Abstract
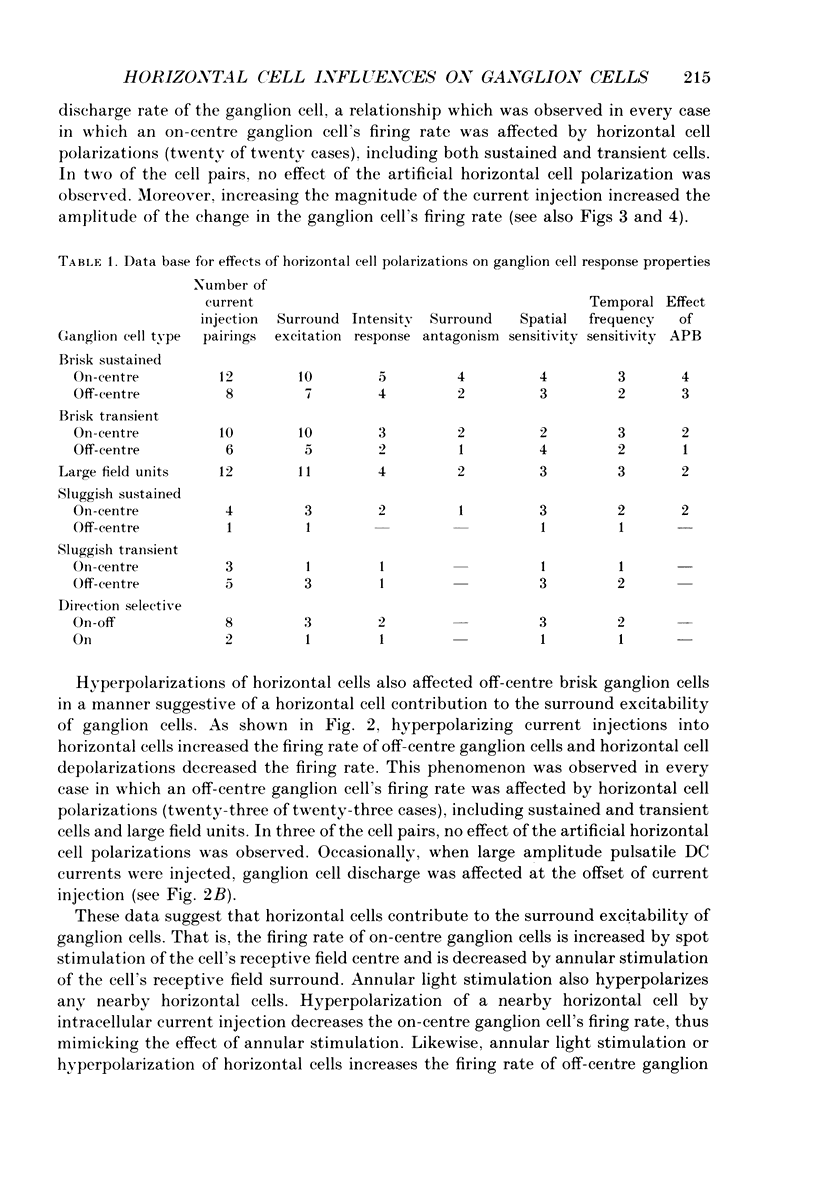
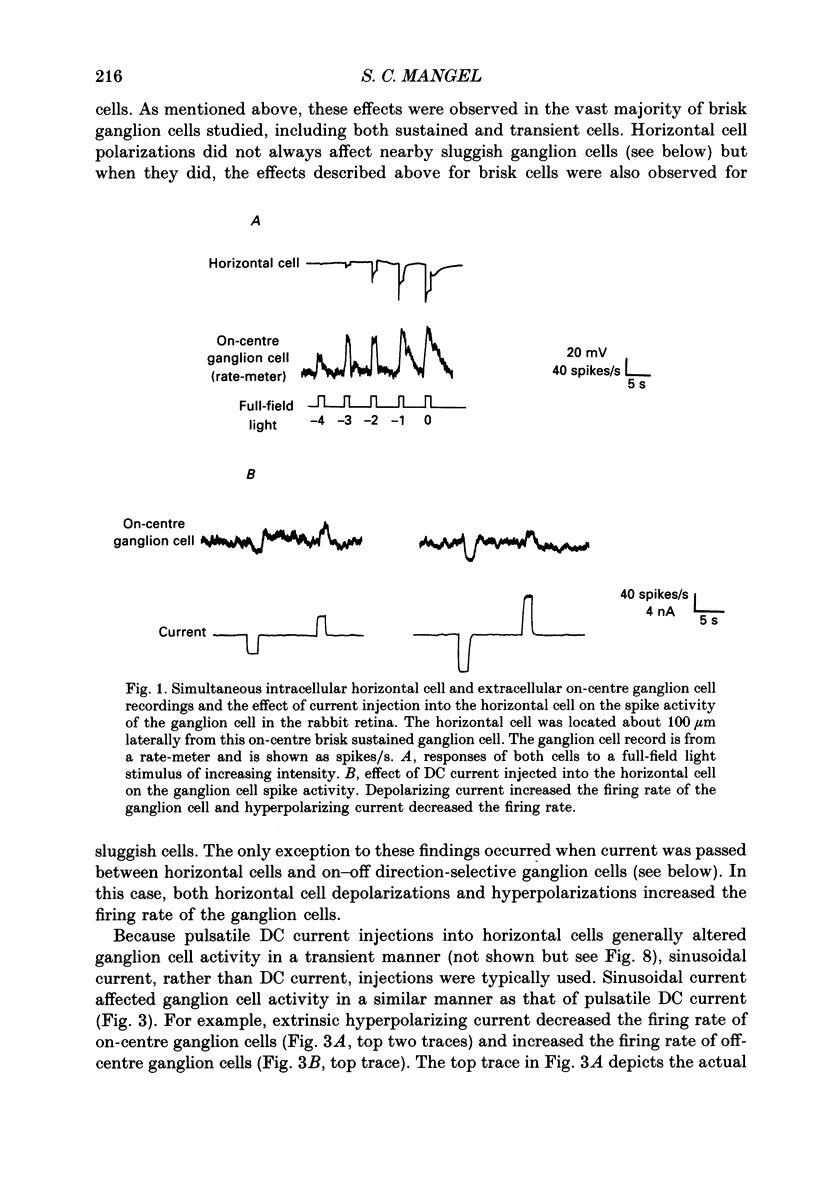
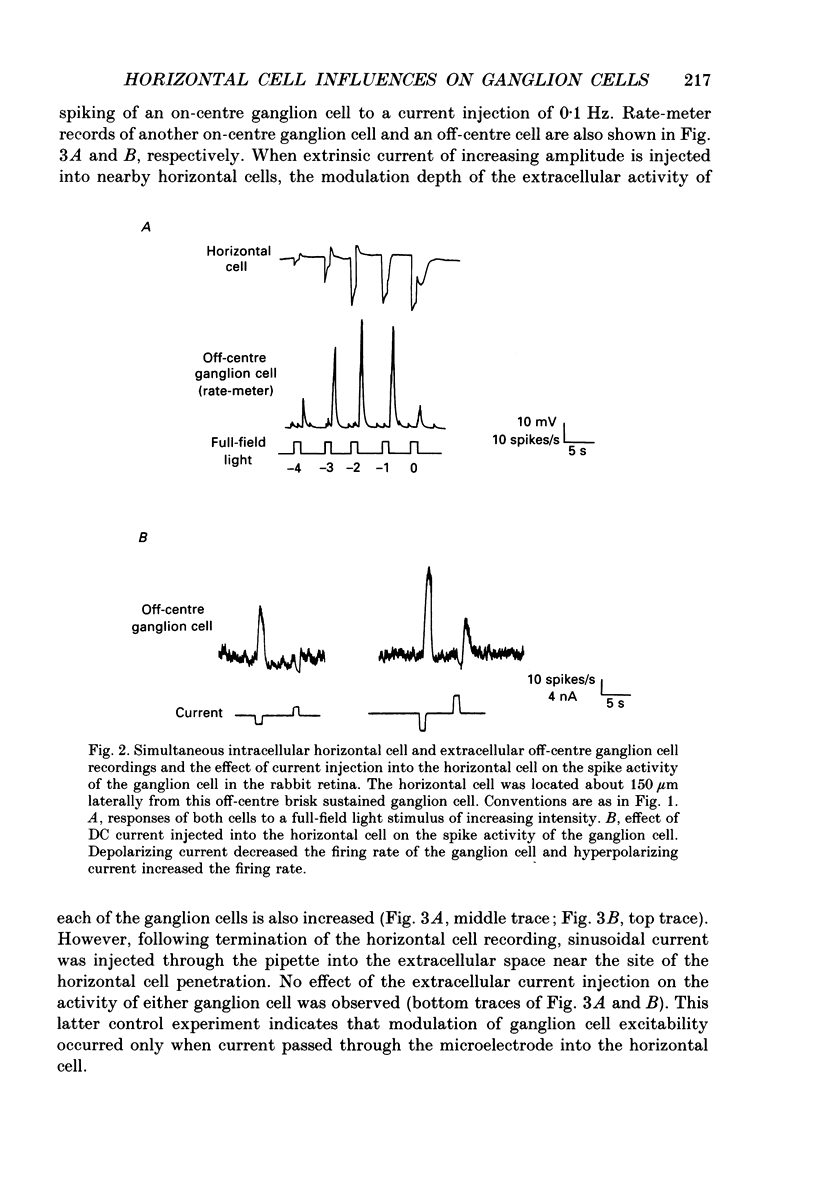
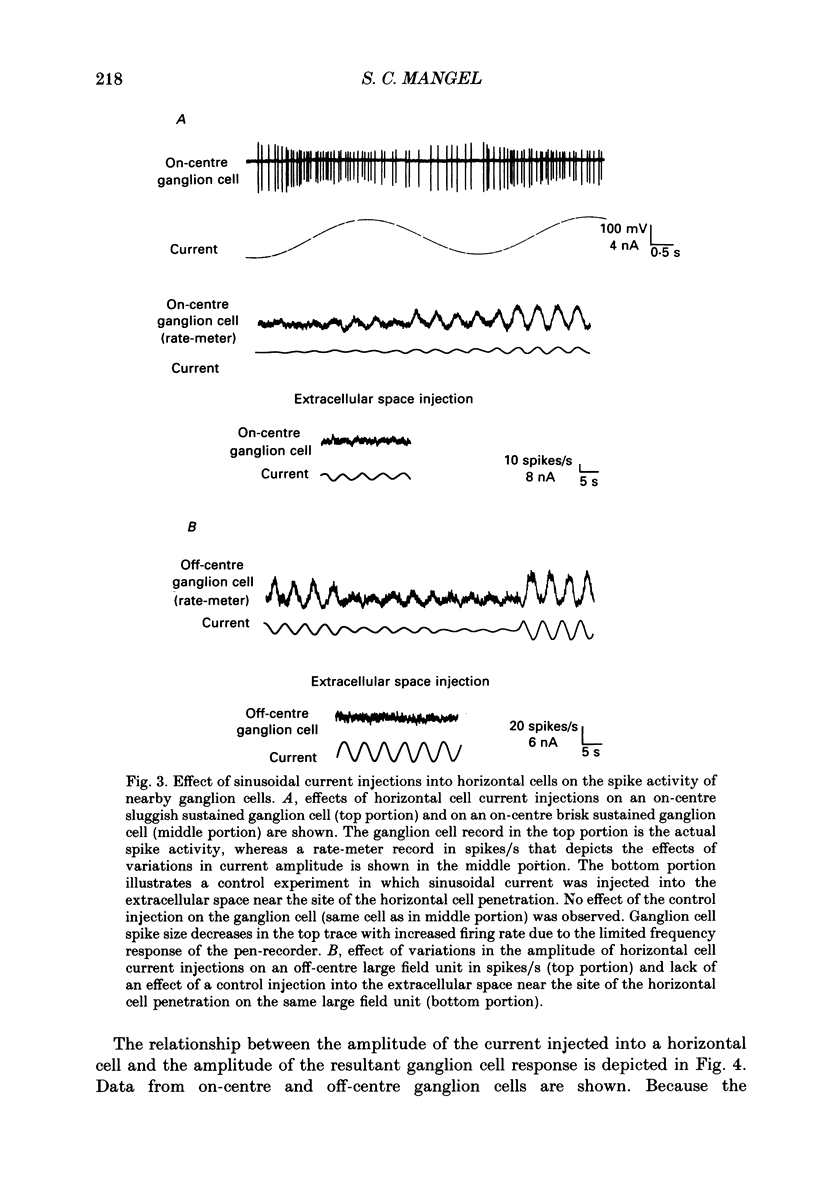
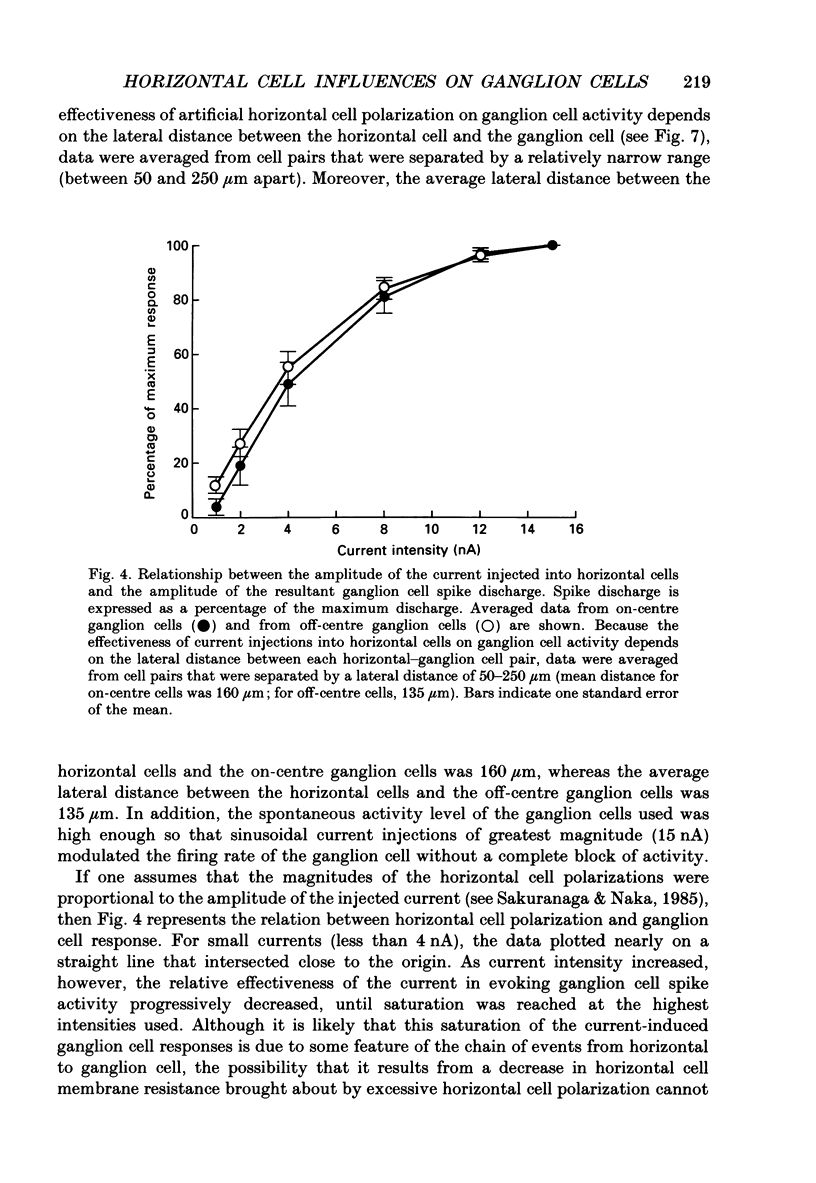
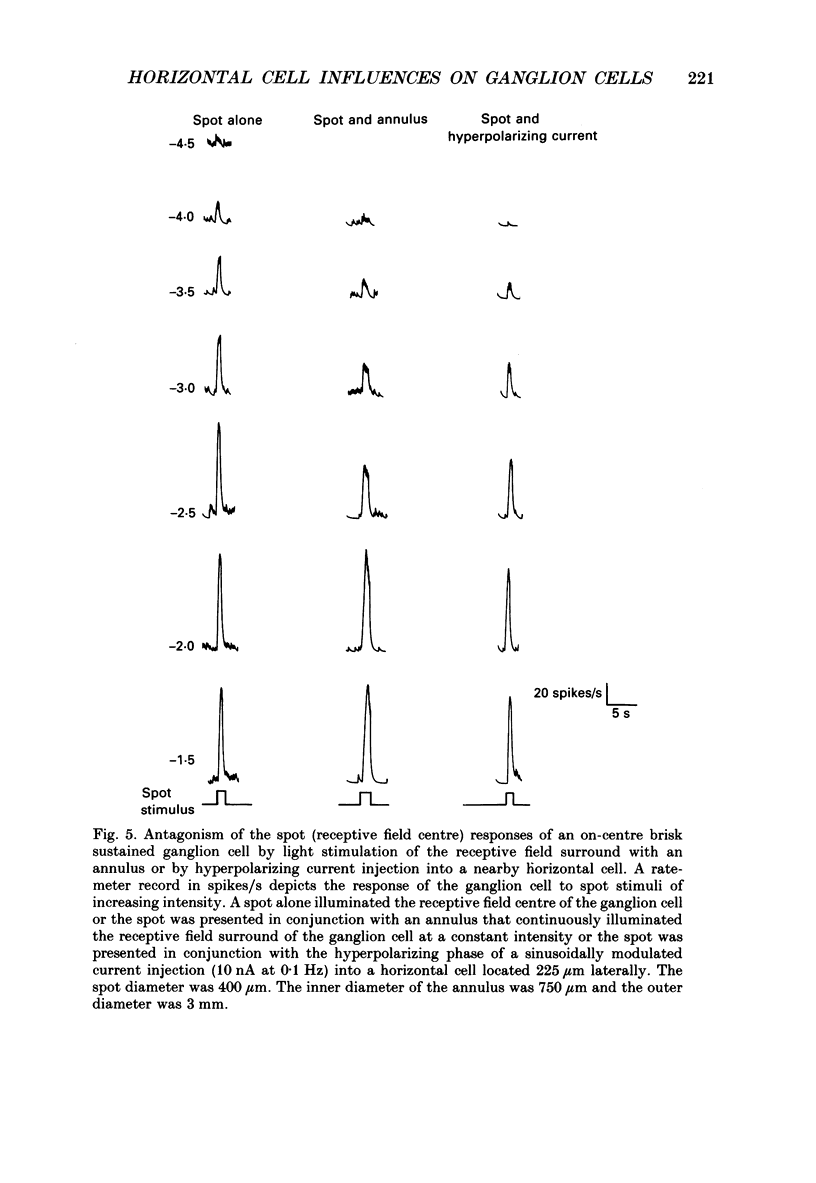
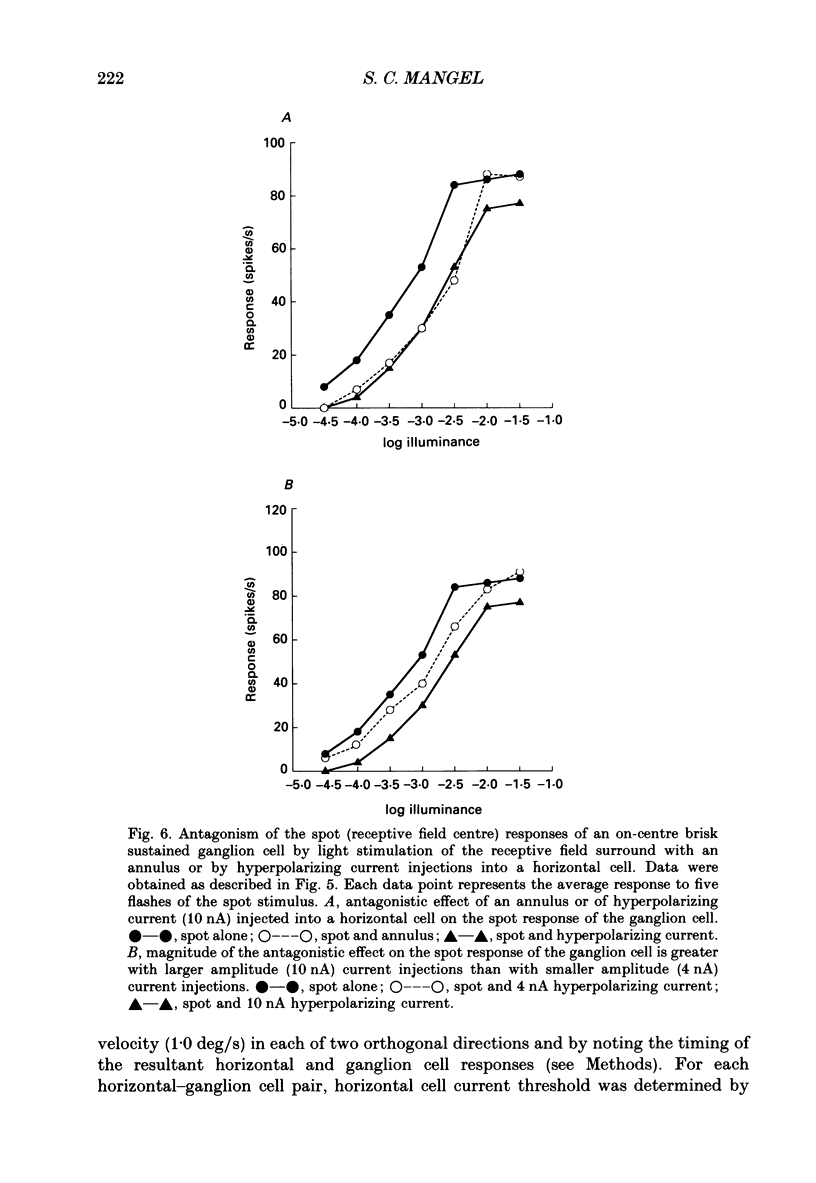
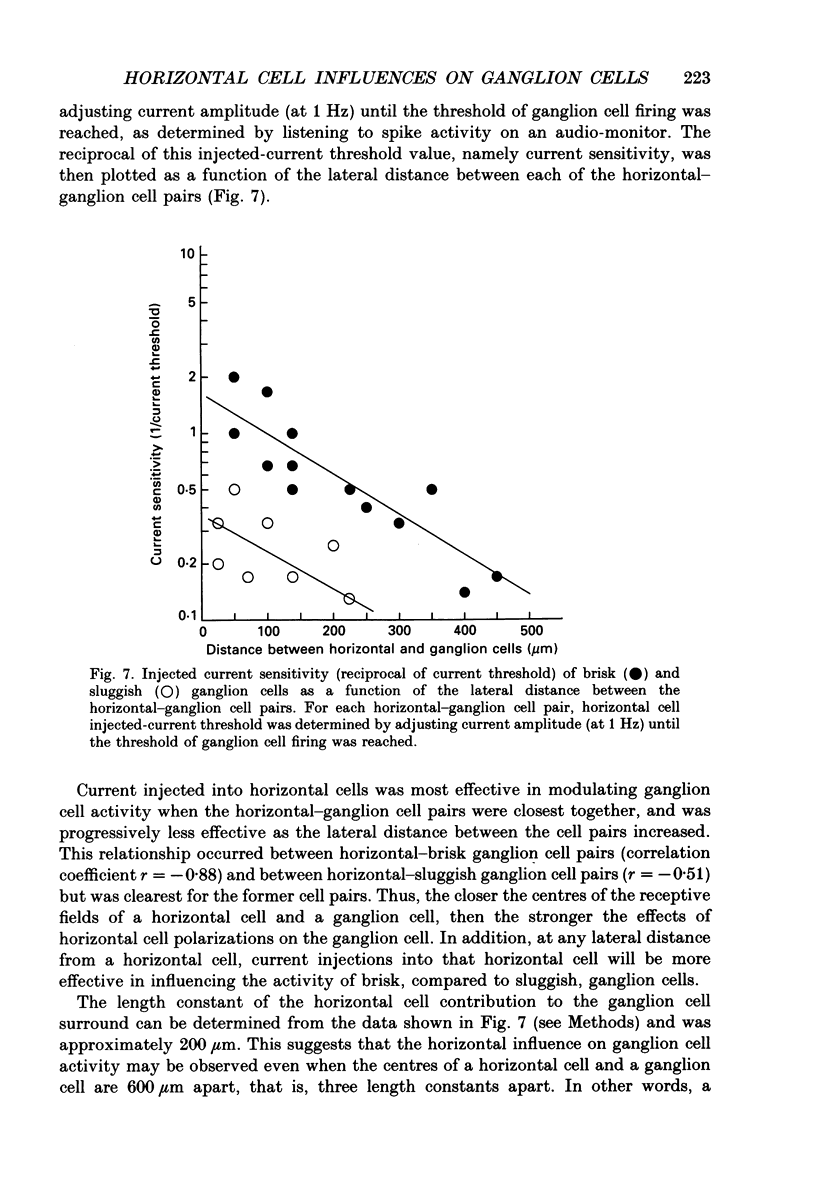
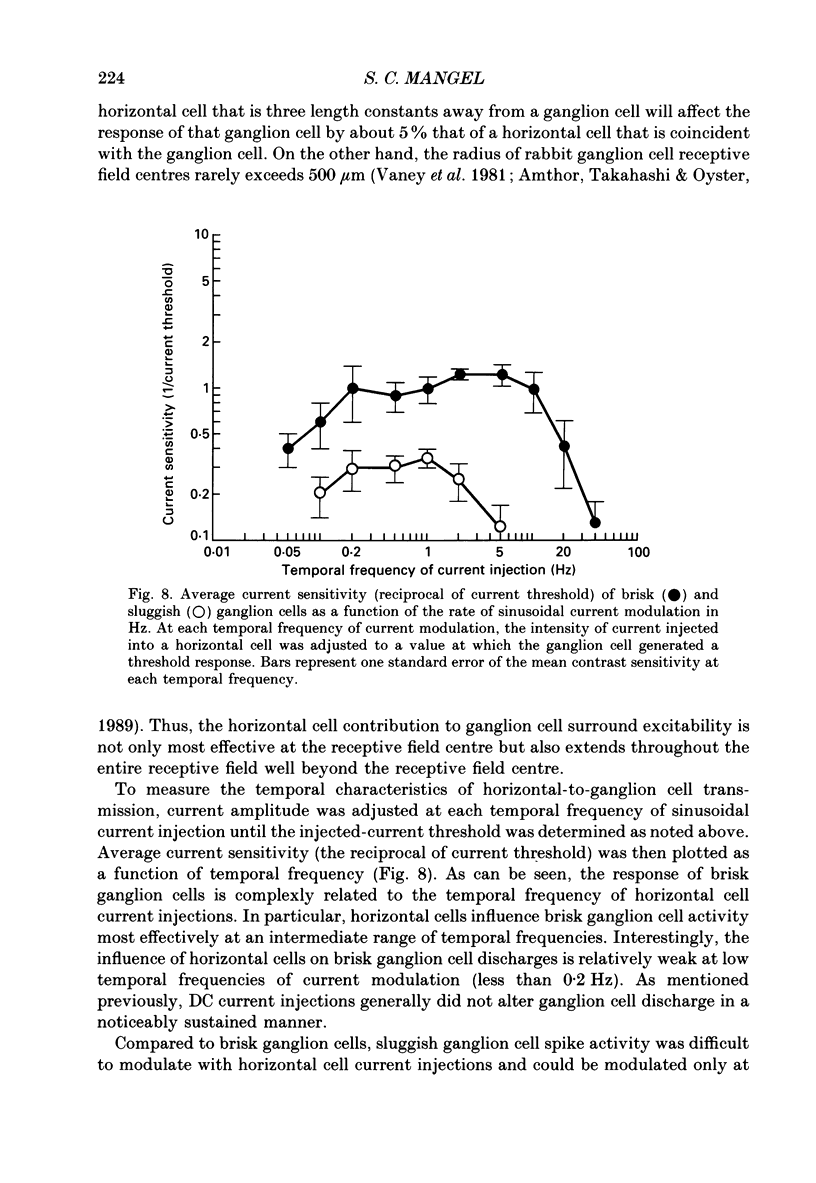
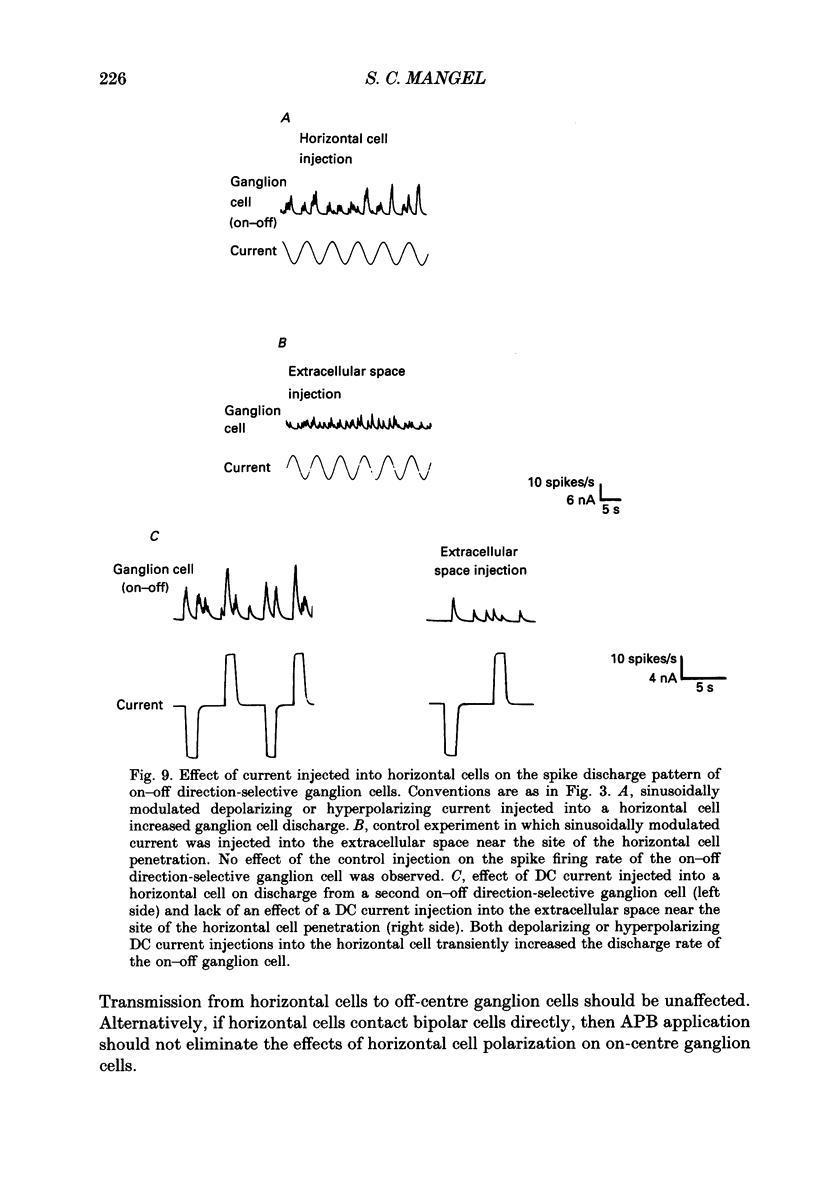
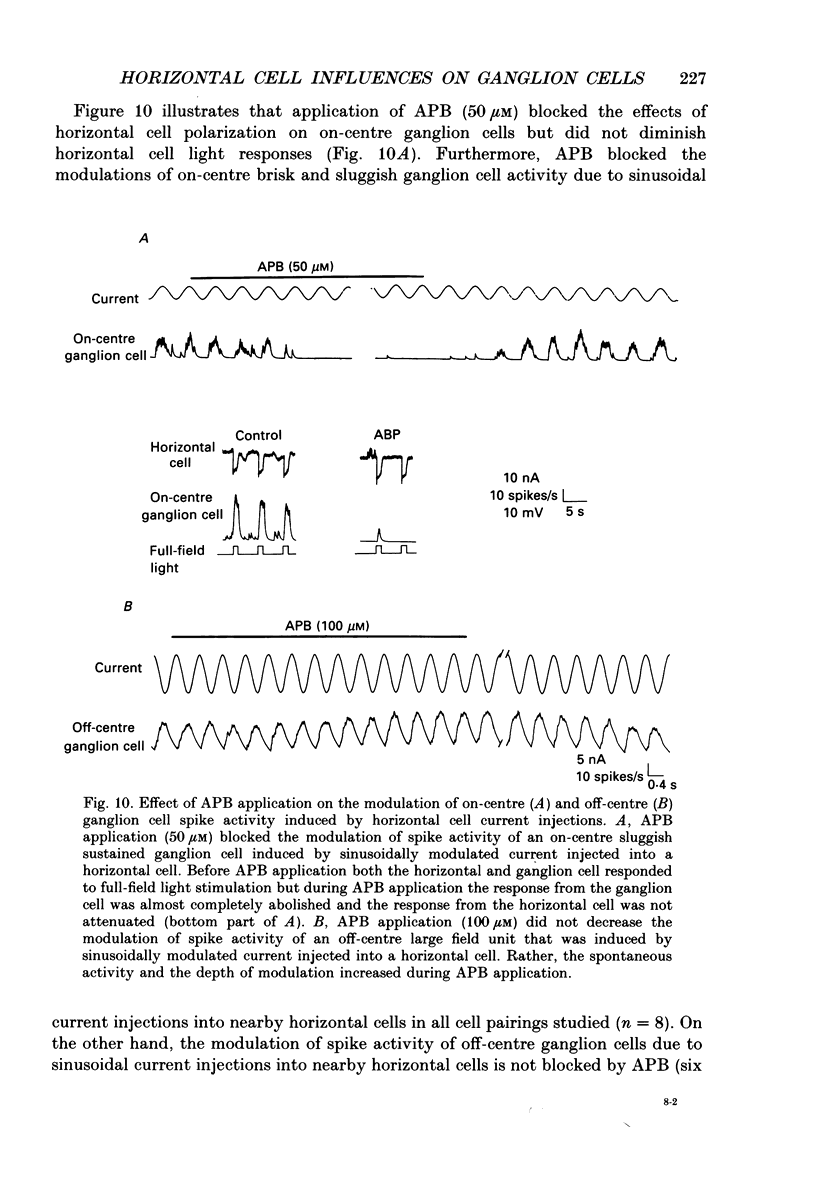
1. The influence of horizontal cells on ganglion cells, the output neuron of the retina, was examined in an in vitro rabbit eyecup preparation. The extracellular spike activity of ganglion cells was monitored while pulsatile DC or sinusoidally modulated current was injected intracellularly into nearby horizontal cells. Interactions between the effects of light stimulation and horizontal cell current injections on ganglion cell responses were also examined. 2. Horizontal cells were found to contribute to the receptive field surround of ganglion cells. In particular, horizontal cells contributed to surround excitability and to surround antagonism of the centre light response. 3. Brisk, sluggish and direction-selective ganglion cells were all affected by current injections into horizontal cells. However, brisk ganglion cells responded to lower amplitude currents than did sluggish or direction-selective cells. 4. Horizontal cells with receptive fields that overlap those of ganglion cells were able to affect ganglion cell discharge. Moreover, the closer a horizontal cell was to the receptive field centre of a ganglion cell, the more effective were current injections in modulating ganglion cell discharge rate. The length constant of the horizontal cell contribution to the ganglion cell receptive field was approximately 200 microns. These results indicate that horizontal cells which are located within or outside of a ganglion cell's receptive field centre can influence that ganglion cell's activity. 5. The influence of horizontal cells on ganglion cell discharges was relatively weak at low temporal frequencies of sinusoidally modulated current. 6. Application of 2-amino-4-phosphonobutyrate (APB), a glutamate analogue, blocked the modulation of spike activity of on-centre ganglion cells that was induced by sinusoidally modulated current injected into nearby horizontal cells. The spike activity of off-centre ganglion cells was not blocked. 7. These findings suggest that horizontal cells contribute to the surround of ganglion cells and bipolar cells primarily through a feedback pathway onto cone photoreceptor cells.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Nesbett F. B. In vitro retina as an experimental model of the central nervous system. J Neurochem. 1981 Oct;37(4):867–877. doi: 10.1111/j.1471-4159.1981.tb04473.x. [DOI] [PubMed] [Google Scholar]
- Amthor F. R., Takahashi E. S., Oyster C. W. Morphologies of rabbit retinal ganglion cells with concentric receptive fields. J Comp Neurol. 1989 Feb 1;280(1):72–96. doi: 10.1002/cne.902800107. [DOI] [PubMed] [Google Scholar]
- Arkin M. S., Miller R. F. Subtle actions of 2-amino-4-phosphonobutyrate (APB) on the Off pathway in the mudpuppy retina. Brain Res. 1987 Nov 17;426(1):142–148. doi: 10.1016/0006-8993(87)90433-1. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield S. A., Dowling J. E. Roles of aspartate and glutamate in synaptic transmission in rabbit retina. I. Outer plexiform layer. J Neurophysiol. 1985 Mar;53(3):699–713. doi: 10.1152/jn.1985.53.3.699. [DOI] [PubMed] [Google Scholar]
- Bloomfield S. A., Miller R. F. A physiological and morphological study of the horizontal cell types of the rabbit retina. J Comp Neurol. 1982 Jul 1;208(3):288–303. doi: 10.1002/cne.902080306. [DOI] [PubMed] [Google Scholar]
- Burkhardt D. A. Responses and receptive-field organization of cones in perch retinas. J Neurophysiol. 1977 Jan;40(1):53–62. doi: 10.1152/jn.1977.40.1.53. [DOI] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W. New properties of rabbit retinal ganglion cells. J Physiol. 1978 Mar;276:257–276. doi: 10.1113/jphysiol.1978.sp012232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W., Wyatt H. J. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interactions for cells with more complex receptive fields. J Physiol. 1978 Mar;276:277–298. doi: 10.1113/jphysiol.1978.sp012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux R. F., Miller R. F. An intracellular electrophysiological study of the ontogeny of functional synapses in the rabbit retina. I. Receptors, horizontal, and bipolar cells. J Comp Neurol. 1981 May 10;198(2):307–326. doi: 10.1002/cne.901980209. [DOI] [PubMed] [Google Scholar]
- Dacheux R. F., Raviola E. Horizontal cells in the retina of the rabbit. J Neurosci. 1982 Oct;2(10):1486–1493. doi: 10.1523/JNEUROSCI.02-10-01486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Brown J. E., Major D. Synapses of horizontal cells in rabbit and cat retinas. Science. 1966 Sep 30;153(3744):1639–1641. doi: 10.1126/science.153.3744.1639. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Lennie P. The control of retinal ganglion cell discharge by receptive field surrounds. J Physiol. 1975 Jun;247(3):551–578. doi: 10.1113/jphysiol.1975.sp010947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. K., Boycott B. B. Synaptic connections made by horizontal cells within the outer plexiform layer of the retina of the cat and the rabbit. Proc R Soc Lond B Biol Sci. 1974 Jul 30;186(1085):317–331. doi: 10.1098/rspb.1974.0052. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. The organization of the outer plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1977 Apr;6(2):131–153. doi: 10.1007/BF01261502. [DOI] [PubMed] [Google Scholar]
- Lamb T. D. Spatial properties of horizontal cell responses in the turtle retina. J Physiol. 1976 Dec;263(2):239–255. doi: 10.1113/jphysiol.1976.sp011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Linberg K. A., Fisher S. K. Ultrastructural evidence that horizontal cell axon terminals are presynaptic in the human retina. J Comp Neurol. 1988 Feb 8;268(2):281–297. doi: 10.1002/cne.902680211. [DOI] [PubMed] [Google Scholar]
- Mangel S. C., Miller R. F. Horizontal cells contribute to the receptive field surround of ganglion cells in the rabbit retina. Brain Res. 1987 Jun 23;414(1):182–186. doi: 10.1016/0006-8993(87)91344-8. [DOI] [PubMed] [Google Scholar]
- Marchiafava P. L. Horizontal cells influence membrane potential of bipolar cells in the retina of the turtle. Nature. 1978 Sep 14;275(5676):141–142. doi: 10.1038/275141a0. [DOI] [PubMed] [Google Scholar]
- Marshak D. W., Dowling J. E. Synapses of cone horizontal cell axons in goldfish retina. J Comp Neurol. 1987 Feb 15;256(3):430–443. doi: 10.1002/cne.902560310. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Dacheux R. F. Synaptic organization and ionic basis of on and off channels in mudpuppy retina. I. Intracellular analysis of chloride-sensitive electrogenic properties of receptors, horizontal cells, bipolar cells, and amacrine cells. J Gen Physiol. 1976 Jun;67(6):639–659. doi: 10.1085/jgp.67.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. F., Zalutsky R. A., Massey S. C. A perfused rabbit retina preparation suitable for pharmacological studies. J Neurosci Methods. 1986 Jun;16(4):309–322. doi: 10.1016/0165-0270(86)90056-7. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Nye P. W. Role of horizontal cells in organization of the catfish retinal receptive field. J Neurophysiol. 1971 Sep;34(5):785–801. doi: 10.1152/jn.1971.34.5.785. [DOI] [PubMed] [Google Scholar]
- Naka K. I. Receptive field mechanism in the vertebrate retina. Science. 1971 Feb 19;171(3972):691–693. doi: 10.1126/science.171.3972.691. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Witkovsky P. Dogfish ganglion cell discharge resulting from extrinsic polarization of the horizontal cells. J Physiol. 1972 Jun;223(2):449–460. doi: 10.1113/jphysiol.1972.sp009857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. Functional organization of catfish retina. J Neurophysiol. 1977 Jan;40(1):26–43. doi: 10.1152/jn.1977.40.1.26. [DOI] [PubMed] [Google Scholar]
- Nelson R. Cat cones have rod input: a comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. J Comp Neurol. 1977 Mar 1;172(1):109–135. doi: 10.1002/cne.901720106. [DOI] [PubMed] [Google Scholar]
- Nelson R., Kolb H., Robinson M. M., Mariani A. P. Neural circuitry of the cat retina: cone pathways to ganglion cells. Vision Res. 1981;21(11):1527–1536. doi: 10.1016/0042-6989(81)90028-6. [DOI] [PubMed] [Google Scholar]
- Nelson R., Kolb H. Synaptic patterns and response properties of bipolar and ganglion cells in the cat retina. Vision Res. 1983;23(10):1183–1195. doi: 10.1016/0042-6989(83)90032-9. [DOI] [PubMed] [Google Scholar]
- Nelson R., von Litzow A., Kolb H., Gouras P. Horizontal cells in cat retina with independent dendritic systems. Science. 1975 Jul 11;189(4197):137–139. doi: 10.1126/science.1138370. [DOI] [PubMed] [Google Scholar]
- Oyster C. W. The analysis of image motion by the rabbit retina. J Physiol. 1968 Dec;199(3):613–635. doi: 10.1113/jphysiol.1968.sp008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M., Witkovsky P., Trimarchi C. Dopaminergic mechanisms underlying the reduction of electrical coupling between horizontal cells of the turtle retina induced by d-amphetamine, bicuculline, and veratridine. J Neurosci. 1987 Aug;7(8):2273–2284. [PMC free article] [PubMed] [Google Scholar]
- Raviola E., Dacheux R. F. Variations in structure and response properties of horizontal cells in the retina of the rabbit. Vision Res. 1983;23(11):1221–1227. doi: 10.1016/0042-6989(83)90097-4. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res. 1965 Dec;5(11):583–601. doi: 10.1016/0042-6989(65)90033-7. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Sakai H. M., Naka K. Synaptic organization of the cone horizontal cells in the catfish retina. J Comp Neurol. 1986 Mar 1;245(1):107–115. doi: 10.1002/cne.902450108. [DOI] [PubMed] [Google Scholar]
- Sakuranaga M., Naka K. Signal transmission in the catfish retina. I. Transmission in the outer retina. J Neurophysiol. 1985 Feb;53(2):373–389. doi: 10.1152/jn.1985.53.2.373. [DOI] [PubMed] [Google Scholar]
- Schaeffer S. F., Raviola E., Heuser J. E. Membrane specializations in the outer plexiform layer of the turtle retina. J Comp Neurol. 1982 Jan 20;204(3):253–267. doi: 10.1002/cne.902040305. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Calcium-independent release of GABA from isolated horizontal cells of the toad retina. J Physiol. 1982 Feb;323:211–227. doi: 10.1113/jphysiol.1982.sp014069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987 Oct 16;238(4825):350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Organization of on-off cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):1–14. doi: 10.1113/jphysiol.1973.sp010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981 Jan 9;211(4478):182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Tachibana M., Kaneko A. gamma-Aminobutyric acid acts at axon terminals of turtle photoreceptors: difference in sensitivity among cell types. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7961–7964. doi: 10.1073/pnas.81.24.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M., Kaneko A. gamma-Aminobutyric acid exerts a local inhibitory action on the axon terminal of bipolar cells: evidence for negative feedback from amacrine cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3501–3505. doi: 10.1073/pnas.84.10.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibos L. N., Werblin F. S. The properties of surround antagonism elicited by spinning windmill patterns in the mudpuppy retina. J Physiol. 1978 May;278:101–116. doi: 10.1113/jphysiol.1978.sp012295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibos L. N., Werblin F. S. The response properties of the steady antagonistic surround in the mudpuppy retina. J Physiol. 1978 May;278:79–99. doi: 10.1113/jphysiol.1978.sp012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda J. I., Tonosaki K. Effect of polarisation of horizontal cells on the on-centre bipolar cell of carp retina. Nature. 1978 Nov 23;276(5686):399–400. doi: 10.1038/276399a0. [DOI] [PubMed] [Google Scholar]
- Vaney D. I., Levick W. R., Thibos L. N. Rabbit retinal ganglion cells. Receptive field classification and axonal conduction properties. Exp Brain Res. 1981;44(1):27–33. doi: 10.1007/BF00238746. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Yagi T. Interaction between the soma and the axon terminal of retinal horizontal cells in Cyprinus carpio. J Physiol. 1986 Jun;375:121–135. doi: 10.1113/jphysiol.1986.sp016109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazulla S., Kleinschmidt J. Carrier-mediated release of GABA from retinal horizontal cells. Brain Res. 1983 Mar 14;263(1):63–75. doi: 10.1016/0006-8993(83)91201-5. [DOI] [PubMed] [Google Scholar]


