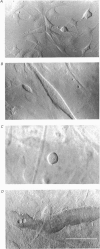
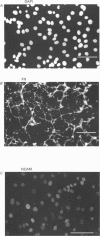
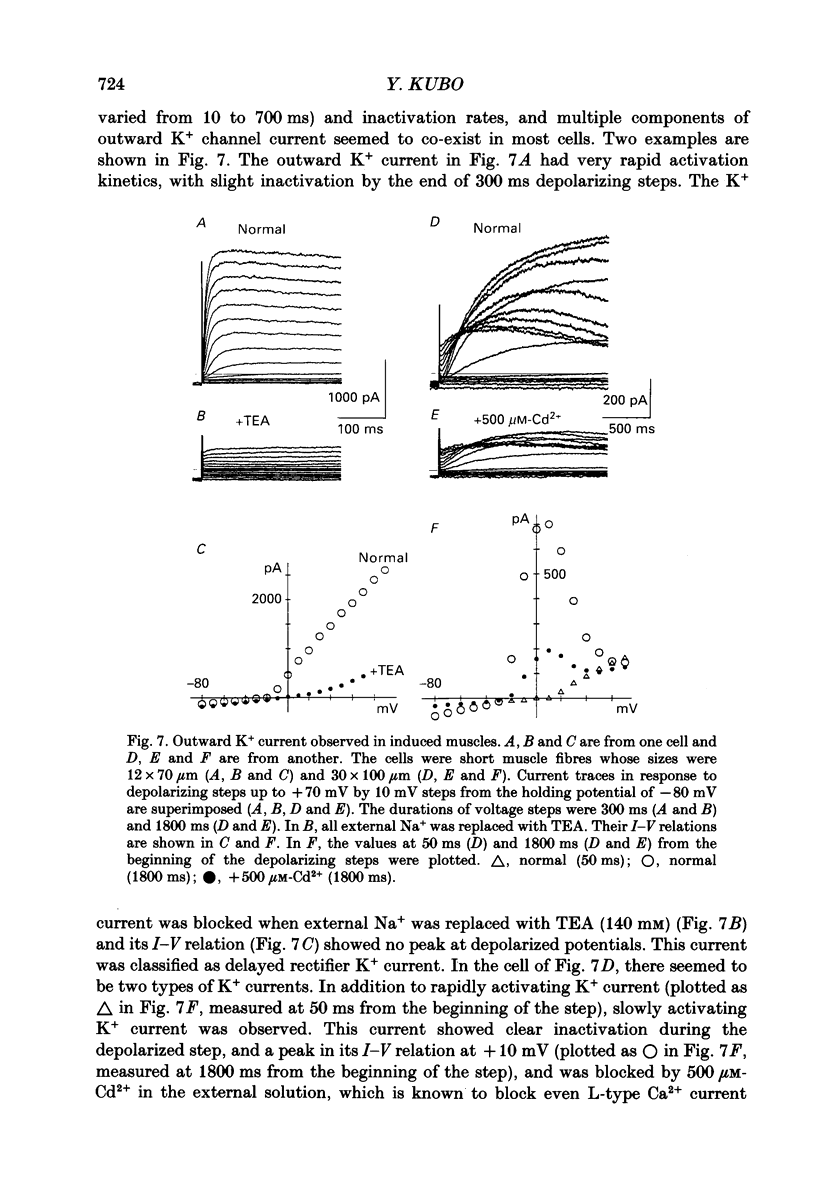
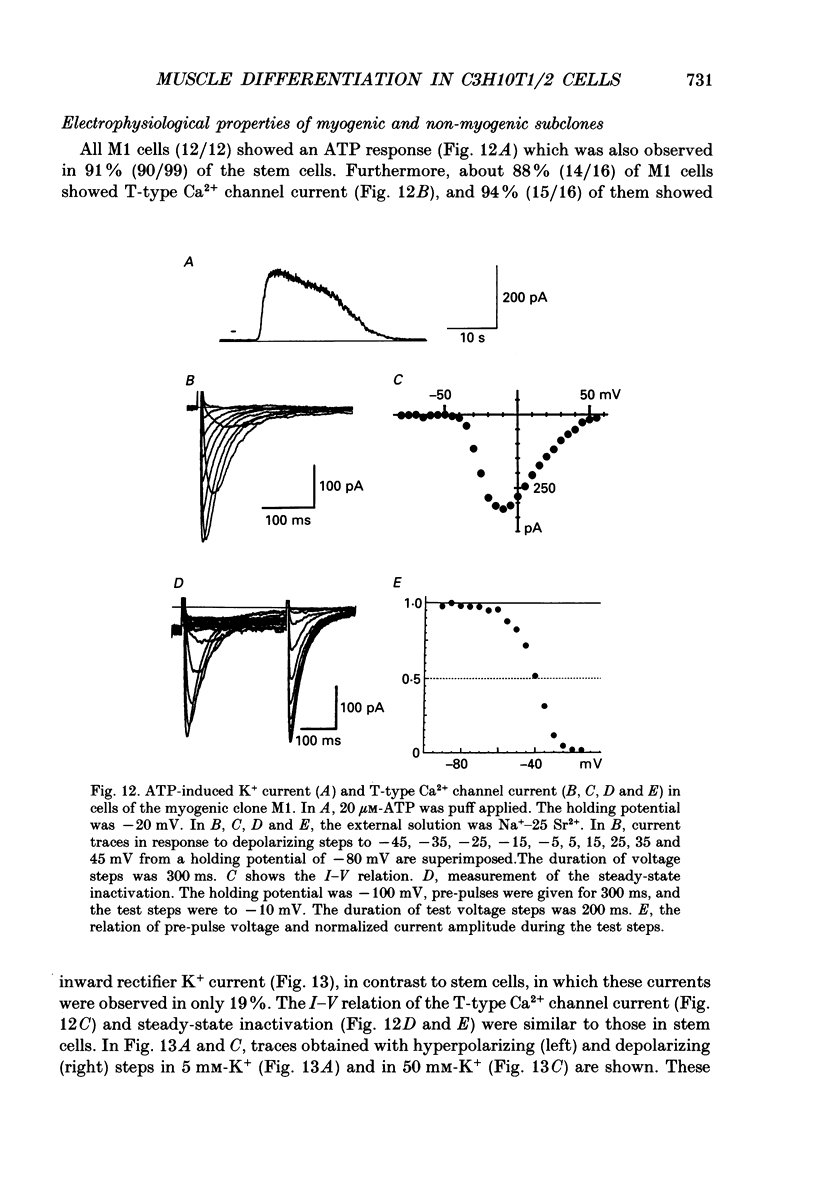
Abstract
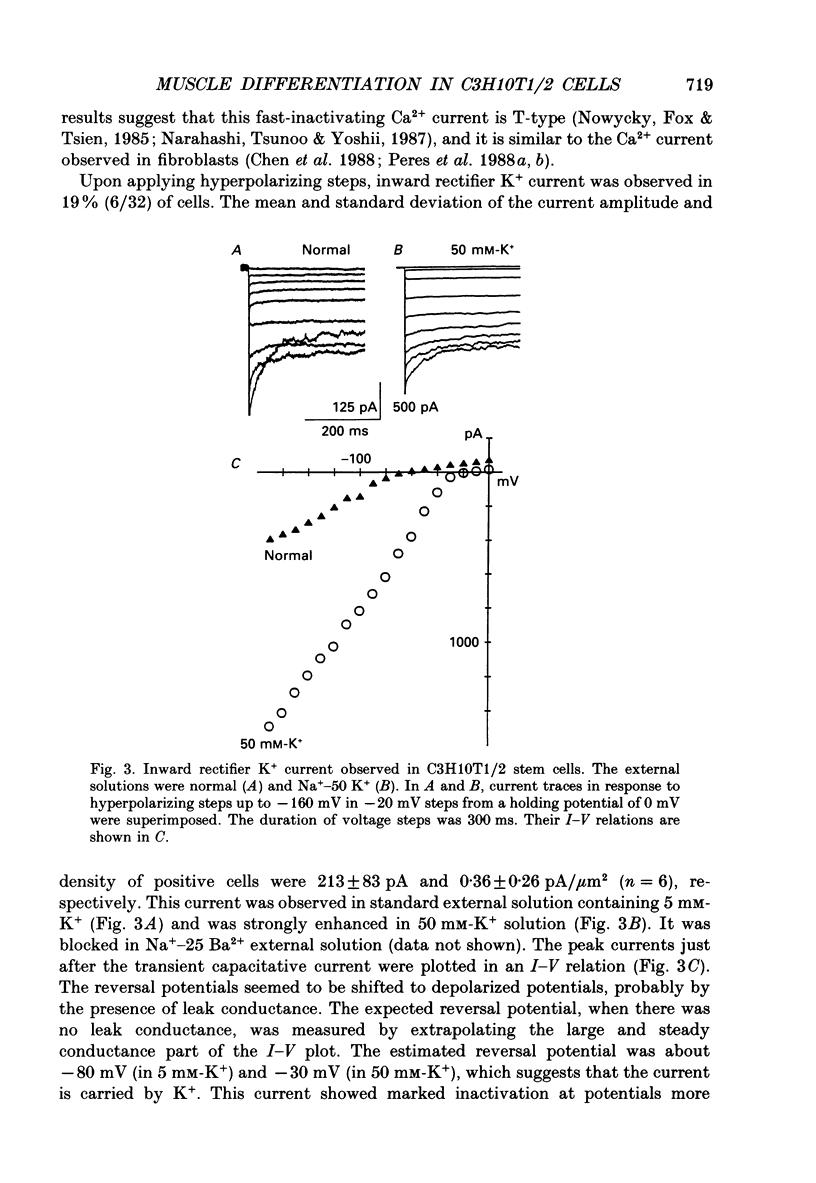
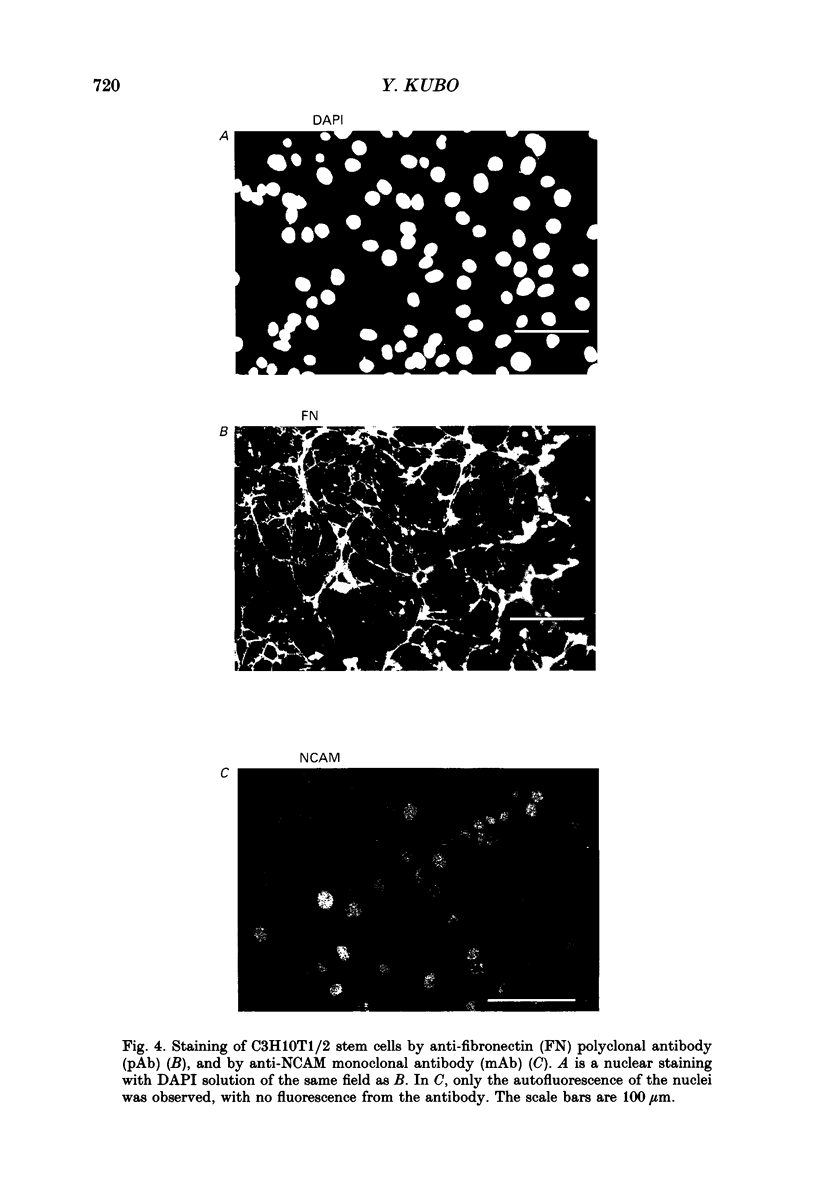
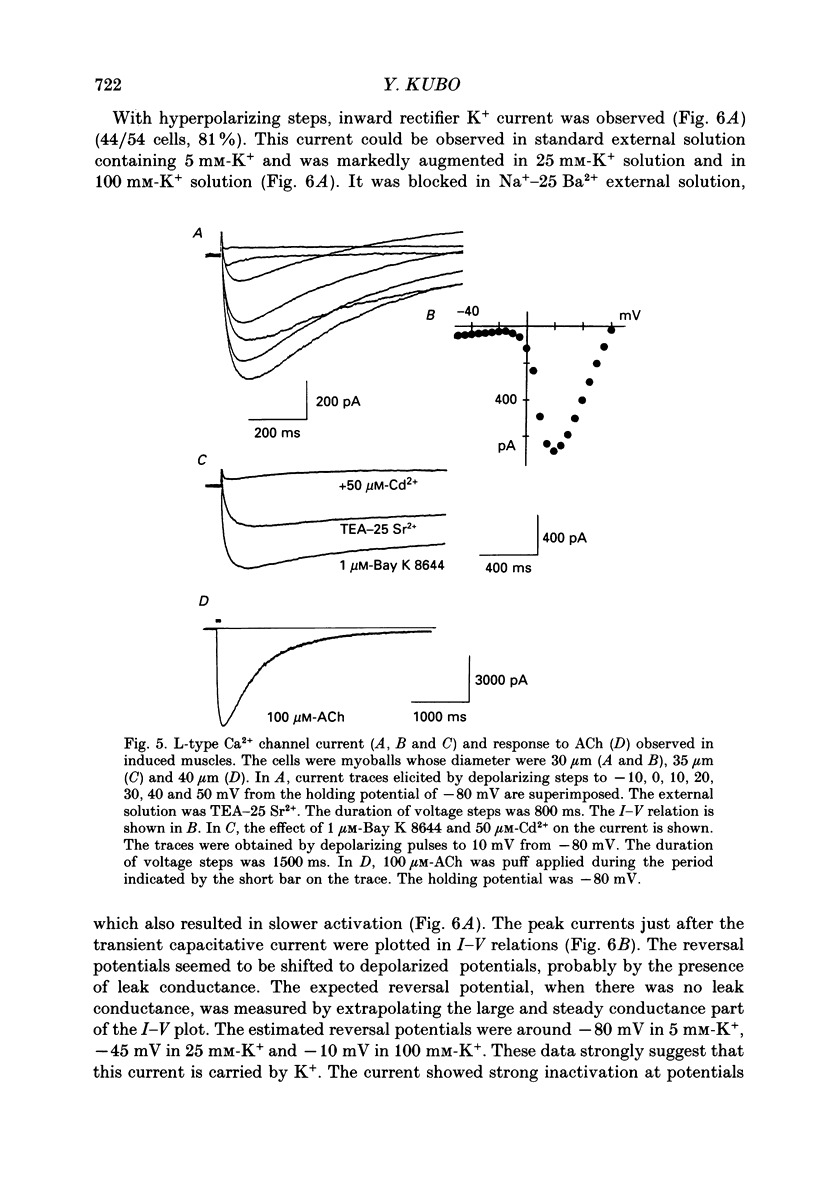
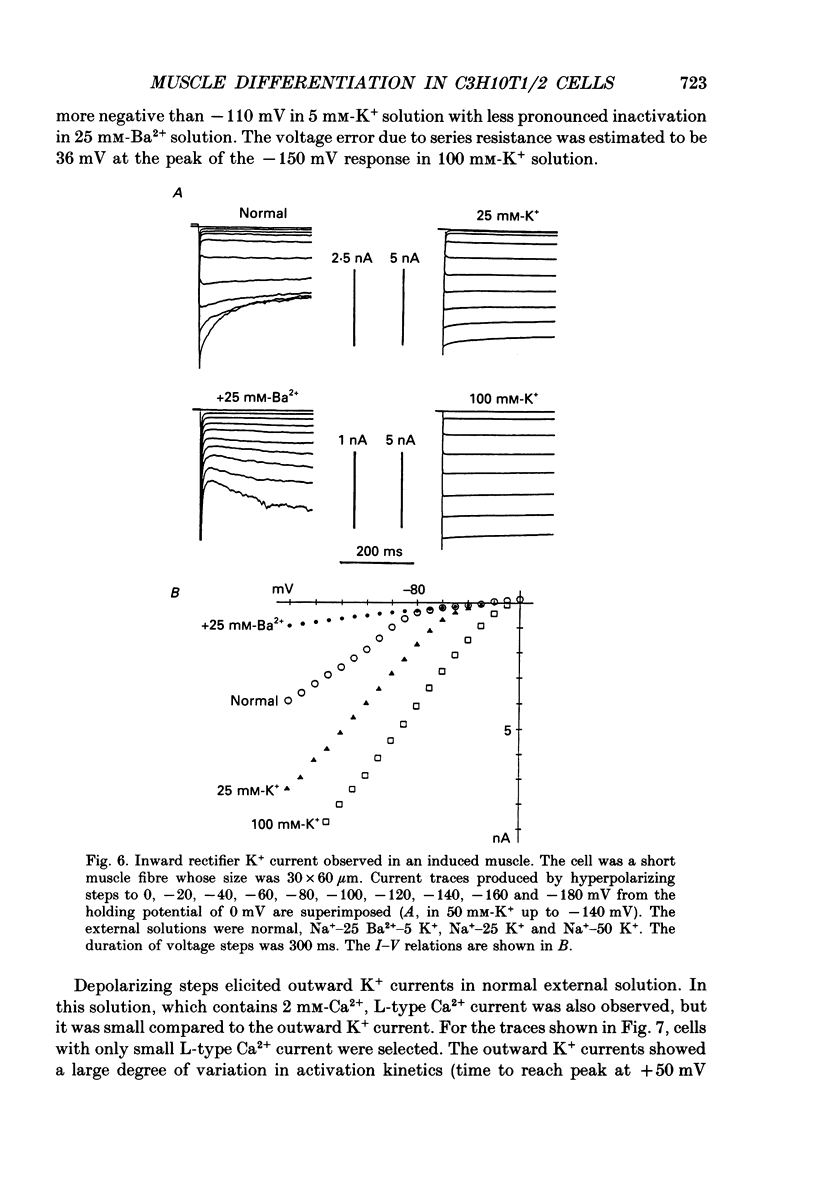
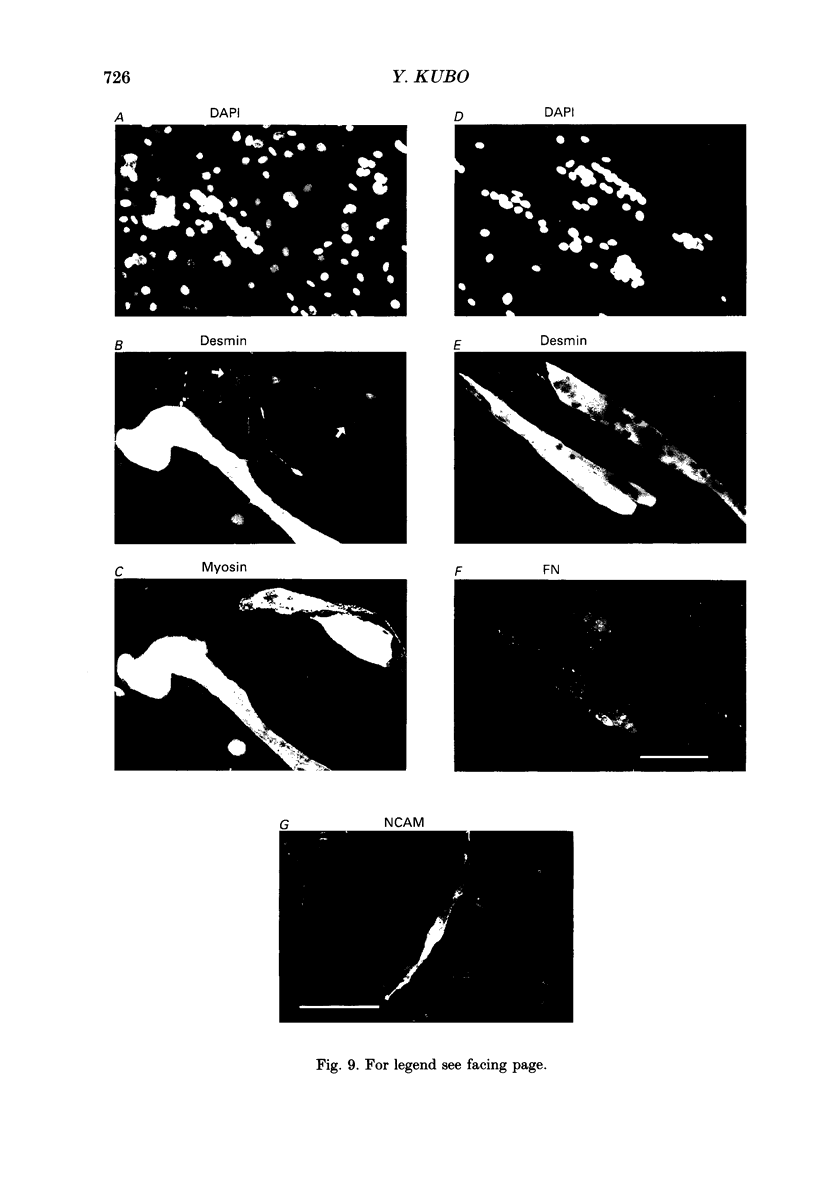
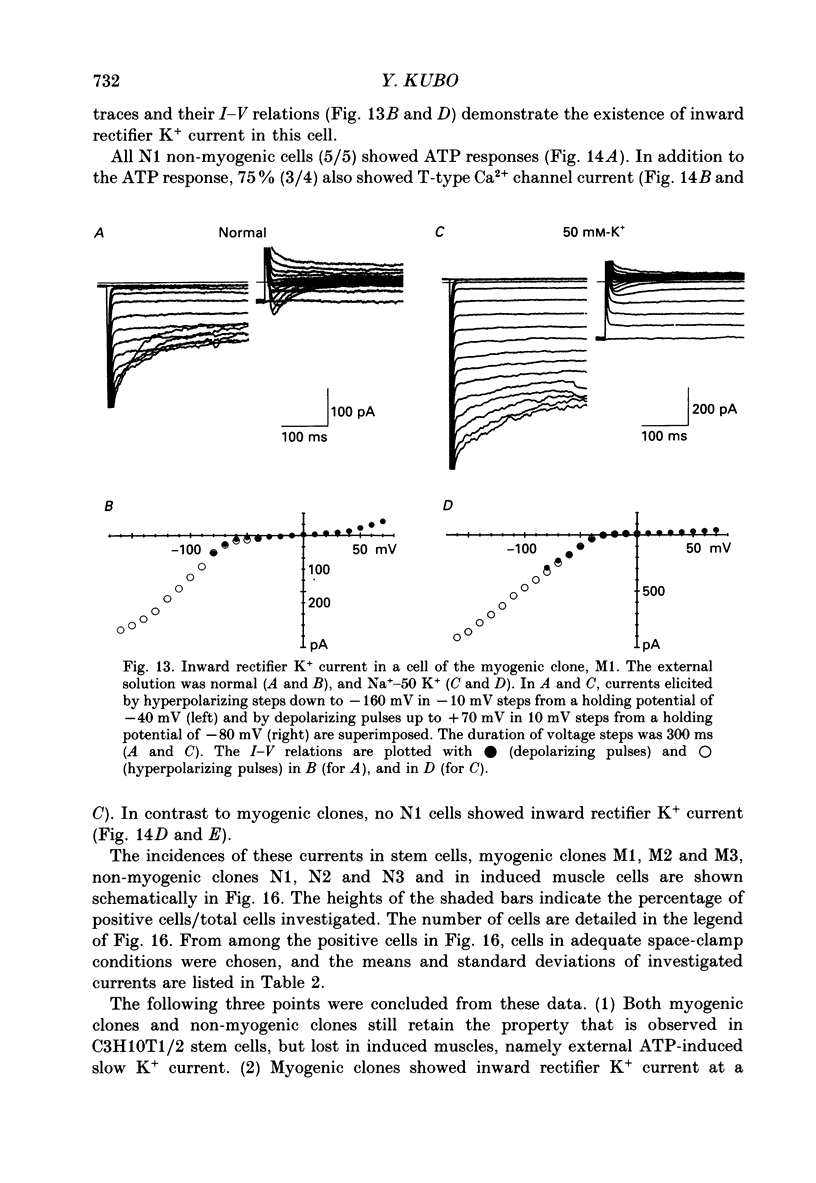
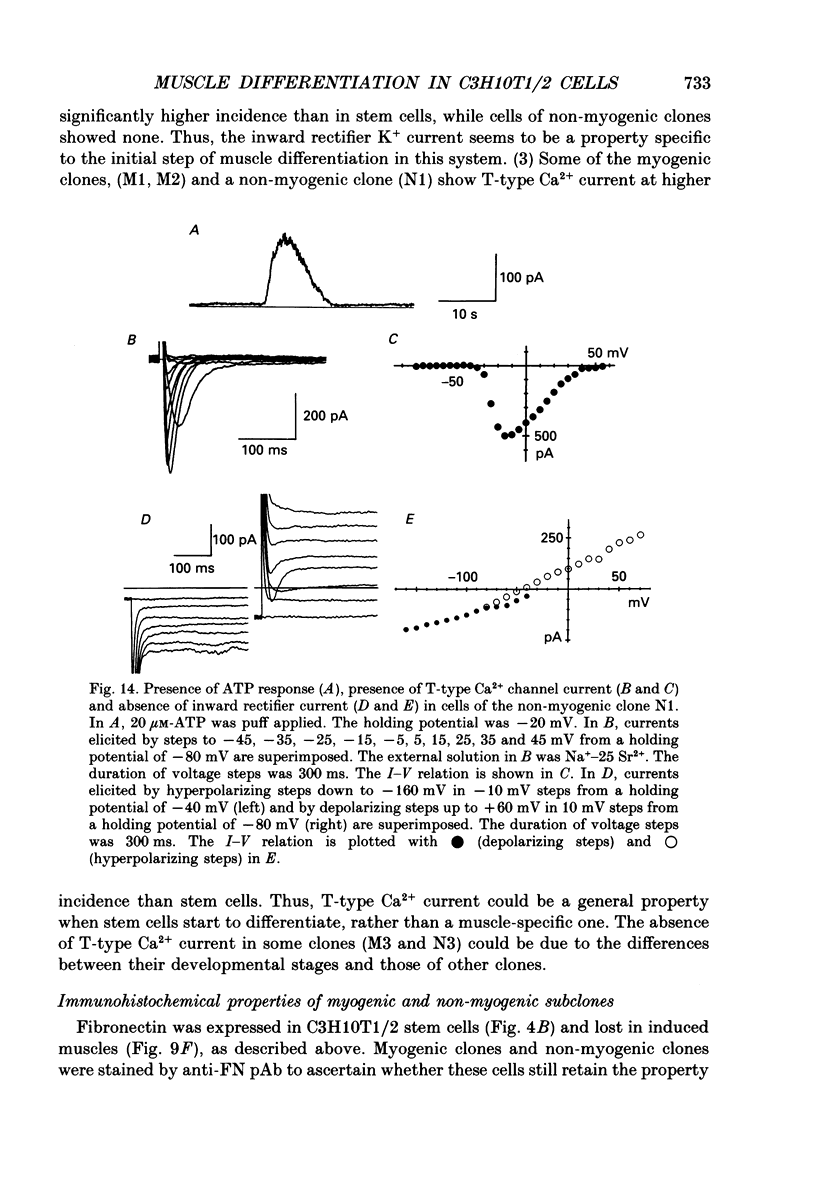
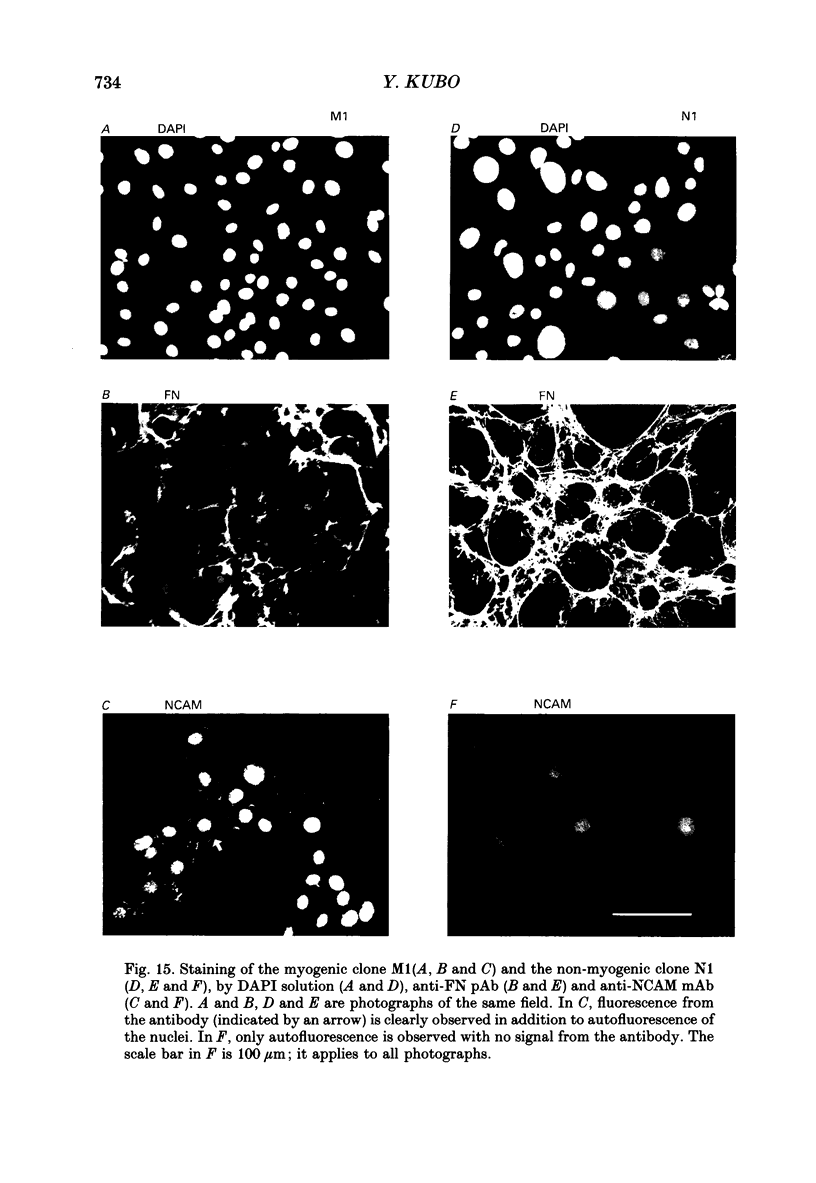
1. A mesodermal stem cell line C3H10T1/2 was induced to differentiate to muscle by adding 0.3 microM-5-aza-2'-deoxy-cytidine to the medium for 24 h. The changes in membrane currents during differentiation were studied by whole-cell recording and changes in the expression of fibronectin, Neural Cell Adhesion Molecule (NCAM), myosin and desmin were studied immunohistochemically. 2. The stem cells showed the morphology of fibroblastic cells. Most of the stem cells showed ATP-induced slow K+ current. T-type Ca2+ current and inward rectifier K+ current were observed in 19% of the stem cells. The stem cells expressed fibronectin, but not NCAM, myosin or desmin. 3. About 2 weeks after the addition of 5-aza-2'-deoxy-cytidine, large multinucleated skeletal muscle-like cells appeared. Most of the induced muscles showed L-type Ca2+ current, responses to acetylcholine, outward K+ current, inward rectifier K+ current and contraction upon depolarizing stimulation. They expressed NCAM, myosin and desmin, but not fibronectin, and showed no ATP response. 4. In some batches (2/14), the induced muscles showed spontaneous twitches, and possessed tetrodotoxin (TTX)-sensitive Na+ current in addition to the currents described above. Furthermore clear striation was observed in some of the twitching muscles under Nomarski optics. 5. To ascertain the properties of cells at the initial step of muscle differentiation, whose differentiation is determined but not yet evident morphologically or electrophysiologically, subcloning was performed from the heterogeneous cells 10 days after induction. Three myogenic clones were obtained, which proliferated at low cell densities but differentiated to muscle with a high incidence at high cell densities, as well as ten non-myogenic clones. 6. Most myogenic clones still showed ATP-induced K+ current and fibronectin. In addition, most of them showed T-type Ca2+ current and inward rectifier K+ current. They had already expressed NCAM. No other properties observed in muscles had yet been expressed. Most cells of the non-myogenic clones showed ATP-induced K+ current and fibronectin. T-type Ca2+ current was also expressed, but not inward rectifier K+ current or NCAM. 7. The properties of the observed ionic currents were studied. The TTX-sensitive Na+ current could be completely blocked by 0.1 microM-TTX. It could be evoked by depolarizing steps to a level above -40 mV, while steady-state inactivation was detectable around -75 mV and reached half by -52 mV. T-type Ca2+ current could be evoked by a depolarizing pulse to a level above -45 mV, with a maximum amplitude around -15 mV.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






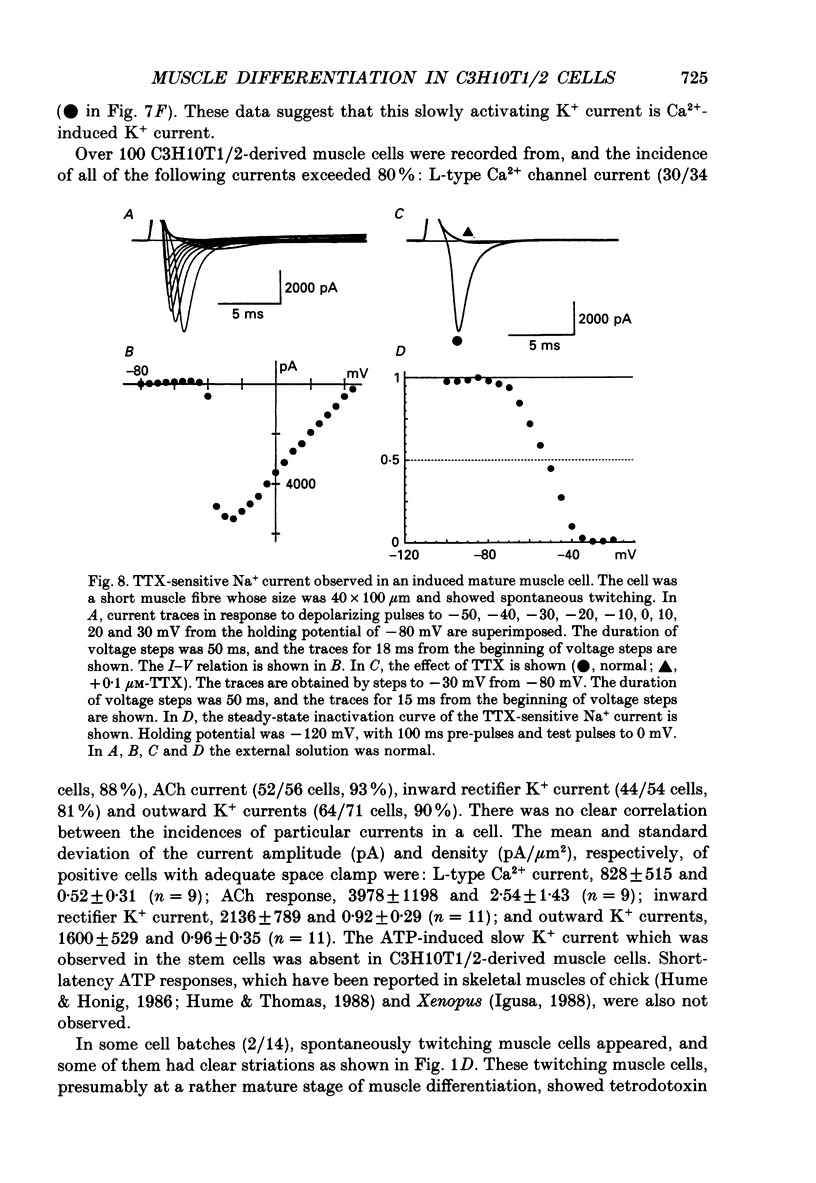



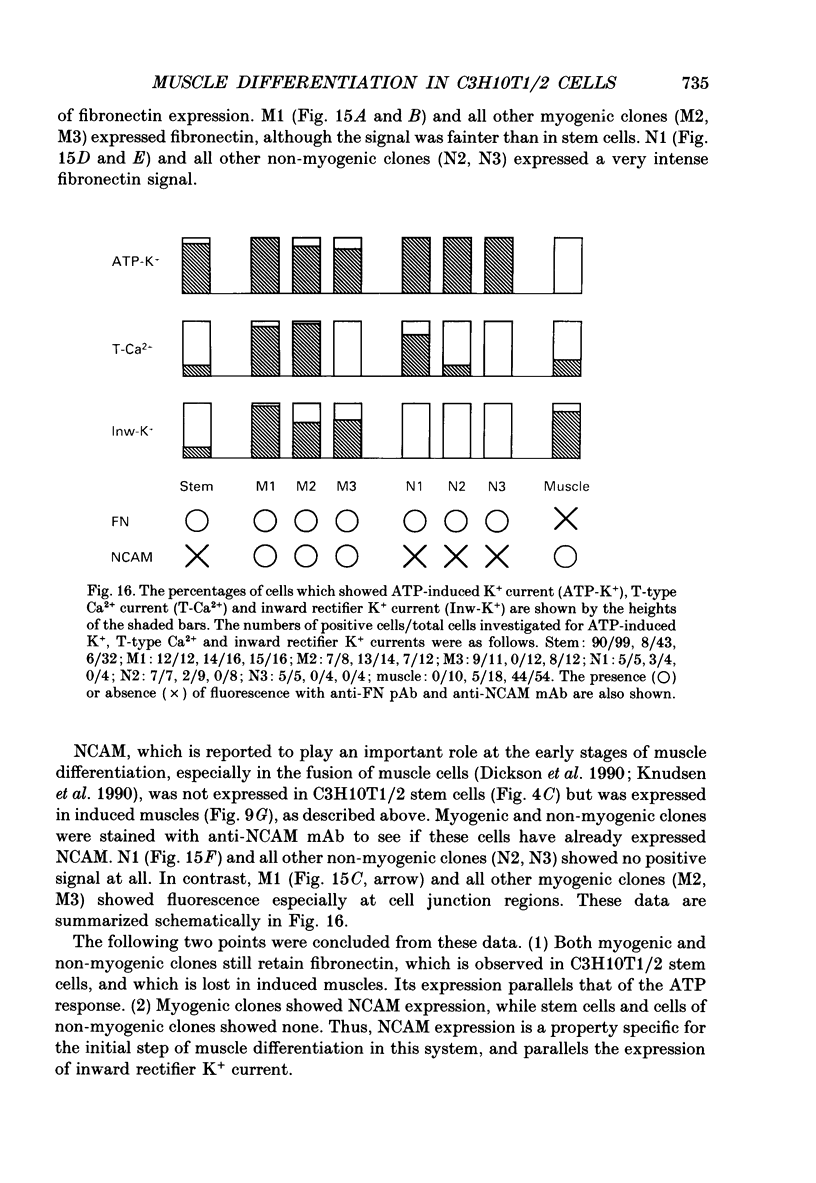
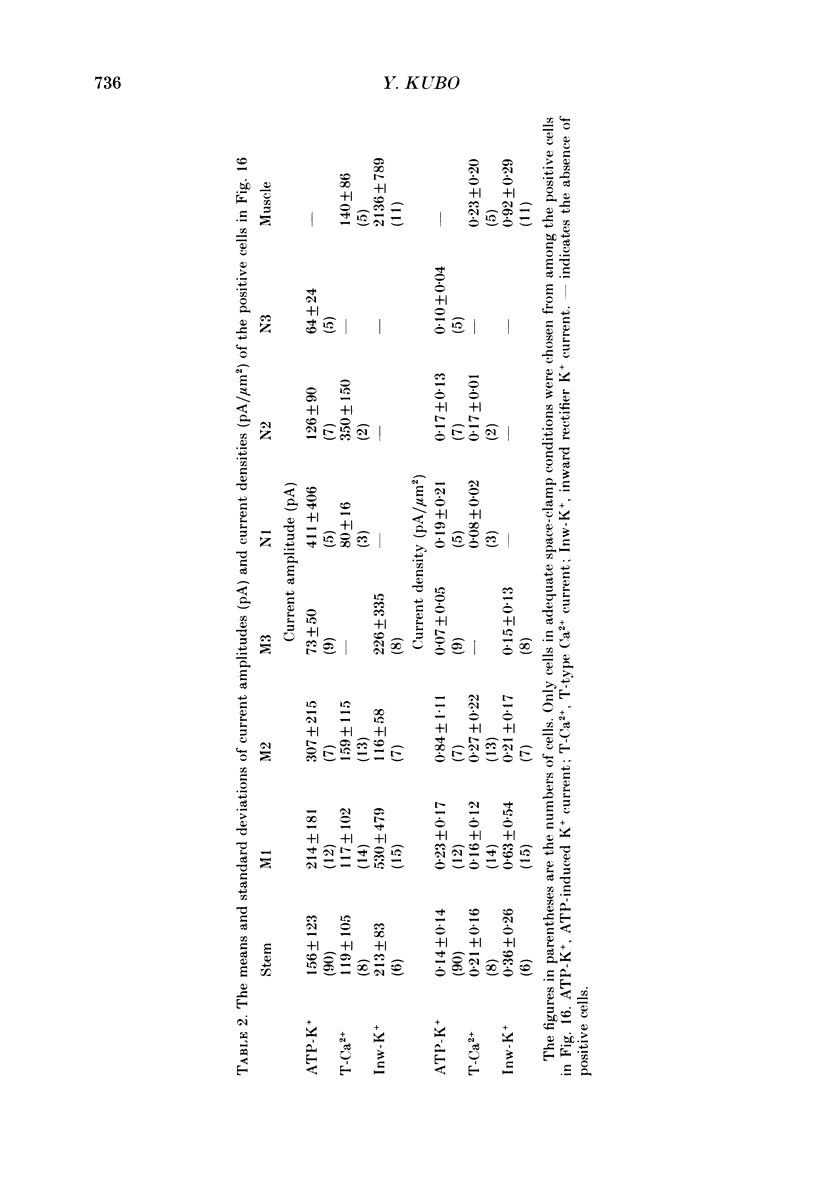

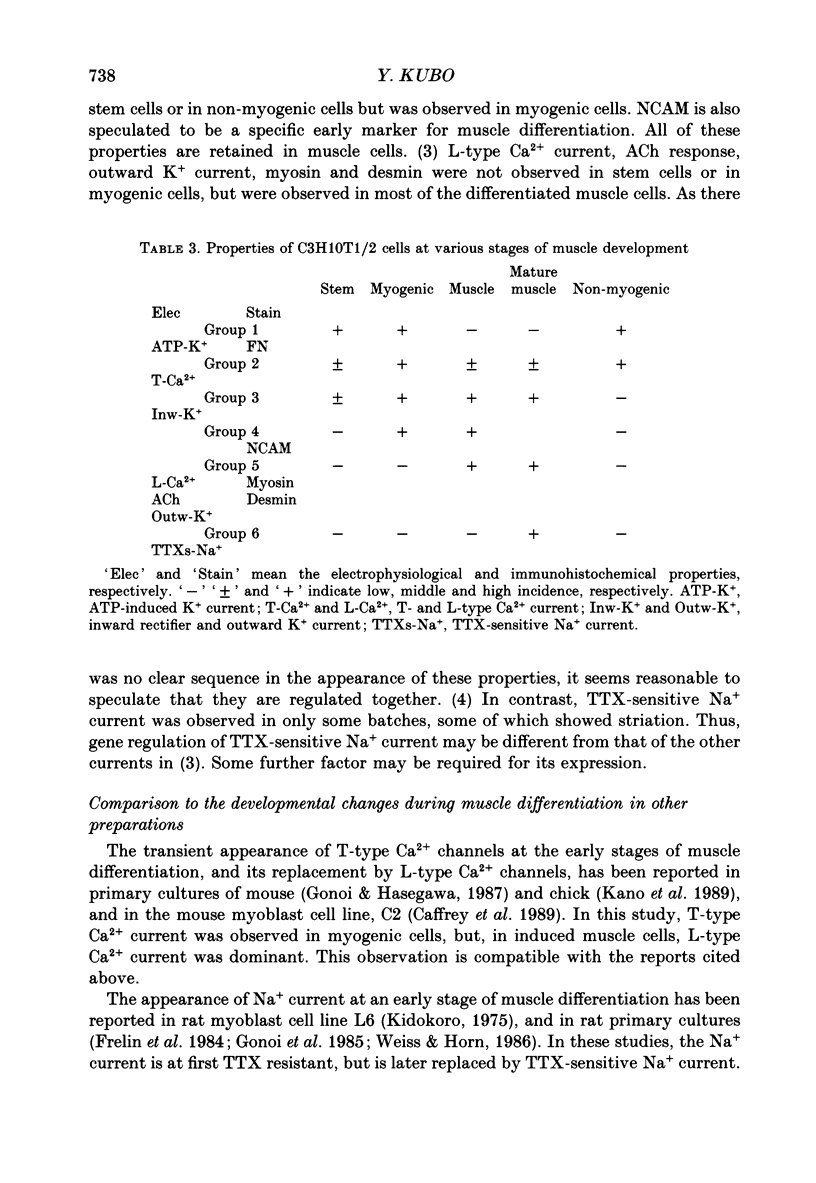



Images in this article
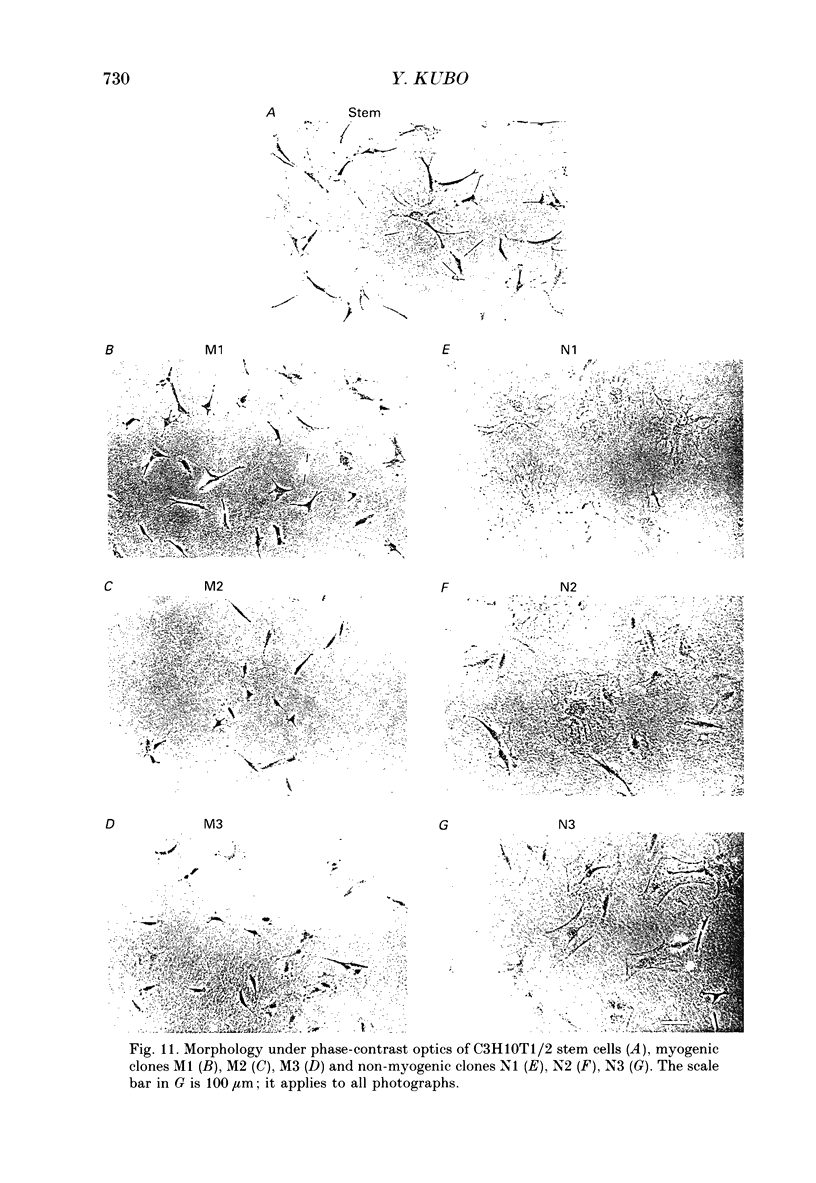
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
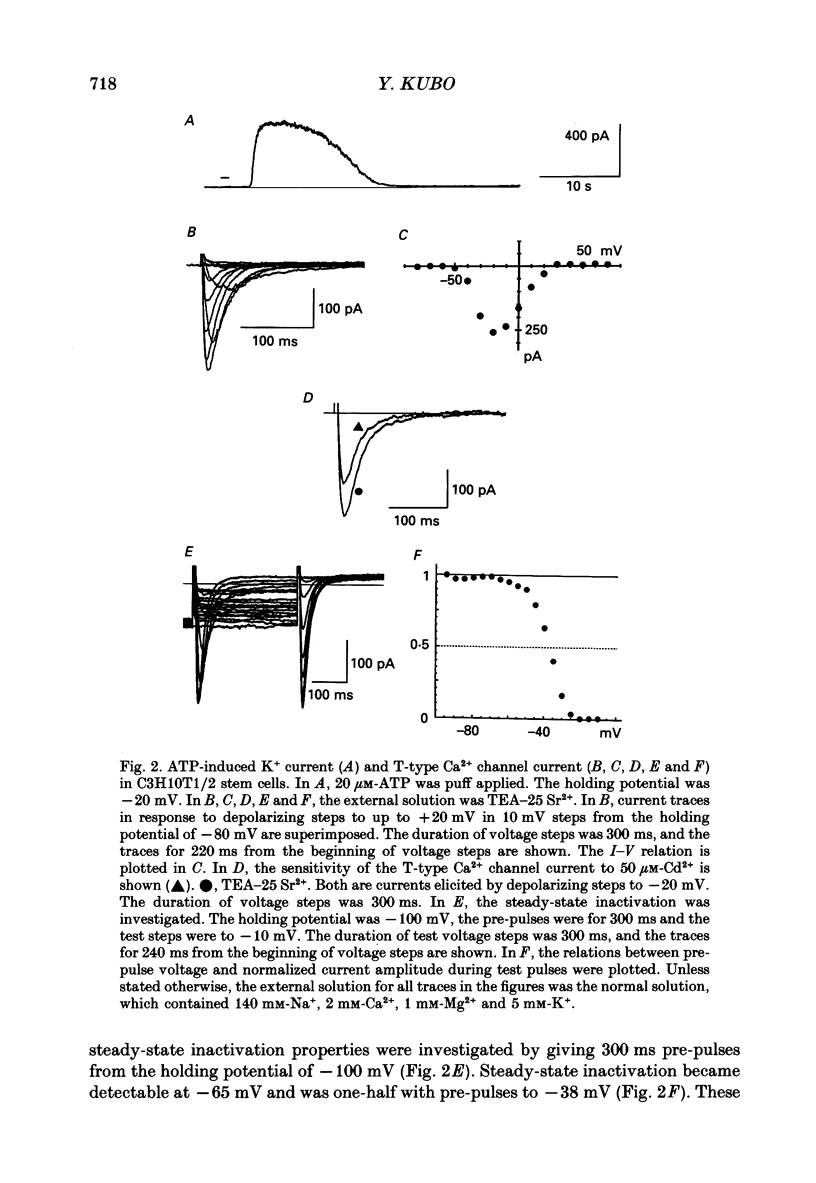
- Beam K. G., Knudson C. M. Calcium currents in embryonic and neonatal mammalian skeletal muscle. J Gen Physiol. 1988 Jun;91(6):781–798. doi: 10.1085/jgp.91.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Knudson C. M. Effect of postnatal development on calcium currents and slow charge movement in mammalian skeletal muscle. J Gen Physiol. 1988 Jun;91(6):799–815. doi: 10.1085/jgp.91.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey J. M., Brown A. M., Schneider M. D. Ca2+ and Na+ currents in developing skeletal myoblasts are expressed in a sequential program: reversible suppression by transforming growth factor beta-1, an inhibitor of the myogenic pathway. J Neurosci. 1989 Oct;9(10):3443–3453. doi: 10.1523/JNEUROSCI.09-10-03443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. F., Corbley M. J., Roberts T. M., Hess P. Voltage-sensitive calcium channels in normal and transformed 3T3 fibroblasts. Science. 1988 Feb 26;239(4843):1024–1026. doi: 10.1126/science.2449730. [DOI] [PubMed] [Google Scholar]
- Cognard C., Lazdunski M., Romey G. Different types of Ca2+ channels in mammalian skeletal muscle cells in culture. Proc Natl Acad Sci U S A. 1986 Jan;83(2):517–521. doi: 10.1073/pnas.83.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides P. G., Jones P. A., Gevers W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 1977 May 26;267(5609):364–366. doi: 10.1038/267364a0. [DOI] [PubMed] [Google Scholar]
- Dickson G., Peck D., Moore S. E., Barton C. H., Walsh F. S. Enhanced myogenesis in NCAM-transfected mouse myoblasts. Nature. 1990 Mar 22;344(6264):348–351. doi: 10.1038/344348a0. [DOI] [PubMed] [Google Scholar]
- Frelin C., Vijverberg H. P., Romey G., Vigne P., Lazdunski M. Different functional states of tetrodotoxin sensitive and tetrodotoxin resistant Na+ channels occur during the in vitro development of rat skeletal muscle. Pflugers Arch. 1984 Oct;402(2):121–128. doi: 10.1007/BF00583323. [DOI] [PubMed] [Google Scholar]
- Gonoi T., Hasegawa S. Post-natal disappearance of transient calcium channels in mouse skeletal muscle: effects of denervation and culture. J Physiol. 1988 Jul;401:617–637. doi: 10.1113/jphysiol.1988.sp017183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonoi T., Sherman S. J., Catterall W. A. Voltage clamp analysis of tetrodotoxin-sensitive and -insensitive sodium channels in rat muscle cells developing in vitro. J Neurosci. 1985 Sep;5(9):2559–2564. doi: 10.1523/JNEUROSCI.05-09-02559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume R. I., Honig M. G. Excitatory action of ATP on embryonic chick muscle. J Neurosci. 1986 Mar;6(3):681–690. doi: 10.1523/JNEUROSCI.06-03-00681.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume R. I., Thomas S. A. A calcium- and voltage-dependent chloride current in developing chick skeletal muscle. J Physiol. 1989 Oct;417:241–261. doi: 10.1113/jphysiol.1989.sp017799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume R. I., Thomas S. A. Multiple actions of adenosine 5'-triphosphate on chick skeletal muscle. J Physiol. 1988 Dec;406:503–524. doi: 10.1113/jphysiol.1988.sp017393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igusa Y. Adenosine 5'-triphosphate activates acetylcholine receptor channels in cultured Xenopus myotomal muscle cells. J Physiol. 1988 Nov;405:169–185. doi: 10.1113/jphysiol.1988.sp017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M., Wakuta K., Satoh R. Calcium channel components of action potential in chick skeletal muscle cells developing in culture. Brain Res. 1987 Apr;429(2):233–240. doi: 10.1016/0165-3806(87)90103-9. [DOI] [PubMed] [Google Scholar]
- Kano M., Wakuta K., Satoh R. Two components of calcium channel current in embryonic chick skeletal muscle cells developing in culture. Brain Res Dev Brain Res. 1989 May 1;47(1):101–112. doi: 10.1016/0165-3806(89)90112-0. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y. Sodium and calcium components of the action potential in a developing skeletal muscle cell line. J Physiol. 1975 Jan;244(1):145–159. doi: 10.1113/jphysiol.1975.sp010788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K. A., McElwee S. A., Myers L. A role for the neural cell adhesion molecule, NCAM, in myoblast interaction during myogenesis. Dev Biol. 1990 Mar;138(1):159–168. doi: 10.1016/0012-1606(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Kubo Y. Comparison of initial stages of muscle differentiation in rat and mouse myoblastic and mouse mesodermal stem cell lines. J Physiol. 1991 Oct;442:743–759. doi: 10.1113/jphysiol.1991.sp018817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y. Development of ion channels and neurofilaments during neuronal differentiation of mouse embryonal carcinoma cell lines. J Physiol. 1989 Feb;409:497–523. doi: 10.1113/jphysiol.1989.sp017510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y. Properties of ionic currents induced by external ATP in a mouse mesodermal stem cell line. J Physiol. 1991 Oct;442:691–710. doi: 10.1113/jphysiol.1991.sp018815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovisolo D., Alloatti G., Bonelli G., Tessitore L., Baccino F. M. Potassium and calcium currents and action potentials in mouse Balb/c 3T3 fibroblasts. Pflugers Arch. 1988 Oct;412(5):530–534. doi: 10.1007/BF00582543. [DOI] [PubMed] [Google Scholar]
- Mitani S. The reduction of calcium current associated with early differentiation of the murine embryo. J Physiol. 1985 Jun;363:71–86. doi: 10.1113/jphysiol.1985.sp015696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake M. The development of action potential mechanism in a mouse neuronal cell line in vitro. Brain Res. 1978 Mar 24;143(2):349–354. doi: 10.1016/0006-8993(78)90574-7. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987 Feb;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Okada Y., Tsuchiya W., Yada T. Calcium channel and calcium pump involved in oscillatory hyperpolarizing responses of L-strain mouse fibroblasts. J Physiol. 1982 Jun;327:449–461. doi: 10.1113/jphysiol.1982.sp014242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Yada T., Ohno-Shosaku T., Oiki S., Ueda S., Machida K. Exogenous ATP induces electrical membrane responses in fibroblasts. Exp Cell Res. 1984 Jun;152(2):552–557. doi: 10.1016/0014-4827(84)90657-8. [DOI] [PubMed] [Google Scholar]
- Peres A., Sturani E., Zippel R. Properties of the voltage-dependent calcium channel of mouse Swiss 3T3 fibroblasts. J Physiol. 1988 Jul;401:639–655. doi: 10.1113/jphysiol.1988.sp017184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres A., Zippel R., Sturani E., Mostacciuolo G. A voltage-dependent calcium current in mouse Swiss 3T3 fibroblasts. Pflugers Arch. 1988 May;411(5):554–557. doi: 10.1007/BF00582377. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Trautmann A., Delaporte C., Marty A. Voltage-dependent channels of human muscle cultures. Pflugers Arch. 1986 Feb;406(2):163–172. doi: 10.1007/BF00586678. [DOI] [PubMed] [Google Scholar]
- Weiss R. E., Horn R. Functional differences between two classes of sodium channels in developing rat skeletal muscle. Science. 1986 Jul 18;233(4761):361–364. doi: 10.1126/science.2425432. [DOI] [PubMed] [Google Scholar]
- Yamashita N. Enhancement of ionic currents through voltage-gated channels in the mouse oocyte after fertilization. J Physiol. 1982 Aug;329:263–280. doi: 10.1113/jphysiol.1982.sp014302. [DOI] [PMC free article] [PubMed] [Google Scholar]