Abstract
1. Endothelin (ET) has been shown to reduce glomerular filtration rate (GFR) and renal blood flow (RBF) and may therefore be a possible mediator of the reduction of GFR and RBF observed in post-ischaemic acute renal failure. 2. We infused a specific ET antibody, i.v., for 1 h before and 1 h after a 60 min period of renal ischemia by clamping the renal artery, and observed the changes in renal function (acute clearance and long-term metabolic cage studies) compared with rats infused with non-immune rabbit serum. 3. In acute and long-term studies, better renal function, as judged by GFR and RBF was observed in rats treated with the ET antibody. Furthermore, ischaemic rats showed higher levels of plasma immunoreactive ET (7.02 +/- 1.17 pg ET (ml plasma)-1; mean +/- S.E.M.) than normal rats where it was undetected. 4. We previously reported that glomeruli and renal platelet-activating factor (PAF) production were increased after renal ischaemia. So, we studied the possible relationship between ET and glomeruli or renal PAF production in post-ischaemic acute renal failure. 5. Glomeruli from ischaemic rats produced greater amounts of PAF than glomeruli from normal or anti-ET antibody-treated ischaemic rats. In addition, total renal PAF production was higher in ischaemic-untreated than in non-ischaemic or anti-ET-treated rats. Glomeruli incubated with 10(-7) M-endothelin produced much more PAF than those incubated in control conditions (138.4 +/- 10.5 vs. 80.2 +/- 9.4 pg PAF (mg protein)-1; means +/- S.E.M.; n = 10). 6. In conclusion, the present study suggests that endothelin plays a role in the persistent renal vasoconstriction that characterizes post-ischaemic acute renal failure. In addition, the observed increase in glomerular PAF production after renal ischaemia may be due to the action of endothelin.
Full text
PDF



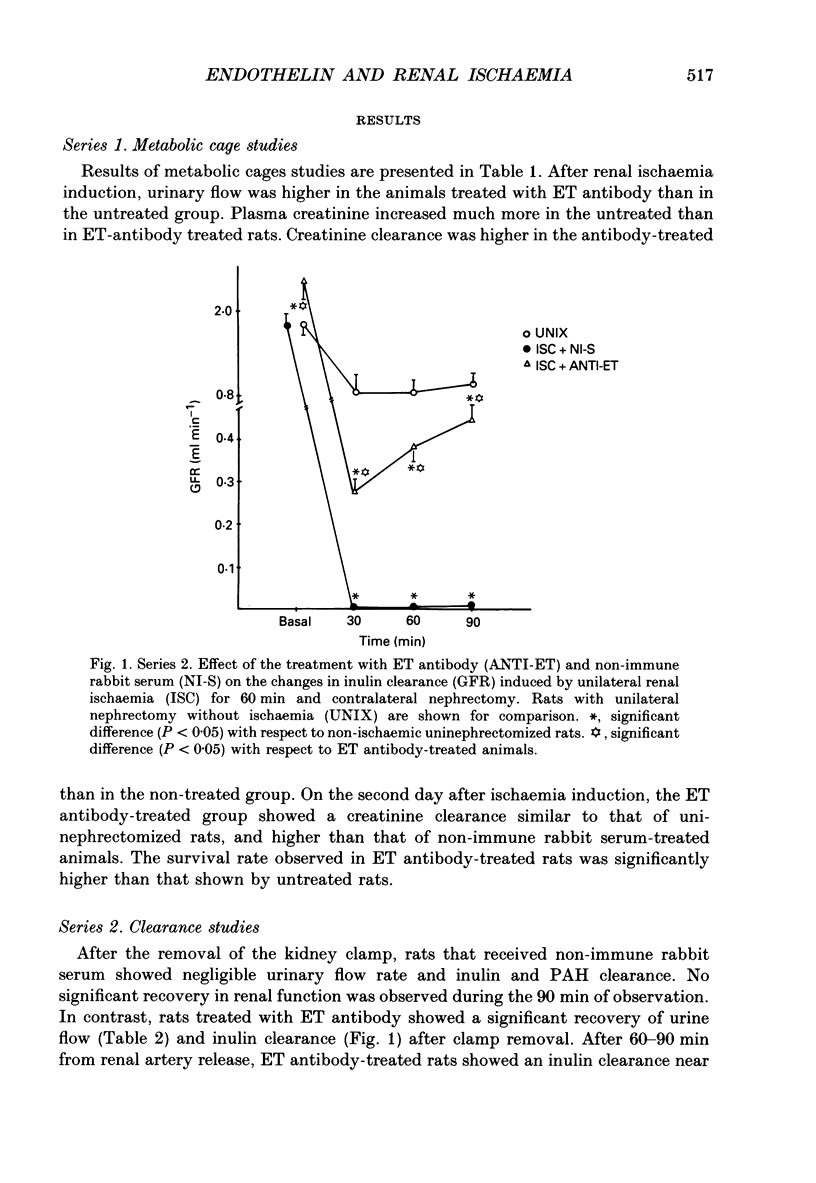
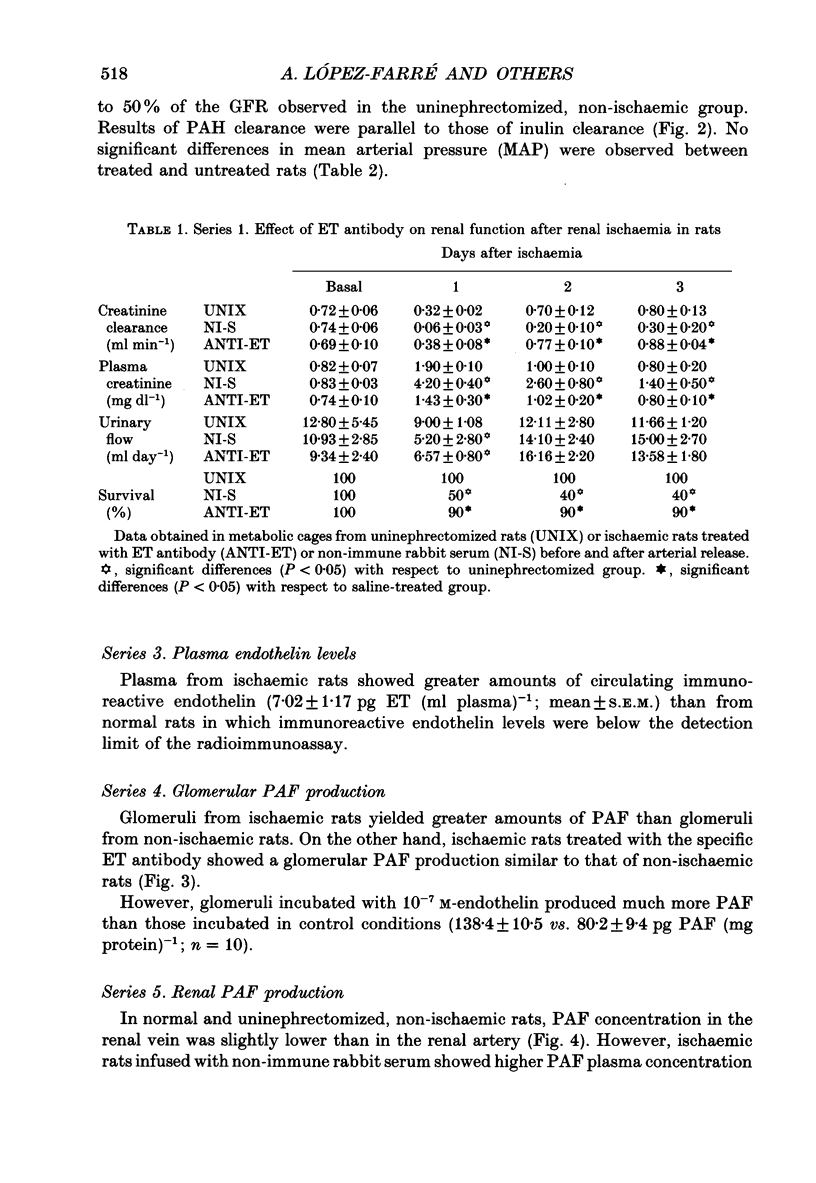
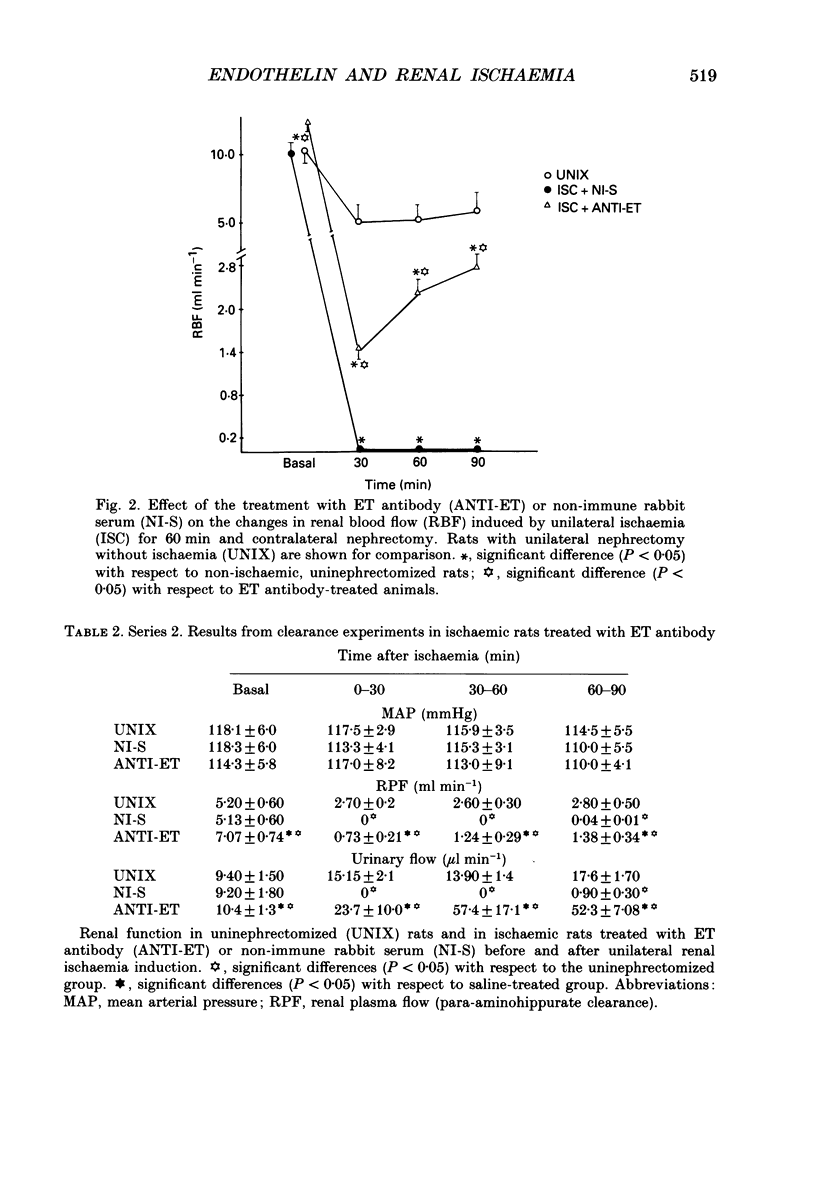
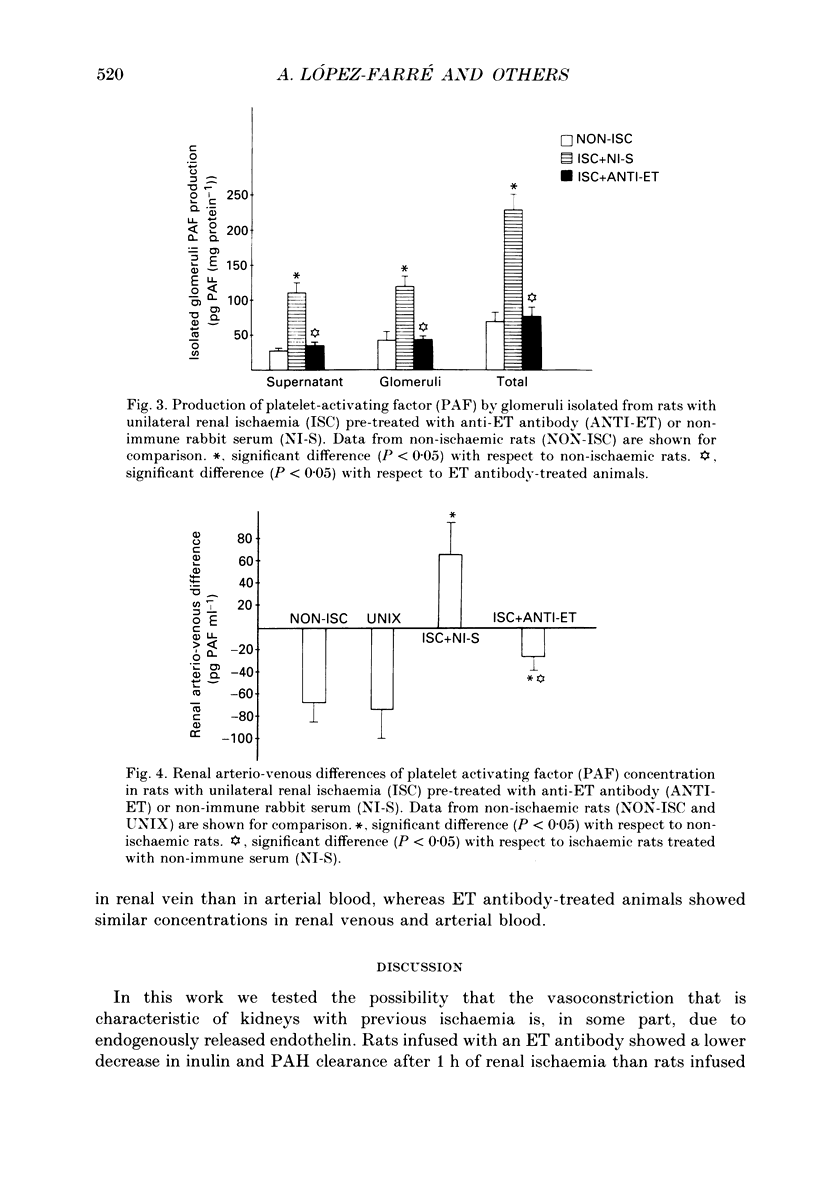


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Kon V., Yoshioka T., Fogo A., Ichikawa I. Glomerular actions of endothelin in vivo. J Clin Invest. 1989 May;83(5):1762–1767. doi: 10.1172/JCI114079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Churchill P. C., Bidani A. K. Effect of theophylline on the initiation phase of postischemic acute renal failure in rats. J Lab Clin Med. 1986 Aug;108(2):150–154. [PubMed] [Google Scholar]
- Liu J. J., Casley D. J., Nayler W. G. Ischaemia causes externalization of endothelin-1 binding sites in rat cardiac membranes. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1220–1225. doi: 10.1016/0006-291x(89)91799-3. [DOI] [PubMed] [Google Scholar]
- López-Farré A., Bernabeu F., Gómez-Garre D., Ramon y Cajal S., Braquet P., López-Novoa J. M. Platelet-activating factor antagonists treatment protects against postischemic acute renal failure in rats. J Pharmacol Exp Ther. 1990 Apr;253(1):328–333. [PubMed] [Google Scholar]
- López-Farré A., Montañs I., Millás I., López-Novoa J. M. Effect of endothelin on renal function in rats. Eur J Pharmacol. 1989 Apr 12;163(1):187–189. doi: 10.1016/0014-2999(89)90417-2. [DOI] [PubMed] [Google Scholar]
- López-Farré A., Torralbo M., López-Novoa J. M. Glomeruli from ischemic rat kidneys produce increased amounts of platelet activating factor. Biochem Biophys Res Commun. 1988 Apr 15;152(1):129–135. doi: 10.1016/s0006-291x(88)80689-2. [DOI] [PubMed] [Google Scholar]
- Pirotzky E., Ninio E., Bidault J., Pfister A., Benveniste J. Biosynthesis of platelet-activating factor. VI. Precursor of platelet-activating factor and acetyltransferase activity in isolated rat kidney cells. Lab Invest. 1984 Nov;51(5):567–572. [PubMed] [Google Scholar]
- Rodríguez-Puyol D., Arriba G., Blanchart A., Santos J. C., Caramelo C., Fernández-Cruz A., Hernando L., López-Novoa J. M. Lack of a direct regulatory effect of atrial natriuretic factor on prostaglandins and renin release by isolated rat glomeruli. Biochem Biophys Res Commun. 1986 Jul 16;138(1):496–501. doi: 10.1016/0006-291x(86)90308-6. [DOI] [PubMed] [Google Scholar]
- Rosmalen F. M., Tan A. C., Tan H. S., Benraad T. J. A sensitive radioimmunoassay of atrial natriuretic peptide in human plasma, using a tracer with an immobilized glycouril agent. Clin Chim Acta. 1987 Jun 15;165(2-3):331–340. doi: 10.1016/0009-8981(87)90178-1. [DOI] [PubMed] [Google Scholar]
- Shichiri M., Hirata Y., Emori T., Ohta K., Nakajima T., Sato K., Sato A., Marumo F. Secretion of endothelin and related peptides from renal epithelial cell lines. FEBS Lett. 1989 Aug 14;253(1-2):203–206. doi: 10.1016/0014-5793(89)80959-7. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Matsumoto H., Kitada C., Yanagisawa M., Miyauchi T., Masaki T., Fujino M. Immunoreactive endothelin-1 in plasma detected by a sandwich-type enzyme immunoassay. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S151–S152. doi: 10.1097/00005344-198900135-00039. [DOI] [PubMed] [Google Scholar]
- Tomita K., Ujiie K., Nakanishi T., Tomura S., Matsuda O., Ando K., Shichiri M., Hirata Y., Marumo F. Plasma endothelin levels in patients with acute renal failure. N Engl J Med. 1989 Oct 19;321(16):1127–1127. doi: 10.1056/NEJM198910193211615. [DOI] [PubMed] [Google Scholar]
- Touqui L., Hatmi M., Vargaftig B. B. Human platelets stimulated by thrombin produce platelet-activating factor (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when the degrading enzyme acetyl hydrolase is blocked. Biochem J. 1985 Aug 1;229(3):811–816. doi: 10.1042/bj2290811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]


