Abstract
1. L-type Ca2+ currents and Ca2+ channel gating currents were studied in isolated guinea-pig ventricular heart cells using the whole-cell patch-clamp technique, in order to investigate the mechanism of Ca(2+)-dependent inactivation. The effect of altering the intracellular Ca2+ concentration ([Ca2+]i) on these currents was studied through photorelease of intracellular Ca2+ ions using the photolabile Ca2+ chelators DM-nitrophen and nitr-5. 2. We found that step increases in [Ca2+]i produced by photorelease could either increase or decrease the L-type Ca2+ current. Specifically, Ca2+ photorelease from DM-nitrophen almost exclusively caused inactivation of the Ca2+ current. In contrast, Ca2+ photorelease from nitr-5 had a biphasic effect: a small, rapid inactivation of the Ca2+ current was followed by a slow potentiation. These two Ca(2+)-dependent processes seemed to differ in their Ca2+ dependence, as small Ca2+ photoreleases elicited potentiation without a preceding inactivation, whereas larger photoreleases elicited both inactivation and potentiation. 3. The mechanism of the Ca(2+)-dependent inactivation of Ca2+ channels was explored by comparing the effects of voltage and photoreleased Ca2+ on the Ca2+ current and the Ca2+ channel gating current. Voltage was found to reduce both the Ca2+ current and the gating current proportionally. However, Ca2+ photorelease from intracellular DM-nitrophen inactivated the Ca2+ current without having any effect on the gating current. 4. The dephosphorylation hypothesis for Ca(2+)-dependent inactivation was tested by applying isoprenaline to the cells before eliciting a maximal rise of [Ca2+]i (maximal flash intensity, zero external [Na+]i). Isoprenaline could completely prevent Ca(2+)-dependent inactivation under these conditions, even when [Ca2+]i rose so high as to cause an irreversible contracture of the cell. 5. We concluded from these experiments that voltage and Ca2+ ions inactivate the L-type Ca2+ channel through separate, independent mechanisms. In addition, we found that Ca(2+)-dependent inactivation does not result in the immobilization of gating charge, and apparently closes the Ca2+ permeation pathway through a mechanism that does not involve the voltage-sensing region of the channel. Furthermore, we found that Ca(2+)-dependent inactivation is entirely sensitive to beta-adrenergic stimulation. These facts suggest that either Ca(2+)-dependent inactivation results from Ca(2+)-dependent dephosphorylation of the Ca2+ channel, or that Ca(2+)-dependent inactivation is modulated by protein kinase A.
Full text
PDF


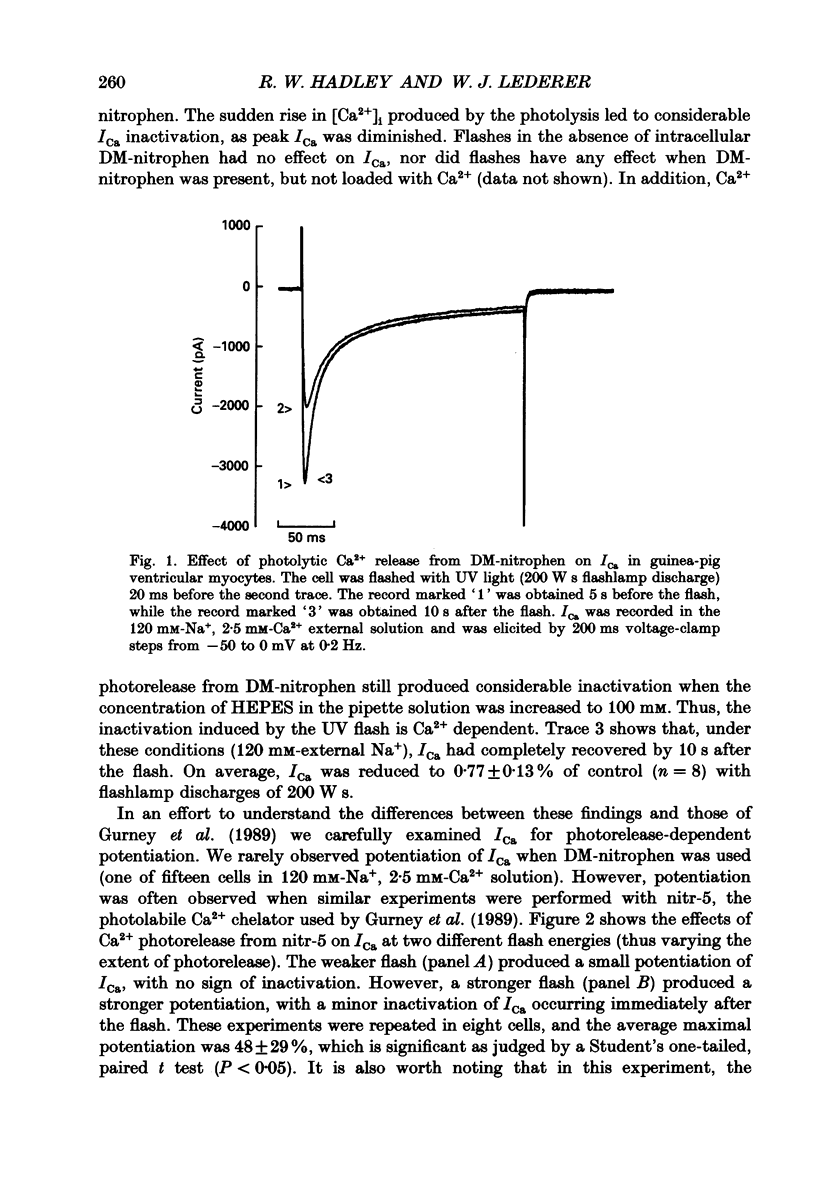


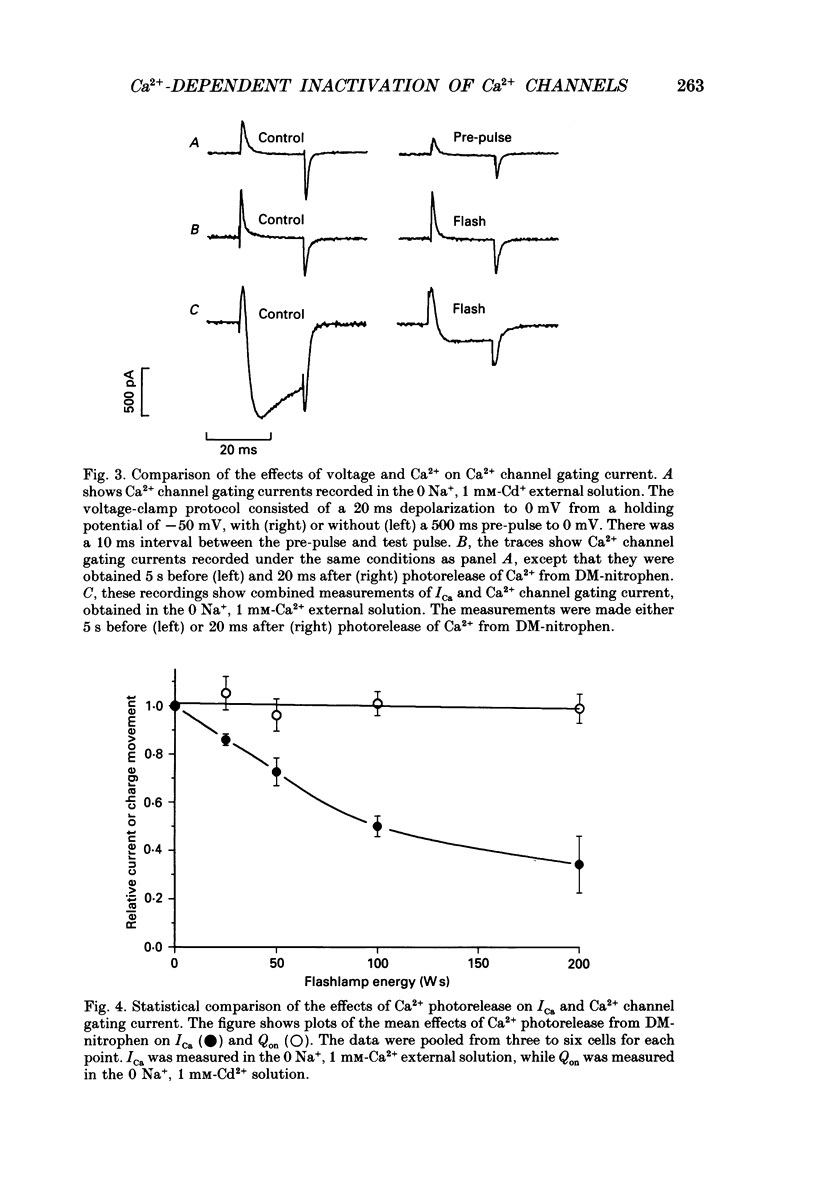
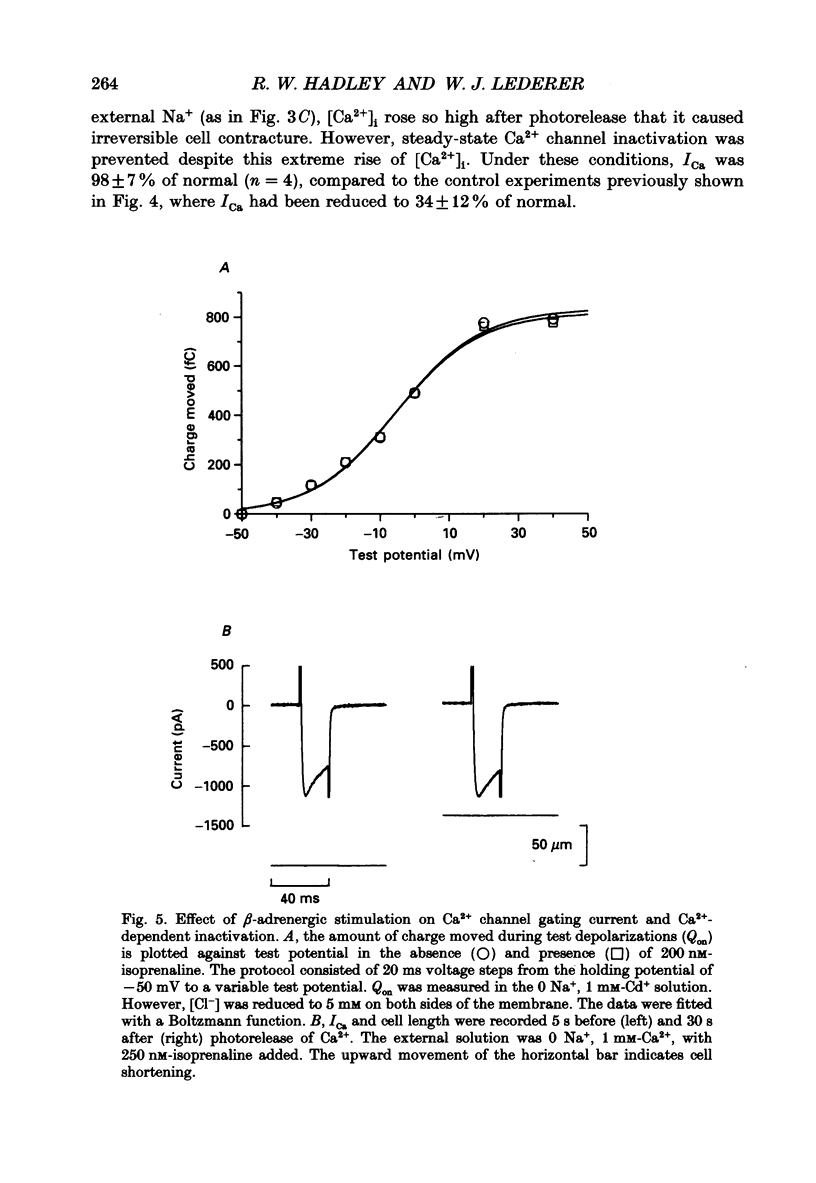

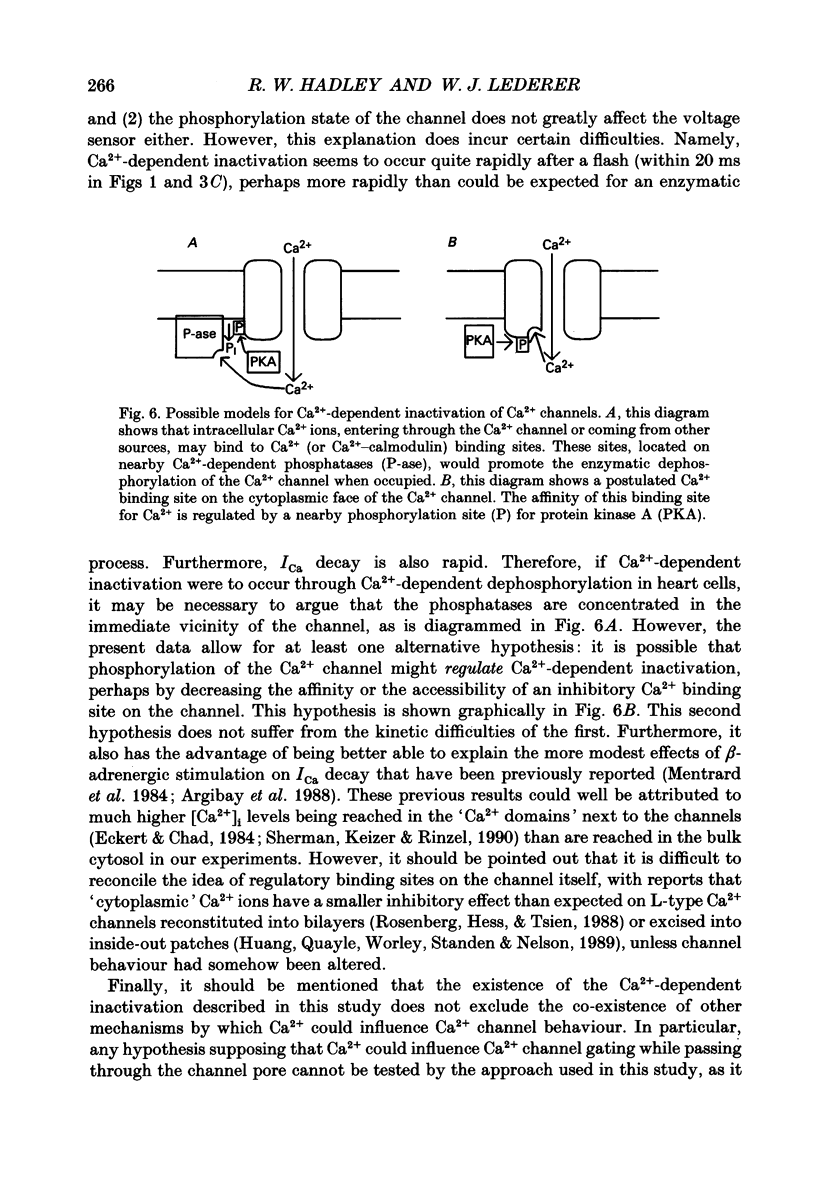


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argibay J. A., Fischmeister R., Hartzell H. C. Inactivation, reactivation and pacing dependence of calcium current in frog cardiocytes: correlation with current density. J Physiol. 1988 Jul;401:201–226. doi: 10.1113/jphysiol.1988.sp017158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D., Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Nowycky M. C., Tsien R. W. Beta-adrenergic modulation of calcium channels in frog ventricular heart cells. 1984 Jan 26-Feb 1Nature. 307(5949):371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Rios E. Nonlinear charge movement in mammalian cardiac ventricular cells. Components from Na and Ca channel gating. J Gen Physiol. 1989 Jul;94(1):65–93. doi: 10.1085/jgp.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Gurney A. M., Charnet P., Pye J. M., Nargeot J. Augmentation of cardiac calcium current by flash photolysis of intracellular caged-Ca2+ molecules. Nature. 1989 Sep 7;341(6237):65–68. doi: 10.1038/341065a0. [DOI] [PubMed] [Google Scholar]
- Hadley R. W., Lederer W. J. Intramembrane charge movement in guinea-pig and rat ventricular myocytes. J Physiol. 1989 Aug;415:601–624. doi: 10.1113/jphysiol.1989.sp017738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. W., Lederer W. J. Properties of L-type calcium channel gating current in isolated guinea pig ventricular myocytes. J Gen Physiol. 1991 Aug;98(2):265–285. doi: 10.1085/jgp.98.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Huang Y., Quayle J. M., Worley J. F., Standen N. B., Nelson M. T. External cadmium and internal calcium block of single calcium channels in smooth muscle cells from rabbit mesenteric artery. Biophys J. 1989 Nov;56(5):1023–1028. doi: 10.1016/S0006-3495(89)82747-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymel L., Striessnig J., Glossmann H., Schindler H. Purified skeletal muscle 1,4-dihydropyridine receptor forms phosphorylation-dependent oligomeric calcium channels in planar bilayers. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4290–4294. doi: 10.1073/pnas.85.12.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986 Aug;407(2):123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Mieskes G., Trautwein W. The protein-specific phosphatase 1 antagonizes the beta-adrenergic increase of the cardiac Ca current. Pflugers Arch. 1986 Oct;407(4):461–463. doi: 10.1007/BF00652635. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H. Photochemical manipulation of divalent cation levels. Annu Rev Physiol. 1990;52:897–914. doi: 10.1146/annurev.ph.52.030190.004225. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentrard D., Vassort G., Fischmeister R. Calcium-mediated inactivation of the calcium conductance in cesium-loaded frog heart cells. J Gen Physiol. 1984 Jan;83(1):105–131. doi: 10.1085/jgp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985 Nov;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Morad M., Davies N. W., Kaplan J. H., Lux H. D. Inactivation and block of calcium channels by photo-released Ca2+ in dorsal root ganglion neurons. Science. 1988 Aug 12;241(4867):842–844. doi: 10.1126/science.2457253. [DOI] [PubMed] [Google Scholar]
- Niggli E., Lederer W. J. Voltage-independent calcium release in heart muscle. Science. 1990 Oct 26;250(4980):565–568. doi: 10.1126/science.2173135. [DOI] [PubMed] [Google Scholar]
- Nunoki K., Florio V., Catterall W. A. Activation of purified calcium channels by stoichiometric protein phosphorylation. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6816–6820. doi: 10.1073/pnas.86.17.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant T. D., Standen N. B., Ward T. A. The effects of injection of calcium ions and calcium chelators on calcium channel inactivation in Helix neurones. J Physiol. 1983 Jan;334:189–212. doi: 10.1113/jphysiol.1983.sp014489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. L., Hess P., Tsien R. W. Cardiac calcium channels in planar lipid bilayers. L-type channels and calcium-permeable channels open at negative membrane potentials. J Gen Physiol. 1988 Jul;92(1):27–54. doi: 10.1085/jgp.92.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman A., Keizer J., Rinzel J. Domain model for Ca2(+)-inactivation of Ca2+ channels at low channel density. Biophys J. 1990 Oct;58(4):985–995. doi: 10.1016/S0006-3495(90)82443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N., Schneider J. A. A metabolic control mechanism for calcium ion influx that may protect the ventricular myocardial cell. Am J Cardiol. 1976 Jun;37(7):1079–1085. doi: 10.1016/0002-9149(76)90428-8. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Hescheler J. Regulation of cardiac L-type calcium current by phosphorylation and G proteins. Annu Rev Physiol. 1990;52:257–274. doi: 10.1146/annurev.ph.52.030190.001353. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Backx P. H., Imredy J. P. Calcium-sensitive inactivation in the gating of single calcium channels. Science. 1990 Dec 21;250(4988):1735–1738. doi: 10.1126/science.2176745. [DOI] [PubMed] [Google Scholar]


