Abstract
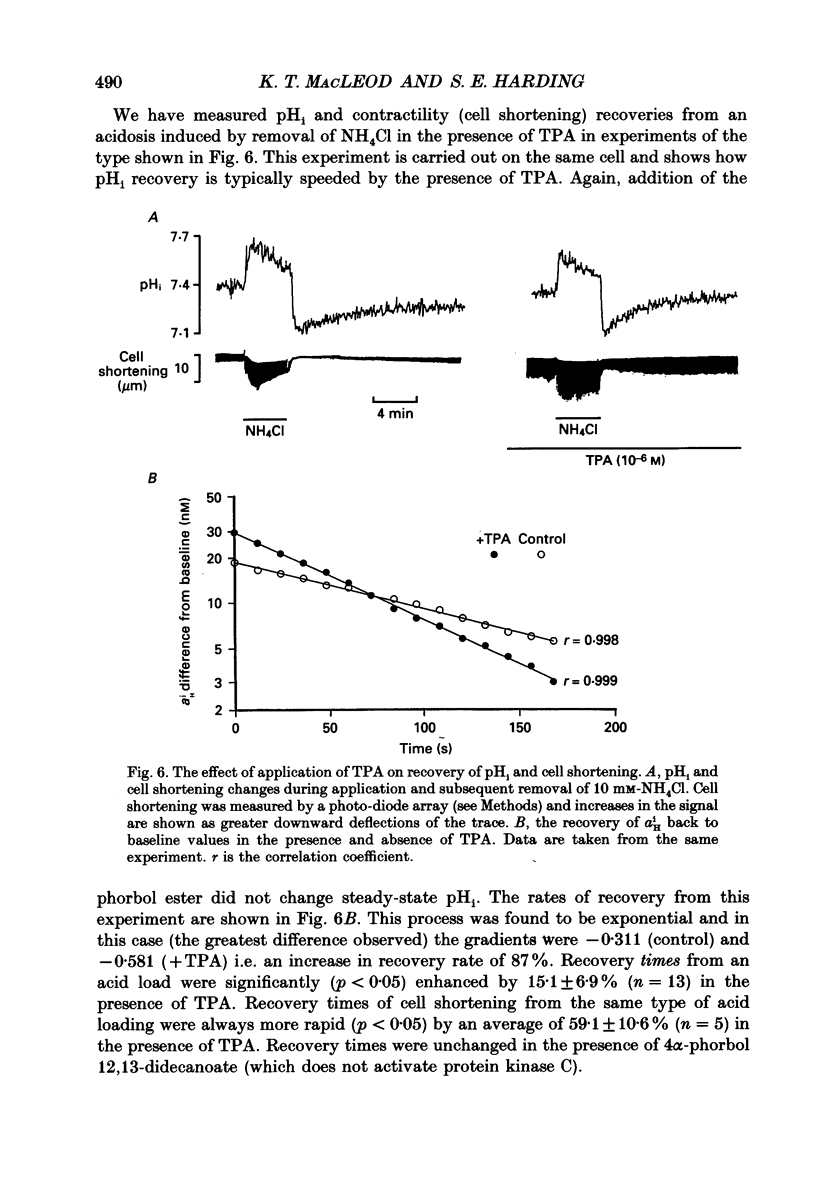
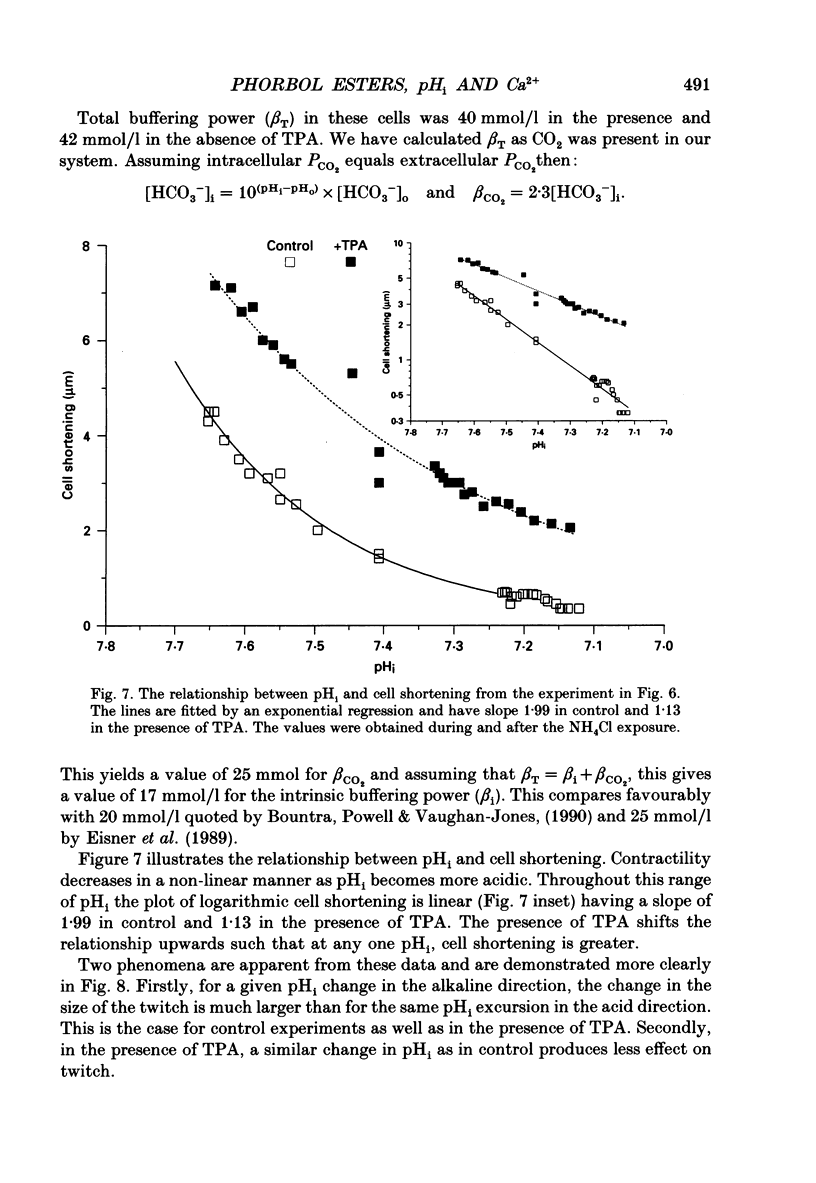
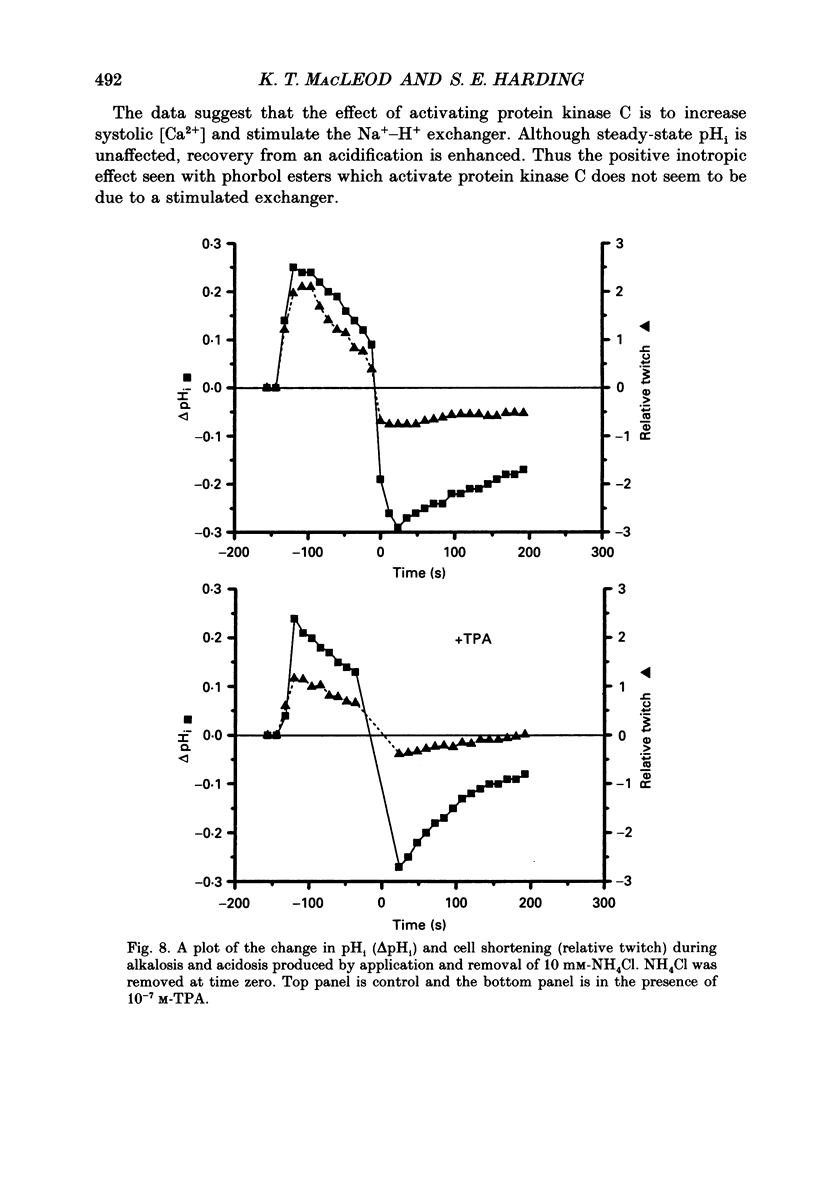
1. We have investigated the actions of certain phorbol esters on the intracellular pH, intracellular Ca2+ and contractility of isolated rat and guinea-pig cardiac myocytes. Intracellular pH was measured using 2',7'-bis(carboxyethyl)-5(6)-carboxyfluorescein (BCECF) and intracellular Ca2+ was measured using Fura-2. 2. Application of the phorbol ester 12-O-tetradecanoylphorbol 13-acetate (also called phorbol 12-myristate 13-acetate) (TPA) (which activates protein kinase C) to rat cardiac myocytes significantly increased cell shortening by 116 +/- 34% (n = 8) (p less than 0.02). The rate of change of cell length during contraction (i.e. +dL/dt) increased from 67.2 +/- 8.7 microns/s to 127.7 +/- 14.1 microns/s (n = 7). The rate of change of cell length during relaxation (-dL/dt) increased from 55.8 +/- 7.4 microns/s to 118.9 +/- 12.1 microns/s (n = 7). Time to peak shortening was unchanged. 3. Application of 4 alpha-phorbol 12,13-didecanoate, which does not activate protein kinase C, did not affect rat myocyte contractility. An insignificant decrease in contractility (by 7.5 +/- 7.5%) was observed (n = 5). The positive inotropic effect of TPA may therefore be evoked through an activation of protein kinase C. 4. In rat myocytes we have measured the changes of pHi and contractility (cell shortening) during an alkalosis and acidosis induced by exposure to and subsequent removal of NH4Cl both in the presence and absence of TPA. Recovery times from an acid load were significantly (p less than 0.05) enhanced by 15.1 +/- 6.9% (n = 13) in the presence of TPA. Recovery times of cell shortening were also more rapid (p less than 0.05) by an average of 59.1 +/- 10.6% (n = 5) in the presence of TPA. Recovery times were unchanged in the presence of 4-phorbol 12,13-didecanoate (which does not activate protein kinase C). 5. Since pHi recovery of an isolated myocyte from an acid load is partially inhibited by the presence of 1 mM-amiloride and inhibited by removing extracellular Na+ then it is suggested that, like pHi regulation in sheep heart Purkinje fibres, pHi recovery in rat cardiac ventricular myocytes is mainly through sarcolemmal Na(+)-H+ exchange. We suggest that in the presence of TPA the Na(+)-H+ exchange is stimulated. 6. The relationship between pHi and cell shortening is non-linear as has been observed by others in whole tissue preparations. The presence of TPA shifts the relationship upwards such that at any one pHi, cell shortening is greater.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


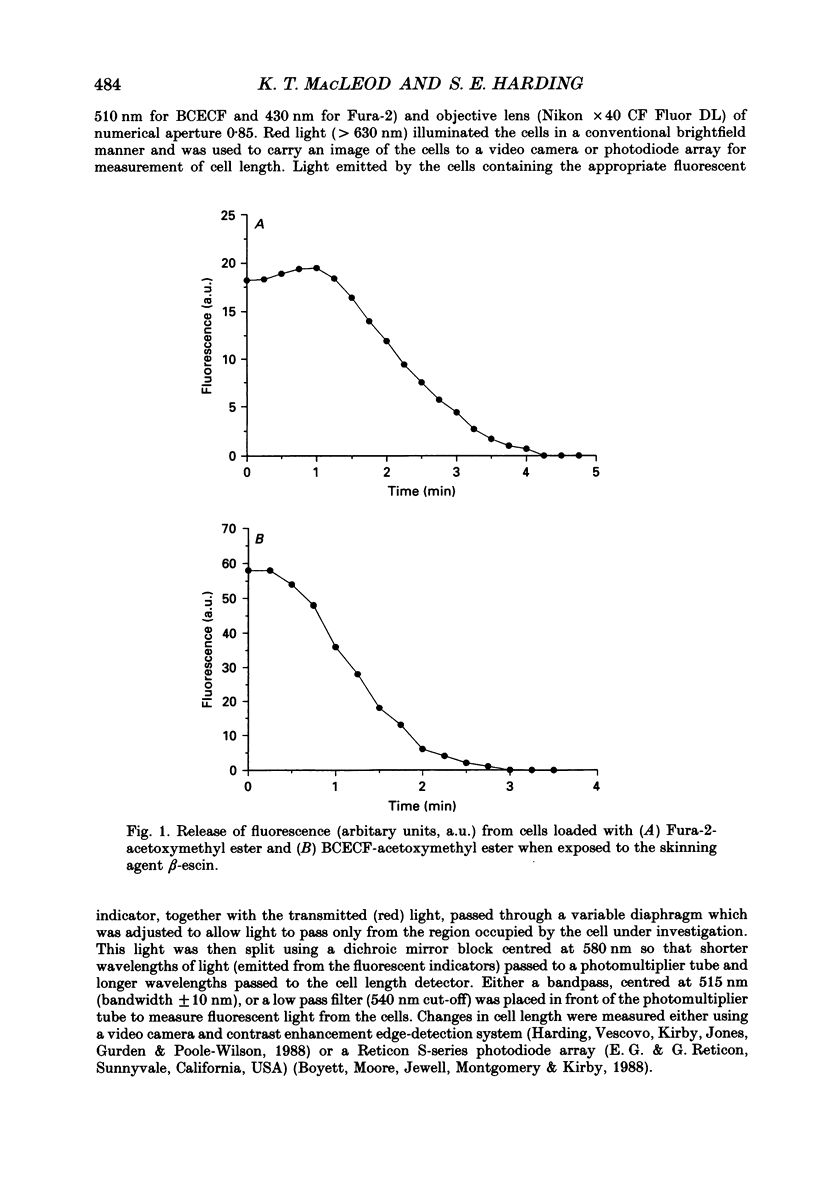


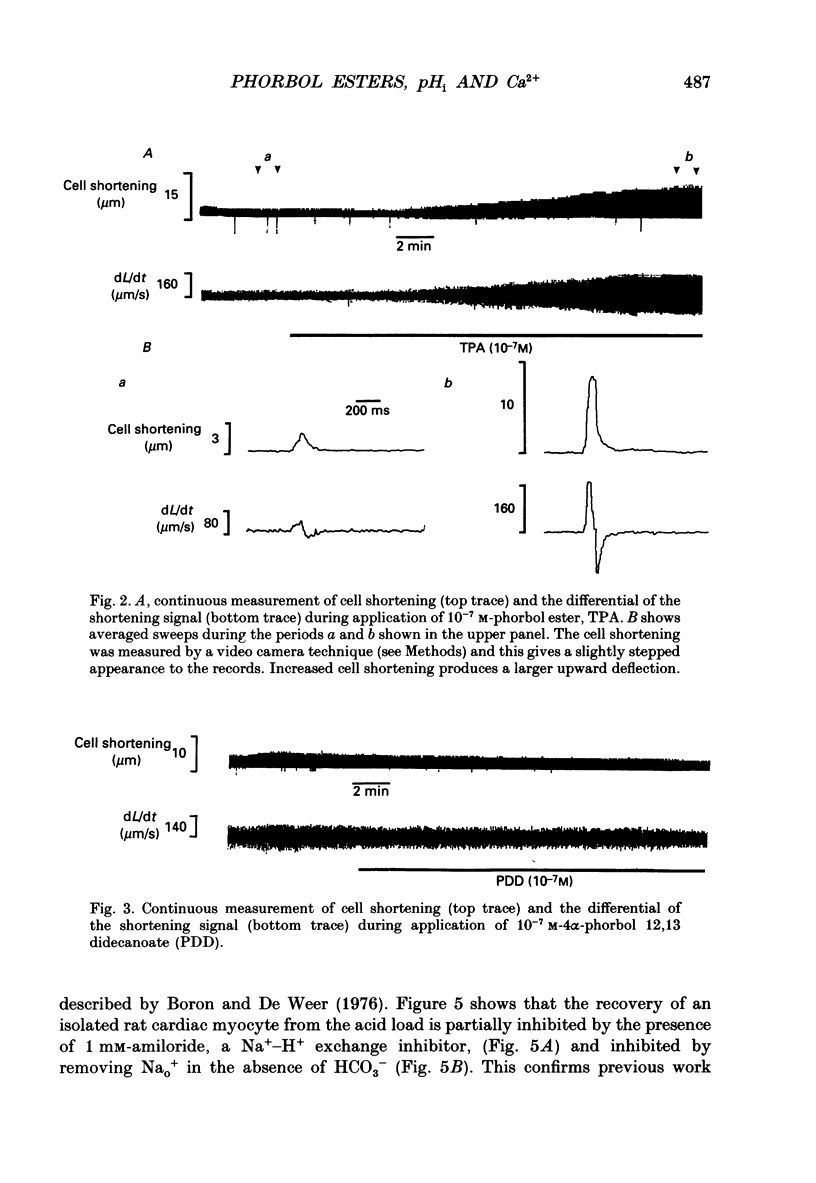
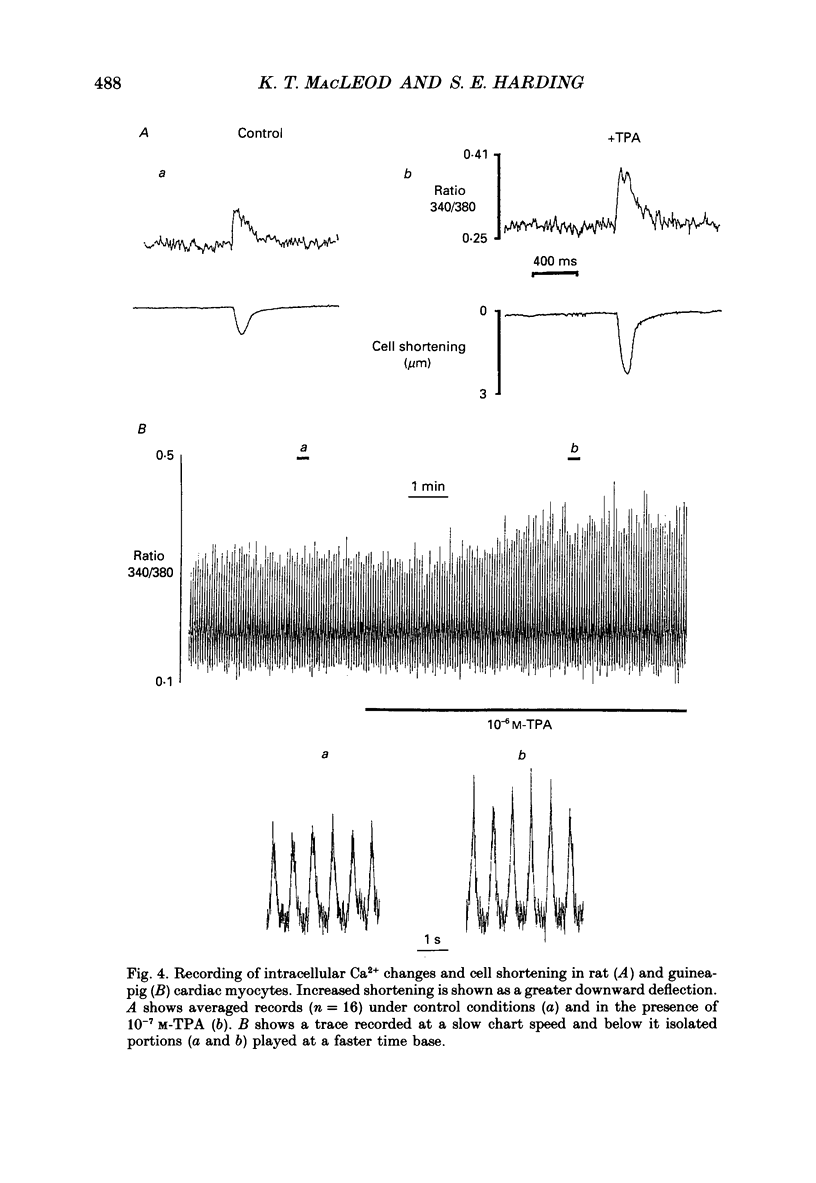
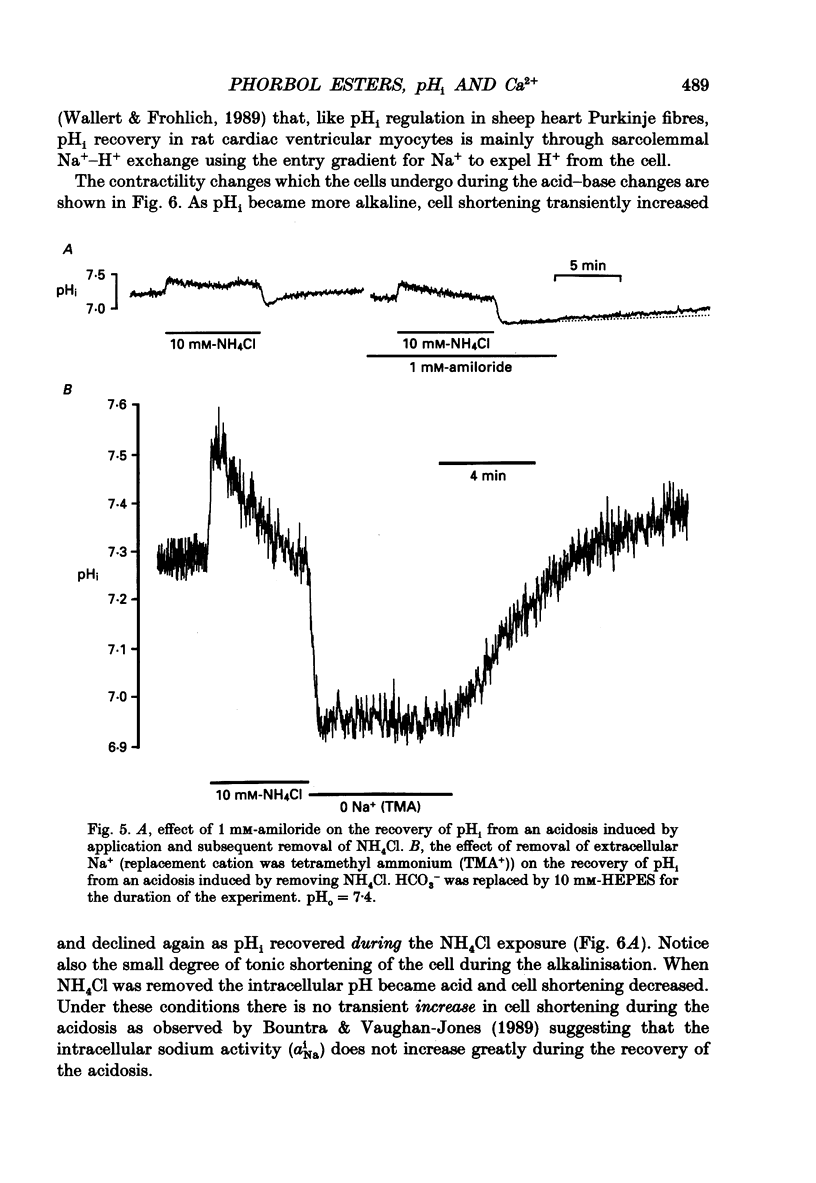






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry W. H., Smith T. W. Mechanisms of transmembrane calcium movement in cultured chick embryo ventricular cells. J Physiol. 1982 Apr;325:243–260. doi: 10.1113/jphysiol.1982.sp014148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Ellis D. Intracellular calcium and sodium activity in sheep heart Purkinje fibres. Effect of changes of external sodium and intracellular pH. Pflugers Arch. 1982 Apr;393(2):171–178. doi: 10.1007/BF00582941. [DOI] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C., Powell T., Vaughan-Jones R. D. Comparison of intracellular pH transients in single ventricular myocytes and isolated ventricular muscle of guinea-pig. J Physiol. 1990 May;424:343–365. doi: 10.1113/jphysiol.1990.sp018071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C., Vaughan-Jones R. D. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J Physiol. 1989 Nov;418:163–187. doi: 10.1113/jphysiol.1989.sp017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Moore M., Jewell B. R., Montgomery R. A., Kirby M. S., Orchard C. H. An improved apparatus for the optical recording of contraction of single heart cells. Pflugers Arch. 1988 Dec;413(2):197–205. doi: 10.1007/BF00582531. [DOI] [PubMed] [Google Scholar]
- Bristow M. R., Ginsburg R., Minobe W., Cubicciotti R. S., Sageman W. S., Lurie K., Billingham M. E., Harrison D. C., Stinson E. B. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982 Jul 22;307(4):205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- Brum G., Osterrieder W., Trautwein W. Beta-adrenergic increase in the calcium conductance of cardiac myocytes studied with the patch clamp. Pflugers Arch. 1984 Jun;401(2):111–118. doi: 10.1007/BF00583870. [DOI] [PubMed] [Google Scholar]
- Böhm M., Diet F., Feiler G., Kemkes B., Erdmann E. Alpha-adrenoceptors and alpha-adrenoceptor-mediated positive inotropic effects in failing human myocardium. J Cardiovasc Pharmacol. 1988 Sep;12(3):357–364. doi: 10.1097/00005344-198809000-00015. [DOI] [PubMed] [Google Scholar]
- Capogrossi M. C., Kaku T., Filburn C. R., Pelto D. J., Hansford R. G., Spurgeon H. A., Lakatta E. G. Phorbol ester and dioctanoylglycerol stimulate membrane association of protein kinase C and have a negative inotropic effect mediated by changes in cytosolic Ca2+ in adult rat cardiac myocytes. Circ Res. 1990 Apr;66(4):1143–1155. doi: 10.1161/01.res.66.4.1143. [DOI] [PubMed] [Google Scholar]
- Döemeci A., Dhallan R. S., Cohen N. M., Lederer W. J., Rogers T. B. Phorbol ester increases calcium current and simulates the effects of angiotensin II on cultured neonatal rat heart myocytes. Circ Res. 1988 Feb;62(2):347–357. doi: 10.1161/01.res.62.2.347. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Nichols C. G., O'Neill S. C., Smith G. L., Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989 Apr;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin C., Vigne P., Ladoux A., Lazdunski M. The regulation of the intracellular pH in cells from vertebrates. Eur J Biochem. 1988 May 16;174(1):3–14. doi: 10.1111/j.1432-1033.1988.tb14055.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harding S. E., Vescovo G., Kirby M., Jones S. M., Gurden J., Poole-Wilson P. A. Contractile responses of isolated adult rat and rabbit cardiac myocytes to isoproterenol and calcium. J Mol Cell Cardiol. 1988 Jul;20(7):635–647. doi: 10.1016/s0022-2828(88)80121-4. [DOI] [PubMed] [Google Scholar]
- Henrich C. J., Simpson P. C. Differential acute and chronic response of protein kinase C in cultured neonatal rat heart myocytes to alpha 1-adrenergic and phorbol ester stimulation. J Mol Cell Cardiol. 1988 Dec;20(12):1081–1085. doi: 10.1016/0022-2828(88)90588-3. [DOI] [PubMed] [Google Scholar]
- Jakob H., Nawrath H., Rupp J. Adrenoceptor-mediated changes of action potential and force of contraction in human isolated ventricular heart muscle. Br J Pharmacol. 1988 Jun;94(2):584–590. doi: 10.1111/j.1476-5381.1988.tb11564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K., Vaughan-Jones R. D. Influence of sodium-hydrogen exchange on intracellular pH, sodium and tension in sheep cardiac Purkinje fibres. J Physiol. 1987 Sep;390:93–118. doi: 10.1113/jphysiol.1987.sp016688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Kitazawa T., Somlyo A. V., Somlyo A. P. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem. 1989 Oct 25;264(30):17997–18004. [PubMed] [Google Scholar]
- Kohmoto O., Spitzer K. W., Movsesian M. A., Barry W. H. Effects of intracellular acidosis on [Ca2+]i transients, transsarcolemmal Ca2+ fluxes, and contraction in ventricular myocytes. Circ Res. 1990 Mar;66(3):622–632. doi: 10.1161/01.res.66.3.622. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Rampe D., Brown A. M. Effects of protein kinase C activators on cardiac Ca2+ channels. Nature. 1988 Sep 15;335(6187):249–251. doi: 10.1038/335249a0. [DOI] [PubMed] [Google Scholar]
- Leatherman G. F., Kim D., Smith T. W. Effect of phorbol esters on contractile state and calcium flux in cultured chick heart cells. Am J Physiol. 1987 Jul;253(1 Pt 2):H205–H209. doi: 10.1152/ajpheart.1987.253.1.H205. [DOI] [PubMed] [Google Scholar]
- Liu J. D., Wood J. G., Raynor R. L., Wang Y. C., Noland T. A., Jr, Ansari A. A., Kuo J. F. Subcellular distribution and immunocytochemical localization of protein kinase C in myocardium, and phosphorylation of troponin in isolated myocytes stimulated by isoproterenol or phorbol ester. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1105–1110. doi: 10.1016/0006-291x(89)90787-0. [DOI] [PubMed] [Google Scholar]
- Moody C. J., Dashwood M. R., Sykes R. M., Chester M., Jones S. M., Yacoub M. H., Harding S. E. Functional and autoradiographic evidence for endothelin 1 receptors on human and rat cardiac myocytes. Comparison with single smooth muscle cells. Circ Res. 1990 Sep;67(3):764–769. doi: 10.1161/01.res.67.3.764. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Boonstra J., van der Saag P. T., de Laat S. W. Sodium/proton exchange in mouse neuroblastoma cells. J Biol Chem. 1981 Dec 25;256(24):12883–12887. [PubMed] [Google Scholar]
- Moolenaar W. H., Tsien R. Y., van der Saag P. T., de Laat S. W. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983 Aug 18;304(5927):645–648. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- Moore R. D. Stimulation of Na:H exchange by insulin. Biophys J. 1981 Feb;33(2):203–210. doi: 10.1016/S0006-3495(81)84881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro J. Modulation of [3H]dihydropyridine receptors by activation of protein kinase C in chick muscle cells. J Biol Chem. 1987 Apr 5;262(10):4649–4652. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Callahan C. M., Ptasienski J., Hosey M. M. Phosphorylation of the 165-kDa dihydropyridine/phenylalkylamine receptor from skeletal muscle by protein kinase C. J Biol Chem. 1988 Nov 25;263(33):17342–17349. [PubMed] [Google Scholar]
- Okumura K., Kawai T., Hashimoto H., Ito T., Ogawa K., Satake T. Sustained diacylglycerol formation in norepinephrine-stimulated rat heart is associated with alpha 1-adrenergic receptor. J Cardiovasc Pharmacol. 1988 Jun;11(6):651–656. doi: 10.1097/00005344-198806000-00004. [DOI] [PubMed] [Google Scholar]
- Paradiso A. M., Tsien R. Y., Machen T. E. Na+-H+ exchange in gastric glands as measured with a cytoplasmic-trapped, fluorescent pH indicator. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7436–7440. doi: 10.1073/pnas.81.23.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe M. W., Lemasters J. J., Herman B. Assessment of Fura-2 for measurements of cytosolic free calcium. Cell Calcium. 1990 Feb-Mar;11(2-3):63–73. doi: 10.1016/0143-4160(90)90060-8. [DOI] [PubMed] [Google Scholar]
- Satoh H., Hashimoto K. On electrophysiological responses to phorbol esters which stimulate protein kinase C in rabbit sino-atrial node cells. Naunyn Schmiedebergs Arch Pharmacol. 1988 Mar;337(3):308–315. doi: 10.1007/BF00168844. [DOI] [PubMed] [Google Scholar]
- Smith K. B., Losonczy I., Sahai A., Pannerselvam M., Fehnel P., Salomon D. S. Effect of 12-O-tetradecanoylphorbol-13-acetate (TPA) on the growth inhibitory and increased phosphatidylinositol (PI) responses induced by epidermal growth factor (EGF) in A431 cells. J Cell Physiol. 1983 Oct;117(1):91–100. doi: 10.1002/jcp.1041170113. [DOI] [PubMed] [Google Scholar]
- Strong J. A., Fox A. P., Tsien R. W., Kaczmarek L. K. Stimulation of protein kinase C recruits covert calcium channels in Aplysia bag cell neurons. Nature. 1987 Feb 19;325(6106):714–717. doi: 10.1038/325714a0. [DOI] [PubMed] [Google Scholar]
- Takasago T., Imagawa T., Shigekawa M. Phosphorylation of the cardiac ryanodine receptor by cAMP-dependent protein kinase. J Biochem. 1989 Nov;106(5):872–877. doi: 10.1093/oxfordjournals.jbchem.a122945. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Eisner D. A., Lederer W. J. Effects of changes of intracellular pH on contraction in sheep cardiac Purkinje fibers. J Gen Physiol. 1987 Jun;89(6):1015–1032. doi: 10.1085/jgp.89.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Wallert M. A., Fröhlich O. Na+-H+ exchange in isolated myocytes from adult rat heart. Am J Physiol. 1989 Aug;257(2 Pt 1):C207–C213. doi: 10.1152/ajpcell.1989.257.2.C207. [DOI] [PubMed] [Google Scholar]
- Walsh K. B., Kass R. S. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988 Oct 7;242(4875):67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- Whiteley B., Cassel D., Zhuang Y. X., Glaser L. Tumor promoter phorbol 12-myristate 13-acetate inhibits mitogen-stimulated Na+/H+ exchange in human epidermoid carcinoma A431 cells. J Cell Biol. 1984 Sep;99(3):1162–1166. doi: 10.1083/jcb.99.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock E. A., McLeod J. K., Smith A. I., Clark M. G. Study of receptor-stimulated phosphatidylinositol hydrolysis in intact, perfused rat hearts. Clin Exp Pharmacol Physiol. 1987 Mar;14(3):209–213. doi: 10.1111/j.1440-1681.1987.tb00377.x. [DOI] [PubMed] [Google Scholar]


