Abstract
1. The currents underlying the graded impulses in small cultured hippocampal neurons from rat embryos were analysed under voltage-clamp conditions with the tight-seal whole-cell recording technique. 2. The leak and capacitative currents induced by a potential step were linearly related to the potential in the range studied (-60 to -100 mV). 3. With steps to potentials more positive than -40 mV, at least two different potential-activated currents were detected: an initial transient current and a delayed sustained one. In addition, 40% of the cells studied showed a delayed transient current. 4. The initial transient current showed sigmoid activation and roughly exponential inactivation. Its reversal potential depended on the Na+ concentration and was close to the Na+ equilibrium potential. Further, it was blocked by 3.0 microM-tetrodotoxin, and was abolished when choline was substituted for Na+ in the extracellular solution. We concluded that this current was carried mainly by Na+ ions. 5. The delayed sustained current showed sigmoid activation and almost no inactivation within 40 ms. The reversal potential was close to the K+ equilibrium potential. We concluded that this current was carried mainly by K+ ions. 6. The delayed transient current was outward in the potential range studied (-50 to +120 mV) and did not depend on the pipette Cl- concentration. It was assumed that this current was carried mainly by K+ ions. 7. A quantitative description of the initial transient and the delayed sustained currents was developed on the basis of earlier descriptions of excitable membranes.
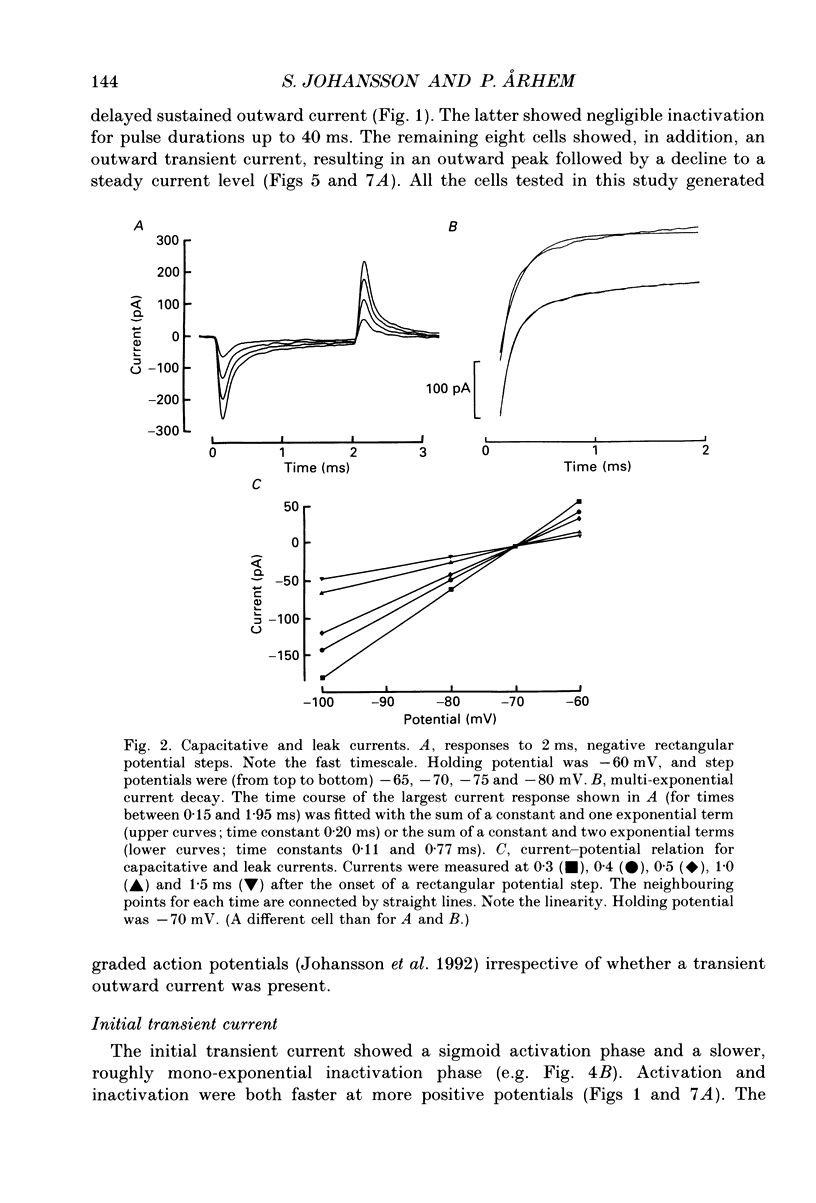
Full text
PDF

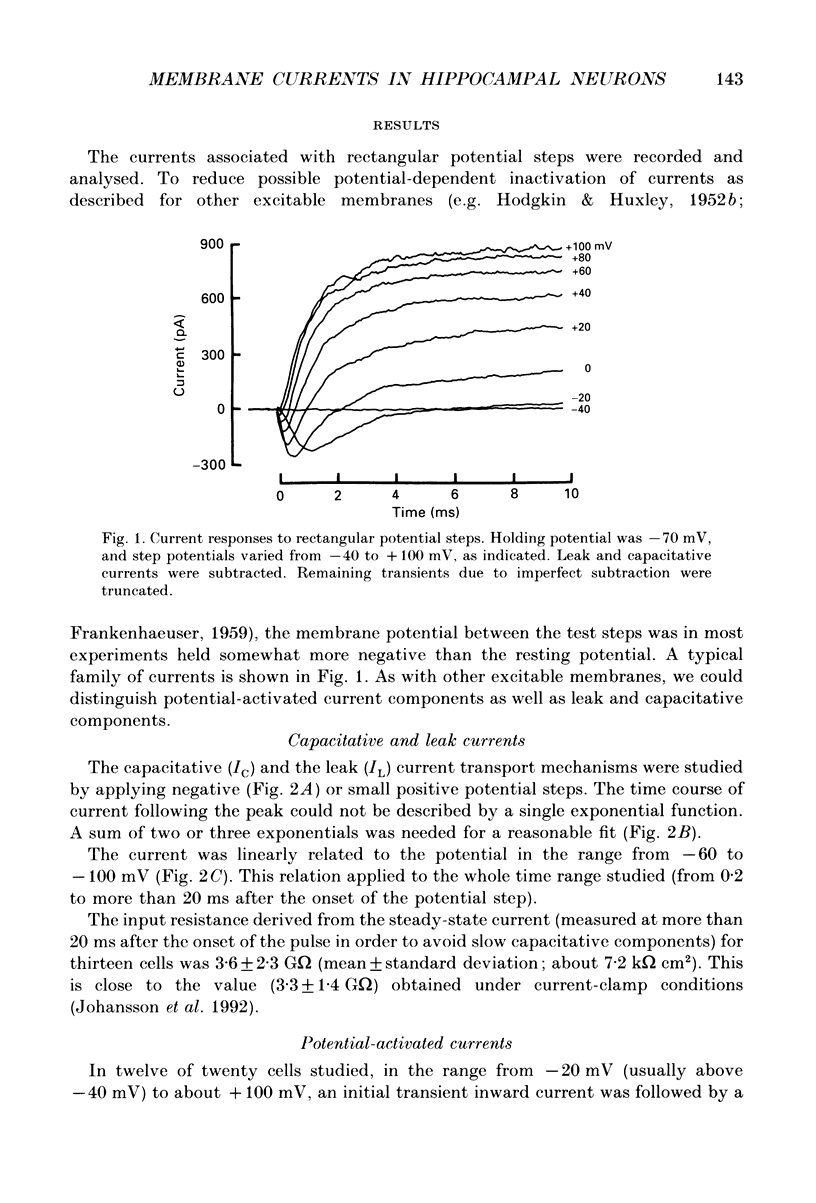
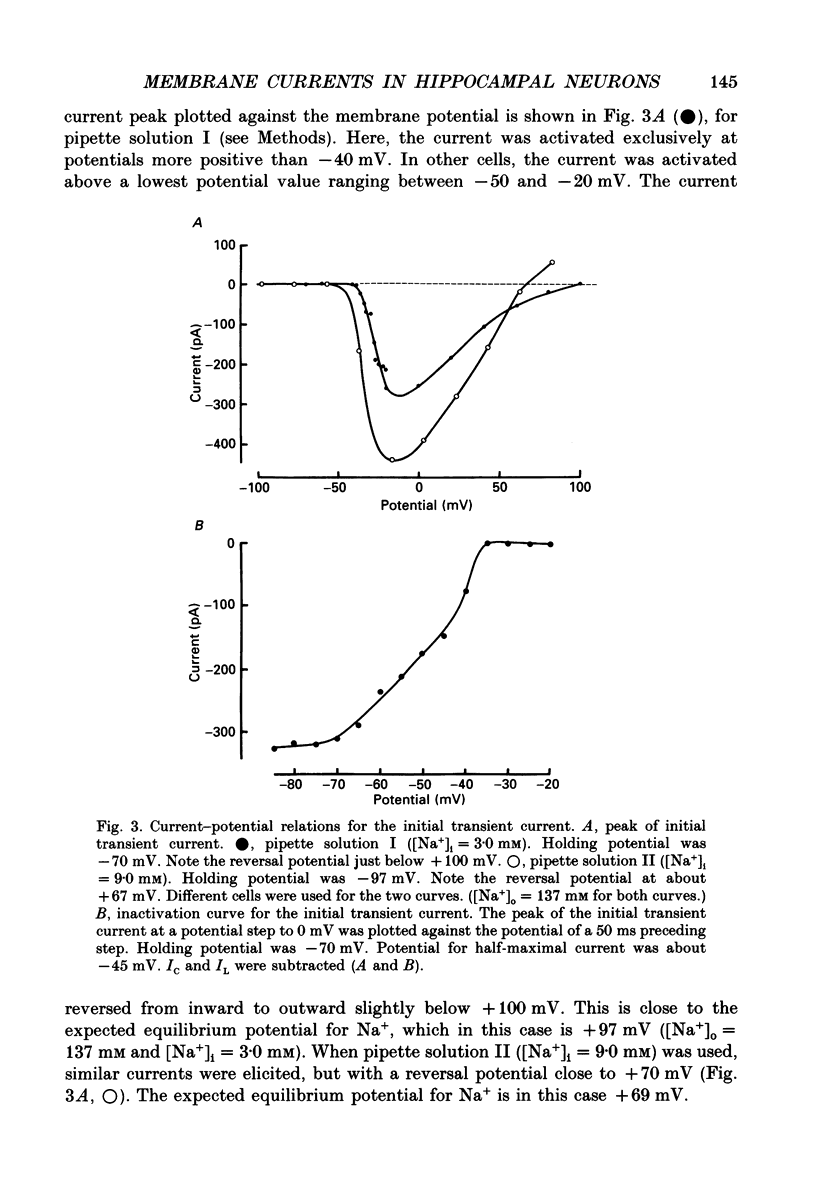
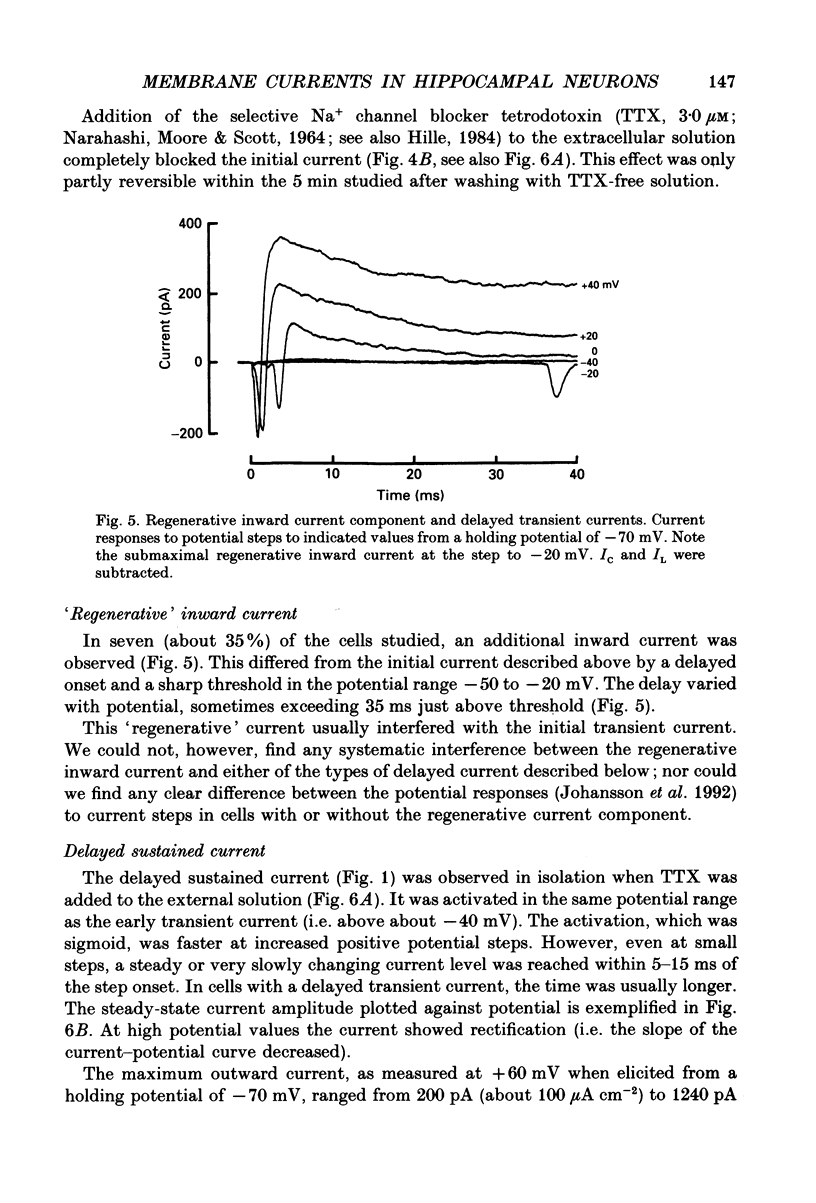






Selected References
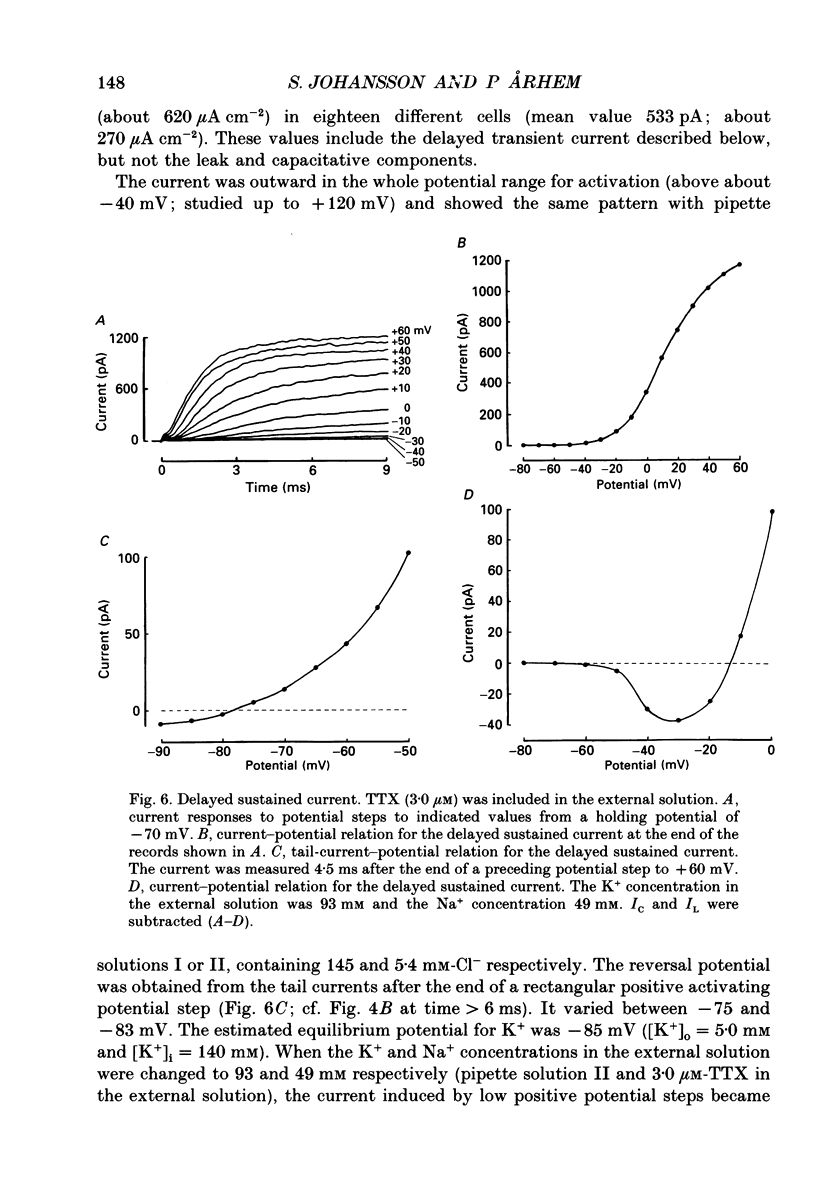
These references are in PubMed. This may not be the complete list of references from this article.
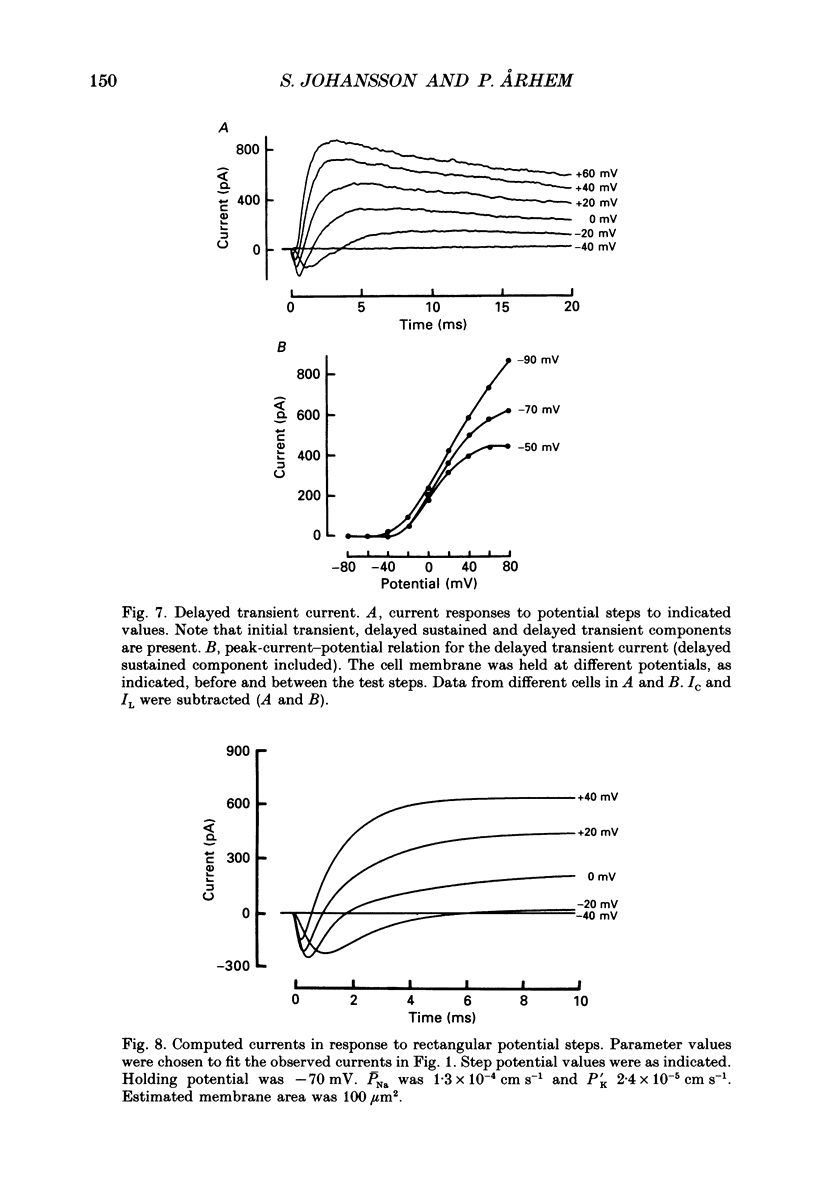
- Arhem P., Frankenhaeuser B., Moore L. E. Ionic currents at resting potential in nerve fibres from Xenopus laevis. Potential clamp experiments. Acta Physiol Scand. 1973 Aug;88(4):446–454. doi: 10.1111/j.1748-1716.1973.tb05474.x. [DOI] [PubMed] [Google Scholar]
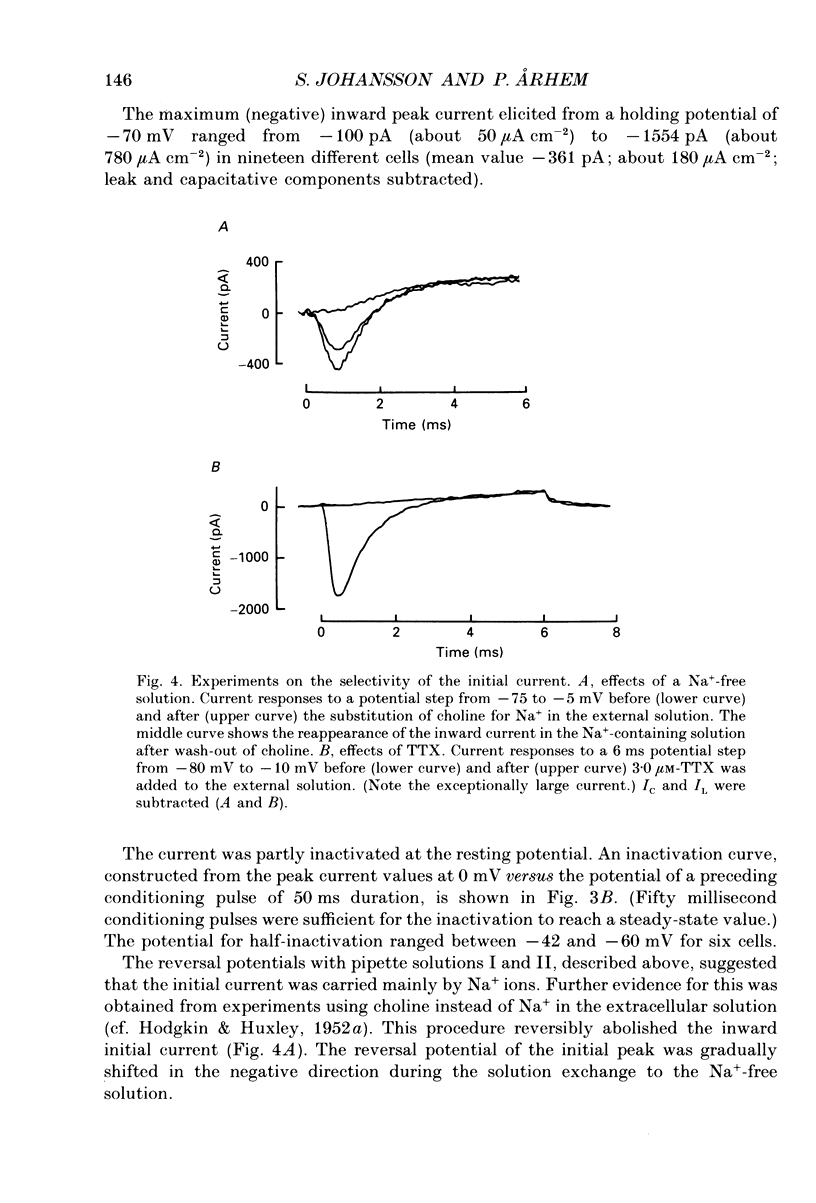
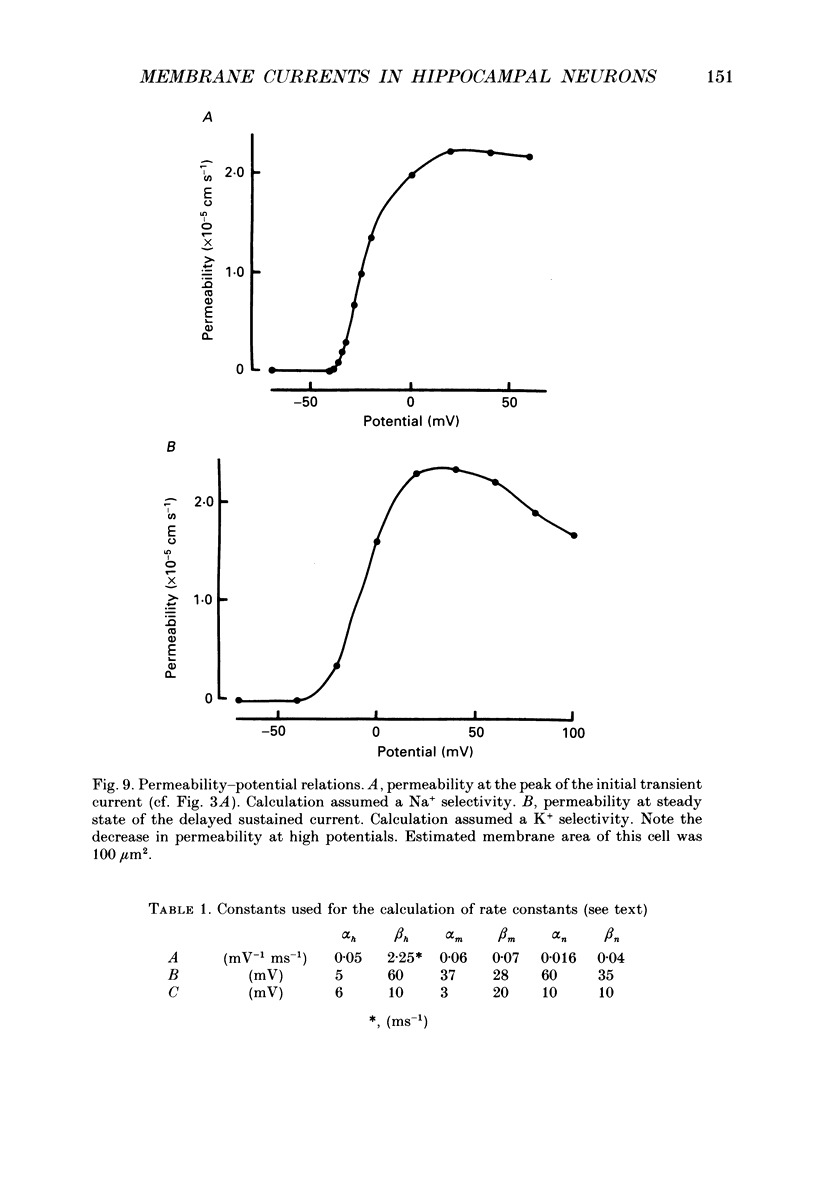
- Barrett E. F., Barrett J. N., Crill W. E. Voltage-sensitive outward currents in cat motoneurones. J Physiol. 1980 Jul;304:251–276. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Crill W. E. Voltage clamp of cat motoneurone somata: properties of the fast inward current. J Physiol. 1980 Jul;304:231–249. doi: 10.1113/jphysiol.1980.sp013322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Marshall C. G., Ogden D. Voltage-activated membrane currents in rat cerebellar granule neurones. J Physiol. 1989 Jul;414:179–199. doi: 10.1113/jphysiol.1989.sp017683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Sodium currents in the myelinated nerve fibre of Xenopus laevis investigated with the voltage clamp technique. J Physiol. 1959 Oct;148:188–200. doi: 10.1113/jphysiol.1959.sp006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. A QUANTITATIVE DESCRIPTION OF POTASSIUM CURRENTS IN MYELINATED NERVE FIBRES OF XENOPUS LAEVIS. J Physiol. 1963 Nov;169:424–430. doi: 10.1113/jphysiol.1963.sp007268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Delayed currents in myelinated nerve fibres of Xenopus laevis investigated with voltage clamp technique. J Physiol. 1962 Jan;160:40–45. doi: 10.1113/jphysiol.1962.sp006832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HUXLEY A. F. THE ACTION POTENTIAL IN THE MYELINATED NERVE FIBER OF XENOPUS LAEVIS AS COMPUTED ON THE BASIS OF VOLTAGE CLAMP DATA. J Physiol. 1964 Jun;171:302–315. doi: 10.1113/jphysiol.1964.sp007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Quantitative description of sodium currents in myelinated nerve fibres of Xenopus laevis. J Physiol. 1960 Jun;151:491–501. doi: 10.1113/jphysiol.1960.sp006455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Steady state inactivation of sodium permeability in myelinated nerve fibres of Xenopus laevis. J Physiol. 1959 Oct;148:671–676. doi: 10.1113/jphysiol.1959.sp006316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Galvan M., Grafe P., Wigström H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982 Sep 16;299(5880):252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockberger P. E., Tseng H. Y., Connor J. A. Immunocytochemical and electrophysiological differentiation of rat cerebellar granule cells in explant cultures. J Neurosci. 1987 May;7(5):1370–1383. doi: 10.1523/JNEUROSCI.07-05-01370.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard J. R., Hamill O. P., Prince D. A. Developmental changes in Na+ conductances in rat neocortical neurons: appearance of a slowly inactivating component. J Neurophysiol. 1988 Mar;59(3):778–795. doi: 10.1152/jn.1988.59.3.778. [DOI] [PubMed] [Google Scholar]
- Johansson S., Arhem P. Computed potential responses of small cultured rat hippocampal neurons. J Physiol. 1992 Jan;445:157–167. doi: 10.1113/jphysiol.1992.sp018917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S., Arhem P. Graded action potentials in small cultured rat hippocampal neurons. Neurosci Lett. 1990 Oct 16;118(2):155–158. doi: 10.1016/0304-3940(90)90615-g. [DOI] [PubMed] [Google Scholar]
- Johansson S., Friedman W., Arhem P. Impulses and resting membrane properties of small cultured rat hippocampal neurons. J Physiol. 1992 Jan;445:129–140. doi: 10.1113/jphysiol.1992.sp018915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S., Rydqvist B., Swerup C., Heilbronn E., Arhem P. Action potentials of cultured human oat cells: whole-cell measurements with the patch-clamp technique. Acta Physiol Scand. 1989 Apr;135(4):573–578. doi: 10.1111/j.1748-1716.1989.tb08619.x. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Westbrook G. L. Early development of voltage-dependent sodium currents in cultured mouse spinal cord neurons. Dev Biol. 1986 Feb;113(2):317–326. doi: 10.1016/0012-1606(86)90167-3. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numann R. E., Wadman W. J., Wong R. K. Outward currents of single hippocampal cells obtained from the adult guinea-pig. J Physiol. 1987 Dec;393:331–353. doi: 10.1113/jphysiol.1987.sp016826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: potassium conductances. J Neurophysiol. 1984 Jun;51(6):1409–1433. doi: 10.1152/jn.1984.51.6.1409. [DOI] [PubMed] [Google Scholar]


