 1999;255:491–494. doi: 10.1006/bbrc.1999.0224. [DOI] [PubMed] [Google Scholar]
- Van Hal NL, Vorst O, van Houwelingen AM, Kok EJ, Peijnenberg A, Aharoni A, van Tunen AJ, Keijper J. The application of DNA microarrays in gene expression analysis. J Biotechnol. 2000;78:271–280. doi: 10.1016/s0168-1656(00)00204-2. [DOI] [PubMed] [Google Scholar]
- Weigel D, Ahn JH, Blázquez MA, Borevitz JO, Christensen SK, Frankhauser C, Ferrándiz C, Kardailsky I, Malancharuvil EJ, Neff MM. Activation tagging in Arabidopsis. Plant Physiol. 2000;122:1003–1013. doi: 10.1104/pp.122.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer MR, Balke NE, Buhler DD. Herbicide clomazone does not inhibit in vitro geranylgeranyl synthesis from mevalonate. Plant Physiol. 1992;98:427–432. doi: 10.1104/pp.98.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler J, Schwender J, Muller C, Wiesner J, Weidemeyer C, Beck E, Jomaa H, Lichtenthaler HK. Inhibition of the non-mevalonate 1-deoxy-d-xylulose 5-phosphate pathway of plant isoprenoid biosynthesis by fosmidomycin. Z Naturforsch. 1998;53c:980–986. [Google Scholar]