Abstract
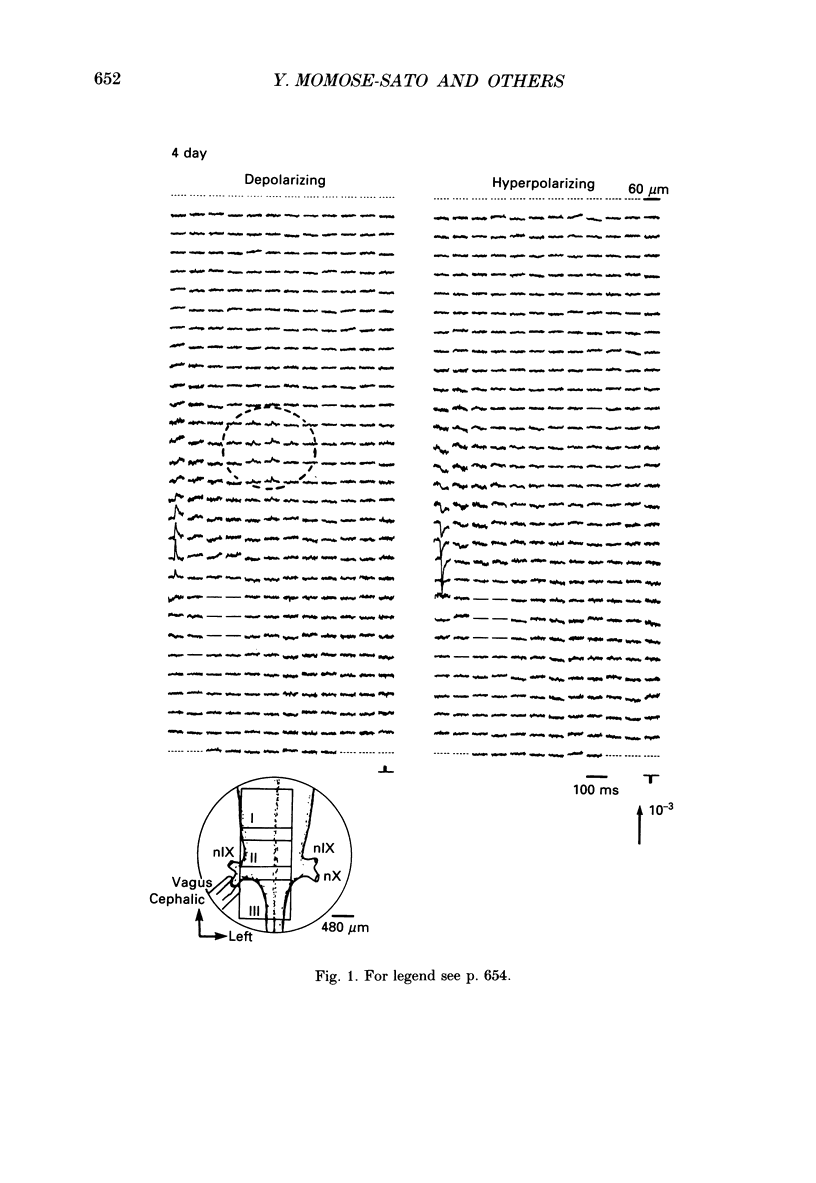
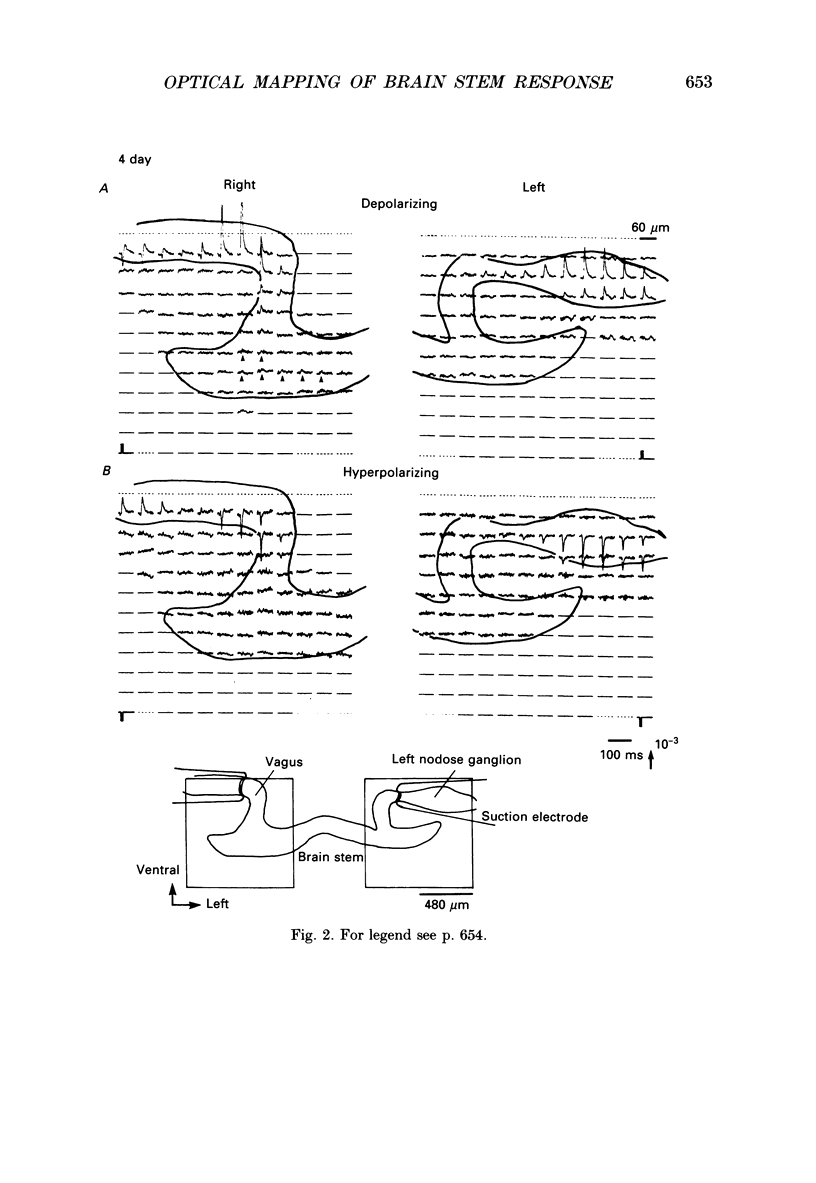
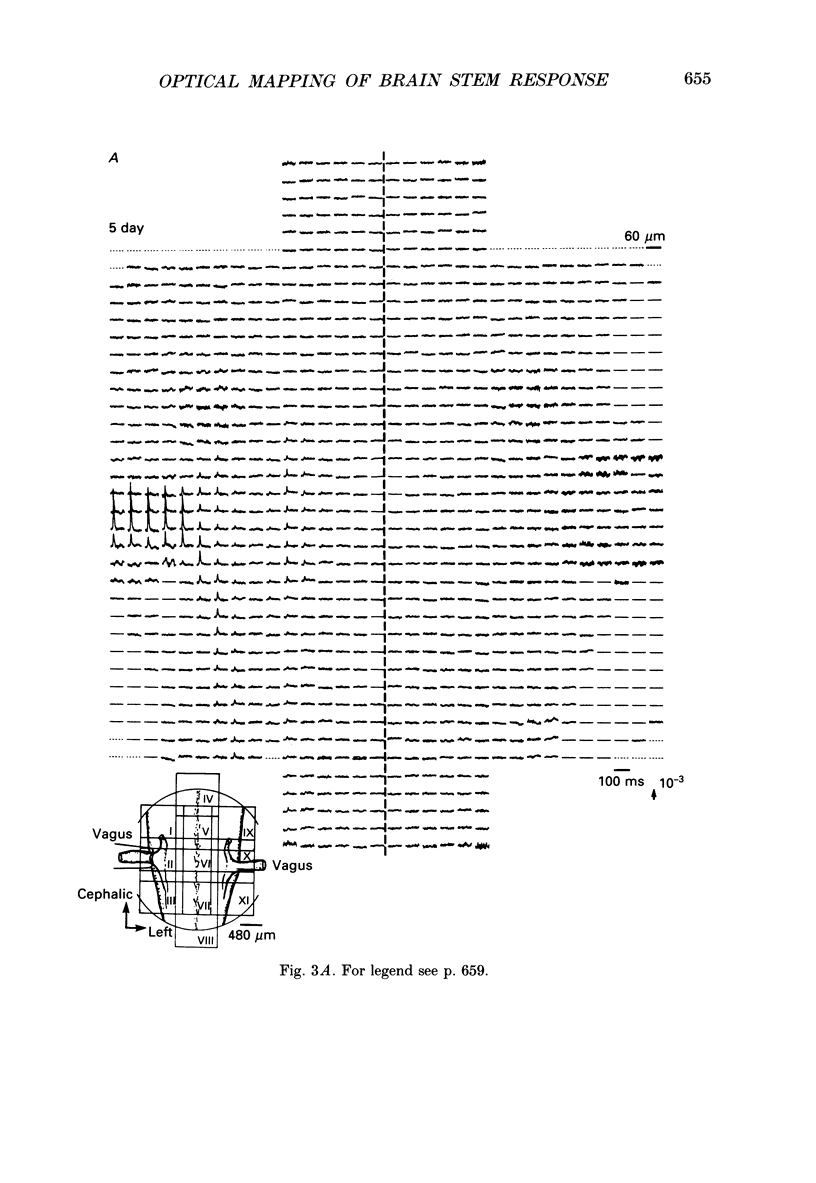
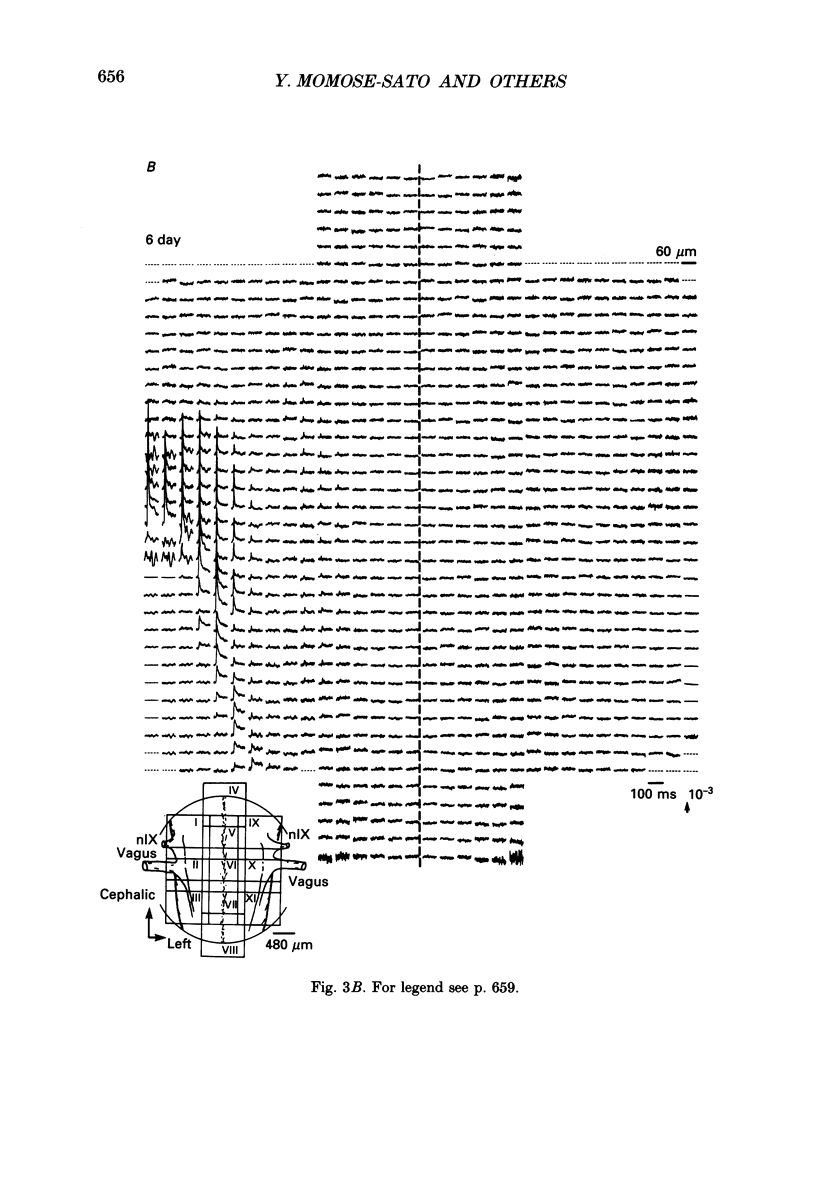
1. In both intact and slice preparations of vagus-brain stem isolated from 3- to 8-day-old chick embryos, the spatial pattern of neural responses to vagal stimulation and its development were assessed by means of multiple-site optical recording of electrical activity, using a voltage-sensitive merocyanine-rhodanine dye (NK2761) and a 12 x 12-element photodiode array. 2. The first neural responses, viz. fast optical signals (related to the action potential), were recorded in the 4-day-old brain stem preparation, and slow optical signals (related to excitatory postsynaptic potentials) were detected from late 7- and 8-day-old brain stem preparations. 3. The evoked optical signals appeared to be concentrated longitudinally in the central region of the stimulated side of the intact brain stem preparation and in a limited dorsal area in the slice preparation. The signal size gradually increased and the response area expanded as development proceeded. 4. Based on the above results, we have constructed developmental maps of the spatial patterns of the fast and slow optical responses. In the maps, the positions of the peak-size regions of the fast and slow signals were assessed and we have found that there were differences in the location of these areas for the fast vs. the slow signals in the late 7- and 8-day-old embryonic brain stem preparations. 5. In the maps for the late 7- and 8-day-old embryonic brain stems, the fast signal response area seems to correspond to the dorsal motor nucleus of the vagus nerve and the slow response area to the nucleus tractus solitarii.
Full text
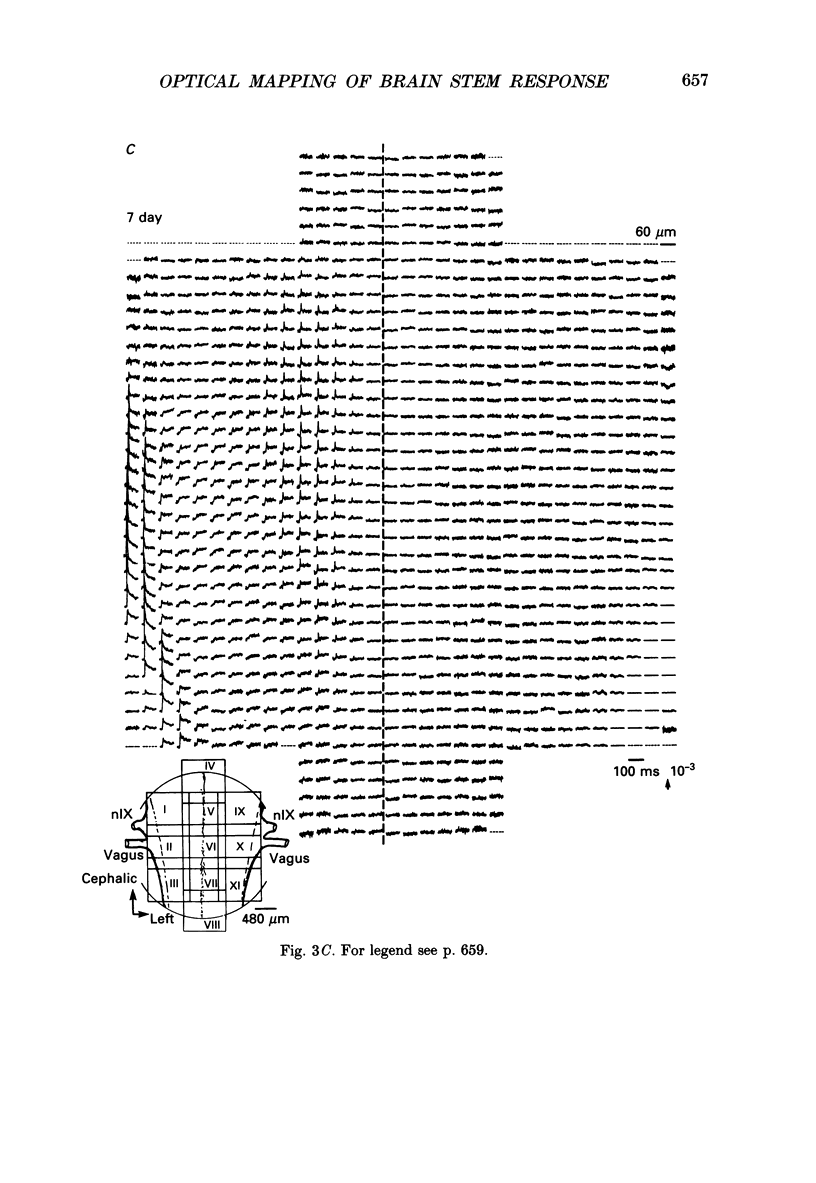
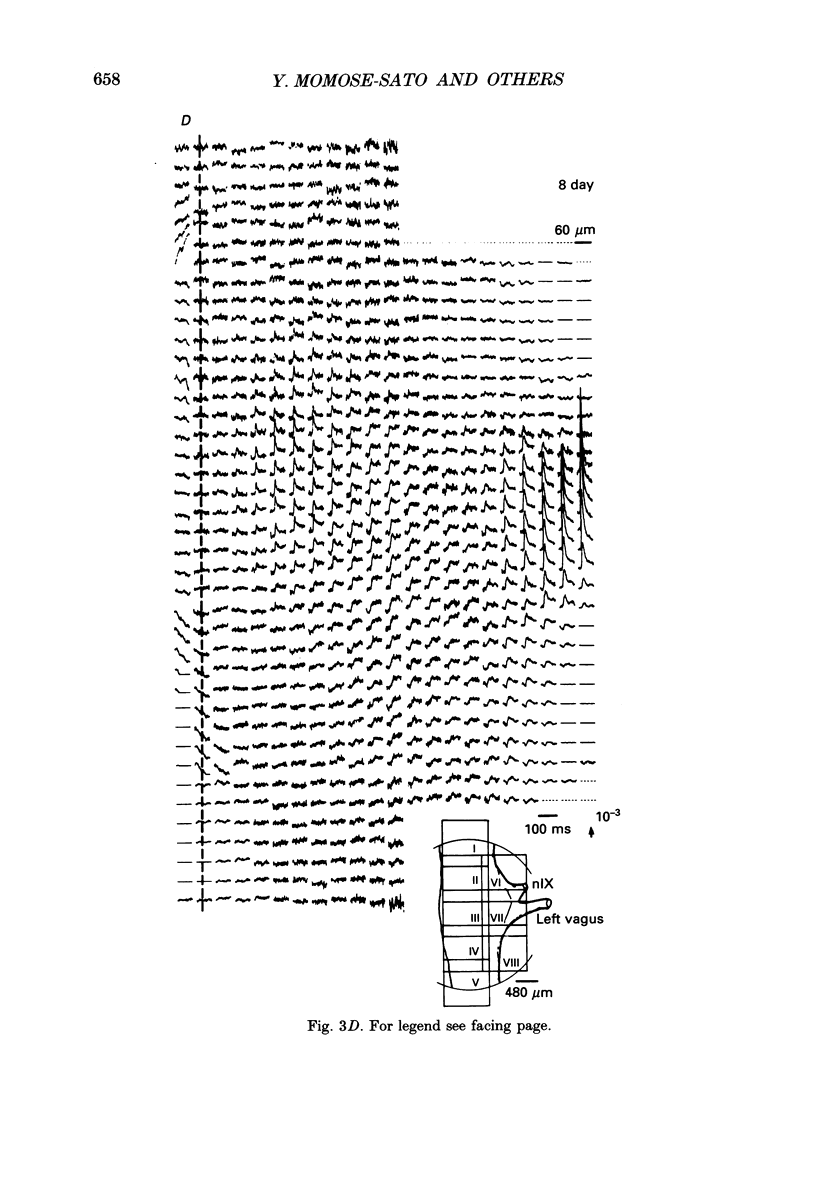
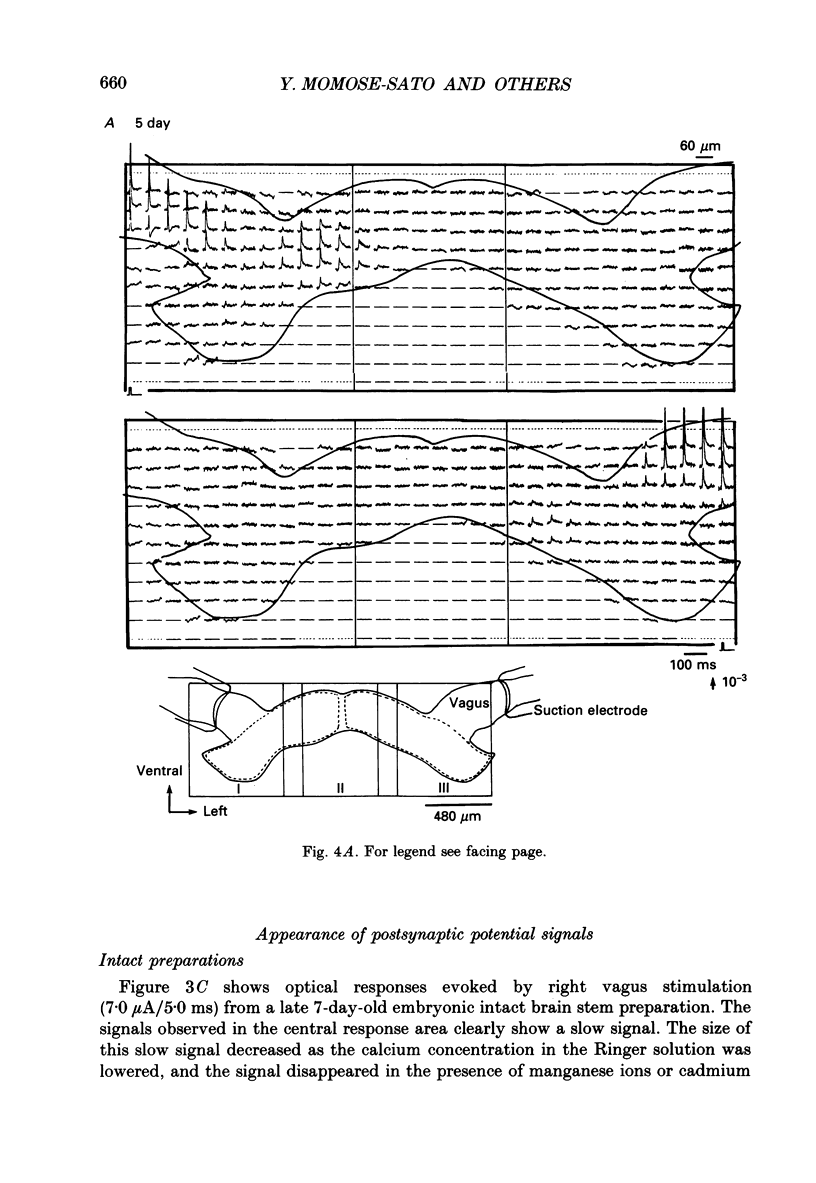
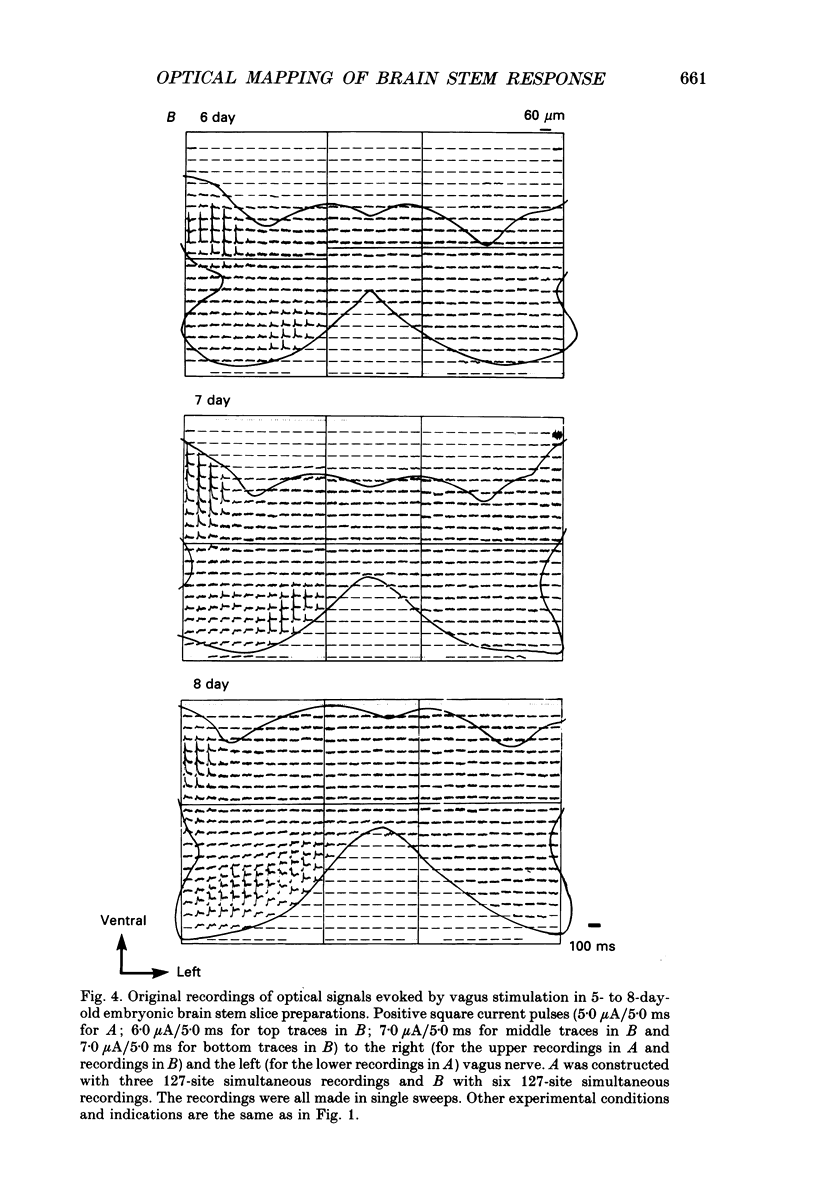
PDF




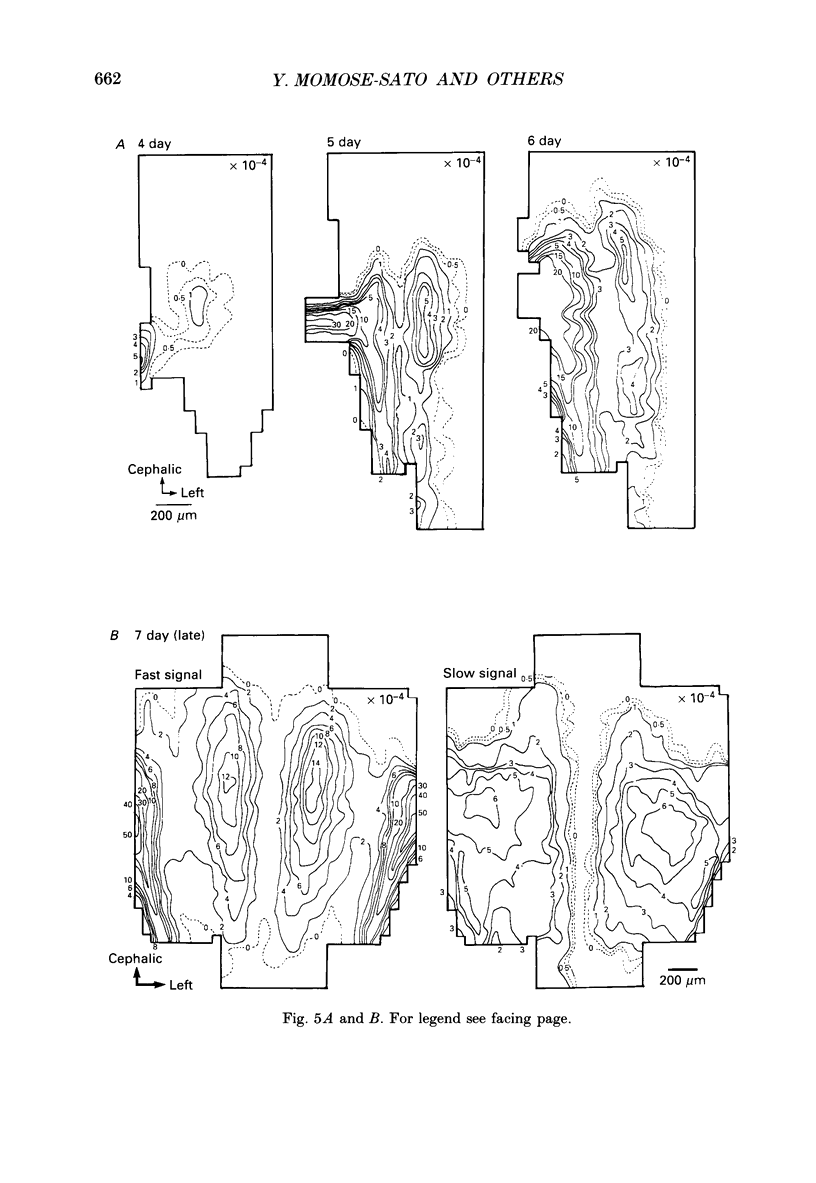
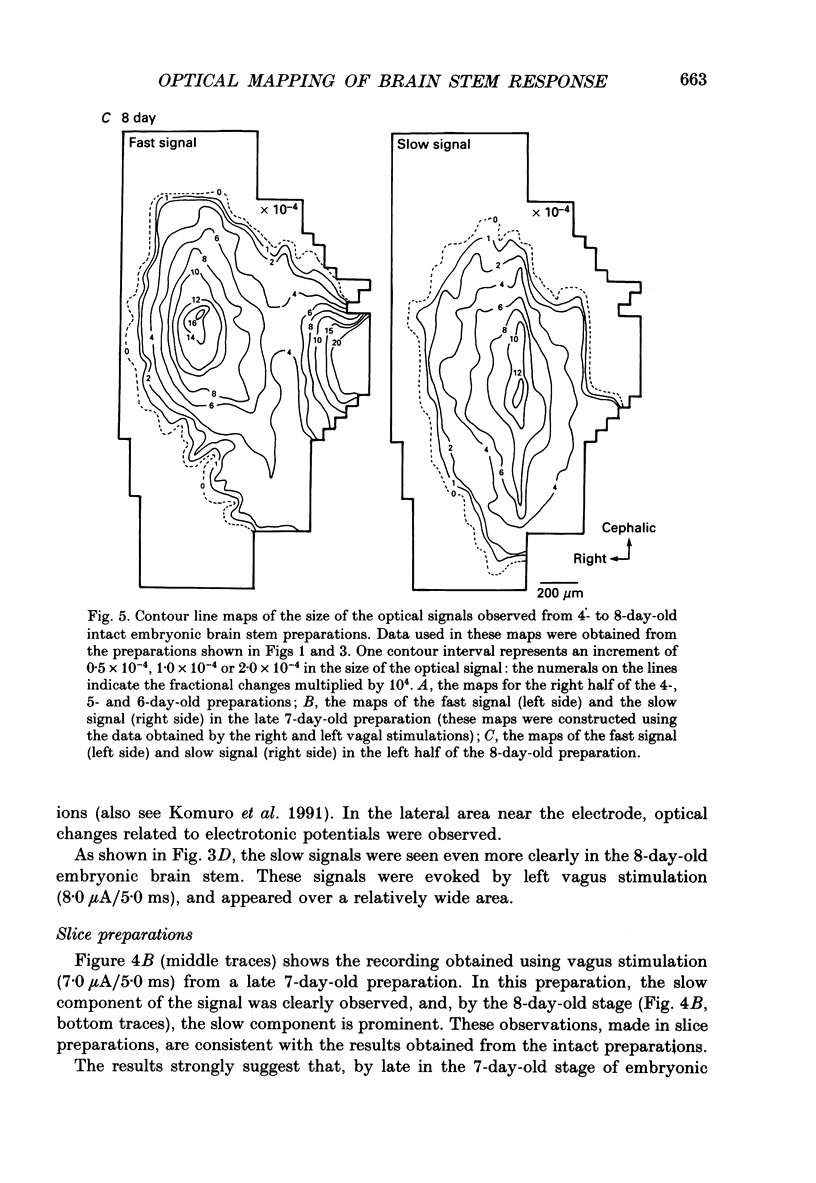

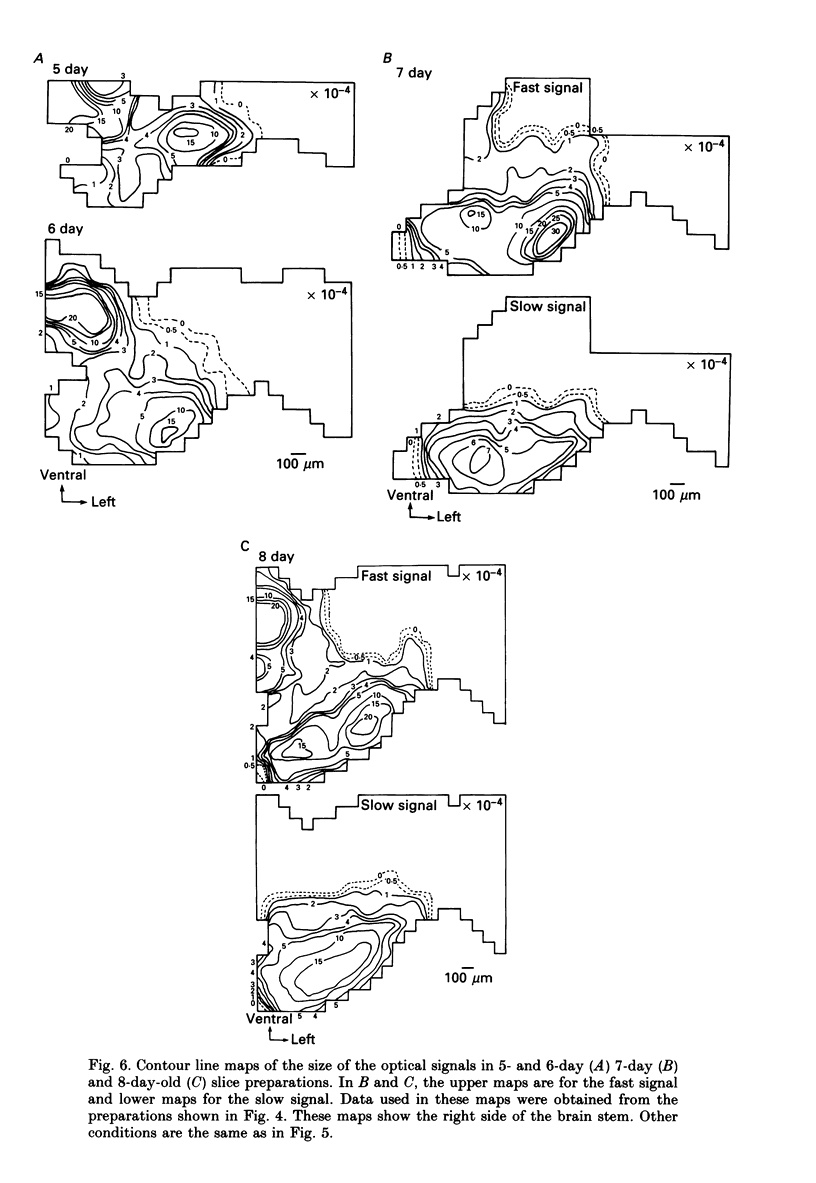



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen D. H., Schnall A. M. Medullary cells of origin of vagal cardioinhibitory fibers in the pigeon. II. Electrical stimulation of the dorsal motor nucleus. J Comp Neurol. 1970 Nov;140(3):321–342. doi: 10.1002/cne.901400306. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M. Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Action potential synchrony in embryonic precontractile chick heart: optical monitoring with potentiometric dyes. J Physiol. 1981;319:529–541. doi: 10.1113/jphysiol.1981.sp013924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamino K., Hirota A., Komuro H. Optical indications of electrical activity and excitation-contraction coupling in the early embryonic heart. Adv Biophys. 1989;25:45–93. doi: 10.1016/0065-227x(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Kamino K., Katoh Y., Komuro H., Sato K. Multiple-site optical monitoring of neural activity evoked by vagus nerve stimulation in the embryonic chick brain stem. J Physiol. 1989 Feb;409:263–283. doi: 10.1113/jphysiol.1989.sp017496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamino K., Komuro H., Sakai T., Sato K. Optical assessment of spatially ordered patterns of neural response to vagal stimulation in the early embryonic chick brainstem. Neurosci Res. 1990 Aug;8(4):255–271. doi: 10.1016/0168-0102(90)90032-a. [DOI] [PubMed] [Google Scholar]
- Kamino K. Optical approaches to ontogeny of electrical activity and related functional organization during early heart development. Physiol Rev. 1991 Jan;71(1):53–91. doi: 10.1152/physrev.1991.71.1.53. [DOI] [PubMed] [Google Scholar]
- Komuro H., Sakai T., Momose-Sato Y., Hirota A., Kamino K. Optical detection of postsynaptic potentials evoked by vagal stimulation in the early embryonic chick brain stem slice. J Physiol. 1991 Oct;442:631–648. doi: 10.1113/jphysiol.1991.sp018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid A. L., Orkand R. K., Gainer H., Salzberg B. M. Active calcium responses recorded optically from nerve terminals of the frog neurohypophysis. J Gen Physiol. 1985 Apr;85(4):481–489. doi: 10.1085/jgp.85.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach H. S., Cohen L. B., Grinvald A. Optical mapping of electrical activity in rat somatosensory and visual cortex. J Neurosci. 1985 Jul;5(7):1886–1895. doi: 10.1523/JNEUROSCI.05-07-01886.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg B. M., Obaid A. L., Senseman D. M., Gainer H. Optical recording of action potentials from vertebrate nerve terminals using potentiometric probes provides evidence for sodium and calcium components. Nature. 1983 Nov 3;306(5938):36–40. doi: 10.1038/306036a0. [DOI] [PubMed] [Google Scholar]


