Abstract
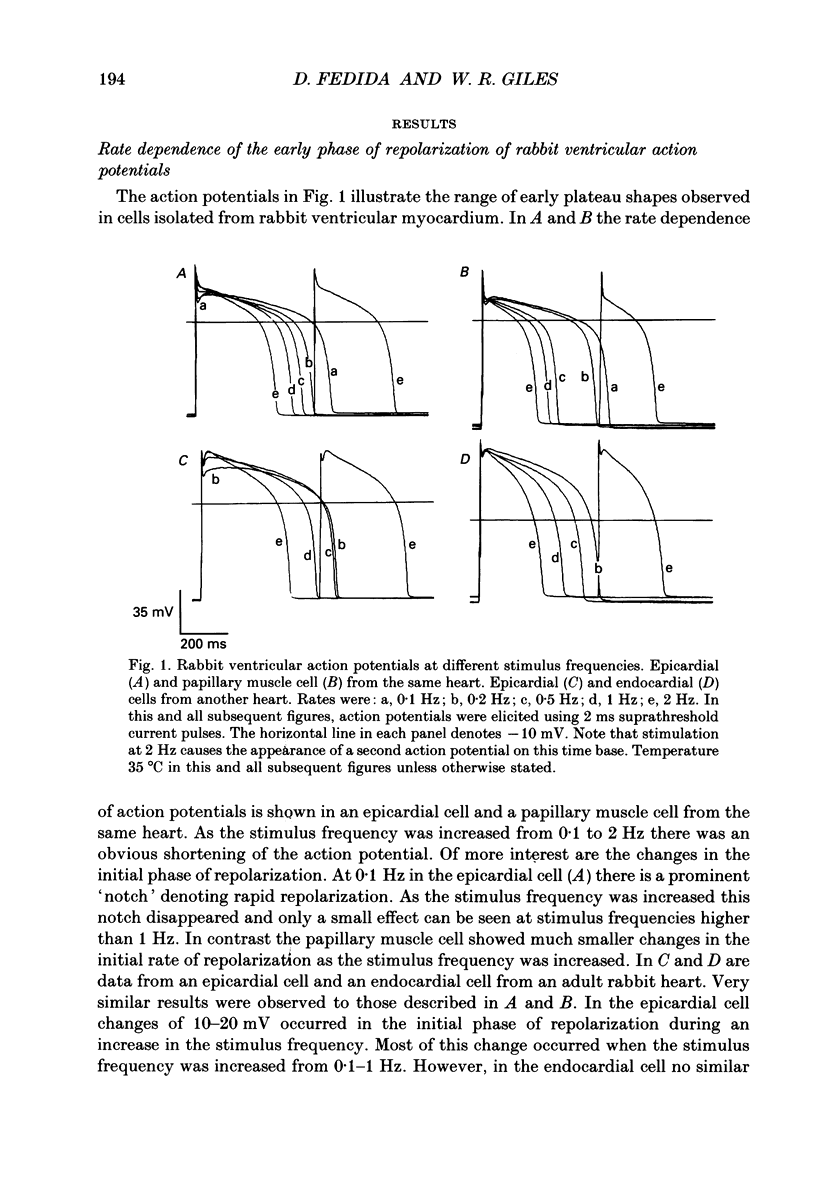
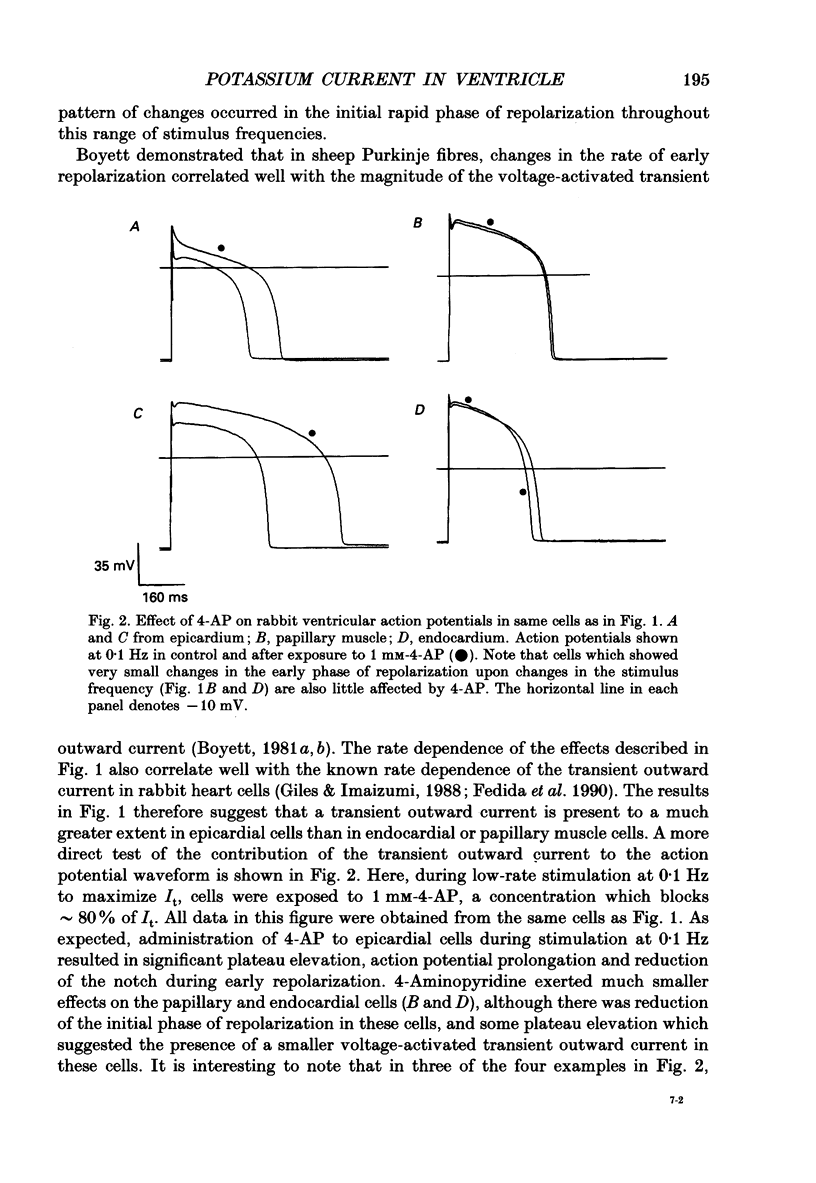
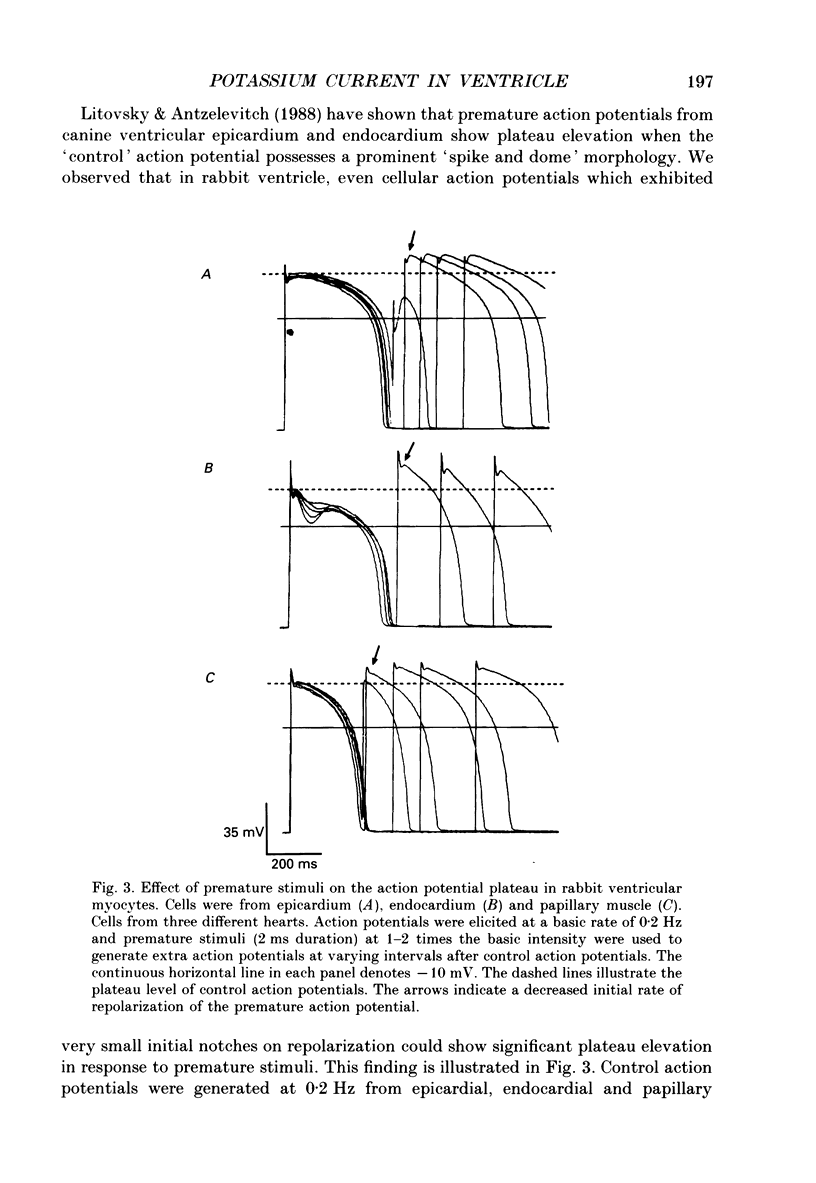
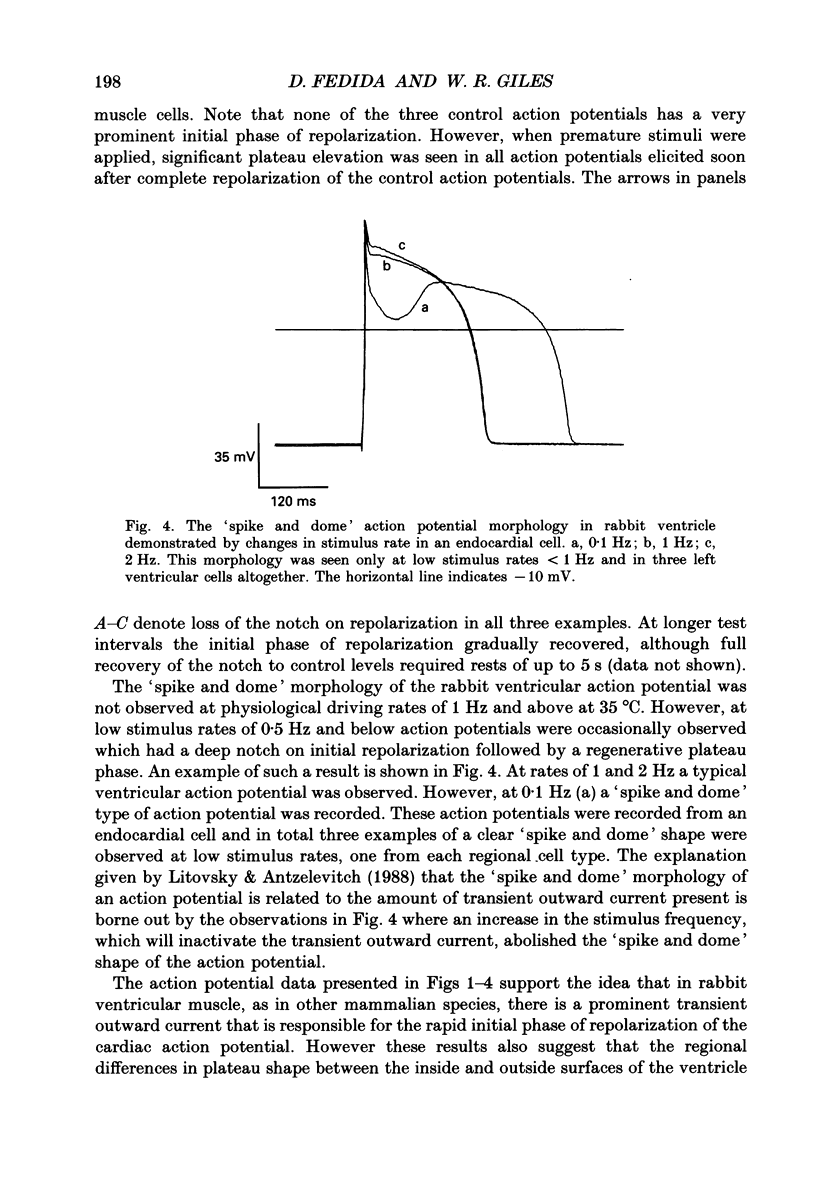
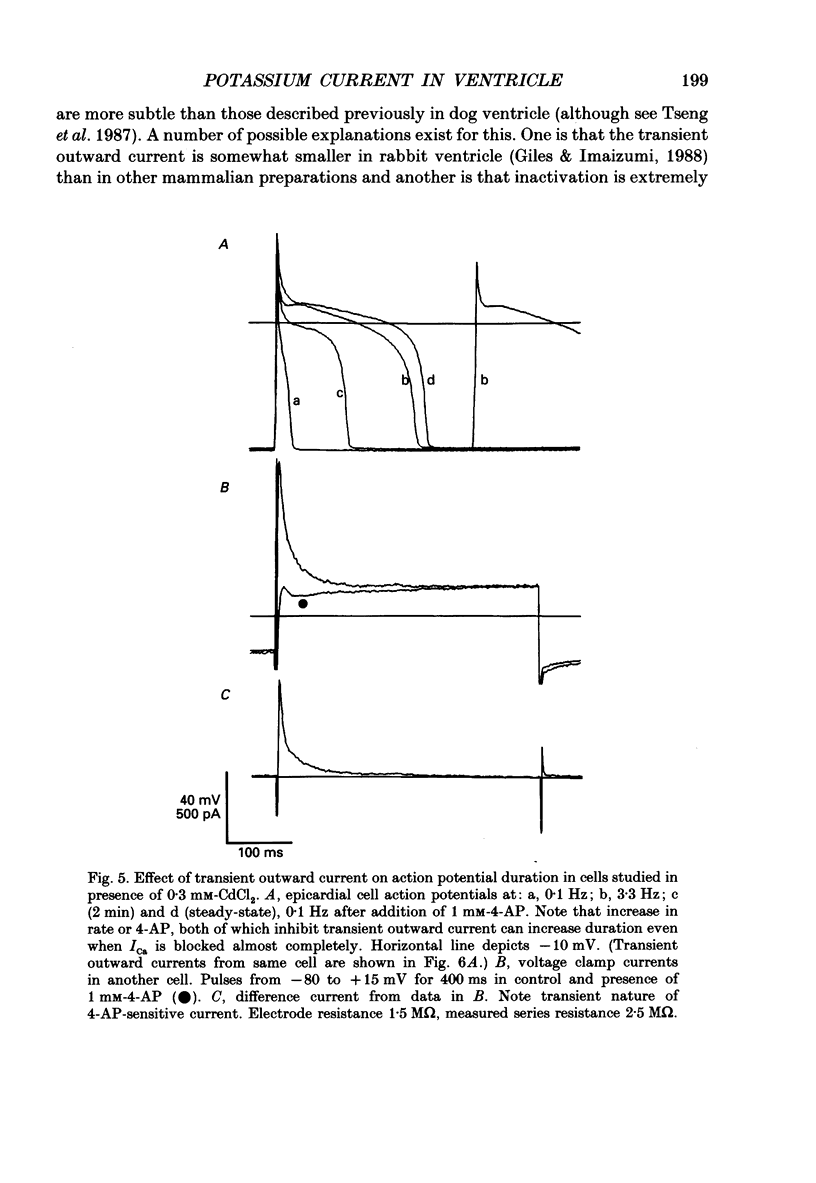
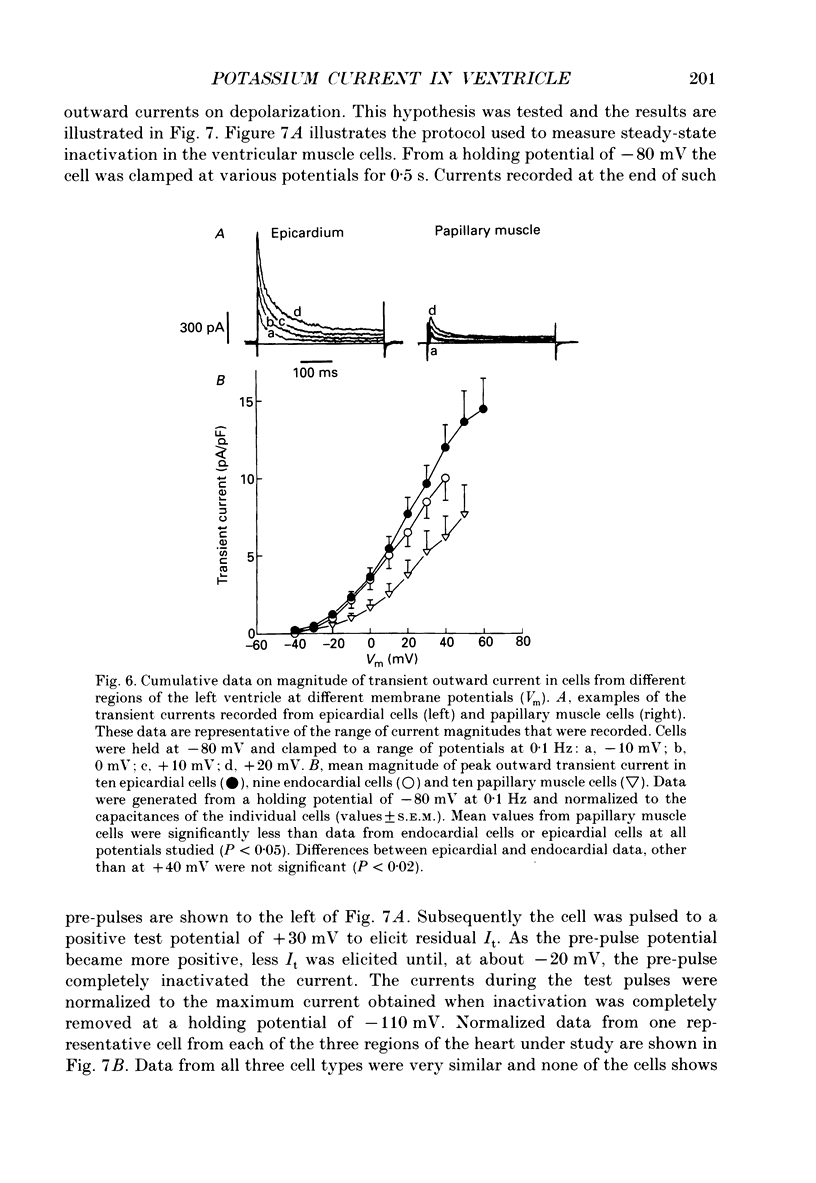
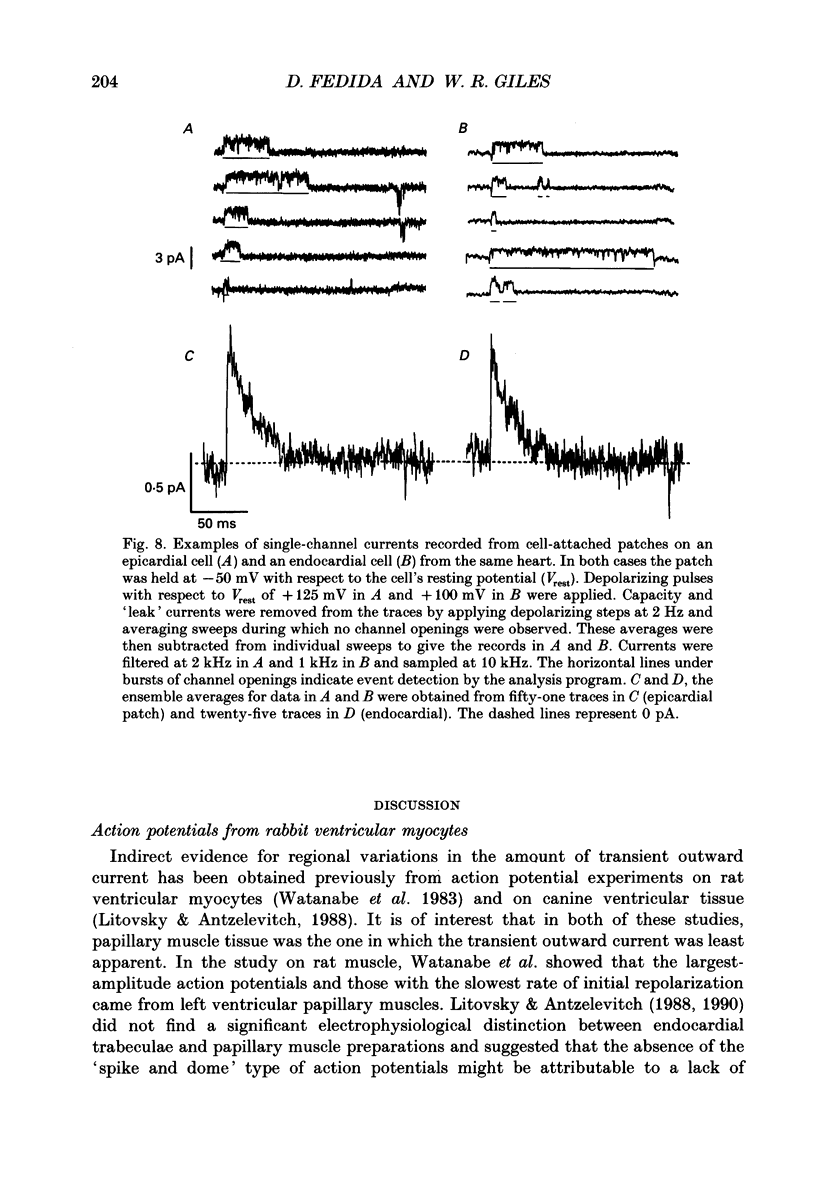
1. Regional variations in the shape of early repolarization of the action potential have been correlated to differences in transient outward K+ current, I(t), in myocytes isolated from the epicardial surface, the endocardial trabeculae and the papillary muscles of rabbit left ventricles. Temperature was 35 degrees C during whole-cell, and 22-23 degrees C during cell-attached experiments. 2. Membrane resting potentials were very similar regionally. At 0.1 Hz stimulation the action potential plateau amplitude in papillary muscle cells was significantly higher (104.7 mV) than in epicardial cells (96.47 mV). Exposure to 4-aminopyridine or increases in the rate of stimulation from 0.1 Hz to 3.3 Hz increased plateau height and diminished the initial notch on repolarization. These effects were correlated to the magnitude of I(t) in these cells. At low rates of stimulation I(t) caused a 'spike and dome' morphology of the action potential. 3. Voltage clamp experiments confirmed a higher current density of I(t) in epicardial cells (7.66 pA/pF at +20 mV) than in endocardial (6.45 pA/pF) or papillary muscle cells (3.69 pA/pF). I(t) at 35 degrees C was faster and larger than previously reported and individual currents inactivated almost completely during 100 ms pulses to plateau potentials. No differences in the kinetics or voltage dependence of whole-cell currents were found. Thus, the half-inactivation potential was -37.8 mV in cells from all three regions. 4. Cell-attached recordings from endocardial and epicardial cells showed very similar single-channel amplitudes, burst open probabilities and ensemble averages. The peak channel open probability soon after the start of depolarizing voltage clamp pulses did not change between cell types (P approximately 0.8). The slope conductance of I(t) channels was 13.0 pS with an intercept near the resting potential of the cell. 5. We conclude that regional variations in the shape of initial repolarization in cells from rabbit left ventricle are caused by variations in the magnitude of the transient outward K+ current, I(t). Epicardial cells have the largest, and papillary muscle cells the smallest I(t). The differences are not explained by alterations in the whole-cell kinetics or single-channel kinetics and conductance. The most likely explanation for variations in whole-cell current density is therefore a decrease in channel density in endocardium and papillary muscle compared with epicardial tissue. We estimate the density of I(t) channels per cell to be 1495 (one per 3-4 micron2) in epicardium, 1175 (one per 4-5 micron2) in endocardium, and 875 (one per 6 micron2) in papillary muscle cells.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benndorf K., Markwardt F., Nilius B. Two types of transient outward currents in cardiac ventricular cells of mice. Pflugers Arch. 1987 Aug;409(6):641–643. doi: 10.1007/BF00584667. [DOI] [PubMed] [Google Scholar]
- Boyett M. R. A study of the effect of the rate of stimulation on the transient outward current in sheep cardiac Purkinje fibres. J Physiol. 1981;319:1–22. doi: 10.1113/jphysiol.1981.sp013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R. Effect of rate-dependent changes in the transient outward current on the action potential in sheep Purkinje fibres. J Physiol. 1981;319:23–41. doi: 10.1113/jphysiol.1981.sp013889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Jewell B. R. Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart. Prog Biophys Mol Biol. 1980;36(1):1–52. doi: 10.1016/0079-6107(81)90003-1. [DOI] [PubMed] [Google Scholar]
- Braun A. P., Fedida D., Clark R. B., Giles W. R. Intracellular mechanisms for alpha 1-adrenergic regulation of the transient outward current in rabbit atrial myocytes. J Physiol. 1990 Dec;431:689–712. doi: 10.1113/jphysiol.1990.sp018355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess M. J. Relation of ventricular repolarization to electrocardiographic T wave-form and arrhythmia vulnerability. Am J Physiol. 1979 Mar;236(3):H391–H402. doi: 10.1152/ajpheart.1979.236.3.H391. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Vereecke J., Carmeliet E. Existence of a calcium-dependent potassium channel in the membrane of cow cardiac Purkinje cells. Pflugers Arch. 1986 Apr;406(4):424–426. doi: 10.1007/BF00590947. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Cardiac transmembrane potentials and metabolism. Circ Res. 1978 May;42(5):577–587. doi: 10.1161/01.res.42.5.577. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Giles W. R., Imaizumi Y. Properties of the transient outward current in rabbit atrial cells. J Physiol. 1988 Nov;405:147–168. doi: 10.1113/jphysiol.1988.sp017326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E., Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Feb;392(4):352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- Fedida D., Shimoni Y., Giles W. R. Alpha-adrenergic modulation of the transient outward current in rabbit atrial myocytes. J Physiol. 1990 Apr;423:257–277. doi: 10.1113/jphysiol.1990.sp018021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Myerburg R. J., Furukawa N., Bassett A. L., Kimura S. Differences in transient outward currents of feline endocardial and epicardial myocytes. Circ Res. 1990 Nov;67(5):1287–1291. doi: 10.1161/01.res.67.5.1287. [DOI] [PubMed] [Google Scholar]
- GIBBS C. L., JOHNSON E. A. Effect of changes in frequency of stimulation upon rabbit ventricular action potential. Circ Res. 1961 Jan;9:165–170. doi: 10.1161/01.res.9.1.165. [DOI] [PubMed] [Google Scholar]
- Giles W. R., Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988 Nov;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W. R., van Ginneken A. C. A transient outward current in isolated cells from the crista terminalis of rabbit heart. J Physiol. 1985 Nov;368:243–264. doi: 10.1113/jphysiol.1985.sp015856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan K., Edmands R. E., Fisch C. Effects of cycle-length alteration on canine cardiac action potentials. Am J Physiol. 1967 Jun;212(6):1416–1420. doi: 10.1152/ajplegacy.1967.212.6.1416. [DOI] [PubMed] [Google Scholar]
- HOFFMAN B. F., SUCKLING E. E. Effect of heart rate on cardiac membrane potentials and the unipolar electrogram. Am J Physiol. 1954 Oct;179(1):123–130. doi: 10.1152/ajplegacy.1954.179.1.123. [DOI] [PubMed] [Google Scholar]
- Hiraoka M., Kawano S. Calcium-sensitive and insensitive transient outward current in rabbit ventricular myocytes. J Physiol. 1989 Mar;410:187–212. doi: 10.1113/jphysiol.1989.sp017528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iinuma H., Kato K. Mechanism of augmented premature responses in canine ventricular muscle. Circ Res. 1979 May;44(5):624–629. doi: 10.1161/01.res.44.5.624. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y., Giles W. R. Quinidine-induced inhibition of transient outward current in cardiac muscle. Am J Physiol. 1987 Sep;253(3 Pt 2):H704–H708. doi: 10.1152/ajpheart.1987.253.3.H704. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. Early outward current in rat single ventricular cells. Circ Res. 1984 Feb;54(2):157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- Kenyon J. L., Sutko J. L. Calcium- and voltage-activated plateau currents of cardiac Purkinje fibers. J Gen Physiol. 1987 Jun;89(6):921–958. doi: 10.1085/jgp.89.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukushkin N. I., Gainullin R. Z., Sosunov E. A. Transient outward current and rate dependence of action potential duration in rabbit cardiac ventricular muscle. Pflugers Arch. 1983 Oct;399(2):87–92. doi: 10.1007/BF00663902. [DOI] [PubMed] [Google Scholar]
- Levine J. H., Spear J. F., Guarnieri T., Weisfeldt M. L., de Langen C. D., Becker L. C., Moore E. N. Cesium chloride-induced long QT syndrome: demonstration of afterdepolarizations and triggered activity in vivo. Circulation. 1985 Nov;72(5):1092–1103. doi: 10.1161/01.cir.72.5.1092. [DOI] [PubMed] [Google Scholar]
- Litovsky S. H., Antzelevitch C. Differences in the electrophysiological response of canine ventricular subendocardium and subepicardium to acetylcholine and isoproterenol. A direct effect of acetylcholine in ventricular myocardium. Circ Res. 1990 Sep;67(3):615–627. doi: 10.1161/01.res.67.3.615. [DOI] [PubMed] [Google Scholar]
- Litovsky S. H., Antzelevitch C. Rate dependence of action potential duration and refractoriness in canine ventricular endocardium differs from that of epicardium: role of the transient outward current. J Am Coll Cardiol. 1989 Oct;14(4):1053–1066. doi: 10.1016/0735-1097(89)90490-7. [DOI] [PubMed] [Google Scholar]
- Litovsky S. H., Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res. 1988 Jan;62(1):116–126. doi: 10.1161/01.res.62.1.116. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Wallace A. G., Feezor M. D. A quantitative comparison of the relation between the shape of the action potential and the pattern of stimulation in canine ventricular muscle and Purkinje fibers. J Mol Cell Cardiol. 1971 Mar;2(1):3–19. doi: 10.1016/0022-2828(71)90074-5. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Irisawa H. Transient outward current carried by potassium and sodium in quiescent atrioventricular node cells of rabbits. Circ Res. 1985 Jul;57(1):65–73. doi: 10.1161/01.res.57.1.65. [DOI] [PubMed] [Google Scholar]
- Payet M. D., Schanne O. F., Ruiz-Ceretti E. Frequency dependence of the ionic currents determining the action potential repolarization in rat ventricular muscle. J Mol Cell Cardiol. 1981 Feb;13(2):207–215. doi: 10.1016/0022-2828(81)90217-0. [DOI] [PubMed] [Google Scholar]
- Shibata E. F., Drury T., Refsum H., Aldrete V., Giles W. Contributions of a transient outward current to repolarization in human atrium. Am J Physiol. 1989 Dec;257(6 Pt 2):H1773–H1781. doi: 10.1152/ajpheart.1989.257.6.H1773. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng G. N., Hoffman B. F. Two components of transient outward current in canine ventricular myocytes. Circ Res. 1989 Apr;64(4):633–647. doi: 10.1161/01.res.64.4.633. [DOI] [PubMed] [Google Scholar]
- Tseng G. N., Robinson R. B., Hoffman B. F. Passive properties and membrane currents of canine ventricular myocytes. J Gen Physiol. 1987 Nov;90(5):671–701. doi: 10.1085/jgp.90.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Delbridge L. M., Bustamante J. O., McDonald T. F. Heterogeneity of the action potential in isolated rat ventricular myocytes and tissue. Circ Res. 1983 Mar;52(3):280–290. doi: 10.1161/01.res.52.3.280. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Rautaharju P. M., McDonald T. F. Ventricular action potentials, ventricular extracellular potentials, and the ECG of guinea pig. Circ Res. 1985 Sep;57(3):362–373. doi: 10.1161/01.res.57.3.362. [DOI] [PubMed] [Google Scholar]
- Wohlfart B. Analysis of mechanical alternans in rabbit papillary muscle. Acta Physiol Scand. 1982 Aug;115(4):405–414. doi: 10.1111/j.1748-1716.1982.tb07098.x. [DOI] [PubMed] [Google Scholar]


