Abstract
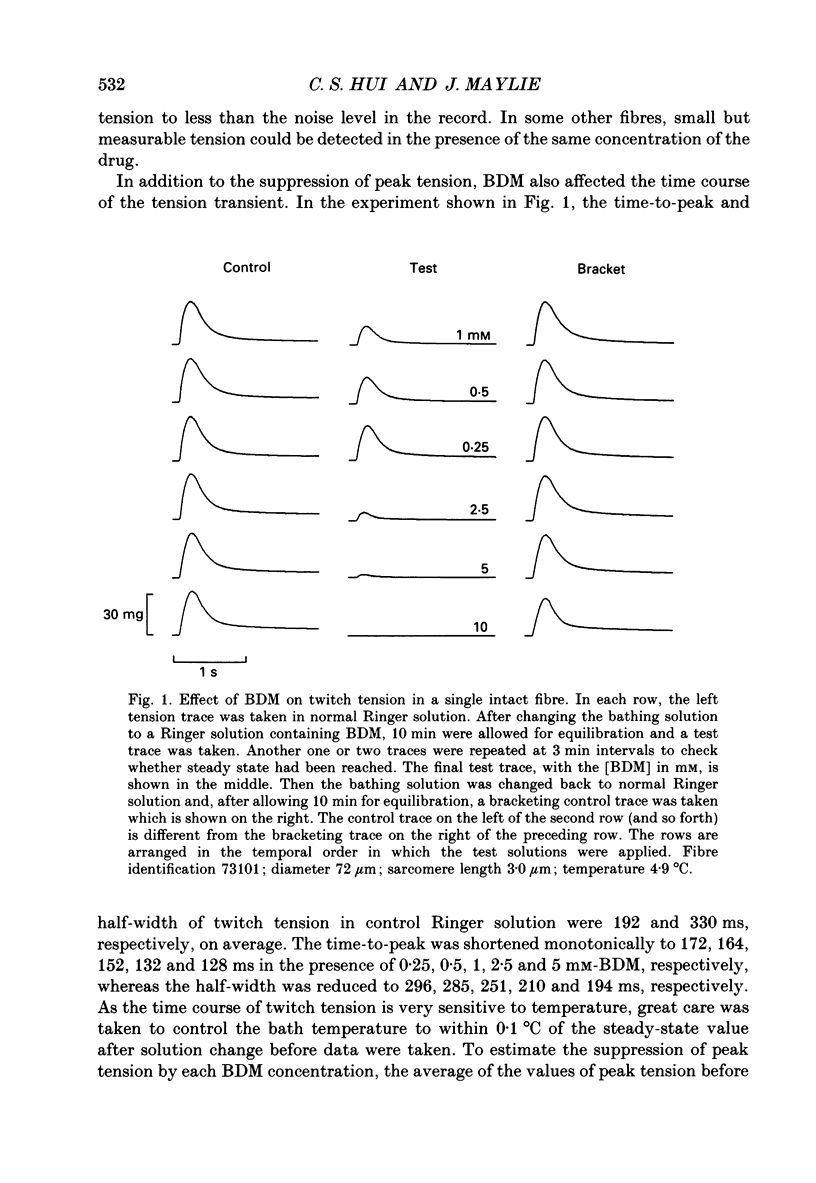
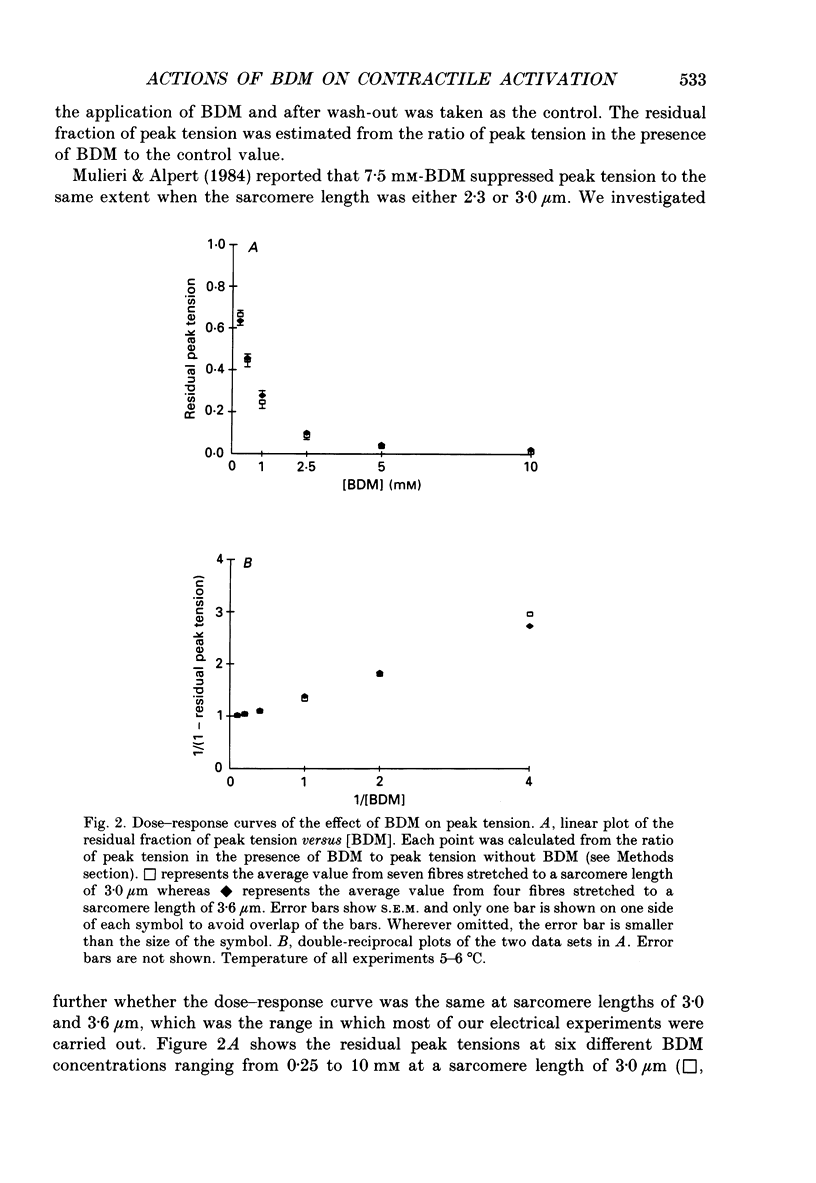
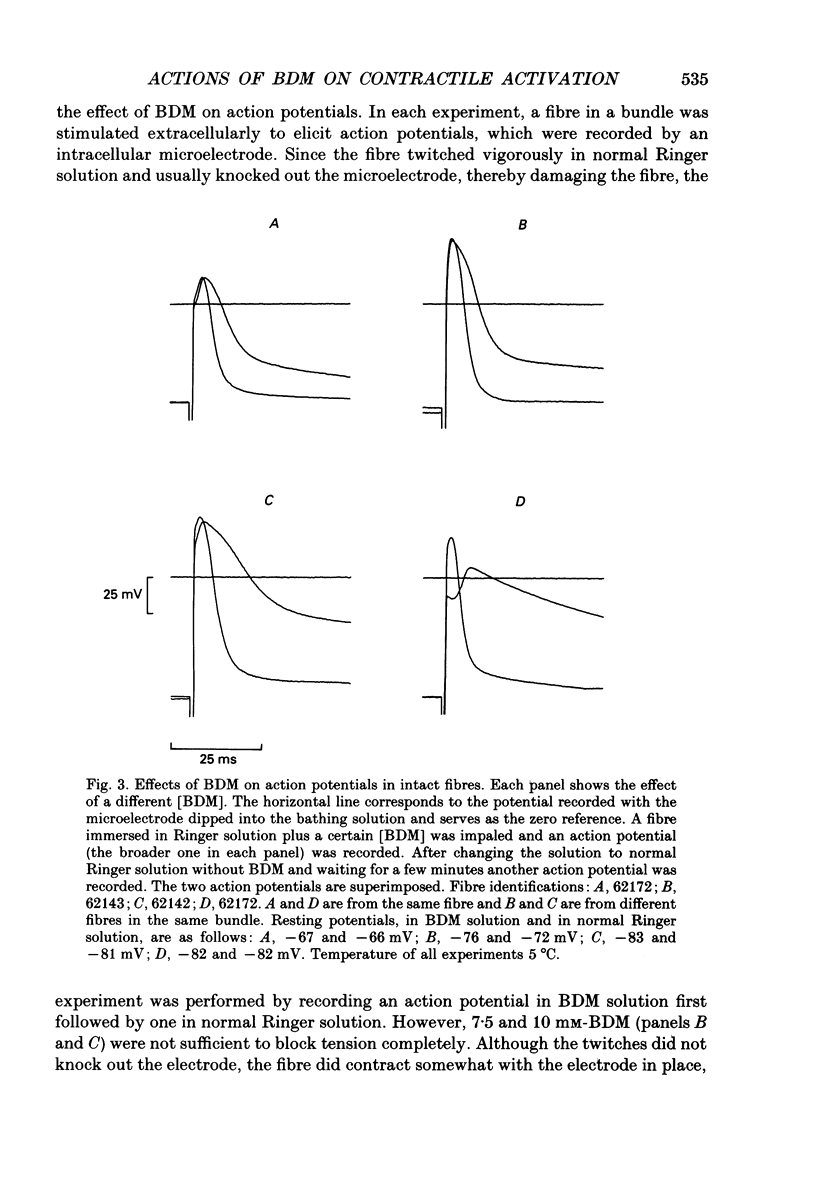
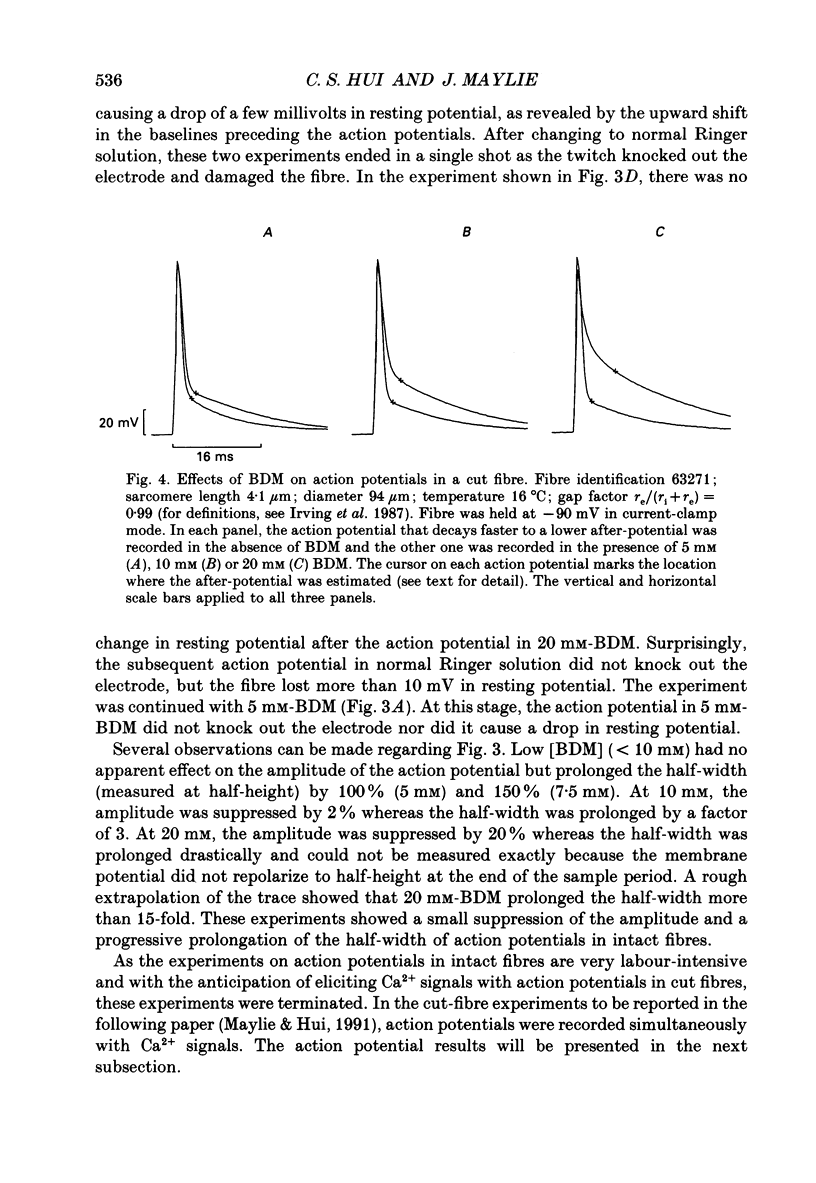
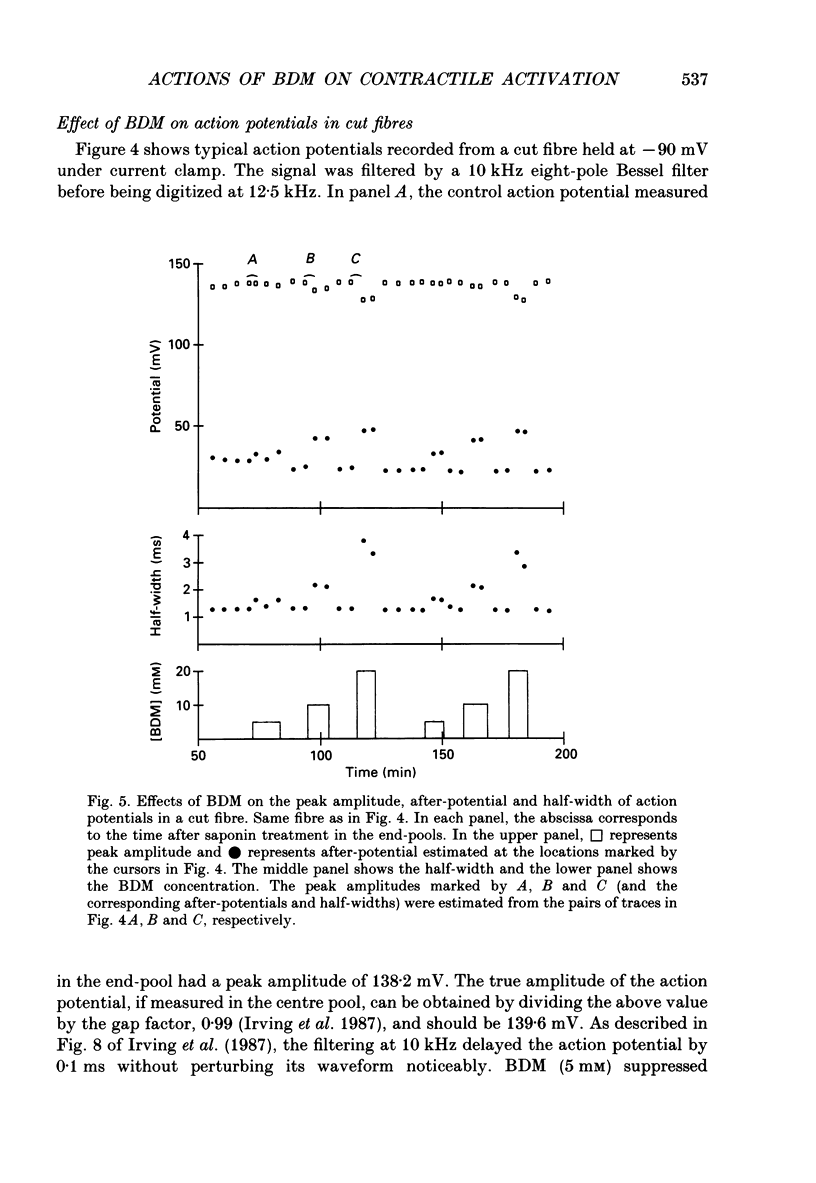
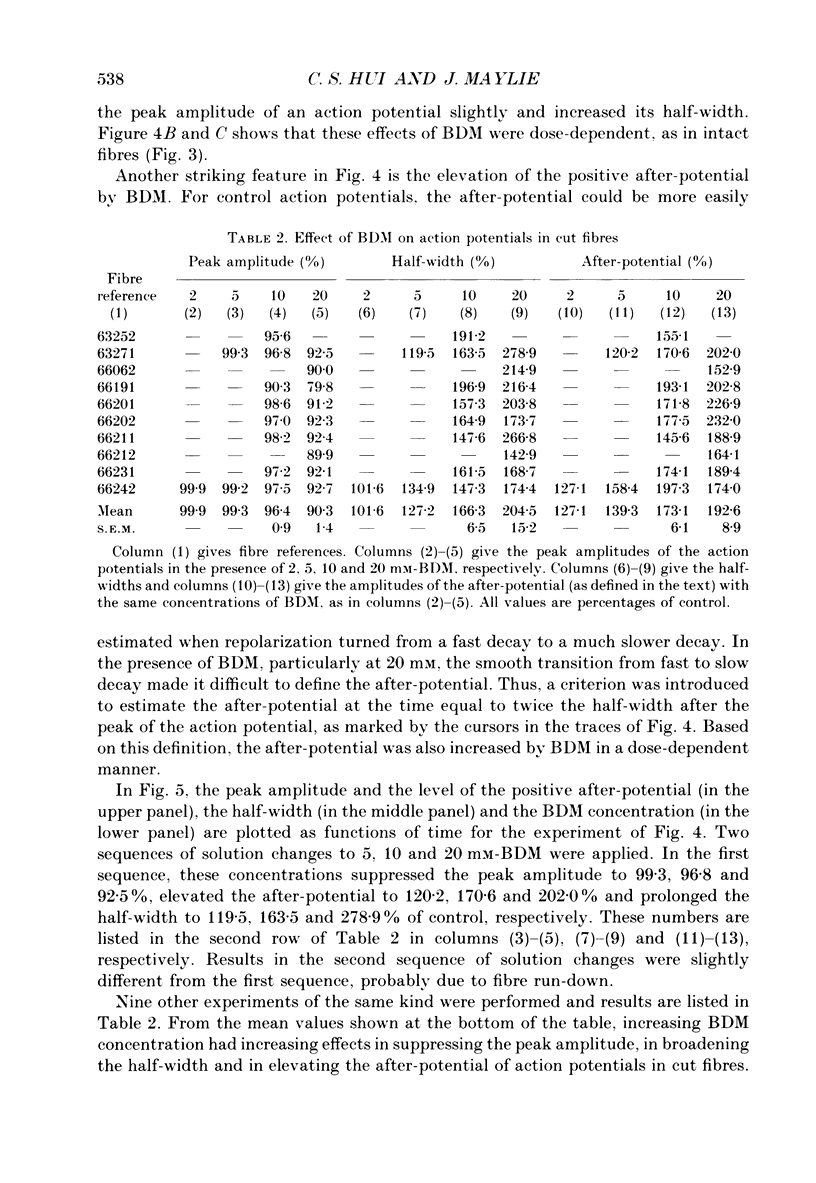
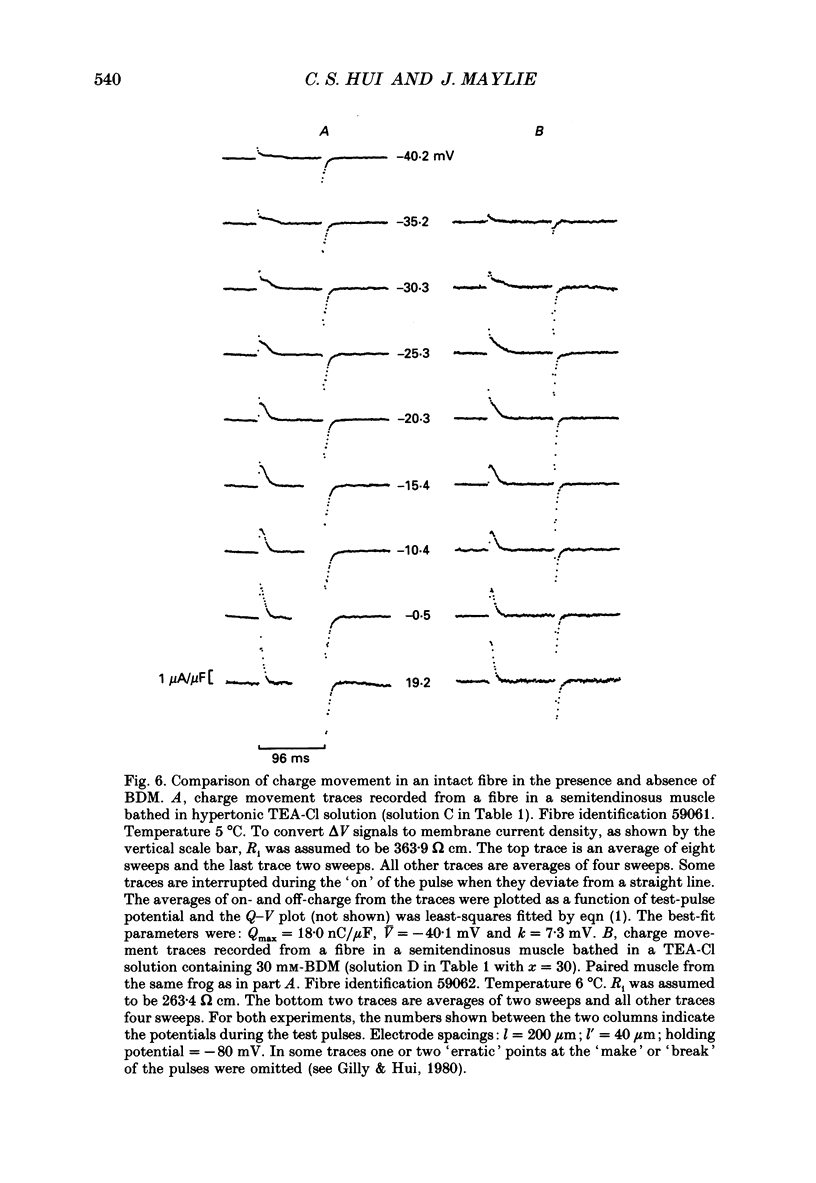
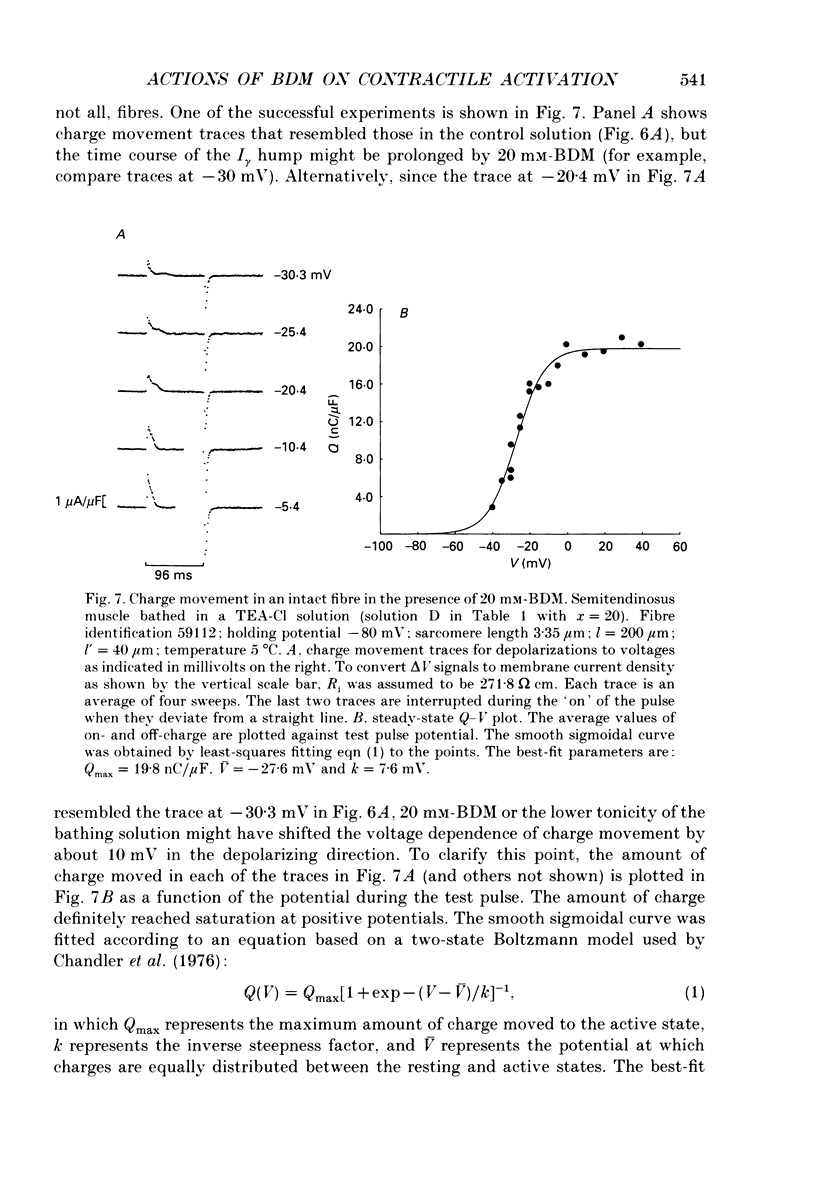
1. The effects of 2,3-butanedione monoxime (BDM) on various steps in the excitation-contraction coupling sequence, including action potential, charge movement and twitch tension, were studied in twitch fibres of Rana temporaria. 2. The resting potential of intact fibres in whole muscle bathed in 20 mM-BDM was the same as control. The resting potential also remained stable after more than 100 min in 20 mM-BDM. 3. The action potential was measured in intact fibres of fibre bundles with an intracellular microelectrode. Applications of 5 and 7.5 mM-BDM had no effect on its amplitude, whereas 10 and 20 mM suppressed its amplitude by about 4 and 10%, respectively. Increasing concentrations of BDM prolonged the half-width and elevated the after-potential of the action potential progressively. The action potential was also measured in cut fibres mounted in a double Vaseline-gap chamber. Results were similar to those in intact fibres. 4. Charge movement was measured in intact fibres of halved muscles with the three-microelectrode voltage-clamp technique. The steady-state Q-V plot of the total charge measured in isotonic tetraethylammonium (TEA) Ringer solution with 20 mM-BDM appeared to be shifted about 10 mV in the depolarizing direction and to be slightly more shallow when compared with the control Q-V plot measured in hypertonic TEA Ringer solution with 350 mM-sucrose. After allowing for the voltage shift, 20 mM-BDM did not appear to affect the kinetics of both components of charge movement, but suppressed the maximum amount of total charge by about one-quarter. 5. Charge movement was also measured in cut fibres with the double Vaseline-gap voltage-clamp technique. In the presence of 20 mM-BDM, charge movement traces resembled those from intact fibres. Twenty millimolar BDM suppressed the maximum amount of total charge by about one-quarter, as in intact fibres. The steady-state Q-V plots from cut fibres were separated into Q beta (early current) and Q gamma (late hump current) components by least-squares fitting with a sum of two Boltzmann distribution functions. On average, 20 mM-BDM suppressed Q beta and Q gamma in roughly equal proportion, but did not affect the individual voltage distributions of Q beta and Q gamma. 6. Twitch tension was measured in single intact fibres stimulated extracellularly. BDM effectively reduced the peak amplitude, the time-to-peak and the half-width of twitch tension. The interaction of BDM with receptors appeared to follow more or less a simple 1:1 binding in fibres stretched to sarcomere lengths of about 3.6 microns.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKEW B. M. Oximes and hydroxamic acids as antidotes in anticholinesterase poisoning. Br J Pharmacol Chemother. 1956 Dec;11(4):417–423. doi: 10.1111/j.1476-5381.1956.tb00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Peres A. Charge movement and membrane capacity in frog muscle. J Physiol. 1979 Apr;289:83–97. doi: 10.1113/jphysiol.1979.sp012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Best P. M. Effects of tetracaine on displacement currents and contraction of frog skeletal muscle. J Physiol. 1976 Nov;262(3):583–611. doi: 10.1113/jphysiol.1976.sp011611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hui C. S. Membrane capacitance in frog cut twitch fibers mounted in a double vaseline-gap chamber. J Gen Physiol. 1990 Aug;96(2):225–256. doi: 10.1085/jgp.96.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cojocaru Z., Serban F., Năstasă V., Ungureanu M., Lazăr M. I. Sinteza diacetilmonoximei, reactiv pentru dozarea ureei din lichide biologice. Rev Med Chir Soc Med Nat Iasi. 1985 Oct-Dec;89(4):661–662. [PubMed] [Google Scholar]
- Fryer M. W., Gage P. W., Neering I. R., Dulhunty A. F., Lamb G. D. Paralysis of skeletal muscle by butanedione monoxime, a chemical phosphatase. Pflugers Arch. 1988 Jan;411(1):76–79. doi: 10.1007/BF00581649. [DOI] [PubMed] [Google Scholar]
- Gilly W. F., Hui C. S. Voltage-dependent charge movement in frog slow muscle fibres. J Physiol. 1980 Apr;301:175–190. doi: 10.1113/jphysiol.1980.sp013197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuti K., Higuchi H., Umazume Y., Konishi M., Okazaki O., Kurihara S. Mechanism of action of 2, 3-butanedione 2-monoxime on contraction of frog skeletal muscle fibres. J Muscle Res Cell Motil. 1988 Apr;9(2):156–164. doi: 10.1007/BF01773737. [DOI] [PubMed] [Google Scholar]
- Huang C. L. Pharmacological separation of charge movement components in frog skeletal muscle. J Physiol. 1982 Mar;324:375–387. doi: 10.1113/jphysiol.1982.sp014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S., Chandler W. K. Intramembranous charge movement in frog cut twitch fibers mounted in a double vaseline-gap chamber. J Gen Physiol. 1990 Aug;96(2):257–297. doi: 10.1085/jgp.96.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S. Contractile properties of frog twitch fibres after D600 paralysis. J Muscle Res Cell Motil. 1989 Dec;10(6):473–487. doi: 10.1007/BF01771823. [DOI] [PubMed] [Google Scholar]
- Hui C. S. D600 binding sites on voltage-sensors for excitation-contraction coupling in frog skeletal muscle are intracellular. J Muscle Res Cell Motil. 1990 Dec;11(6):471–488. doi: 10.1007/BF01745215. [DOI] [PubMed] [Google Scholar]
- Hui C. S. Differential properties of two charge components in frog skeletal muscle. J Physiol. 1983 Apr;337:531–552. doi: 10.1113/jphysiol.1983.sp014640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S., Milton R. L. Suppression of charge movement in frog skeletal muscle by D600. J Muscle Res Cell Motil. 1987 Jun;8(3):195–208. doi: 10.1007/BF01574588. [DOI] [PubMed] [Google Scholar]
- Hui C. S. Pharmacological studies of charge movement in frog skeletal muscle. J Physiol. 1983 Apr;337:509–529. doi: 10.1113/jphysiol.1983.sp014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M., Maylie J., Sizto N. L., Chandler W. K. Intrinsic optical and passive electrical properties of cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):1–40. doi: 10.1085/jgp.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs L., Rios E., Schneider M. F. Measurement and modification of free calcium transients in frog skeletal muscle fibres by a metallochromic indicator dye. J Physiol. 1983 Oct;343:161–196. doi: 10.1113/jphysiol.1983.sp014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Sperelakis N., Teneick R. E., Solaro R. J. Effects of diacetyl monoxime on cardiac excitation-contraction coupling. J Pharmacol Exp Ther. 1985 Mar;232(3):688–695. [PubMed] [Google Scholar]
- Maylie J., Hui C. S. Action of 2,3-butanedione monoxime on calcium signals in frog cut twitch fibres containing antipyrylazo III. J Physiol. 1991 Oct;442:551–567. doi: 10.1113/jphysiol.1991.sp018808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Chandler W. K. Comparison of arsenazo III optical signals in intact and cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):41–81. doi: 10.1085/jgp.89.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Schneider M. F., Simon B. J., Szucs G. Intramembrane charge movement and calcium release in frog skeletal muscle. J Physiol. 1986 Apr;373:481–511. doi: 10.1113/jphysiol.1986.sp016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada H., Sada S., Sperelakis N. Effects of diacetyl monoxime (DAM) on slow and fast action potentials of young and old embryonic chick hearts and rabbit hearts. Eur J Pharmacol. 1985 Jun 7;112(2):145–152. doi: 10.1016/0014-2999(85)90490-x. [DOI] [PubMed] [Google Scholar]
- Taylor S. R., Rüdel R., Blinks J. R. Calcium transients in amphibian muscle. Fed Proc. 1975 Apr;34(5):1379–1381. [PubMed] [Google Scholar]
- Vergara J., Caputo C. Effects of tetracaine on charge movements and calcium signals in frog skeletal muscle fibers. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1477–1481. doi: 10.1073/pnas.80.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins J. R., Reiser J., Fitzpatrick D. F., Bergey J. L. Inotropic actions of diacetyl monoxime in cat ventricular muscle. J Pharmacol Exp Ther. 1980 Feb;212(2):217–224. [PubMed] [Google Scholar]


