Abstract
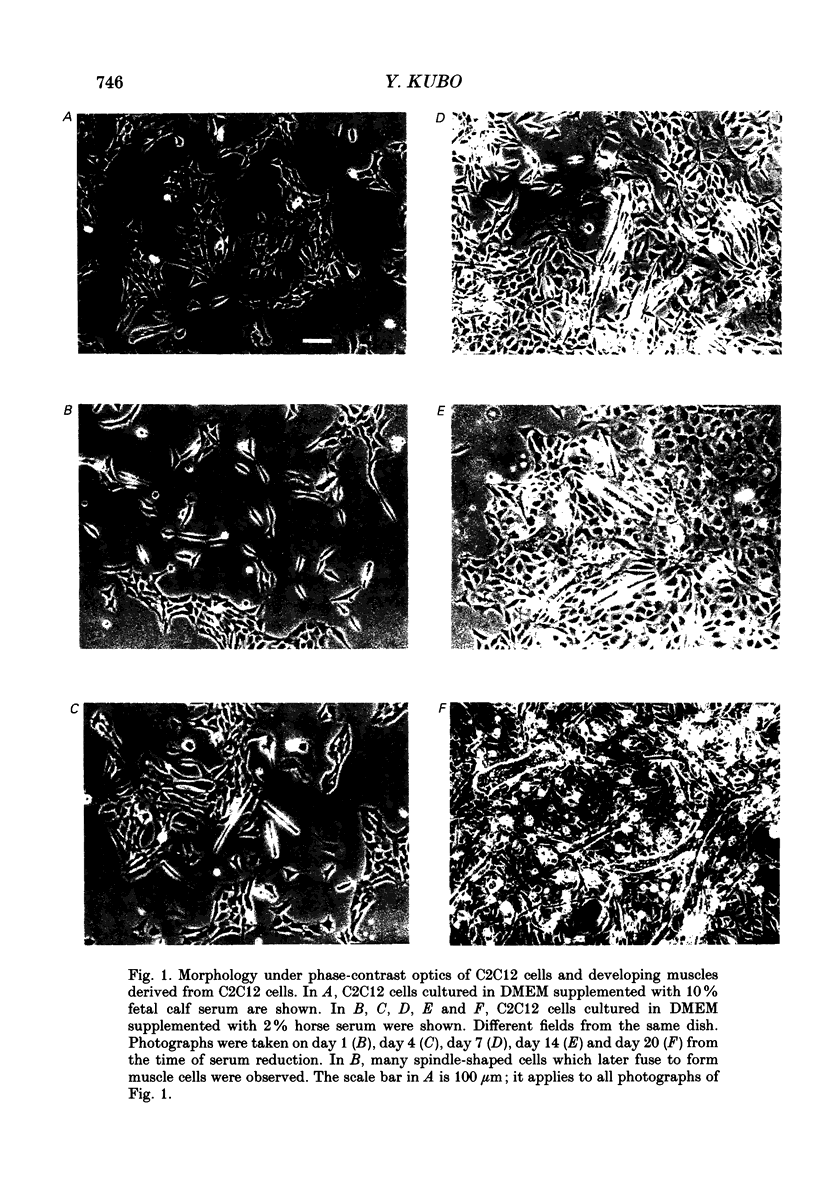
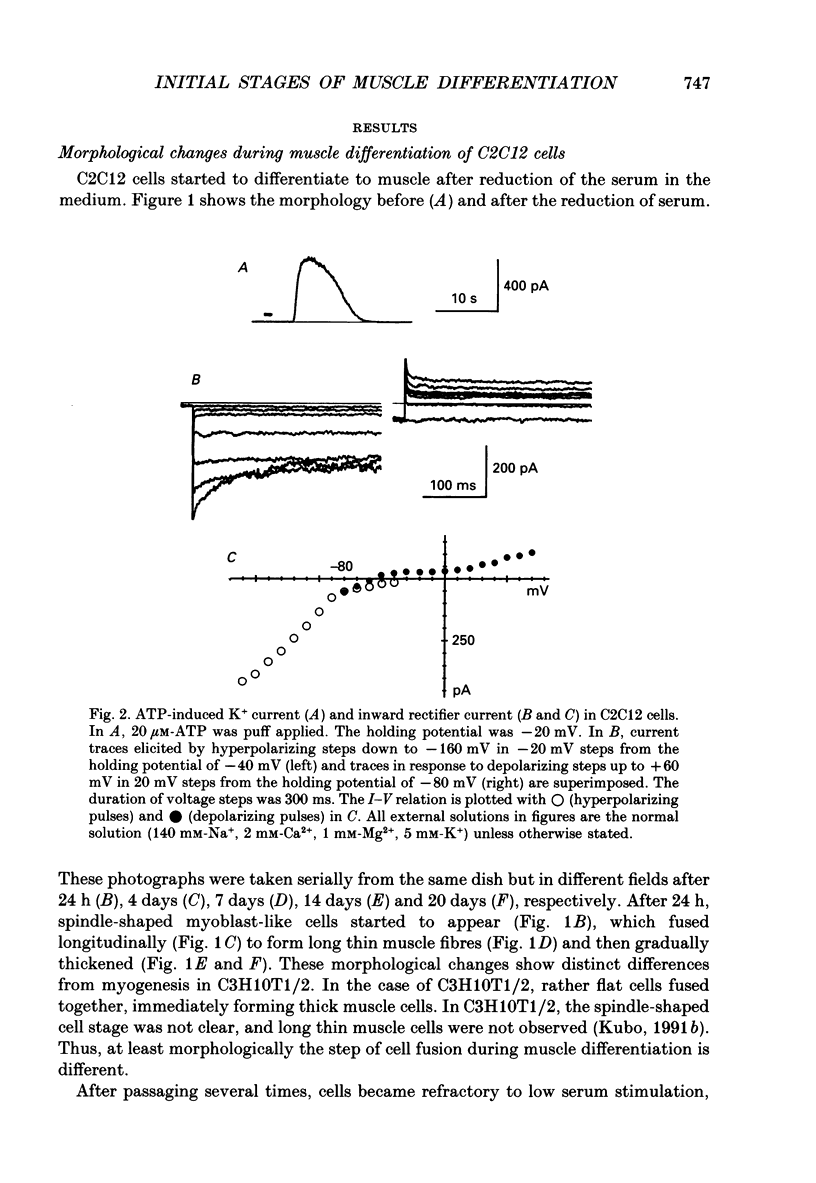
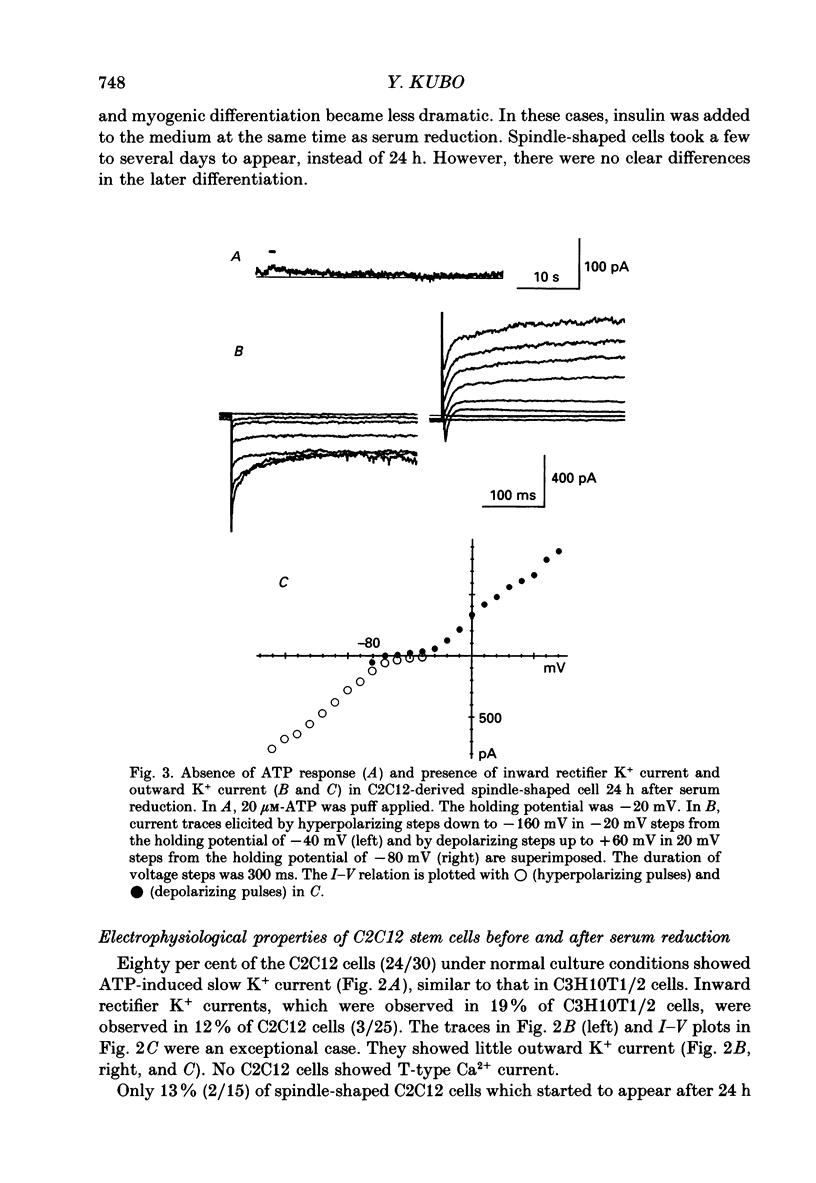
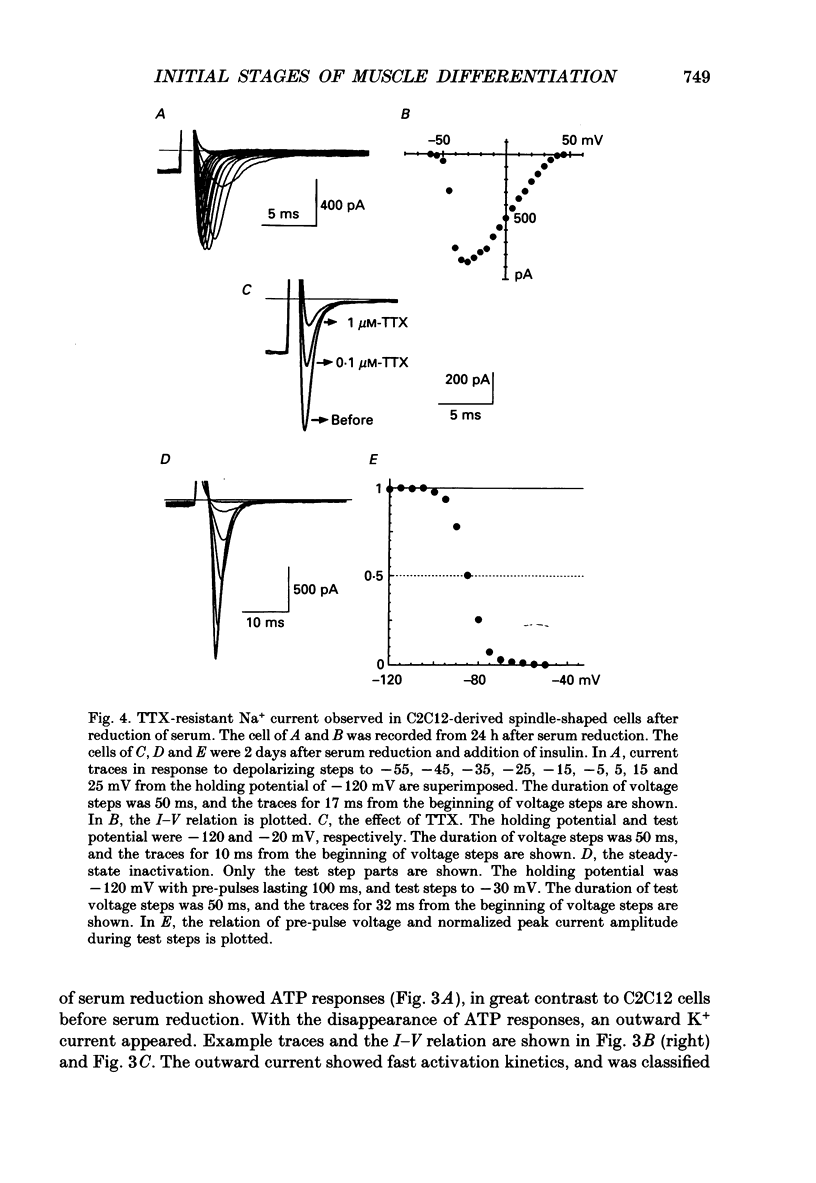
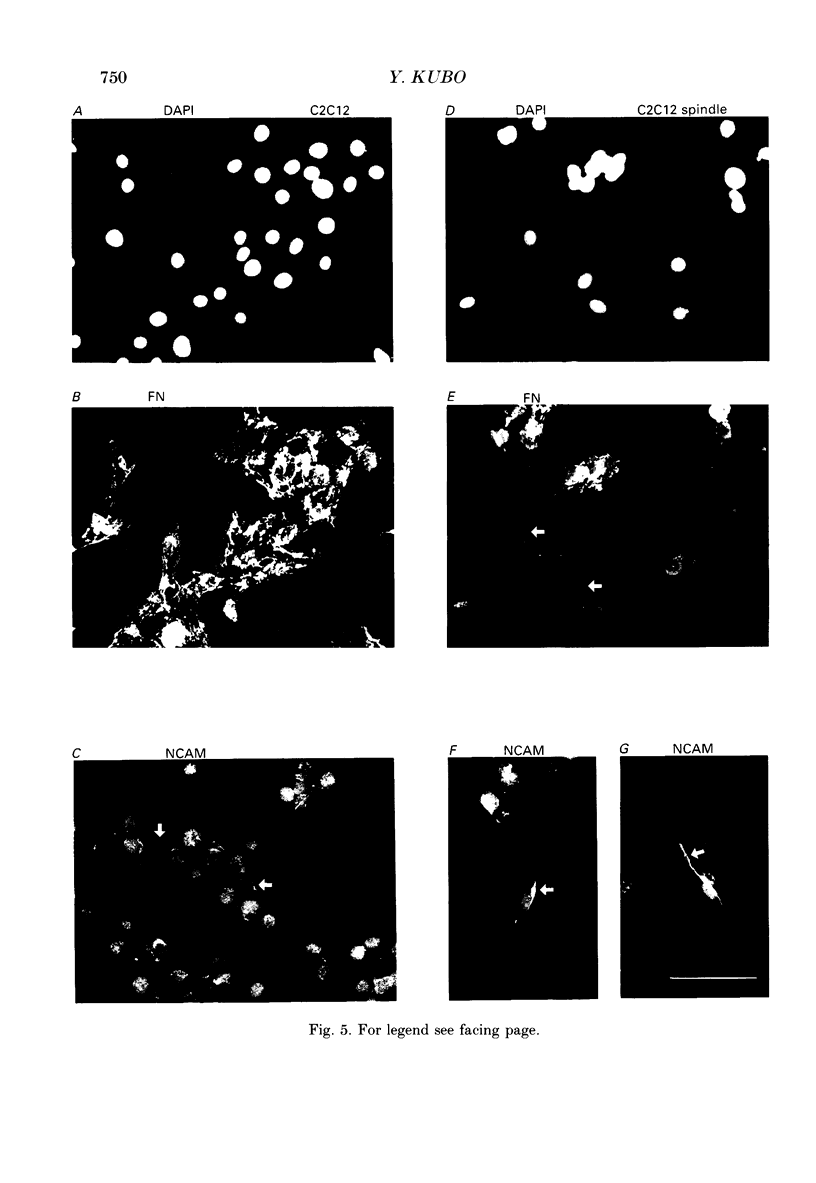
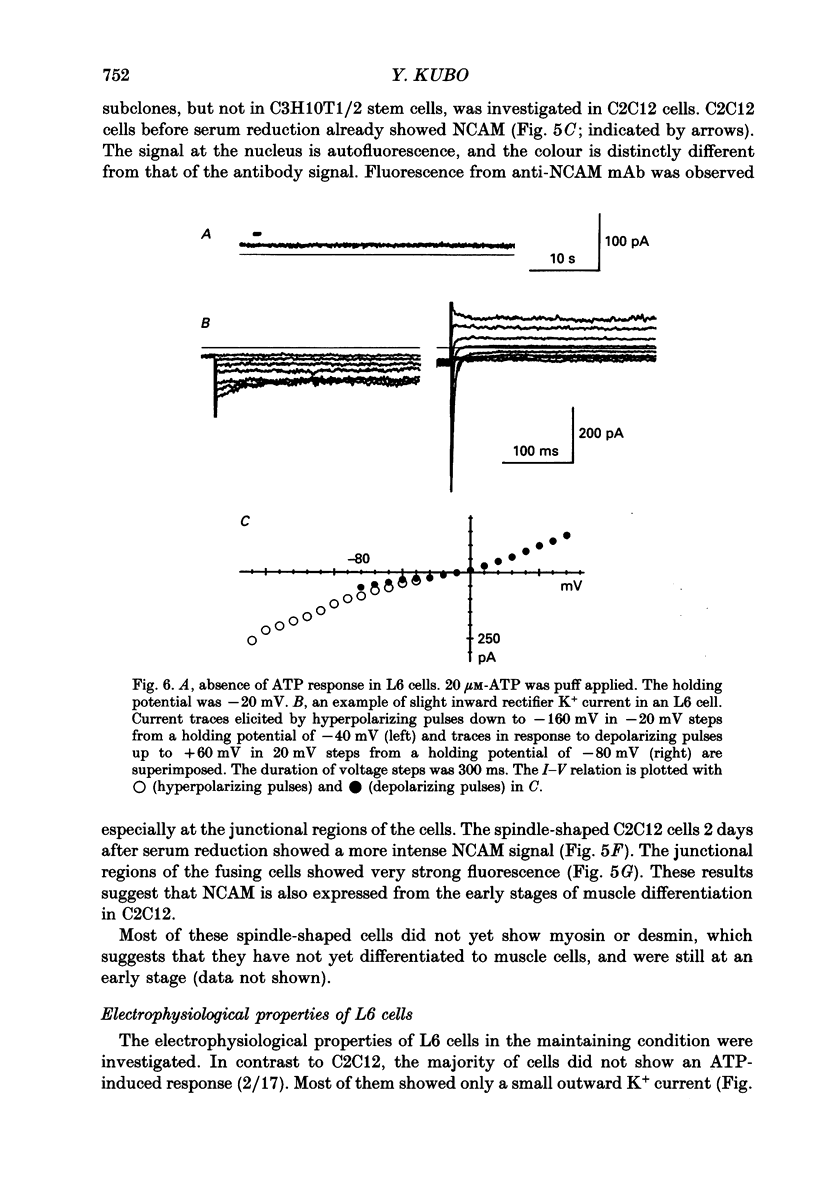
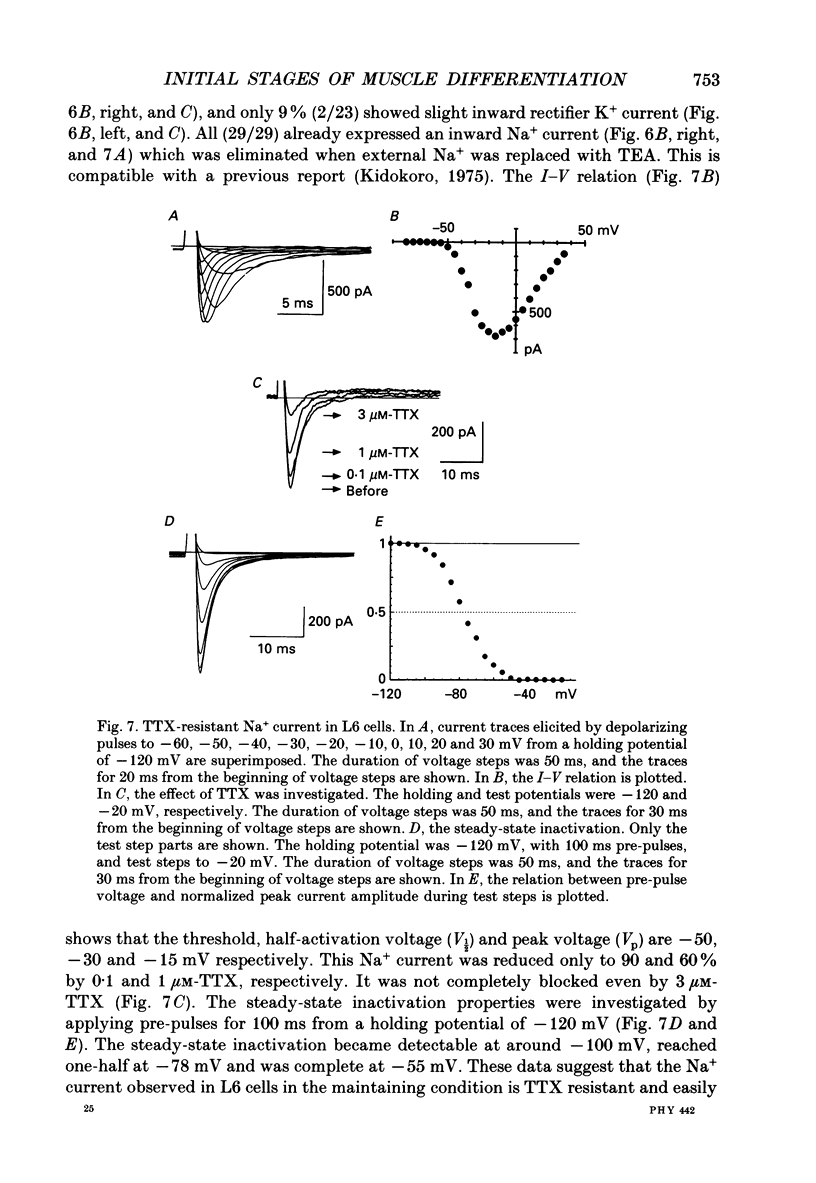
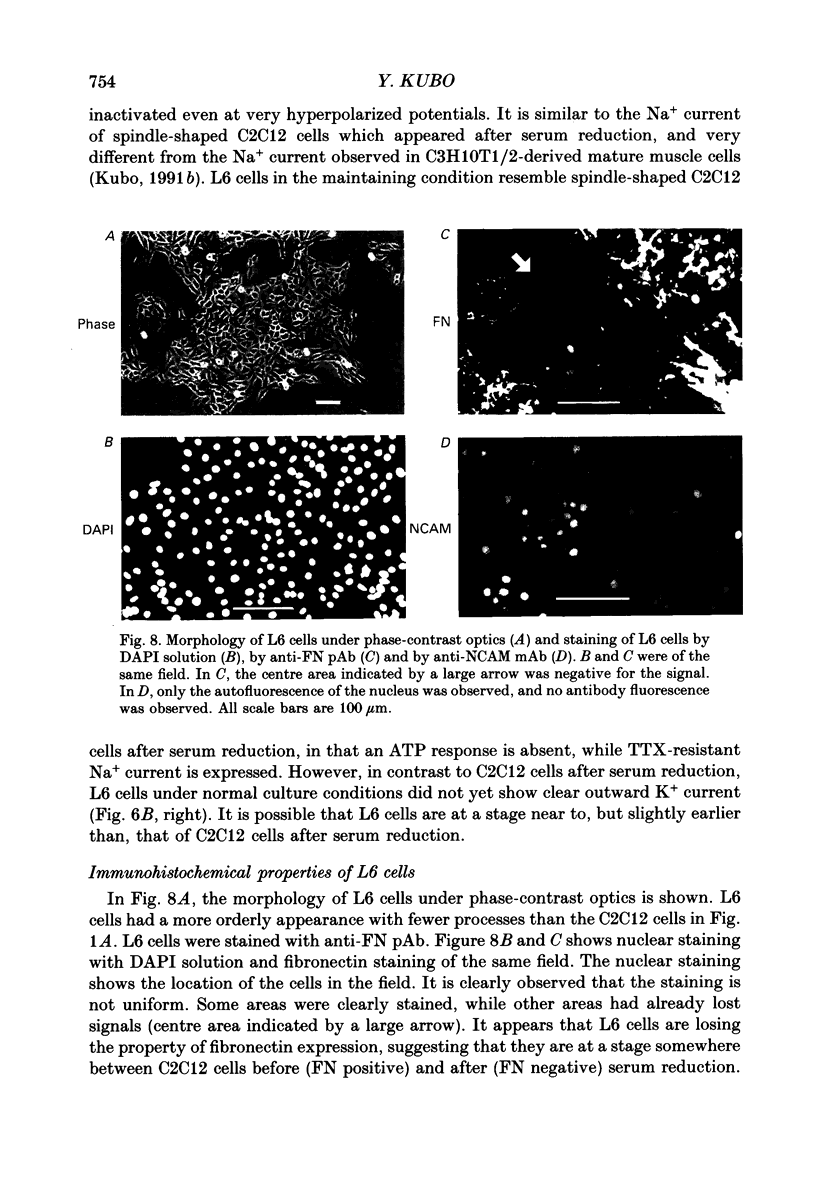
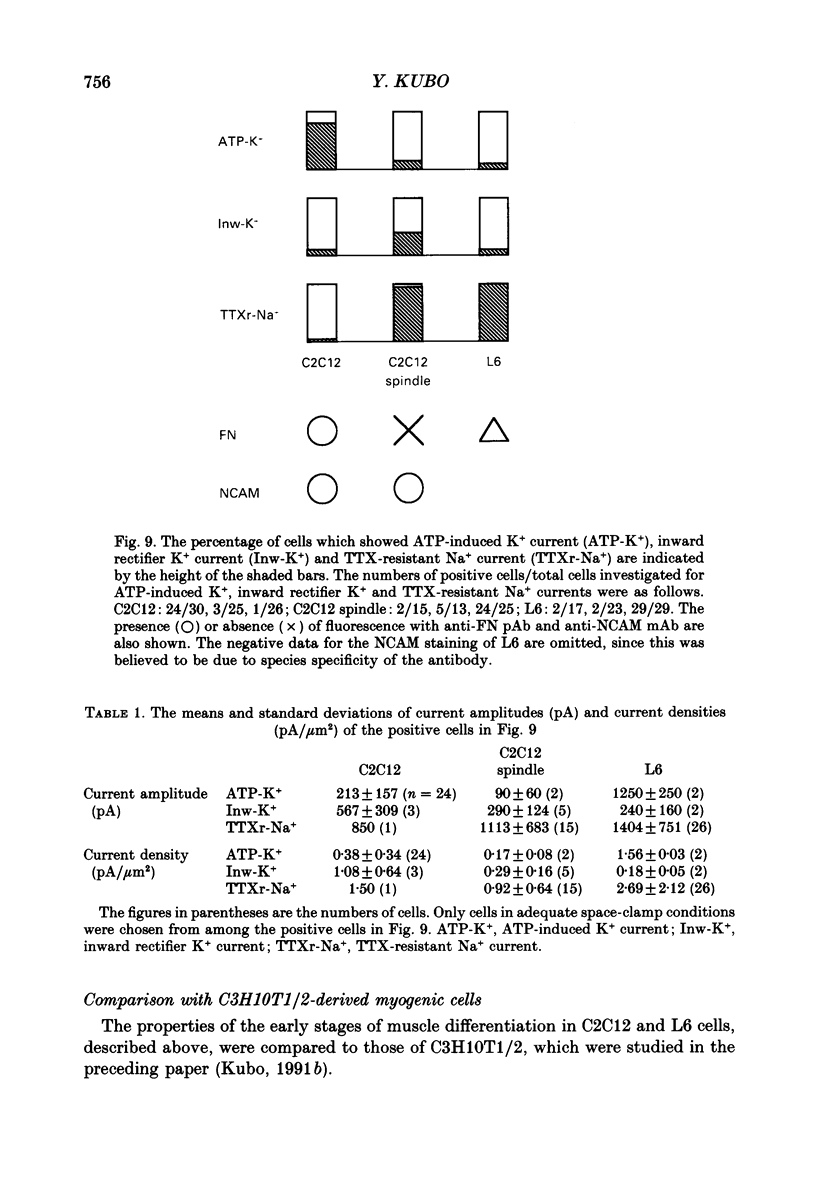
1. Electrophysiological and immunohistochemical properties during the early stages of muscle differentiation were studied in two myoblastic cell lines, mouse C2C12 and rat L6, and compared to those in myogenic clonal cells derived from the mouse mesodermal stem cell line C3H10T1/2, studied in the preceding paper. 2. Mouse C2C12 cells were induced to differentiate to muscle by changing from 10% fetal calf serum to 2% horse serum in the medium. Most of the C2C12 cells before serum reduction showed ATP-induced slow K+ current. Twelve per cent showed inward rectifier K+ current. They expressed fibronectin and Neural Cell Adhesion Molecule (NCAM). Small spindle-shaped cells at an early stage of muscle differentiation began to appear 24 h after serum reduction. In contrast to cells before serum reduction, only 13% of these spindle-shaped cells showed an ATP response. Most showed tetrodotoxin (TTX)-resistant Na+ current and outward K+ current. Thirty-eight per cent had inward rectifier K+ current. They expressed NCAM but not fibronectin. The T-type Ca2+ current was not observed up to the latest stage of differentiation investigated. 3. Rat L6 cells in maintaining culture medium showed only infrequent ATP responses, but already showed TTX-resistant Na+ current. No clear T-type Ca2+, inward rectifier K+ or outward K+ currents were observed. About one-third of the cells did not express fibronectin. From these results, L6 cells appear to be at a stage near to but slightly earlier than that of C2C12 cells after serum reduction. 4. The properties of the early stages of muscle differentiation in C3H10T1/2 cells, such as the disappearance of ATP-induced K+ current and fibronectin, and the appearance of NCAM, were also seen in C2C12 and L6. However, T-type Ca2+ and inward K+ currents, which were found in the initial stages of C3H10T1/2 muscle differentiation, were not clearly observed in C2C12 and L6. Instead, C2C12 and L6 showed a TTX-resistant Na+ current which was never observed in C3H10T1/2 cells. 5. The properties of the TTX-resistant Na+ current were investigated. In L6 cells, it was reduced to 60% by 1 microM-TTX. It could be evoked by depolarizations to a level above -50 mV with a maximum amplitude at around -15 mV. Steady-state inactivation was detectable with pre-pulses to -100 mV for 100 ms and reached half at pre-pulses of -78 mV. These parameters of inactivation are clearly different from those of the TTX-sensitive Na+ current observed in C3H10T1/2-derived mature muscle cells in the preceding paper.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beam K. G., Knudson C. M. Effect of postnatal development on calcium currents and slow charge movement in mammalian skeletal muscle. J Gen Physiol. 1988 Jun;91(6):799–815. doi: 10.1085/jgp.91.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H. M., Pavlath G. K., Hardeman E. C., Chiu C. P., Silberstein L., Webster S. G., Miller S. C., Webster C. Plasticity of the differentiated state. Science. 1985 Nov 15;230(4727):758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Caffrey J. M., Brown A. M., Schneider M. D. Ca2+ and Na+ currents in developing skeletal myoblasts are expressed in a sequential program: reversible suppression by transforming growth factor beta-1, an inhibitor of the myogenic pathway. J Neurosci. 1989 Oct;9(10):3443–3453. doi: 10.1523/JNEUROSCI.09-10-03443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides P. G., Jones P. A., Gevers W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 1977 May 26;267(5609):364–366. doi: 10.1038/267364a0. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Frelin C., Vijverberg H. P., Romey G., Vigne P., Lazdunski M. Different functional states of tetrodotoxin sensitive and tetrodotoxin resistant Na+ channels occur during the in vitro development of rat skeletal muscle. Pflugers Arch. 1984 Oct;402(2):121–128. doi: 10.1007/BF00583323. [DOI] [PubMed] [Google Scholar]
- Gonoi T., Hasegawa S. Post-natal disappearance of transient calcium channels in mouse skeletal muscle: effects of denervation and culture. J Physiol. 1988 Jul;401:617–637. doi: 10.1113/jphysiol.1988.sp017183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonoi T., Sherman S. J., Catterall W. A. Voltage clamp analysis of tetrodotoxin-sensitive and -insensitive sodium channels in rat muscle cells developing in vitro. J Neurosci. 1985 Sep;5(9):2559–2564. doi: 10.1523/JNEUROSCI.05-09-02559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M., Wakuta K., Satoh R. Calcium channel components of action potential in chick skeletal muscle cells developing in culture. Brain Res. 1987 Apr;429(2):233–240. doi: 10.1016/0165-3806(87)90103-9. [DOI] [PubMed] [Google Scholar]
- Kano M., Wakuta K., Satoh R. Two components of calcium channel current in embryonic chick skeletal muscle cells developing in culture. Brain Res Dev Brain Res. 1989 May 1;47(1):101–112. doi: 10.1016/0165-3806(89)90112-0. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y. Sodium and calcium components of the action potential in a developing skeletal muscle cell line. J Physiol. 1975 Jan;244(1):145–159. doi: 10.1113/jphysiol.1975.sp010788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y. Electrophysiological and immunohistochemical analysis of muscle differentiation in a mouse mesodermal stem cell line. J Physiol. 1991 Oct;442:711–741. doi: 10.1113/jphysiol.1991.sp018816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y. Properties of ionic currents induced by external ATP in a mouse mesodermal stem cell line. J Physiol. 1991 Oct;442:691–710. doi: 10.1113/jphysiol.1991.sp018815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarras D., Pinset C., Chelly J., Kahn A., Gros F. Expression of MyoD1 coincides with terminal differentiation in determined but inducible muscle cells. EMBO J. 1989 Aug;8(8):2203–2207. doi: 10.1002/j.1460-2075.1989.tb08343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney D. F., Pearson-White S. H., Konieczny S. F., Latham K. E., Emerson C. P., Jr Myogenic lineage determination and differentiation: evidence for a regulatory gene pathway. Cell. 1988 Jun 3;53(5):781–793. doi: 10.1016/0092-8674(88)90095-5. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Sassoon D., Lyons G., Wright W. E., Lin V., Lassar A., Weintraub H., Buckingham M. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 1989 Sep 28;341(6240):303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Thayer M. J., Tapscott S. J., Davis R. L., Wright W. E., Lassar A. B., Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989 Jul 28;58(2):241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]
- Weiss R. E., Horn R. Functional differences between two classes of sodium channels in developing rat skeletal muscle. Science. 1986 Jul 18;233(4761):361–364. doi: 10.1126/science.2425432. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]