Abstract
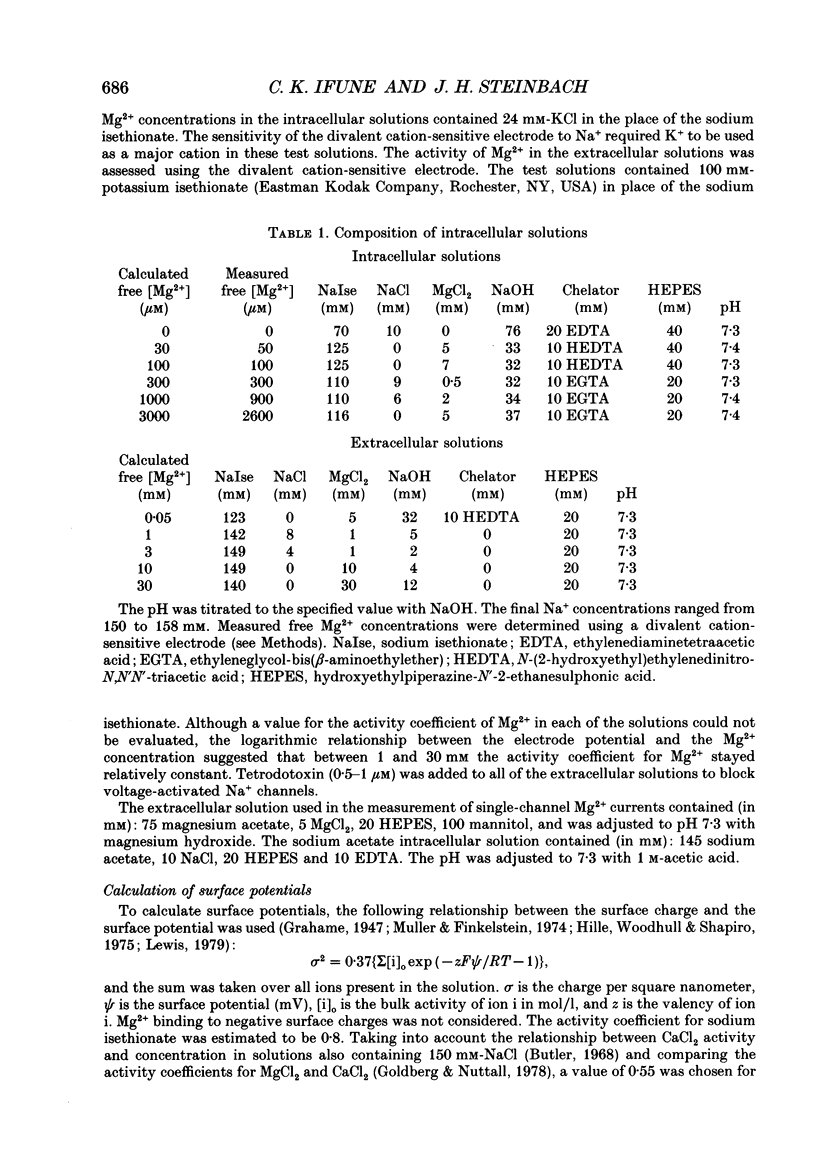
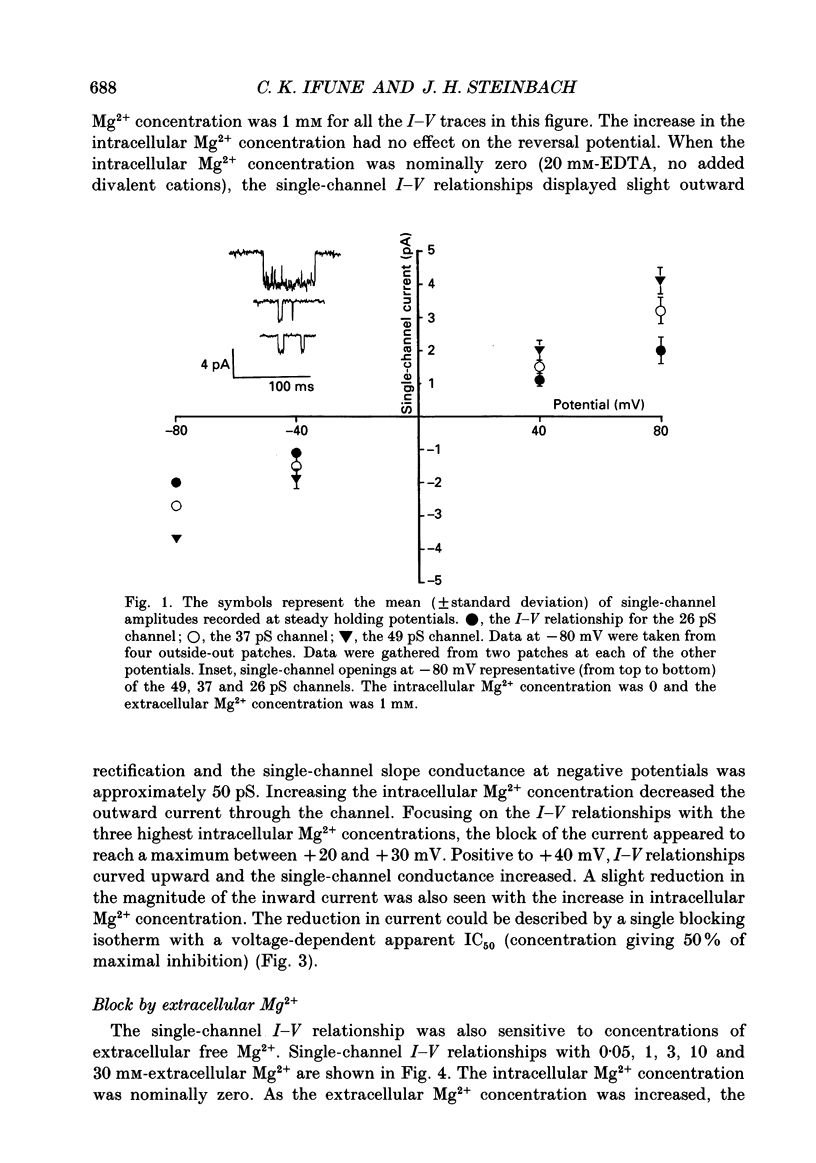
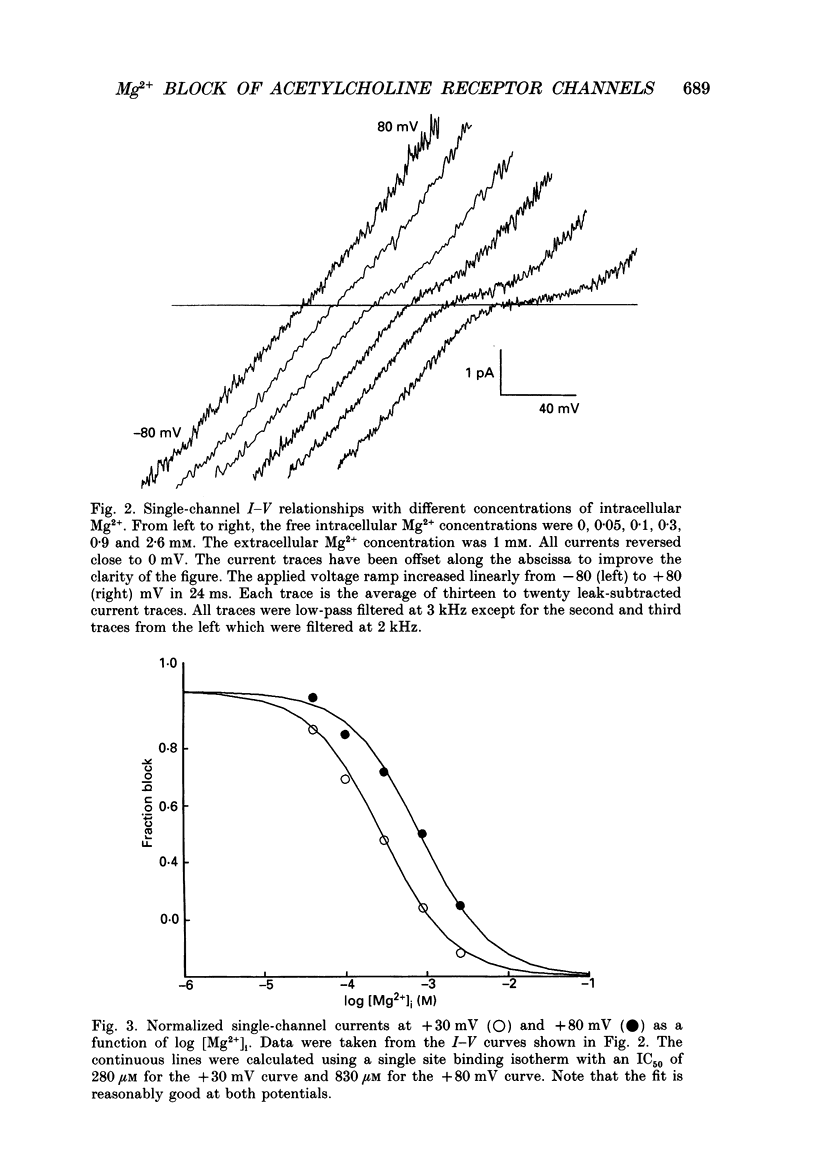
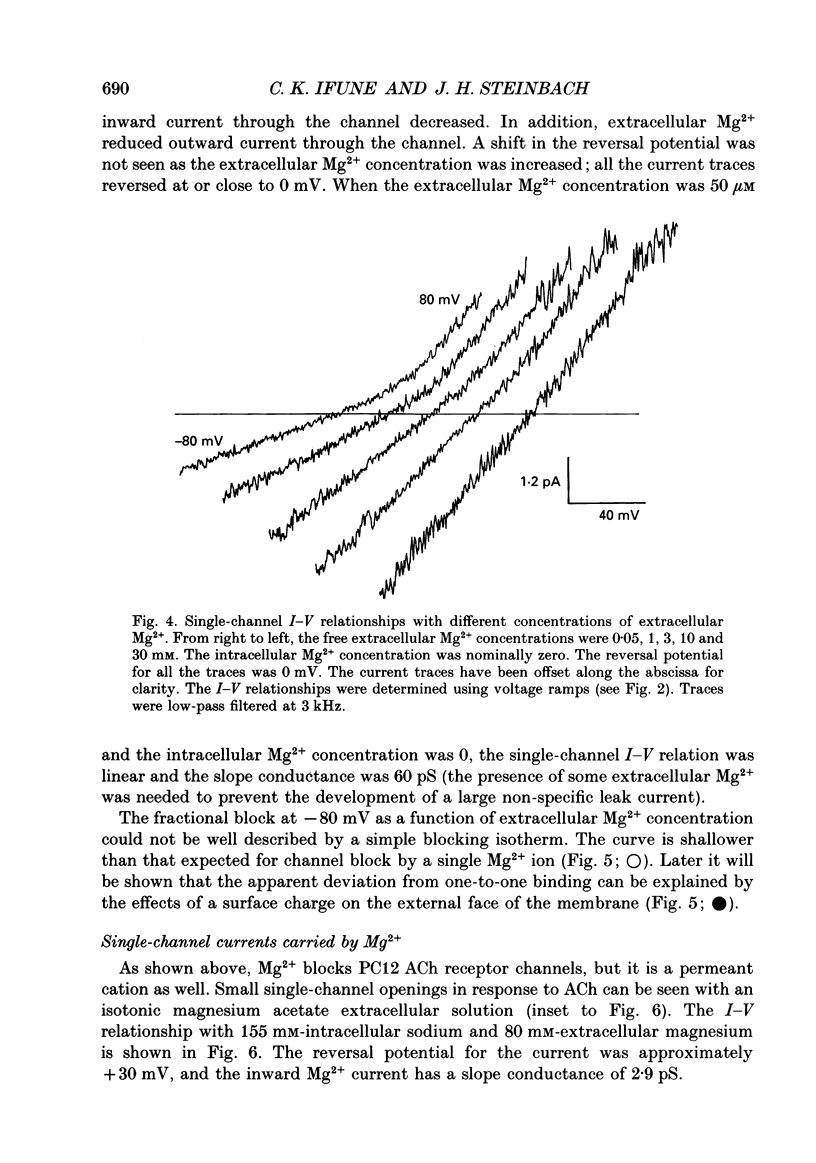
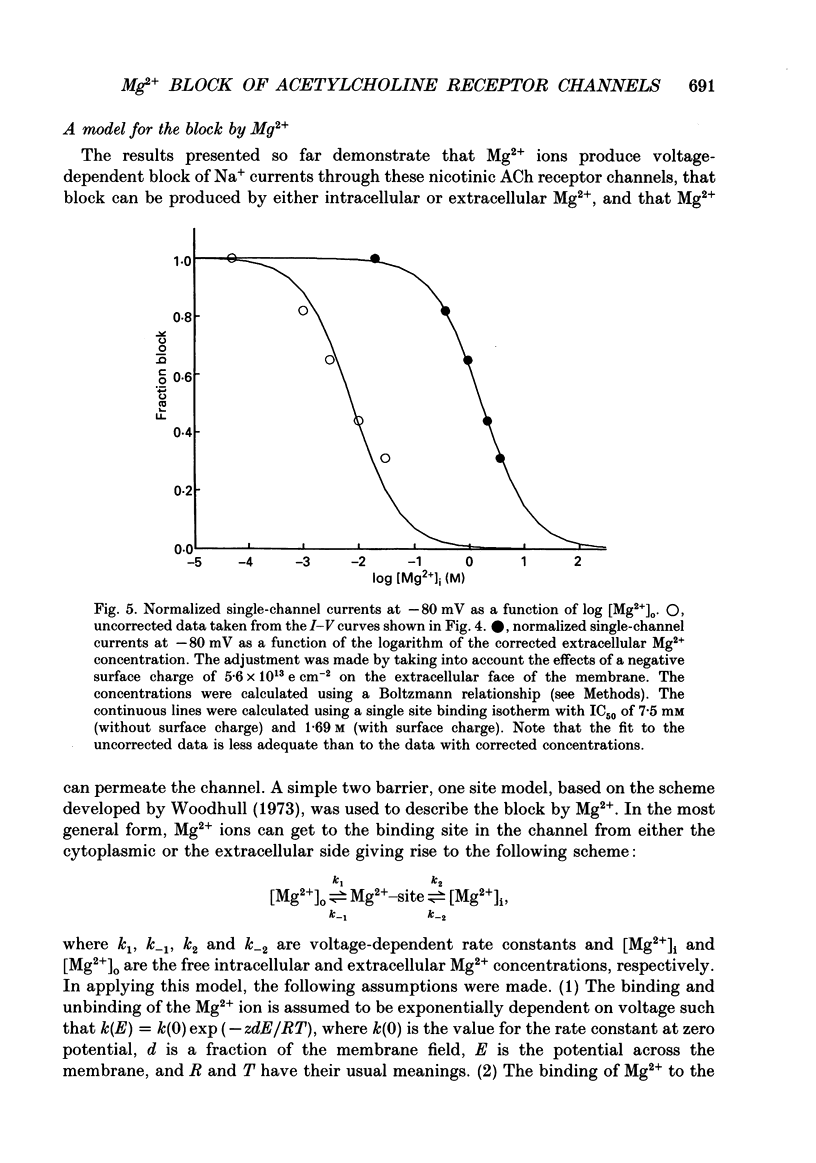
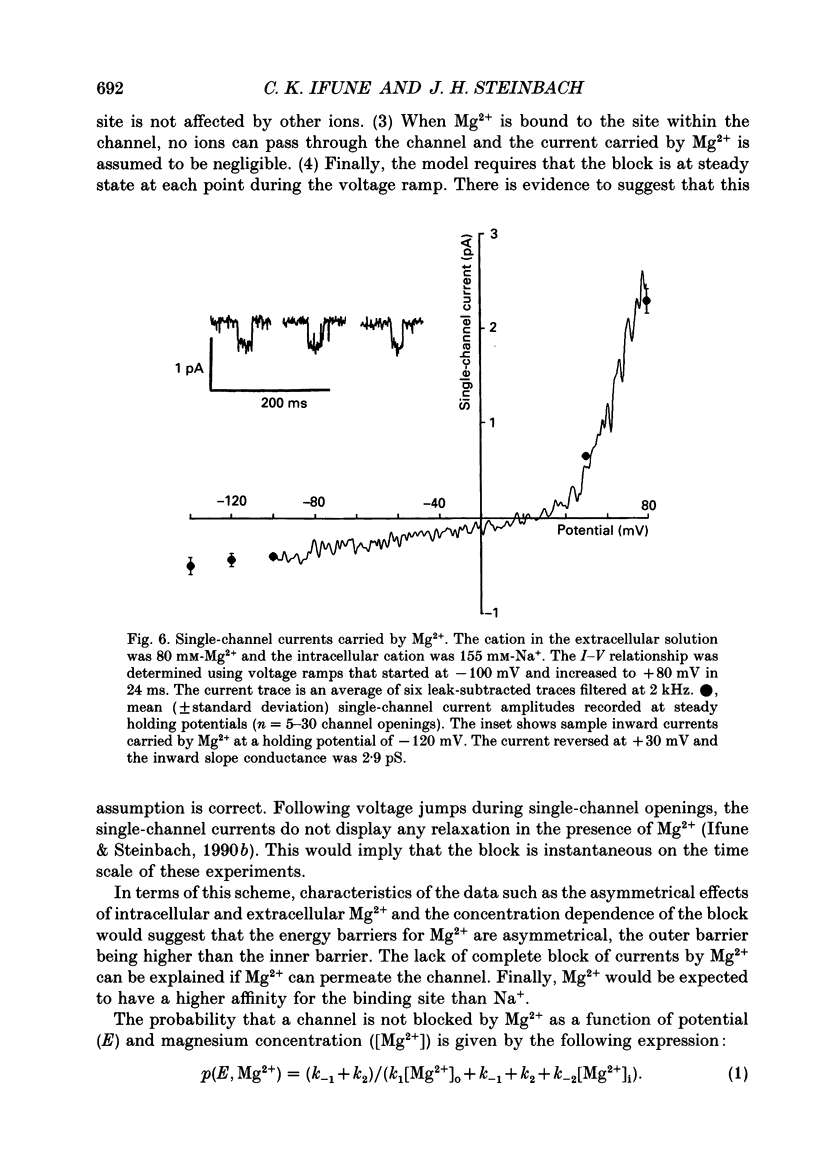
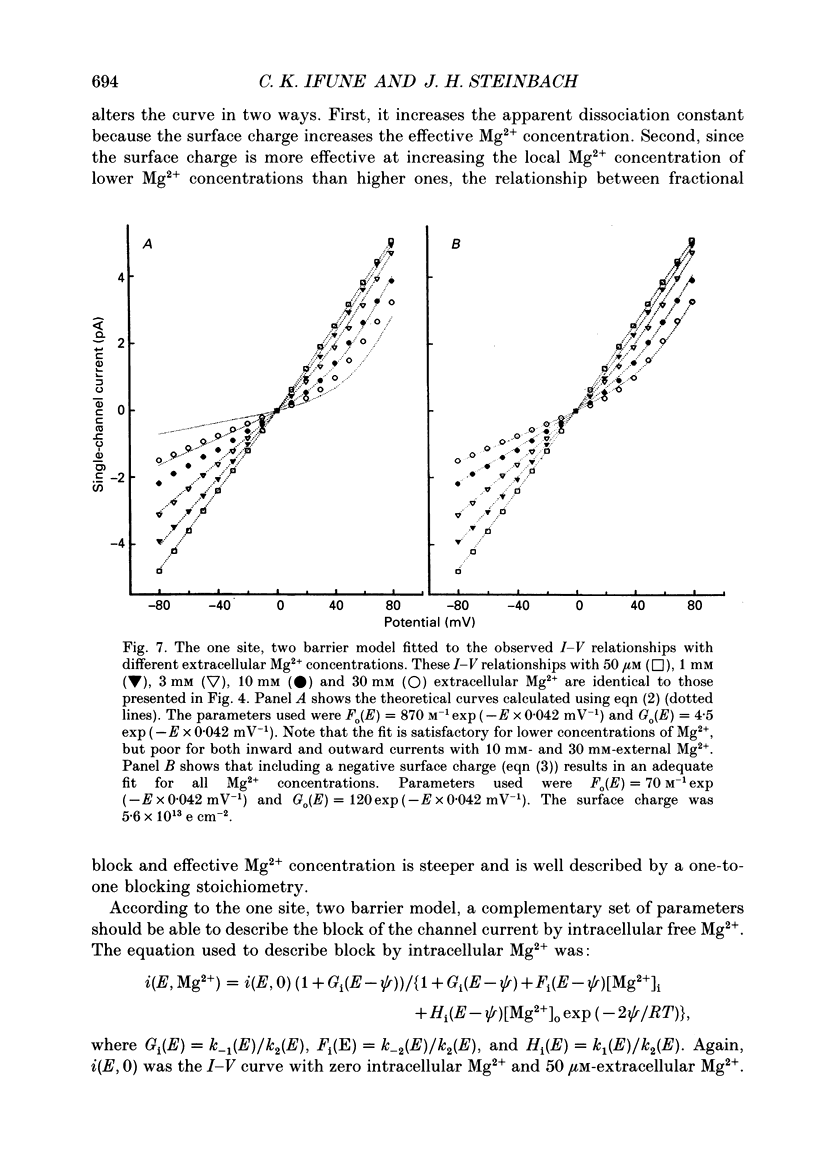
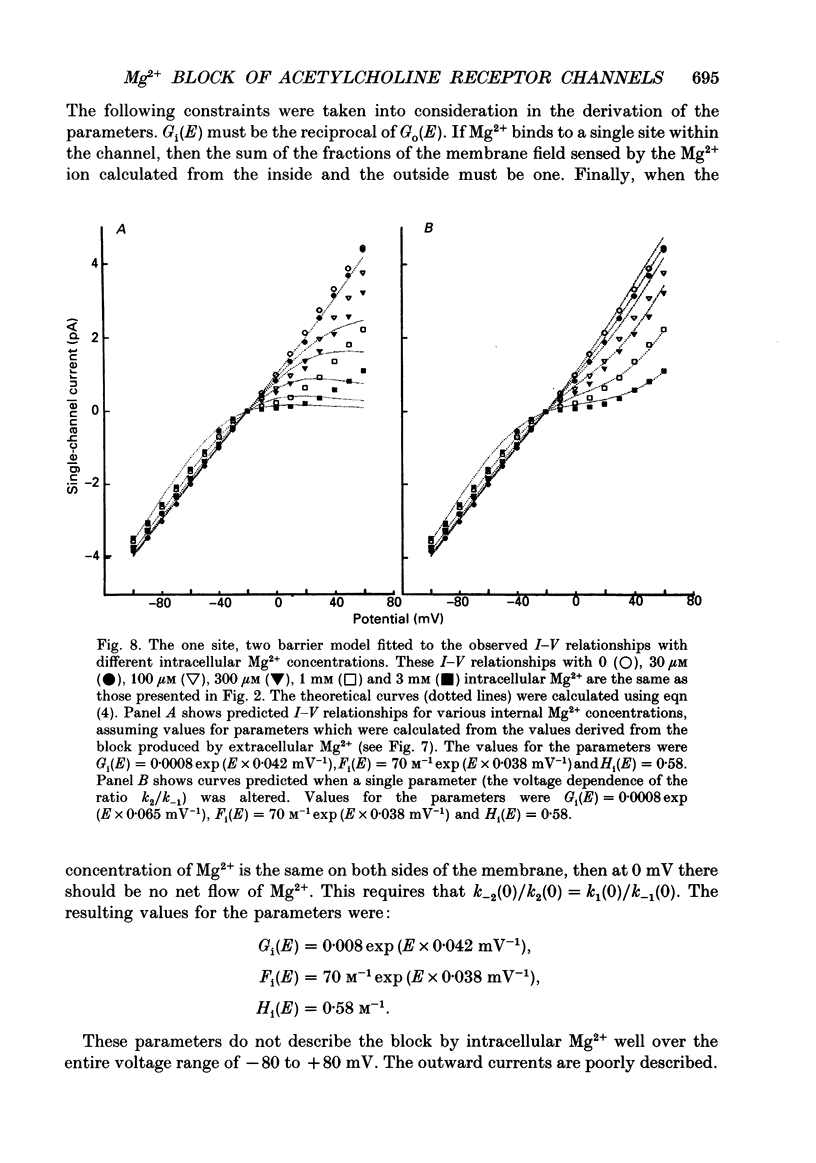
1. The effects of Mg2+ on the single-channel conductance of neuronal nicotinic acetylcholine receptors were examined using receptors expressed by the rat phaeochromocytoma cell line, PC12. PC12 cells express at least three conductance classes of channels that are activated by acetylcholine, the largest conductance class being the most prevalent. This receptor channel is blocked by intracellular and extracellular Mg2+. 2. The effects of Mg2+ are asymmetrical; at a given concentration, internal Mg2+ is more effective at blocking outward currents than external Mg2+ is at blocking inward currents. Receptor channels are blocked at concentrations of Mg2+ that are low compared to the concentration of the main permeant cation, Na+, and the block is voltage dependent. 3. The block by Mg2+ is not complete as Mg2+ can permeate the channel. With 80 mM-extracellular Mg2+ (no extracellular Na+), the channel has an inward slope conductance of 2.9 pS. 4. The block by extracellular Mg2+ can be described by a one site, two barrier model for the channel which includes a negative surface charge on the external surface of the membrane. The parameters of the model place the binding site for Mg2+ at 52% of the membrane field from the outside with an apparent dissociation constant of 14 mM. However, the same parameters cannot describe the block by intracellular Mg2+. The deviations from the model suggest that the receptor channel may have more than one binding site for Mg2+.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., McCleskey E. W., Palade P. T. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J Physiol. 1984 Aug;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballivet M., Nef P., Couturier S., Rungger D., Bader C. R., Bertrand D., Cooper E. Electrophysiology of a chick neuronal nicotinic acetylcholine receptor expressed in Xenopus oocytes after cDNA injection. Neuron. 1988 Nov;1(9):847–852. doi: 10.1016/0896-6273(88)90132-8. [DOI] [PubMed] [Google Scholar]
- Butler J. N. The thermodynamic activity of calcium ion in sodium chloride-calcium chloride electrolytes. Biophys J. 1968 Dec;8(12):1426–1433. doi: 10.1016/S0006-3495(68)86564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Van der Kloot W. Effects of [Ca2+] and [Mg2+] on the decay of miniature endplate currents. Nature. 1978 Jan 5;271(5640):77–79. doi: 10.1038/271077a0. [DOI] [PubMed] [Google Scholar]
- Dani J. A., Eisenman G. Monovalent and divalent cation permeation in acetylcholine receptor channels. Ion transport related to structure. J Gen Physiol. 1987 Jun;89(6):959–983. doi: 10.1085/jgp.89.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A. Site-directed mutagenesis and single-channel currents define the ionic channel of the nicotinic acetylcholine receptor. Trends Neurosci. 1989 Apr;12(4):125–128. doi: 10.1016/0166-2236(89)90049-0. [DOI] [PubMed] [Google Scholar]
- Decker E. R., Dani J. A. Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. J Neurosci. 1990 Oct;10(10):3413–3420. doi: 10.1523/JNEUROSCI.10-10-03413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V. A., Selyanko A. A., Skok V. I. Acetylcholine-induced current fluctuations and fast excitatory post-synaptic currents in rabbit sympathetic neurones. J Physiol. 1983 Mar;336:511–526. doi: 10.1113/jphysiol.1983.sp014595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieber L. A., Adams D. J. Acetylcholine-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991 Mar;434:215–237. doi: 10.1113/jphysiol.1991.sp018466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol. 1985 Jan;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudat J., Dennis M., Heidmann T., Chang J. Y., Changeux J. P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: serine-262 of the delta subunit is labeled by [3H]chlorpromazine. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2719–2723. doi: 10.1073/pnas.83.8.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka H. Nerve growth factor-mediated stimulation of tyrosine hydroxylase activity in a clonal rat pheochromocytoma cell line. Brain Res. 1981 Oct 19;222(2):225–233. doi: 10.1016/0006-8993(81)91029-5. [DOI] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Hirano T., Kidokoro Y., Ohmori H. Acetylcholine dose-response relation and the effect of cesium ions in the rat adrenal chromaffin cell under voltage clamp. Pflugers Arch. 1987 Apr;408(4):401–407. doi: 10.1007/BF00581136. [DOI] [PubMed] [Google Scholar]
- Hucho F., Oberthür W., Lottspeich F. The ion channel of the nicotinic acetylcholine receptor is formed by the homologous helices M II of the receptor subunits. FEBS Lett. 1986 Sep 1;205(1):137–142. doi: 10.1016/0014-5793(86)80881-x. [DOI] [PubMed] [Google Scholar]
- Ifune C. K., Steinbach J. H. Rectification of acetylcholine-elicited currents in PC12 pheochromocytoma cells. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4794–4798. doi: 10.1073/pnas.87.12.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Imoto K., Methfessel C., Sakmann B., Mishina M., Mori Y., Konno T., Fukuda K., Kurasaki M., Bujo H., Fujita Y. Location of a delta-subunit region determining ion transport through the acetylcholine receptor channel. Nature. 1986 Dec 18;324(6098):670–674. doi: 10.1038/324670a0. [DOI] [PubMed] [Google Scholar]
- Leonard R. J., Labarca C. G., Charnet P., Davidson N., Lester H. A. Evidence that the M2 membrane-spanning region lines the ion channel pore of the nicotinic receptor. Science. 1988 Dec 16;242(4885):1578–1581. doi: 10.1126/science.2462281. [DOI] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Weinstock M. M. Nickel and calcium ions modify the characteristics of the acetylcholine receptor-channel complex at the frog neuromuscular junction. J Physiol. 1980 Feb;299:203–218. doi: 10.1113/jphysiol.1980.sp013120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A., Colquhoun D., Cull-Candy S. G. Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurones. J Physiol. 1990 Aug;427:625–655. doi: 10.1113/jphysiol.1990.sp018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A., Cull-Candy S. G., Colquhoun D. Single-channel and whole-cell currents evoked by acetylcholine in dissociated sympathetic neurons of the rat. Proc R Soc Lond B Biol Sci. 1987 Nov 23;232(1267):239–248. doi: 10.1098/rspb.1987.0072. [DOI] [PubMed] [Google Scholar]
- Mulle C., Changeux J. P. A novel type of nicotinic receptor in the rat central nervous system characterized by patch-clamp techniques. J Neurosci. 1990 Jan;10(1):169–175. doi: 10.1523/JNEUROSCI.10-01-00169.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R. U., Finkelstein A. The electrostatic basis of Mg++ inhibition of transmitter release. Proc Natl Acad Sci U S A. 1974 Mar;71(3):923–926. doi: 10.1073/pnas.71.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus R., Cachelin A. B. Changes in the conductance of the neuronal nicotinic acetylcholine receptor channel induced by magnesium. Proc Biol Sci. 1990 Aug 22;241(1301):78–84. doi: 10.1098/rspb.1990.0069. [DOI] [PubMed] [Google Scholar]
- Shatkay A. Individual activity of calcium ions in pure solutions of CaCl2 and in mixtures. Biophys J. 1968 Aug;8(8):912–919. doi: 10.1016/S0006-3495(68)86528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H. Rectification of synaptic and acetylcholine currents in the mouse submandibular ganglion cells. J Physiol. 1989 Oct;417:307–322. doi: 10.1113/jphysiol.1989.sp017803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. W., Feltz P. Nicotinic acetylcholine receptors in porcine hypophyseal intermediate lobe cells. J Physiol. 1990 Mar;422:83–101. doi: 10.1113/jphysiol.1990.sp017974. [DOI] [PMC free article] [PubMed] [Google Scholar]


