Abstract
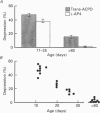
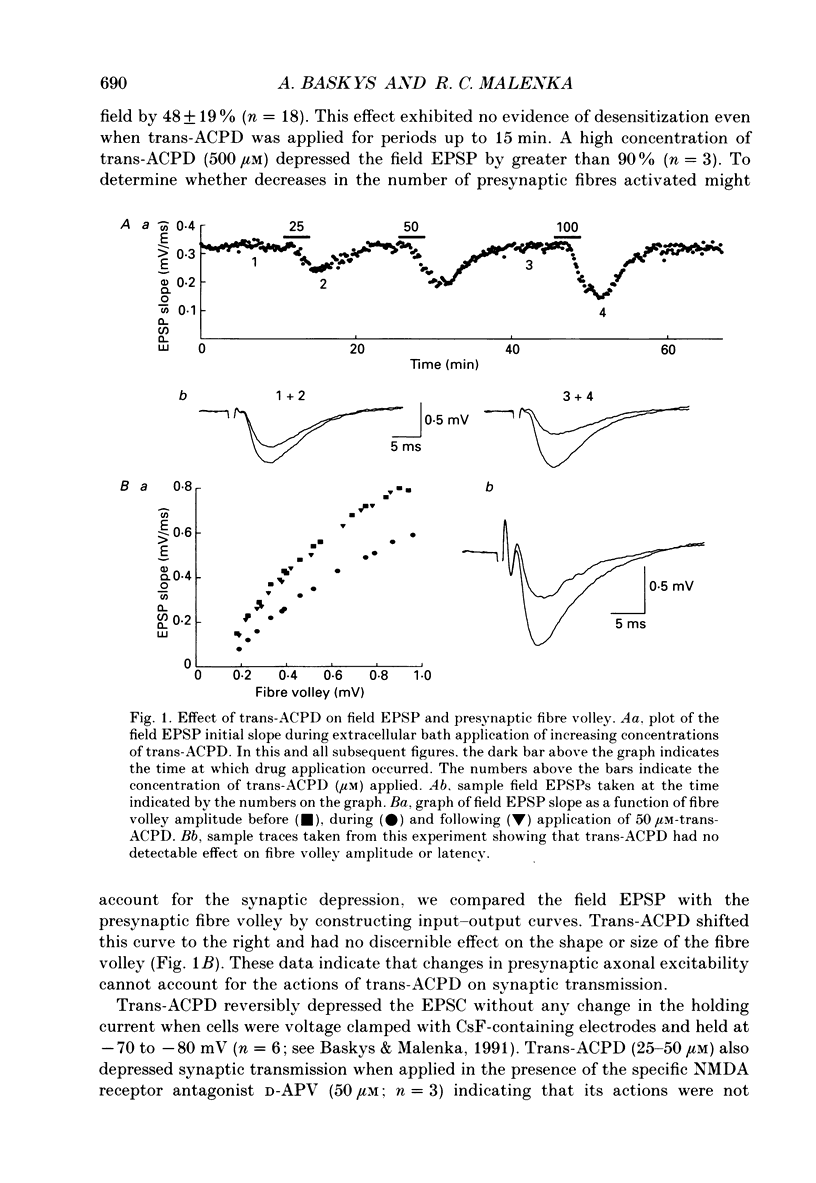
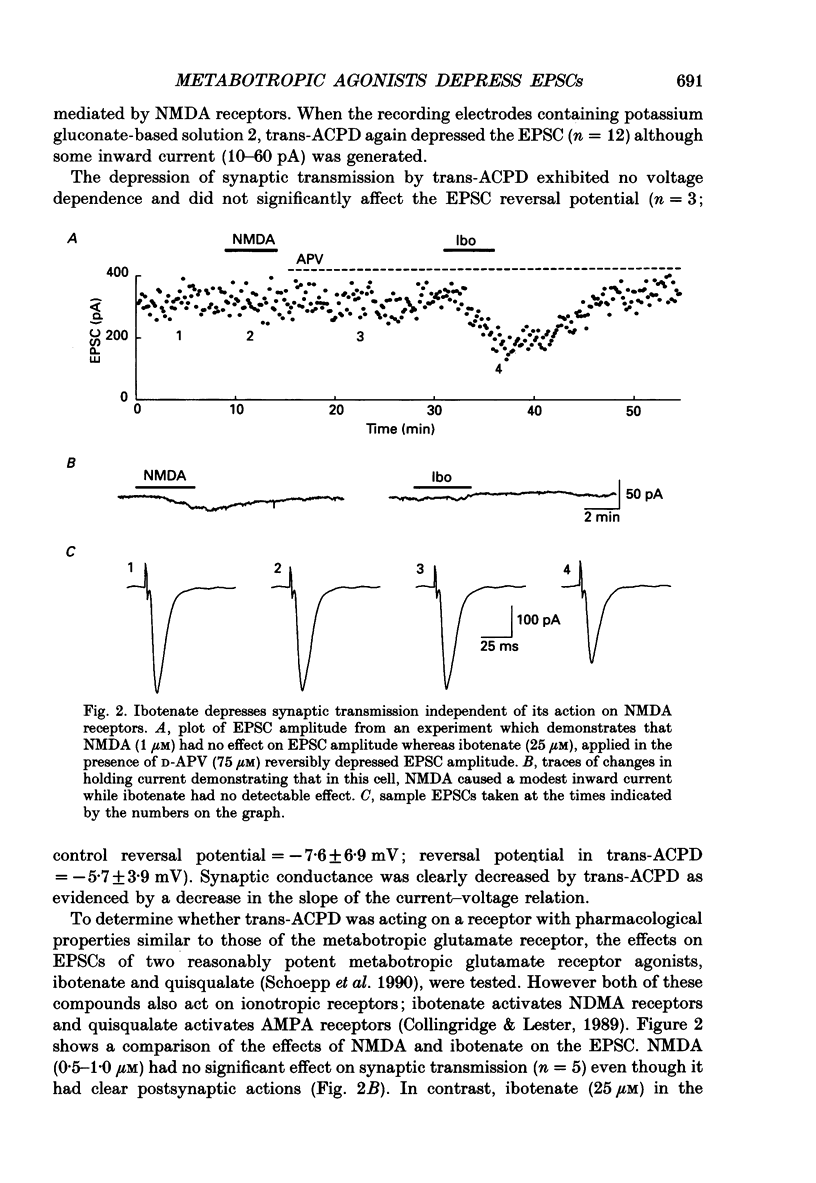
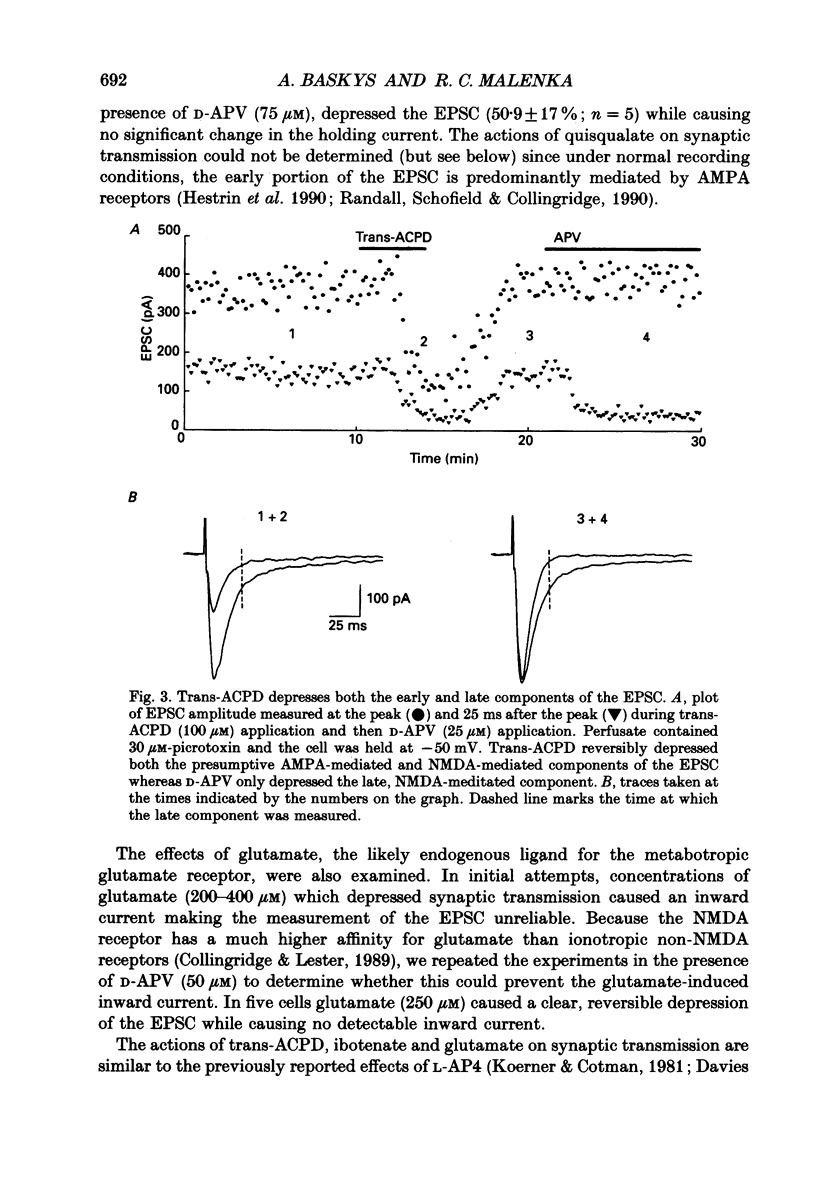
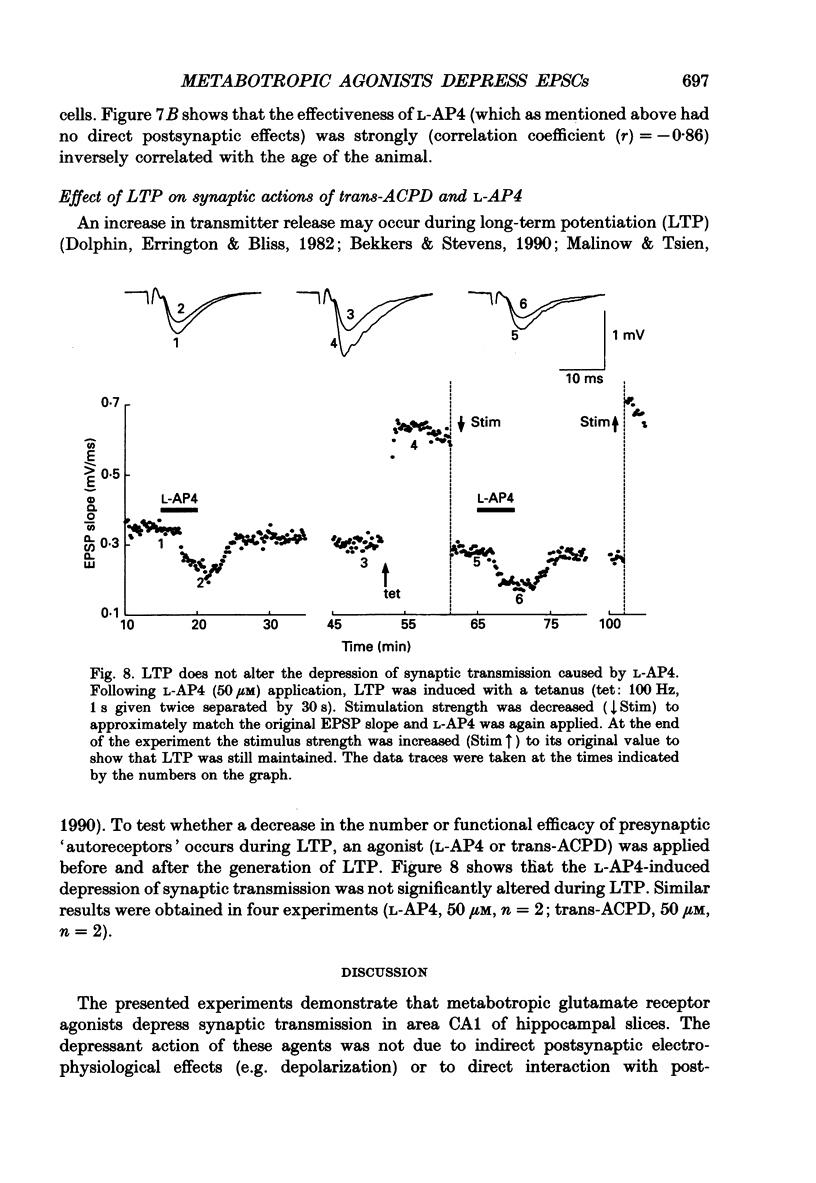
1. The effects of metabotropic glutamate receptor agonists on excitatory synaptic transmission in the CA1 region of rat hippocampal slices (11-30 days) were studied using extracellular and whole-cell patch-clamp recording techniques. 2. Trans-1-amino-1,3-cyclopentanedicarboxylic acid (trans-ACPD; 25-100 microM) reversibly depressed excitatory postsynaptic currents (EPSCs) without affecting presynaptic fibre excitability or EPSC reversal potential. 3. Ibotenate (25 microM) or L-glutamate (250 microM), in the presence of the N-methyl-D-aspartate (NMDA) receptor antagonist, D-2-amino-5-phosphonovaleric acid (APV, 50-75 microM), depressed the EPSC amplitude while inducing no detectable inward current. L-2-Amino-4-phosphonobutyrate (L-AP4, 25-100 microM), the phosphonic derivative of glutamate, also depressed EPSC amplitude and caused no detectable inward current. 4. The NMDA receptor-mediated component of the EPSC recorded in the presence of the non-NMDA receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20-30 microM) was depressed by trans-ACPD, L-AP4, or quisqualate (1-2 microM). 5. The response to ionophoretic application of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) was unaffected by trans-ACPD or L-AP4 although the simultaneously recorded EPSC was strongly depressed. In addition, paired-pulse facilitation (50-75 ms interstimulus interval) was reversibly enhanced by trans-ACPD or L-AP4. These results indicate that the depression of synaptic transmission likely was mediated by a presynaptic 'autoreceptor'. 6. The effects of trans-ACPD or L-AP4 on synaptic transmission decreased significantly over ages 12-30 days and were minimal in adult (greater than 80 days) slices. 7. The depression of synaptic transmission caused by trans-ACPD or L-AP4 was not altered following the induction of long-term potentiation (LTP). 8. The results indicate that metabotropic glutamate receptor agonists suppress excitatory synaptic transmission in CA1 pyramidal cells by an action at a presynaptic site. This effect is developmentally regulated and is maximally expressed during the first postnatal month.
Full text
PDF


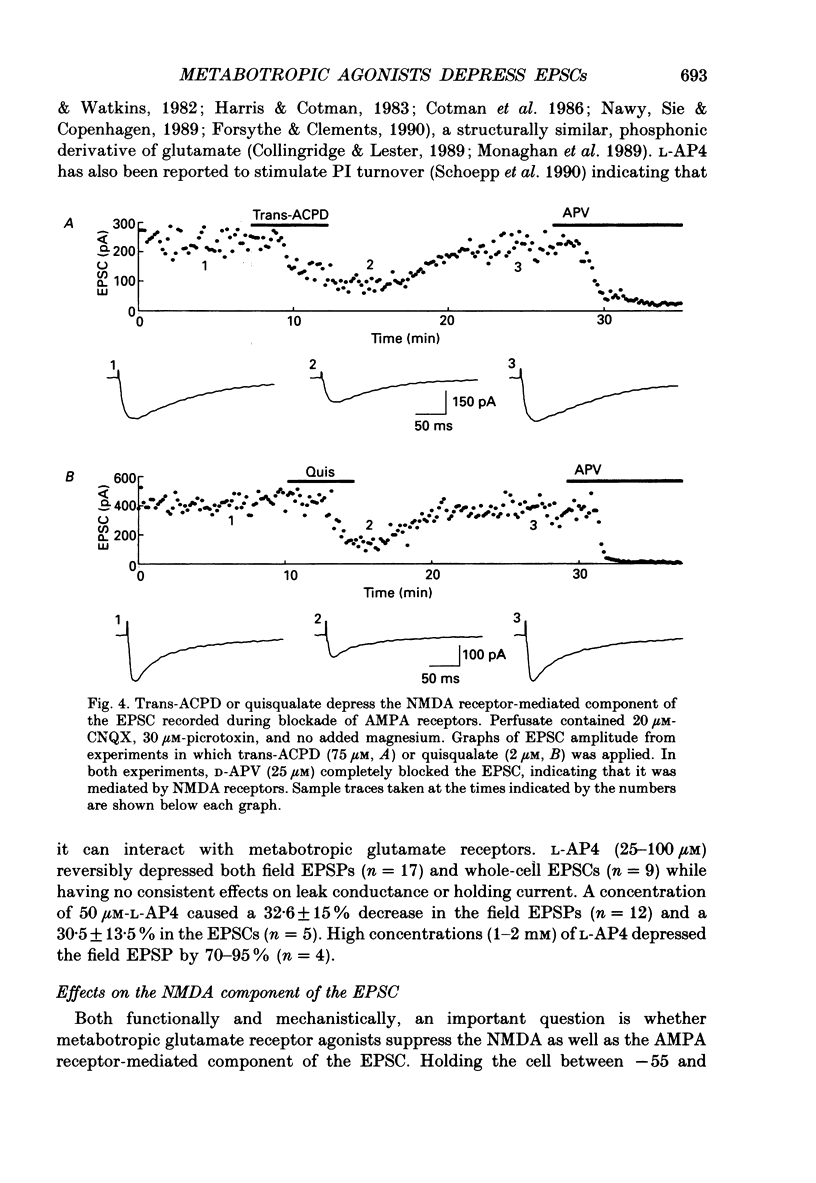
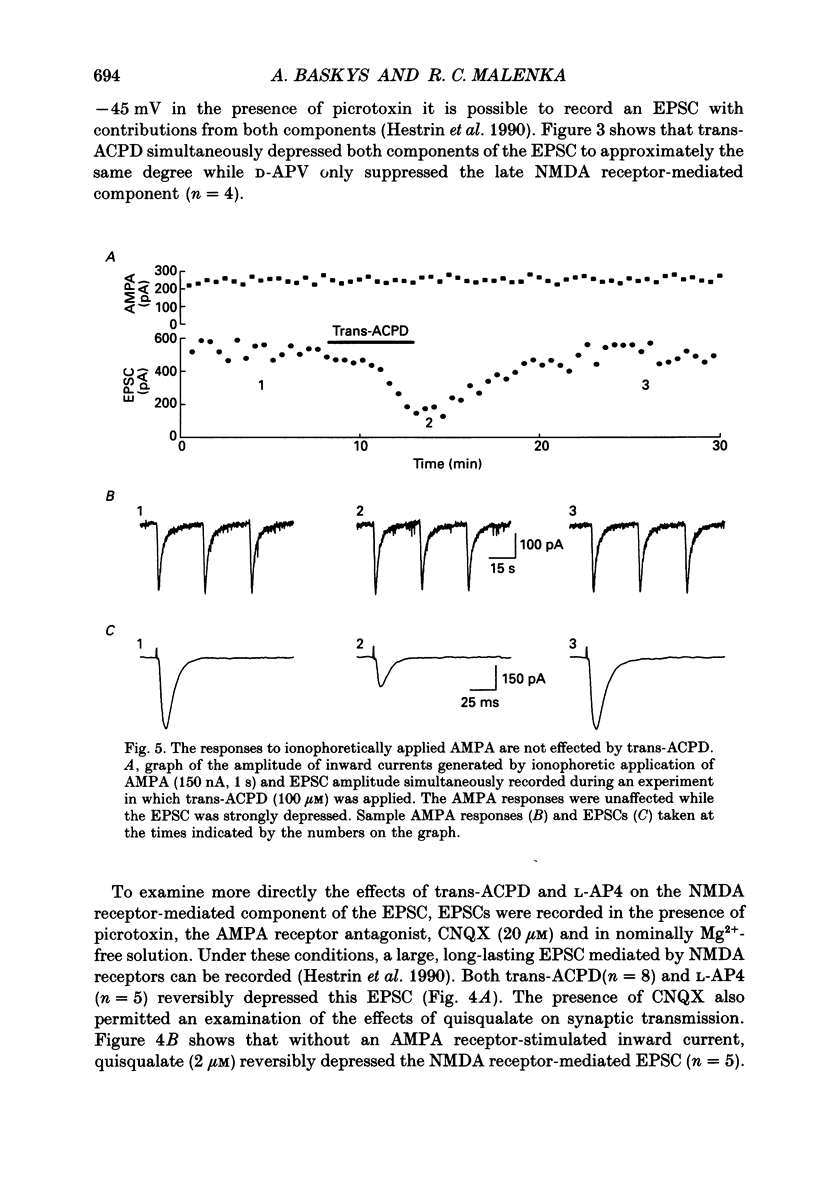
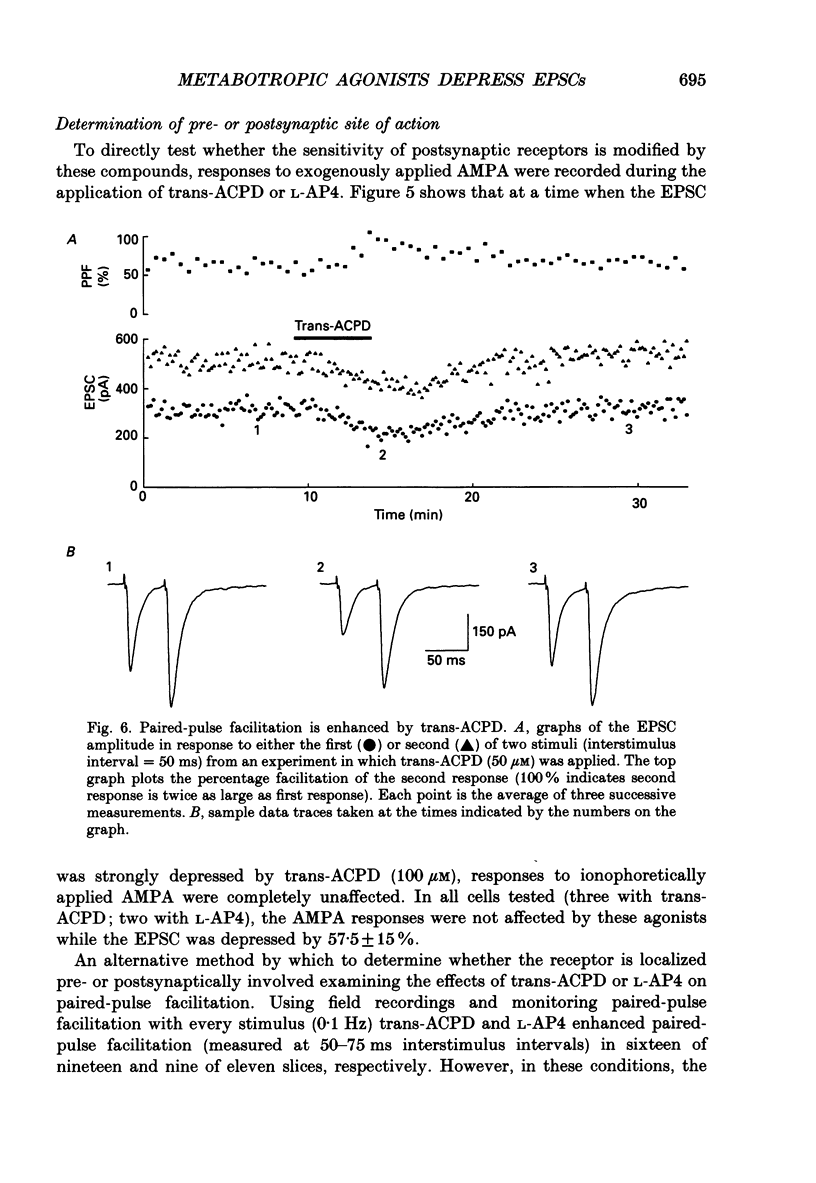
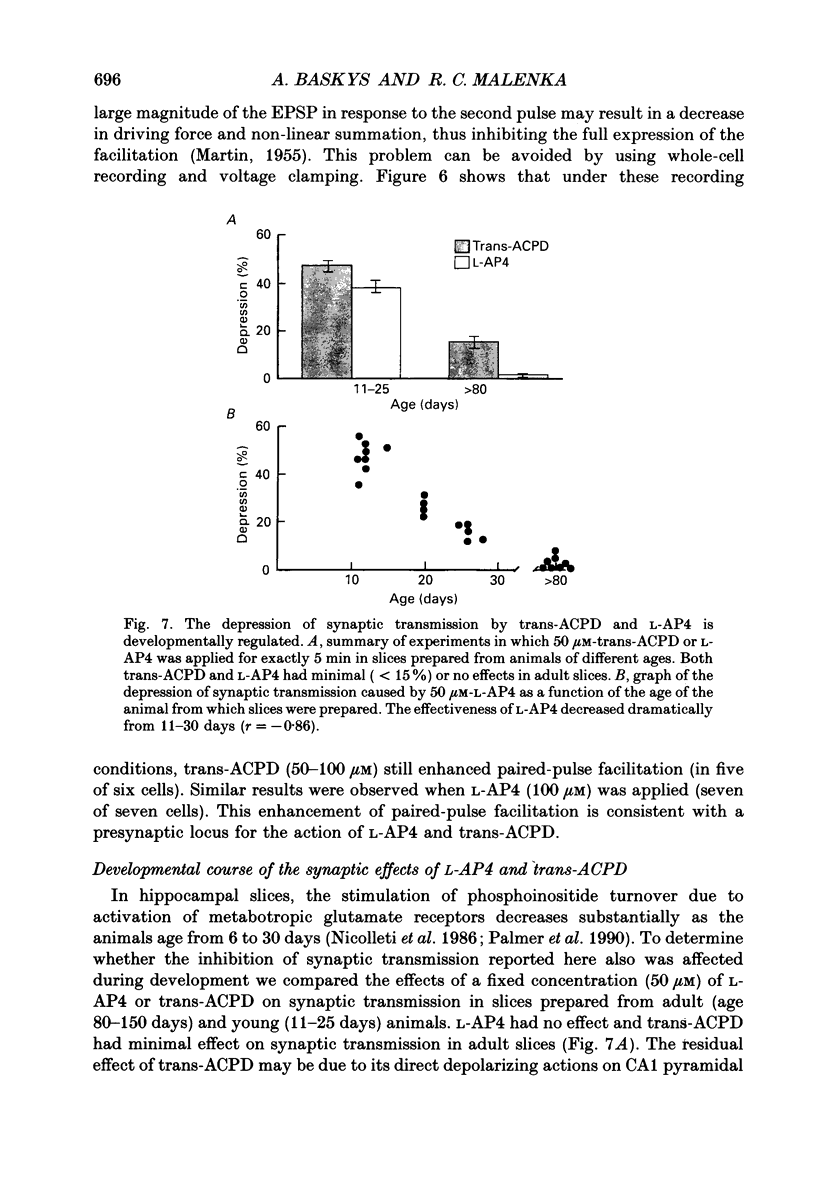




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard E. A., Henley J. M. The non-NMDA receptors: types, protein structure and molecular biology. Trends Pharmacol Sci. 1990 Dec;11(12):500–507. doi: 10.1016/0165-6147(90)90051-9. [DOI] [PubMed] [Google Scholar]
- Baskys A., Bernstein N. K., Barolet A. W., Carlen P. L. NMDA and quisqualate reduce a Ca-dependent K+ current by a protein kinase-mediated mechanism. Neurosci Lett. 1990 Apr 20;112(1):76–81. doi: 10.1016/0304-3940(90)90325-4. [DOI] [PubMed] [Google Scholar]
- Baskys A., Malenka R. C. Trans-ACPD depresses synaptic transmission in the hippocampus. Eur J Pharmacol. 1991 Jan 25;193(1):131–132. doi: 10.1016/0014-2999(91)90213-a. [DOI] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990 Aug 23;346(6286):724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Charpak S., Gähwiler B. H., Do K. Q., Knöpfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990 Oct 25;347(6295):765–767. doi: 10.1038/347765a0. [DOI] [PubMed] [Google Scholar]
- Chernevskaya N. I., Obukhov A. G., Krishtal O. A. NMDA receptor agonists selectively block N-type calcium channels in hippocampal neurons. Nature. 1991 Jan 31;349(6308):418–420. doi: 10.1038/349418a0. [DOI] [PubMed] [Google Scholar]
- Coleman P. A., Miller R. F. Measurement of passive membrane parameters with whole-cell recording from neurons in the intact amphibian retina. J Neurophysiol. 1989 Jan;61(1):218–230. doi: 10.1152/jn.1989.61.1.218. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Cotman C. W., Flatman J. A., Ganong A. H., Perkins M. N. Effects of excitatory amino acid antagonists on evoked and spontaneous excitatory potentials in guinea-pig hippocampus. J Physiol. 1986 Sep;378:403–415. doi: 10.1113/jphysiol.1986.sp016227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Actions of D and L forms of 2-amino-5-phosphonovalerate and 2-amino-4-phosphonobutyrate in the cat spinal cord. Brain Res. 1982 Mar 11;235(2):378–386. doi: 10.1016/0006-8993(82)91017-4. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Errington M. L., Bliss T. V. Long-term potentiation of the perforant path in vivo is associated with increased glutamate release. Nature. 1982 Jun 10;297(5866):496–498. doi: 10.1038/297496a0. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T., Madison D., Lynch G. Synaptic transmission is required for initiation of long-term potentiation. Brain Res. 1978 Jul 14;150(2):413–417. doi: 10.1016/0006-8993(78)90293-7. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Clements J. D. Presynaptic glutamate receptors depress excitatory monosynaptic transmission between mouse hippocampal neurones. J Physiol. 1990 Oct;429:1–16. doi: 10.1113/jphysiol.1990.sp018240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. W., Cotman C. W. Effects of acidic amino acid antagonists on paired-pulse potentiation at the lateral perforant path. Exp Brain Res. 1983;52(3):455–460. doi: 10.1007/BF00238039. [DOI] [PubMed] [Google Scholar]
- Harris E. W., Stevens D. R., Cotman C. W. Hippocampal cells primed with quisqualate are depolarized by AP4 and AP6, ligands for a putative glutamate uptake site. Brain Res. 1987 Aug 25;418(2):361–365. doi: 10.1016/0006-8993(87)90104-1. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Nicoll R. A., Perkel D. J., Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990 Mar;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner J. F., Cotman C. W. Micromolar L-2-amino-4-phosphonobutyric acid selectively inhibits perforant path synapses from lateral entorhinal cortex. Brain Res. 1981 Jul 6;216(1):192–198. doi: 10.1016/0006-8993(81)91288-9. [DOI] [PubMed] [Google Scholar]
- Koerner J. F., Cotman C. W. Response of Schaffer collateral-CA 1 pyramidal cell synapses of the hippocampus to analogues of acidic amino acids. Brain Res. 1982 Nov 11;251(1):105–115. doi: 10.1016/0006-8993(82)91278-1. [DOI] [PubMed] [Google Scholar]
- Lester R. A., Jahr C. E. Quisqualate receptor-mediated depression of calcium currents in hippocampal neurons. Neuron. 1990 May;4(5):741–749. doi: 10.1016/0896-6273(90)90200-y. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson D. S., Curry K., Peet M. J., McLennan H. Structural requirements for activation of excitatory amino acid receptors in the rat spinal cord in vitro. Exp Brain Res. 1988;73(3):541–545. doi: 10.1007/BF00406612. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Zucker R. S., Nicoll R. A. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988 Oct 7;242(4875):81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Malinow R., Tsien R. W. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990 Jul 12;346(6280):177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Nawy S., Sie A., Copenhagen D. R. The glutamate analog 2-amino-4-phosphonobutyrate antagonizes synaptic transmission from cones to horizontal cells in the goldfish retina. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1726–1730. doi: 10.1073/pnas.86.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Iadarola M. J., Wroblewski J. T., Costa E. Excitatory amino acid recognition sites coupled with inositol phospholipid metabolism: developmental changes and interaction with alpha 1-adrenoceptors. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1931–1935. doi: 10.1073/pnas.83.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. A simple chamber for recording from submerged brain slices. J Neurosci Methods. 1981 Aug;4(2):153–156. doi: 10.1016/0165-0270(81)90049-2. [DOI] [PubMed] [Google Scholar]
- Palmer E., Monaghan D. T., Cotman C. W. Trans-ACPD, a selective agonist of the phosphoinositide-coupled excitatory amino acid receptor. Eur J Pharmacol. 1989 Aug 3;166(3):585–587. doi: 10.1016/0014-2999(89)90383-x. [DOI] [PubMed] [Google Scholar]
- Palmer E., Nangel-Taylor K., Krause J. D., Roxas A., Cotman C. W. Changes in excitatory amino acid modulation of phosphoinositide metabolism during development. Brain Res Dev Brain Res. 1990 Jan 1;51(1):132–134. doi: 10.1016/0165-3806(90)90266-2. [DOI] [PubMed] [Google Scholar]
- Randall A. D., Schofield J. G., Collingridge G. L. Whole-cell patch-clamp recordings of an NMDA receptor-mediated synaptic current in rat hippocampal slices. Neurosci Lett. 1990 Jul 3;114(2):191–196. doi: 10.1016/0304-3940(90)90070-p. [DOI] [PubMed] [Google Scholar]
- Robinson M. B., Whittemore E. R., Marks R. L., Koerner J. F. Exposure of hippocampal slices to quisqualate sensitizes synaptic responses to phosphonate-containing analogues of glutamate. Brain Res. 1986 Aug 27;381(1):187–190. doi: 10.1016/0006-8993(86)90711-0. [DOI] [PubMed] [Google Scholar]
- Schoepp D. D., Johnson B. G. Excitatory amino acid agonist-antagonist interactions at 2-amino-4-phosphonobutyric acid-sensitive quisqualate receptors coupled to phosphoinositide hydrolysis in slices of rat hippocampus. J Neurochem. 1988 May;50(5):1605–1613. doi: 10.1111/j.1471-4159.1988.tb03050.x. [DOI] [PubMed] [Google Scholar]
- Schoepp D., Bockaert J., Sladeczek F. Pharmacological and functional characteristics of metabotropic excitatory amino acid receptors. Trends Pharmacol Sci. 1990 Dec;11(12):508–515. doi: 10.1016/0165-6147(90)90052-a. [DOI] [PubMed] [Google Scholar]
- Sladeczek F., Pin J. P., Récasens M., Bockaert J., Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985 Oct 24;317(6039):717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- Stratton K. R., Worley P. F., Baraban J. M. Excitation of hippocampal neurons by stimulation of glutamate Qp receptors. Eur J Pharmacol. 1989 Dec 7;173(2-3):235–237. doi: 10.1016/0014-2999(89)90529-3. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]