Abstract
Infiltration of cellulase (EC 3.2.1.4) from Trichoderma longibrachiatum into melon (Cucumis melo) cotyledons induced several key defense mechanisms and hypersensitive reaction-like symptoms. An oxidative burst was observed 3 hours after treatment and was followed by activation of ethylene and salicylic acid (SA) signaling pathways leading to marked induction of peroxidase and chitinase activities. The treatment of cotyledons by heat-denatured cellulase also led to some induction of peroxidase and chitinase activities, but the oxidative burst and SA production were not observed. Co-infiltration of aminoethoxyvinil-glycine (an ethylene inhibitor) with the active cellulase did not affect the high increase of peroxidase and chitinase activities. In contrast, co-infiltration of aminoethoxyvinil-glycine with the denatured enzyme blocked peroxidase and chitinase activities. Our data suggest that the SA pathway (induced by the cellulase activity) and ethylene pathway (induced by heat-denatured and active protein) together coordinate the activation of defense mechanisms. We found a partial interaction between both signaling pathways since SA caused an inhibition of the ethylene production and a decrease in peroxidase activity when co-infiltrated with denatured cellulase. Treatments with active or denatured cellulase caused a reduction in powdery mildew (Sphaerotheca fuliginea) disease.
Plants have the ability to perceive specific signals resulting from pathogen attack. This recognition triggers a wide range of plant defense mechanisms used for protecting against the invading pathogen. Defense may be induced specifically, as in the gene-for-gene type of interaction (Flor, 1971), or nonspecifically by a range of biotic and abiotic elicitors (Benhamou and Nicole, 1999). During host invasion, fungal and bacterial pathogens secrete hydrolytic enzymes that digest the plant cell wall, allowing the pathogen to have access to plant tissues (Salmond, 1994; Walton, 1994). Some of these cell-degrading enzymes, including cellulases, have been shown to induce plant defense mechanisms, probably in part by releasing cell wall fragments (Bucheli et al., 1990). Different cell wall constituents, originating from the host or even from the invading pathogen, can induce plant defense reactions (Hahn et al., 1981; Nothnagel et al., 1983; Davis and Hahlbrock, 1987). Several enzymes such as pectinase (Vidal et al., 1998), xylanase (Avni et al., 1994), or cellulase (Calderon et al., 1994) have been reported to act directly as elicitor of reactions in plant cells (Sharon et al., 1993; Enkerli et al., 1999; Furman-Matarasso et al., 1999). For example, Hanania and Avni (1997) demonstrated the existence of high-affinity binding sites for xylanase on the plasma membrane of Nicotiana tabacum cultivars. The role of both the protein structure and enzyme activity in triggering plant reactions has not been yet clearly established. Treatments that lead to loss in enzyme activity, such as treatment with protease or heat denaturation, may also abolish elicitor activity. This means that the three-dimensional protein structure may be essential for elicitor activity (Fuchs et al., 1989; Lotan and Fluhr, 1990). However, Enkerli et al. (1999) demonstrated that enzyme activity is not necessary for elicitor activity using site-directed mutagenesis to reduce catalytic activity of xylanase II from Trichoderma reesei.
The signaling pathway leading to plant resistance that is activated by cellulase or other cell-wall-degrading protein still remains undefined. Several authors reported that xylanase, acting as an elicitor of pathogen-related protein synthesis in N. tabacum used a non-ethylene pathway for induction (Lotan and Fluhr, 1990). By contrast, Avni et al. (1994) showed that ethylene biosynthesis induced by xylanase from Trichoderma viride was accompanied by an accumulation of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor in N. tabacum. Yano et al. (1998) have shown that xylanase from T. reesei induced shrinkage of the cytoplasm, condensation of the nucleus, and cell death associated with typical defense responses, including an oxidative burst and expression of defense genes.
Concerning cellulases, Piel et al. (1997) revealed that treatments of Nicotiana plumbaginifolia, lima bean (Phaseolus lunatus), or corn (Zea mays) by cellulases from T. viride induced the biosynthesis of jasmonic acid (JA) followed by a transient emission of ethylene. Local and systemic expression of defense genes were also demonstrated when tobacco was treated by cellulases from Erwinia carotovora (Vidal et al., 1998). Their results indicated that salicylic acid (SA) did not appear to be involved in the defense process, as systemic resistance was induced similarly in transgenic NahG plants that overproduce a salicylate hydroxylase and cannot accumulate SA.
We report an investigation of the signaling pathways leading to expression of defense mechanisms in melon (Cucumis melo) plants after infiltration with cellulases produced by Trichoderma longibrachiatum. Our study revealed that the active cellulase (A-cell.) was able to stimulate early defense mechanisms associated with the hypersensitive reaction (HR) as well as the SA and ethylene/JA pathways. Infiltration of heat denatured, nonactive cellulase (NA-cell.) induced ethylene and jasmonate production, without accumulation of SA or HR-like key reactions. We speculate that treatment of melon cotyledons by cellulase elicits two different pathways, which act in tandem to increase plant defenses. In addition, we suggest that SA may control JA and ethylene production during the stimulation of defense by A-cell.
RESULTS
All of the experiments were done independently at a minimum of three times, and we always obtained similar results.
Peroxidase and Chitinase Activities after Treatments of Melon Cotyledons with the Cellulase from T. longibrachiatum
A dose test experiment was undertaken to determine whether cellulase from T. longibrachiatum induced local induction of peroxidase activity (Fig. 1).
Figure 1.
Dose-dependent effect of A-cell. and NA-cell. on induced peroxidase activity. Cotyledons were infiltrated with water and various concentrations of cellulase preparations. Peroxidase activity in cotyledons was measured 72 h after infiltration of cellulase. Each value is the mean ± se of 10 replicates from different plants.
When cotyledons were infiltrated with A-cell.3 or NA-cell.3, a significant 4-fold increase in peroxidase activity was observed compared with that of water-infiltrated samples (Fig. 1). Infiltration with A-cell.5, NA-cell.5, A-cell.10, and NA-cell.10, as well as NA-cell.20 or NA-cell.50 induced a 7-fold increase in peroxidase activity. It is surprising that the infiltration of A-cell.20 induced a lower peroxidase activity than the NA-cell.20 treatment in cotyledons. A similar phenomenon was observed when A-cell.50 and NA-cell.50 were infiltrated (Fig. 1).
For detailed analysis of the effect of heat-denatured or active cellulase on defense responses, the dose A-cell.5, NA-cell.5, A-cell.50, and NA-cell.50 were chosen.
Peroxidase and chitinase activities began to increase 8 h after infiltration of A-cell.5, NA-cell.50, or NA-cell.5 (Fig. 2, A and B), reaching a maximum between 48 and 72 h postinfiltration. A similar time course of activity was observed after A-cell.50 infiltration, but both chitinase and peroxidase activities were weaker. Cotyledons infiltrated with water showed only a very slight increase of peroxidase and chitinase activities.
Figure 2.
Time course of induction of peroxidase activity (A) and chitinase activity (B) after A-cell. and NA-cell. infiltration into melon cotyledons. ●, Water control; ♦, A-cell.5; ▴, NA-cell.5; ⋄, A-cell.50; ▵, NA-cell.50. Each value is the mean ± se of 10 replicates from different plants.
Ethylene and Nonethylene-Dependent Pathways of Induction of Chitinase and Peroxidase
To test the possible involvement of ethylene as a signal molecule in the induction of chitinase and peroxidase activities, we used the ethylene inhibitor aminoethoxivinyl-Gly (AVG), which acts as a competitive inhibitor of 1-aminocyclopropane-1-carb-oxylicacid synthase, a key enzyme in the ethylene biosynthesis pathway (Fig. 3).
Figure 3.
Effect of AVG on peroxidase activity after A-cell.5 and NA-cell.5 infiltration in melon cotyledons. AVG and cellulase were co-infiltrated in cotyledons and peroxidase activity was measured 72 h postinfiltration in cotyledons. Each value is the mean ± se of five replicates from different plants.
Peroxidase activity was analyzed 72 h after cellulase infiltration. Treatments with A-cell.5 and NA-cell.5 induced a 7-fold increase in peroxidase activity. When AVG was co-infiltrated with NA-cell.5 (Fig. 3), peroxidase activity was strongly reduced, but no reduction was observed in the induction of peroxidase by A-cell.5 (Fig. 3). Similar differential effect was observed with A-Cell.50 and NA-Cell.50 treatments (data not shown).
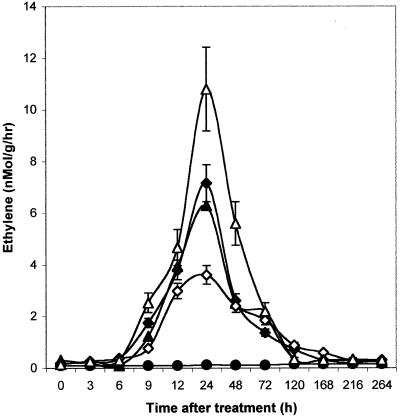
To verify the production of ethylene, following infiltration with A-cell.5, NA-cell.5, A-cell.50, and NA-cell.50, ethylene content was investigated by gas chromatography (GC). A significant production of ethylene was observed 24 h after infiltration of both active and heat-denatured cellulase (A-cell.5, A-cell.50, NA-cell.5, and NA-cell.50; Fig. 4). A similar level of production was detected after infiltration of A-cell.5 and NA-cell.5. A greater accumulation of ethylene was observed when NA-cell.50 was infiltrated in cotyledons, whereas A-cell.50 treatments induced a smaller accumulation of ethylene (Fig. 4).
Figure 4.
Changes in ethylene production levels after A-cell. and NA-cell. infiltration into melon cotyledons. ●, Control; ♦, A-cell.5; ▴, NA-cell.5; ⋄, A-cell.50; ▵, NA-cell.50. Levels of endogenous ethylene were analyzed by gas chromatography. Each value is the mean ± se of 10 replicates from different plants.
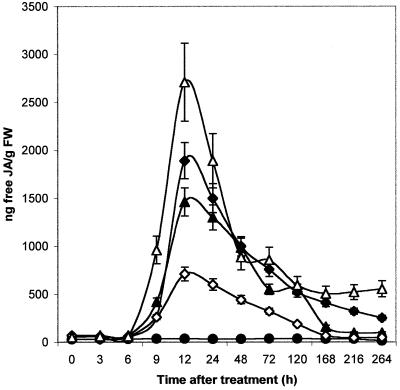
Since a clear interdependence between ethylene and JA has been suggested (Seo et al., 1997; Penninckx et al., 1998), we investigated JA production in melon following treatments with cellulase. Significant production of JA was observed 12 h after infiltration of A-cell.5 and NA-cell.5 (Fig. 5). The increasing concentration of heat-denatured cellulase (NA-cell.50) induced a stronger production of JA, whereas infiltration of a same dose of active cellulase (A-cell.50) caused a diminution of JA. The treatment with the inhibitor diethyldithiocarbamic acid led to a strong reduction of JA production (data not shown).
Figure 5.
Changes in endogenous-free JA content after A-cell. and NA-cell. infiltration into melon cotyledons. ●, Control; ♦, A-cell.5; ▴, NA-cell.5; ⋄, A-cell.50; ▵, NA-cell.50. Levels of free JA were analyzed by gas chromatography. Each value is the mean ± se of five replicates from different plants.
Lipoxygenase (Lox) Activity
After infiltration of cellulase, two peaks of Lox activity were detected (Fig. 6). The first occurred 3 h after infiltration, whereas the second, which was greater, was detected after 6 h. After NA-cell.50 infiltration, a strong increase in Lox activity was detected, whereas the same dose A-cell.50 caused a weaker Lox activity.
Figure 6.
Lox activity after A-cell. and NA-cell. infiltration into melon cotyledons. Control (●); A-cell.5 (♦); NA-cell.5 (▴); A-cell.50 (⋄); NA-cell.50 (▵). Each value is the mean ± se of 10 replicates from different plants.
Phe Amonia Lyase (PAL) Activity
Two peaks of PAL activity, a key enzyme in phenolic synthesis, were detected 6 and 48 h after infiltration of A-cell.5 and A-cell.50 (Fig. 7). Treatments with NA-cell.5 or NA-cell.50 caused an increase in only the second peak of PAL activity.
Figure 7.
Changes in PAL activity after A-cell. and NA-cell. infiltration into melon cotyledons. ●, Control; ♦, A-cell.5; ▴, NA-cell.5; ⋄, A-cell.50; ▵, NA-cell.50. Each value is the mean ± se of 10 replicates from different plants.
SA- and Non-SA-Dependent Pathway
SA production was analyzed by HPLC. Infiltration of A-cell.5 and A-cell.50 induced accumulation of SA, which started between 3 and 6 h after infiltration (Fig. 8). Infiltration of A-cell.50 caused a more sustained accumulation of SA. The results showed that SA remained in cotyledon tissues at a high level even 312 h (13 d) after infiltration of A-cell.50. Infiltration of NA-cell.5, NA-cell.50, or water did not induce significant SA accumulation.
Figure 8.
Effect of A-cell. and NA-cell. infiltration on SA level in melon cotyledons. SA content was analyzed by HPLC. ●, Control; ♦, A-cell.5; ▴, NA-cell.5; ⋄, A-cell.50; ▵, NA-cell.50. Each value is the mean ± se of 10 replicates from different plants.
Effect of SA on Ethylene Content and Peroxidase Activity
Because ethylene accumulated when cotyledons were treated by A-cell. or NA-cell. (Fig. 4), we investigated whether SA may modulate ethylene production. A-cell.5, A-cell.50, NA-cell.5, and NA-cell.50 were each co-infiltrated with 800 μm of SA in melon cotyledons. A significant decrease in ethylene content was observed when SA was co-infiltrated as compared with cellulase treatment alone (Fig. 9A).
Figure 9.
Effect of SA on ethylene content (A) and peroxidase activity (B). Cotyledons were co-infiltrated with SA (800 μm) and cellulase. Ethylene level (A) was measured 24 h after infiltration. Peroxidase activity (B) was measured 72 h later in cotyledons. SA solution was prepared by titration with 0.1 m NaOH to a pH value around 7.0. Each value is the mean ± se of 10 replicates from different plants.
Infiltration with SA (800 μm) alone in melon cotyledons induced a strong increase in peroxidase (Fig. 9B) and chitinase (data not shown) activities compared with controls. Co-infiltration with SA and A-cell. or NA-cell. caused a decrease in peroxidase activity (Fig. 9B) as compared with A-cell. or NA-cell. infiltration alone.
Activation of Early Defense Responses
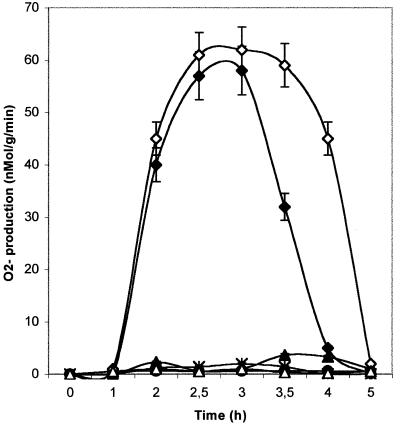
A striking accumulation of O2·− was observed with 2 h of infiltration with A-cell.5 and A-cell.50 infiltration (Fig. 10). The oxidative burst appeared more prolonged with the higher concentration. If the inhibitor diphenyliodonium (DPI) was added in the reaction medium, the production of O2·− was inhibited, indicating that O2·− was generated by an NADPH-oxydase. NA-cell.5 and NA-cell.50 infiltration did not cause any significant O2·− production (Fig. 10).
Figure 10.
Time course of O2·− production in cotyledons after A-cell. and NA-cell. infiltration. ●, Control; ♦, A-cell.5; ∗, A-cell.5 + DPI; ▴, NA-cell.5; ⋄, A-cell.50; ▵, NA-cell.50. Cotyledons discs were incubated in cytochrome C medium, and superoxyde anion production was monitored periodically by the reduction of cytochrome C. DPI was added to the reaction buffer just before immersion of cotyledon discs in cytochrom C buffer. Each value is the mean ± se of 10 replicates from different plants
HR-Like Reaction of Cotyledons after Cellulase Infiltration
Cotyledons infiltrated with A-cell.5 or A-cell.50 showed an HR-like reaction at infiltration sites; these brownish necrotic lesions were visible by 12 h after infiltration. Cotyledons treated by NA-cell.5 or NA-cell.50 did not develop similar local lesions.
Resistance against Powdery Mildew
A strong reduction of disease symptom was observed in cotyledons and in young true leaves after inoculation with powdery mildew when A-cell.5, A-cell.50, NA-cell.5, and NA-cell.50 were infiltrated in cotyledons 72 h before inoculation and in cotyledons and new leaves at 14 d interval (Fig. 11). The most important effect was detected using A-cell.5 infiltration, which allowed plants to produce fruits. Control plants (treated with water) rapidly showed (10 d) severe disease symptoms and failed to set fruits.
Figure 11.
Percentage of powdery mildew in cotyledons and leaves after treatments with A-cell. and NA-cell. Cotyledons were treated with cellulase 72 h before inoculation and then each 14 d. After senescence of cotyledons, the following ranks of leaves were treated similarly. ●, Control; ♦, A-cell.5; ▴, NA-cell.5; ⋄, A-cell.50; ▵, NA-cell.50. Each value is the mean ± se of observations on 50 plants by treatments.
DISCUSSION
Trichoderma species have received considerable attention as potential biocontrol agents for a number of pathogens (Chet, 1987; Ghisalberti and Sivasithamparam, 1991; Haran et al., 1996). The mechanisms by which Trichoderma is able to control pathogen populations has been focused on the production of antibiotics (Ghisalberti and Sivasithamparam, 1991), hydrolytic enzymes leading to mycoparasitism (Haran et al., 1996), associated with possible competition for nutrient in the rhizosphere (Sivan and Chet, 1993).
However, several recent reports indicated that T. viride may also activate plant-resistance responses. Cellulysin, a crude cellulase from T. viride was shown to elicit a massive induction of volatile biosynthesis in several higher plants (Piel et al., 1997). The pattern of emitted volatiles acts via activation of octadecanoid signaling pathway. They demonstrated that treatments with cellulase raises the level of endogenous JA after 30 min, followed by a transient emission of ethylene after 2 to 3 h. Vidal et al. (1998) revealed that cellulase from E. carotovora acted synergistically with pectinase to induce both local and systemic expression of genes involved in tobacco defense responses. They reported that SA did not appear to be involved in the process. Suspension cells of grapevine (Vitis vinifera) treated with the elicitor Onozuka R-10 cellulase from T. viride displayed an HR-like response in addition to a peroxidase-mediated formation of resveratrol oxidation products, which possess a greater antifungal activity than resveratrol itself (Calderon et al., 1993, 1994).
In our work, we have shown that cellulase produced by T. longibrachiatum is a powerful elicitor of resistance process in melon. Significant differences in induced responses were found using active A-cell. and heat denatured (NA-cell.) cellulase preparations. Biochemical indicators of localized and systemic defense responses were investigated, such as peroxidases (Montalbini et al., 1995; Martinez et al., 1996) and chitinases (Sahai and Manocha, 1993). Treatment with A-cell.20 and A-cell.50 caused a weaker induction of peroxidase activity than NA-cell.20 or Na-cell.50 infiltration. Mode of action of active and heat-denatured cellulase in the stimulation of defense responses in melon plants was thus investigated using A-cell.5, NA-cell.5, A-cell.50, and NA-cell.50. Time course experiment revealed that peroxidase and chitinase activities were induced concomitantly by A-cell. or NA-cell. infiltration but at a lower level when the A-cell.50 was used. This observation may indicate that A-cell. was less effective than NA-cell. and suggests that an important dose of active cellulase (A-cell.20, A-cell.50) may severely wound the plant cell wall, thus inducing a derivative healing process.
Induction of defense genes requires generation of endogenous signaling molecules by the challenged cells at the elicited site. It is now well established that ethylene is produced during host-pathogen interactions in many plants (Boller, 1991; Avni et al., 1994) and accumulated rapidly and transiently in leaves after induction by JA (Dong, 1998). Furthermore, JA and its methyl-ester, collectively termed jasmonates, synthesized from linolenic acid by lipoxygenase, are also involved in defense responses against pathogen attack (Melan et al., 1993). To investigate the signaling pathway leading to the increase in peroxidase and chitinase activities in melon cotyledons after cellulase infiltration, production of ethylene, free JA, and increase in Lox activity were analyzed.
Our results revealed that both forms of cellulase protein (A-cell.5, A-cell.50, NA-cell.5, and NA-cell.50) are inducers of ethylene and JA in melon plants. Two peaks of Lox activity were evidenced after A-cell. and NA-cell. treatments, logically preceding the JA and ethylene production. It is surprising that the ethylene inhibitor AVG caused a decrease in peroxidase activity when co-infiltrated with NA-cell.5 or NA-cell.50 but not when co-infiltrated with A-cell.5 or A-cell.50, suggesting that another concomitant transduction signaling pathway than that of ethylene may occur.
SA plays a central role in the local and systemic resistance against pathogens (Delaney et al., 1994; Klessig et al., 1998; Martinez et al., 2000). In melon cotyledons, production of free SA increased in a dose dependent manner after A-cell. infiltration only and was concomitant with ethylene and JA accumulation. Infiltration of A-cell.50 caused a greater and more durable production of SA than the treatment by A-cell.5 but induced a reduction of ethylene and JA accumulation. By contrast, production of JA and ethylene increased in a dose-dependent manner after treatment with NA-cell. This suggests a possible regulation role for SA in ethylene and JA production, which may explain their moderate accumulation with increasing dose of A-cell. To verify this hypothesis, SA (800 μm) was co-infiltrated with A-cell. and NA-cell. in melon cotyledons; a decrease in ethylene production and in peroxidase activity was then observed. Several authors have previously reported an antagonist relationship between SA and JA, demonstrating that SA interferes with the JA-signaling pathway (Peña-Cortés et al., 1993; Seo et al., 1997). The SA and the JA/ethylene pathways may interact antagonistically, with SA inhibiting both synthesis and signal transduction of JA and ethylene (Dong, 1998).
Our data suggest that NA-cell. is recognized by the plant cell, thus stimulating the ethylene and JA pathways, whereas A-cell. may be involved in the induction of both SA and JA/ethylene pathways. This indicates that (a) SA and JA/ethylene may play a concomitant role in defense signaling pathway following elicitation by A-cell., (b) SA may play a negative control on JA/ethylene production, and (c) NA-cell. is not able to stimulate SA pathway. In this way, Vidal et al. (1998) suggested that after treatment with cellulase, SA does not appear to be involved in the systemic resistance to the pathogen, as systemic resistance was induced similarly in trangenic NahG plants that overproduce a salicylate hydroxylase and cannot accumulate SA in transformed plants. They conclude that the lack of SA requirement suggested the presence of a different signal transduction pathway involved in plant-pathogens interactions.
To confirm that defense pathways are differentially elicited by A-cell. and NA-cell. in melon plants, we investigated several key events involved in the establishment of a defense reaction. PAL is responsible for the conversion of Phe to trans-cinnamic acid, a key intermediate in the pathway for production of lignin, SA, and it is believed to be correlated with synthesis of defense phenols (Nicholson and Hammerschmidt, 1992). In response to infiltration with A-cell.5 and A-cell.50 in melon cotyledons, two peaks of PAL activity were observed. One occurred logically just before the production of SA, whereas the second one was found later. In the case of NA-cell.5 and NA-cell.50 infiltration, the second peak of PAL activity was only observed. These results reinforce the idea that two signaling pathways are differentially activated by cellulase.
The earliest reactions of plant cells to elicitors also consist in a rapid production and accumulation of reactive oxygen species, such as O2·− and H2O2, known as the oxidative burst (Bolwell et al., 1995; Doke et al., 1996, 1998; Martinez et al., 1998). In our model, an early strong production of O2·− was observed after infiltration of A-cell.5 and A-cell.50 but not following treatments with NA-cell.5 and NA-cell.50. Infiltration with A-cell.50 elicited the same level of O2·− than that of A-cell.5 but over a longer period. The differences in time course of O2·− productions may explain the longer induction of SA resulting from A-cell.50 treatment.
In light of our data, we propose the following tentative model (Fig. 12) to explain how cellulase from Trichoderma elicits signaling defense pathways in melon. After A-cell. infiltration, generation of free radicals are early events triggered in the defense reaction process. We suggest that active oxygen species stimulated the SA production, which in turn caused the stimulation of a number of defense reactions including PAL, peroxidase, and chitinase activities. Another signaling pathway seems to operate in parallel, corresponding to the activation of Lox, followed by JA and ethylene production. This pathway certainly reinforces the defense responses, since peroxidase and chitinase activities were abolished by co-infiltrating AVG and NA-cell., which stimulated the JA/ethylene pathway only. We evidenced a partial interaction between both signaling pathways, since SA caused an inhibition of the ethylene production, certainly to regulate the expression of defense-related genes.
Figure 12.
Cellulase-induced signaling pathway in melon cotyledons.
To improve resistance of melon plants to powdery mildew, cotyledons were treated by cellulase before inoculation. Our results have shown a significant decrease of powdery mildew symptoms. Treatment by A-cell.5 or A-cell.50 was more effective than NA-cell.5 or NA-cell.50 treatment, suggesting that the both SA and JA/ethylene pathways are necessary for the establishment of resistance. After treatments with A-cell.5, melon plants continue to grow and produce fruits, whereas A-cell.50 infiltration did not permit an equal development.
In natural conditions, we need to confirm if the non-pathogenic fungi Trichoderma is able to stimulate plant defense mechanisms. Recent findings indicated that application of Trichoderma harzianum to the rhizosphere of young cucumber seedlings initiated in the plants a range of morphological as well as biochemical changes, which are considered to be part of plant-defense responses (Yedidia et al., 1999). As with immunization, Trichoderma-inoculated plants may be sensitized to respond more rapidly and efficiently to pathogen attack. The present work reinforces the idea that cellulases produced by Trichoderma act as powerful elicitors of plant resistance, explaining a part of the biological control capacity assigned to Trichoderma. Our data have shown that cellulase treatments could be an interesting tool for the biological control of powdery mildew in field.
MATERIALS AND METHODS
Plant Material
One variety of melon (Cucumis melo), the Clipper variety, susceptible to powdery mildew (Sphaerotheca fuliginea), was used in this study.
Plants were grown in a greenhouse, and the temperature was maintained at 25°C. They were exposed to a normal 12-h light/dark cycle.
Fungal Culture and Inoculation
Powdery mildew was conserved on leaves of susceptible Clipper melon varieties. To maintain virulence, the fungus was periodically inoculated onto susceptible melon leaves. For inoculation, pieces of infected leaves were suspended on top of young 15-d-old plants.
The melon variety and the powdery mildew isolate used in this study were kindly provided by the ASL Society (Avignon, France).
Observation of Symptoms
Disease severity on cotyledons and leaves (percentage of powdery mildew infection) was recorded at various intervals until completion of the test (2 months after inoculation). Cotyledons were treated with cellulase 72 h before inoculation and each 2 weeks to maintain a good resistance level. After senescence of cotyledons, the following ranks of leaves were treated similarly.
Treatments with Cellulase
Cotyledons were syringe-infiltrated with cellulase (EC 3.2.1.4) from Trichoderma longibrachiatum (Megazyme International, Bray, County Wicklow, Ireland). This cellulase presents a single band on SDS-PAGE gels (Mr is 54,000) and a single major band on isoelectric focusing gels (pI = 4.7). Megazyme reports endo-1.3-β-glucanase, α-amylase, α-glucosidase, and β-glucosidase activities to be less than 0.002% of cellulase.
To determine the optimal dose for elicitation, different quantities (mg proteins mL−1 sodium acetate buffer, 0.025 mm, pH 7) of active cellulase (A-cell.) were infiltrated in melon cotyledons corresponding to: A-cell.0.5, 0.005 mg mL−1 (0.5 units mL−1); A-cell.1, 0.01 mg mL−1 (1 unit mL−1); A-cell.2, 0.019 mg mL−1 (2 units mL−1); A-cell.3, 0.029 mg mL−1 (3 units mL−1); A-cell.5, 0.048 mg mL−1 (5 units mL−1); A-cell.10, 0.097 mg mL−1 (10 units mL−1); A-cell.20, 0.194 mg mL−1 (20 units mL−1); A-cell.50, 0.485 mg mL−1 (50 units mL−1). For the nonactive cellulase (NA-cell.), the same quantities of each sample were heated 10 min at 90°C before infiltration. To verify protein denaturation, cellulase activity was measured according to the method of Kapat et al. (1998) after the heat treatment and was always zero.
Peroxidase Assay
Cotyledons were harvested at different times after infiltration of cellulase preparations. One gram of fresh cotyledon was mixed in 2 mL of sodium phosphate buffer (pH 5, 0.05 m). The extract was centrifuged at 10,000g during 5 min. Assays of peroxidase activities in the supernatant were carried out in a citrate-phosphate buffer (pH 6, 0.05 m), using gaïacol as the hydrogen donor. Activities were estimated from increase in A470. Total activity was expressed in nanokatals per milligrams of proteins.
Chitinase Assay
Assay for endochitinase activity was carried out according to Boller et al. (1983) and modified as followed. The reaction mixture contained 0.5 mg of colloidal chitin and various volumes of crude treated cotyledons extract in a final volume of 0.5 mL of 0.1 m sodium acetate (pH 5.2). This mixture was incubated on a test tube rotator at 37°C for 1 h. After incubation, the tubes were centrifuged at 10,000g for 10 min. The mixture was incubated at 37°C for 1 h, and then 0.1 mL of 0.6 m potassium tetraborate was added to the tubes before heating for 3 min. After rapid cooling, 1 mL of the reagent stock solution (10% [w/v] 4-methylamino-benzaldehyde in glacial acetic acid and 11.5 m HCl [87.5:12.5, v/v]) diluted 1:2 with glacial acetic acid was added. After incubation at 37°C for 20 min, the amount of liberated N-acetyl-glucosamine was determined spectrophotometrically at 585 nm. One katal was defined as the enzyme activity producing 1 mol of N-acetyl-glucosamine equivalents per second. Activity was expressed in nanokatals per milligram of apoplastic protein.
Assay of O2·−-Generating Activity of Cotyledon Discs
The O2·−-generating activity of cotyledon discs was assayed spectrophotometrically by measuring the reduction of exogeneously supplied cytochrome C at 550 nm as previously described (Martinez et al., 1998). DPI (20 μm), an inhibitor of the NADPH-oxidase, was added to the reaction buffer just before immersion of cotyledons discs in cytochrome C buffer.
Lox Assay
Fresh cotyledons were harvested at different time after A-cell. and NA-cell. infiltration. They were mixed in 1.5 mL of 0.05 m potassium phosphate (pH 7.0) using a grinder (Ultra-turax, Janke and Kunkel, IKA Labortechnik, Germany). The homogenate was centrifuged 5 min at 10,000g. Lox activity was determined on 1 mL of enzyme solution plus 9 mL of 0.5 mL Tween 20, 6.7 mL of 0.05 m potassium phosphate (pH 9), 0.5 mL of oleic acid (control), 1.3 mL of 1 n NaOH, mixed until the solution was clear and transparent and then was diluted to 200 mL with 0.05 m potassium phosphate (pH 5). The combined enzyme-substrate solution was mixed continuously with O2 for 10 min at 20°C. Then 1 mL of the enzyme substrate reaction solution was combined with 2 mL 100% ethanol, and optical density of the alcoholic solution was measured at 234 nm against oleic acid control.
Quantitative Analysis of JA
At different times after cellulase infiltration, 5 g of fresh treated-cotyledons of melon plants were shock-frozen in liquid N2. The frozen tissues were thawed in 10 mL of ethanol. The different extractions steps were then made according to Gundlach et al. (1992). JA was then analyzed by GC/mass spectrometry (Saturn 2100, Varian, Middleburg, The Netherlands). The separations were performed on a DB-5 (30- × 0.25-mm) column (J&W Scientific, Folsom, CA). Diethyldithiocarbamic acid, an inhibitor of jasmonate biosynthesis dissolved in 15 mm K2HPO4, pH 7, was infiltrated in a same time than cellulase.
GC Analysis of Ethylene
At each time after infiltration of A-cell. and NA-cell., 10 cotyledon-treated plants were enclosed in stoppered 400-mL glass bottles for 24 h. Samples of air were then withdrawn by syringe and injected into a gas chromatograph (Shimadzu CG 8A)/flame ionization detector equipped with a PoraPak T column (2 m × 0.5 mm; Touzard et Matignon, Vitry sur Seine, France). The carrier gas was nitrogen, and the injection temperature was 90°C. Experiments were run in duplicate.
Inhibition of ethylene biosynthesis was achieved by injecting cotyledons with a solution of 0.1 mm of AVG (100 μL cm−2). Ethylene action was inhibited by spraying plants with a solution of 50 μm silver thiosulfate containing 0.01% (v/v) Tween 20.
PAL Analysis
Cotyledons treated by A-cell. and NA-cell. were mixed in 0.1 m borate buffer, pH 8.8, containing 17 mm β-mercaptoethanol. The mixture was centrifuged at 12,000g for 30 min, and 50 to 100 μL of the supernatant was used for the enzymatic assays. PAL activity was assayed as described previously (Pellegrini et al., 1994) and was expressed in nanokatals per gram of fresh weight.
HPLC Analysis of SA
Apoplastic washing fluids (AWF) were prepared by vacuum infiltration of petioles of fresh cotyledons treated with cellulase or water (Rasmussen et al., 1991). Petioles were cut and were washed with distilled water. They were immersed for 15 min in 50 mm sodium acetate buffer, pH 6. Vacuum was applied and slowly released. Petioles were then introduced vertically in Eppendorf tubes and centrifuged at 5,000g for 5 min, and 20 to 30 μL of AWF were obtained per gram of petiole.
An equal volume of methanol was added to the AWF. SA analysis was carried out by HPLC on a C18 column (LiChrospher 100 RP-18, 250 × 4.6 mm; 5 μm; Alltech, Deerfield, IL) equilibrated with 5% (v/v) buffered acetonitrile (50 mm sodium acetate buffer, pH 4.5). SA was eluted isocratically 15 min following injection and detected by fluorescence (excitation, 290 nm; emission, 402 nm). Concentration was determined using a linear range of calibration standards consisting in 0 to 2 μg/50 μL of SA (Sigma, St. Louis). SA concentration was expressed in μg SA g−1 of fresh weight.
Infiltration of SA in Melon Cotyledons
Cotyledons were syringe infiltrated with SA (800 μm). The SA solution was prepared by titration with 0.1 m NaOH to a pH value around 7.0.
ACKNOWLEDGMENTS
The authors which to thank the ASL society (Avignon, France), which provided the melon variety and the powdery mildew isolate used in this study. We kindly acknowledge Professor John W. Mansfield (Imperial College of Science, Technology and Medicine, Department of Biology, University of London, Kent, UK) and Dr. Alain Clerivet (Institut de Recherche pour le Développement, Unité de Recherche Résistance des Plantes, Montpellier, France) for critical reading of the manuscript.
LITERATURE CITED
- Avni A, Bailey BA, Mattoo AK, Anderson JD. Induction of ethylene biosynthesis in Nicotiana tabacum by a Trichoderma viride xylanase is correlated to the accumulation of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase transcripts. Plant Physiol. 1994;106:1049–1055. doi: 10.1104/pp.106.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou N, Nicole M. Cell biology of plant immunization against microbial infection: the potential of induced resistance in controlling plant diseases. Plant Physiol Biochem. 1999;37:703–719. [Google Scholar]
- Boller T. Ethylene in pathogenesis and disease resistance. In: Mattoo AK, JC Suttle AK, editors. The Plant Hormone Ethylene. Boca Raton, FL: CRC Press; 1991. pp. 293–314. [Google Scholar]
- Boller T, Gehri A, Mauch F, Vögeli U. Chitinases in bean leaves: induction by ethylene, purification, properties, and possible function. Planta. 1983;157:22–31. doi: 10.1007/BF00394536. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Butt VS, Davies DI, Zimmerlin A. The origin of the oxidative burst in plants. Free Radic Res. 1995;11:517–532. doi: 10.3109/10715769509065273. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auch CK, Murphy TM. Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distincts mechanisms. Plant Physiol. 1998;116:1379–1385. doi: 10.1104/pp.116.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheli P, Doares SH, Albersheim P, Darvill A. Host-pathogens interactions: XXXVI. Partial purification and characterization of heat-labile molecules secreted by the rice blast pathogens that solubilize plant cell wall fragments that kill plant cells. Physiol Mol Plant Pathol. 1990;36:159–173. [Google Scholar]
- Calderon AA, Zapata JM, Barcelo AR. Peroxidase-mediated formation of resveratrol oxidation products during the hypersensitive-like reaction of grapevine cells to an elicitor from Trichoderma viride. Physiol Mol Plant Pathol. 1994;44:289–299. [Google Scholar]
- Calderon AA, Zapata JM, Munoz R, Pedreno MA, Barcelo AR. Resveratrol production as a part of the hypersensitive-like response of grapevine cells to an elicitor from Trichoderma viride. New Phytol. 1993;124:455–463. [Google Scholar]
- Chet I. Trichoderma: application, mode of action, and potential as biocontrol agent of soilborne plant pathogenic fungi. In: Chet I, editor. Innovative Approaches to Plant Disease Control. New York: John Wiley & Sons; 1987. pp. 137–160. [Google Scholar]
- Davis KR, Hahlbrock K. Induction of defense responses in culture parsley cells by plant cell wall fragments. Plant Physiol. 1987;84:1286–1290. doi: 10.1104/pp.84.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney T, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- Doke N, Miura Y, Sanchez LM, Park HJ, Noritake T, Yoshiora H, Kawakita K. The oxidative burst protects plants against pathogen attack: mechanism and role as an emergency signal for plant bio-defense: a review. Gene. 1996;179:45–51. doi: 10.1016/s0378-1119(96)00423-4. [DOI] [PubMed] [Google Scholar]
- Dong X. SA, JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol. 1998;1:316–323. doi: 10.1016/1369-5266(88)80053-0. [DOI] [PubMed] [Google Scholar]
- Enkerli J, Felix G, Boller T. The enzymatic activity of fungal xylanase is not necessary for its elicitor activity. Plant Physiol. 1999;121:391–397. doi: 10.1104/pp.121.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor HH. Current status of gene-for-gene concept. Annu Rev Phytopathol. 1971;9:275–296. [Google Scholar]
- Fuchs Y, Saxena A, Gamble HR, Anderson JD. Ethylene biosynthesis-inducing protein from cellulysin is an endoxylanase. Plant Physiol. 1989;89:138–143. doi: 10.1104/pp.89.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman-Matarasso N, Cohen E, Du Q, Chejanovsky N, Hanania U, Avni A. A point mutation in the ethylene-inducing xylanase elicitor inhibits the B-1-4-endoxylanase activity but not the elicitation activity. Plant Physiol. 1999;121:345–352. doi: 10.1104/pp.121.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisalberti EL, Sivasithamparam K. Antifungal antibiotics produced by Trichoderma spp. Soil Biol Biochem. 1991;23:1011–1020. [Google Scholar]
- Gundlach H, Müller MJ, Kutchan TM, Zenk MH. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA. 1992;89:2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MG, Darvill AG, Albersheim P. Host-pathogen interactions: XIX. The endogenous elicitor, a fragment of a plant cell wall polysaccharide that elicits phytoalexin accumulation in soybeans. Plant Physiol. 1981;68:1161–1169. doi: 10.1104/pp.68.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanania U, Avni A. High-affinity binding site for ethylene-inducing xylanase elicitor on Nicotiana tabacum membranes. Plant J. 1997;12:113–120. [Google Scholar]
- Haran S, Schickler H, Chet I. Differential expression of Trichoderma harzianum chitinases during mycoparasitism. Phytopathology. 1996;86:980–985. [Google Scholar]
- Kapat A, Zimand G, Elad Y. Effect of two isolates of Trichoderma harzianum on the activity of hydrolytic enzymes produced by Botrytis cinerea. Physiol Mol Plant Pathol. 1998;52:127–137. [Google Scholar]
- Klessig DF, Durner J, Shah J, Yang Y. Salicylic acid-mediated signal transduction in plant disease resistance. In: Romeo JT, Downum KR, Verpoorte R, editors. Phytochemical Signals and Plant-Microbe Interactions. New York: Plenum Press; 1998. pp. 119–137. [Google Scholar]
- Lotan T, Fluhr R. Xylanase, a novel elicitor of pathogenesis-related proteins in tobacco, uses a non-ethylene pathway for induction. Plant Physiol. 1990;93:811–817. doi: 10.1104/pp.93.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Baccou JC, Bresson E, Bessac Y, Daniel JF, Jalloul A, Montillet JL, Geiger JP, Assigbetsé K, Nicole M. Salicylic acid mediated by the oxidative burst is a key molecule in local and systemic response of cotton challenged by an avirulent race of Xanthomonas campestris pv. malvacearum. Plant Physiol. 2000;122:757–766. doi: 10.1104/pp.122.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Geiger JP, Bresson E, Daniel JF, Dai GH, Andary C, Nicole M. Isoperoxidases are associated with resistance of cotton to Xanthomonas campestris pv. malvacearum (race 18) In: Obinger O, Burner U, Ebermann R, Penel C, Greppin H, editors. Plant Peroxidases: Biochemistry and Physiology. Vienna: University of Agriculture; 1996. , University of Geneva, pp 327–332. [Google Scholar]
- Martinez C, Montillet JL, Bresson E, Agnel JP, Dai GH, Daniel JF, Geiger JP, Nicole M. Apoplastic NADH-peroxidase generates superoxide anions in cells of cotton cotyledons undergoing the hypersensitive reaction to Xanthomonas campestris pv. malvacearum race 18. Mol Plant Microbe Interact. 1998;11:1038–1047. [Google Scholar]
- Melan MA, Dong X, Endara ME, Davis KR, Ausubel FM, Peterman TK. An Arabidopsis thaliana lipoxygenase gene can be induced by pathogens, abscisic acid, and methyl jasmonate. Plant Physiol. 1993;101:441–450. doi: 10.1104/pp.101.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalbini P, Buonaurio R, Umesh-Kumar NN. Peroxidase activity and isoperoxidase pattern in tobacco leaves infected with tobacco necrosis virus and other viruses inducing necrotic and non necrotic alterations. J Phytopathol. 1995;143:295–301. [Google Scholar]
- Nicholson RL, Hammerschmidt R. Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol. 1992;30:369–389. [Google Scholar]
- Nothnagel EA, McNeil M, Albersheim P, Dell A. Host-pathogen interactions: XXII. A galacturonic acid oligosaccharide from plant cell walls elicits phytoalexins. Plant Physiol. 1983;71:916–926. doi: 10.1104/pp.71.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L, Rohfritsch O, Fritig B, Legrand M. Phenylalanine ammonia-lyase in tobacco: molecular cloning and gene expression during the hypersensitive reaction to tobacco mosaic virus and the response to a fungal elicitor. Plant Physiol. 1994;106:877–886. doi: 10.1104/pp.106.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Cortés H, Albrecht T, Prat S, Weiler EW, Willmitzer L. Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta. 1993;191:123–128. [Google Scholar]
- Penninckx IAMA, Thomma BPHJ, Buchala A, Métraux JP, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel J, Atzorn R, Gabler R, Kühnemann F, Boland W. Cellulysin from the plant parasitic fungus Trichoderma viride elicits volatile biosynthesis in higher plants via the octadecanoid signalling cascade. FEBS Lett. 1997;416:143–148. doi: 10.1016/s0014-5793(97)01169-1. [DOI] [PubMed] [Google Scholar]
- Rasmussen JB, Hammerschmidt R, Zook MN. Systemic induction of salicylic acid accumulation in cucumber after inoculation with Pseudomonas syringae pv. syringae. Plant Physiol. 1991;97:1342–1347. doi: 10.1104/pp.97.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai AS, Manocha MS. Chitinases of fungi and plants: their involvement in morphogenesis and host-parasite interaction. FEMS Microbiol Rev. 1993;11:317–338. [Google Scholar]
- Salmond GPC. Secretion of extracellular virulence factors by plant pathogenic bacteria. Annu Rev Phytopathol. 1994;32:181–200. [Google Scholar]
- Seo S, Sano H, Ohashi Y. Jasmonic acid in wound signal transduction pathways. Physiol Plant. 1997;101:740–745. [Google Scholar]
- Sharon A, Fuchs Y, Anderson JD. The elicitation of ethylene biosynthesis by a Trichoderma xylanase is not related to the cell wall degradation activity of the enzyme. Plant Physiol. 1993;102:1325–1329. doi: 10.1104/pp.102.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan A, Chet I. Integrated control of Fusarium crown and root rot of tomato with Trichoderma harzianum in combination with methyl bromide or soil solarization. Crop Protection. 1993;12:380–386. [Google Scholar]
- Vidal S, Erikson ARB, Montesano M, Denecke J, Palva ET. Cell wall-degrading enzyme from Erwinia carotovora cooperate in the salicylic acid-independant induction of a plant defense response. Mol Plant-Microbe Interact. 1998;11:23–32. [Google Scholar]
- Walton JD. Deconstructing the cell wall. Plant Physiol. 1994;104:1113–1118. doi: 10.1104/pp.104.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano A, Suzuki K, Uchimiya H, Shinshi H. Induction of hypersensitive cell death by a fungal protein in culture of tobacco cells. Mol Plant-Microbe Interact. 1998;11:115–123. [Google Scholar]
- Yedidia I, Benhamou N, Chet I. Induction of defense responses in cucumber plants (Cucumis sativus L) by the biocontrol agent Trichoderma harzianum. Appl Environ Microbiol. 1999;65:1061–1070. doi: 10.1128/aem.65.3.1061-1070.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]