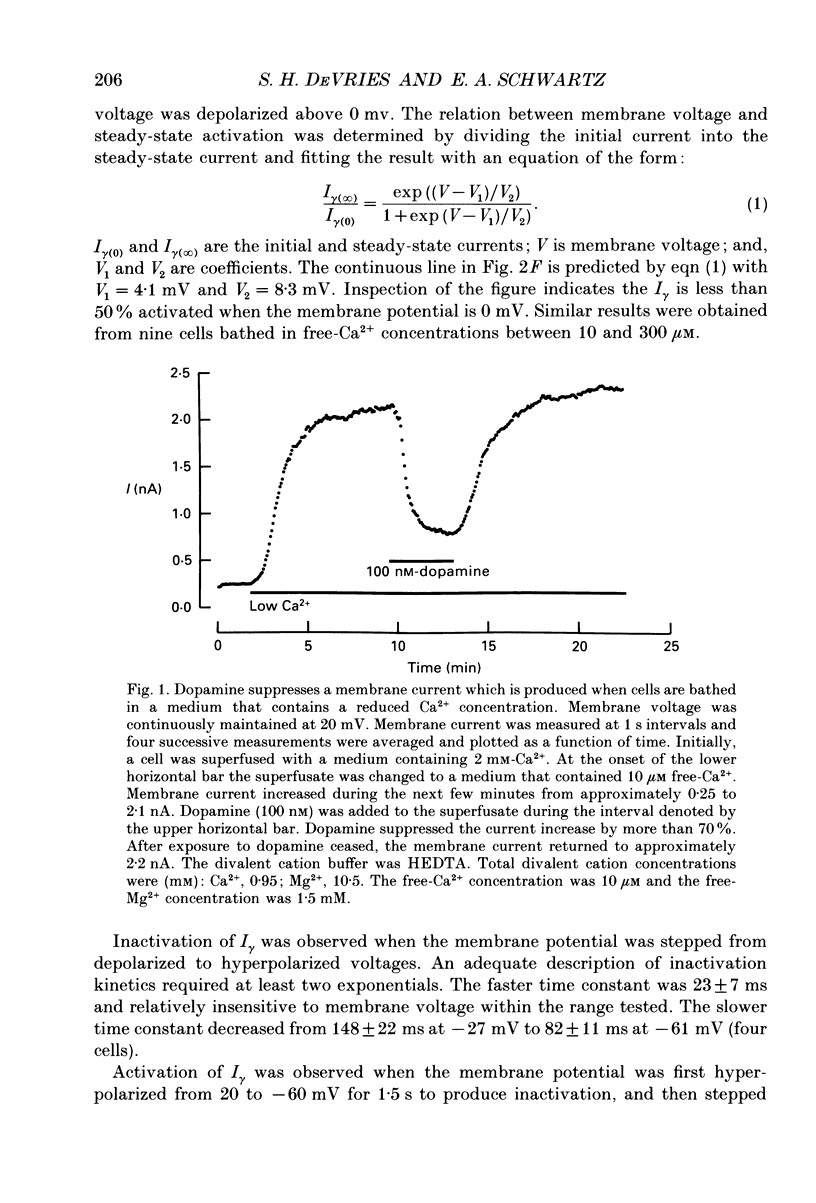
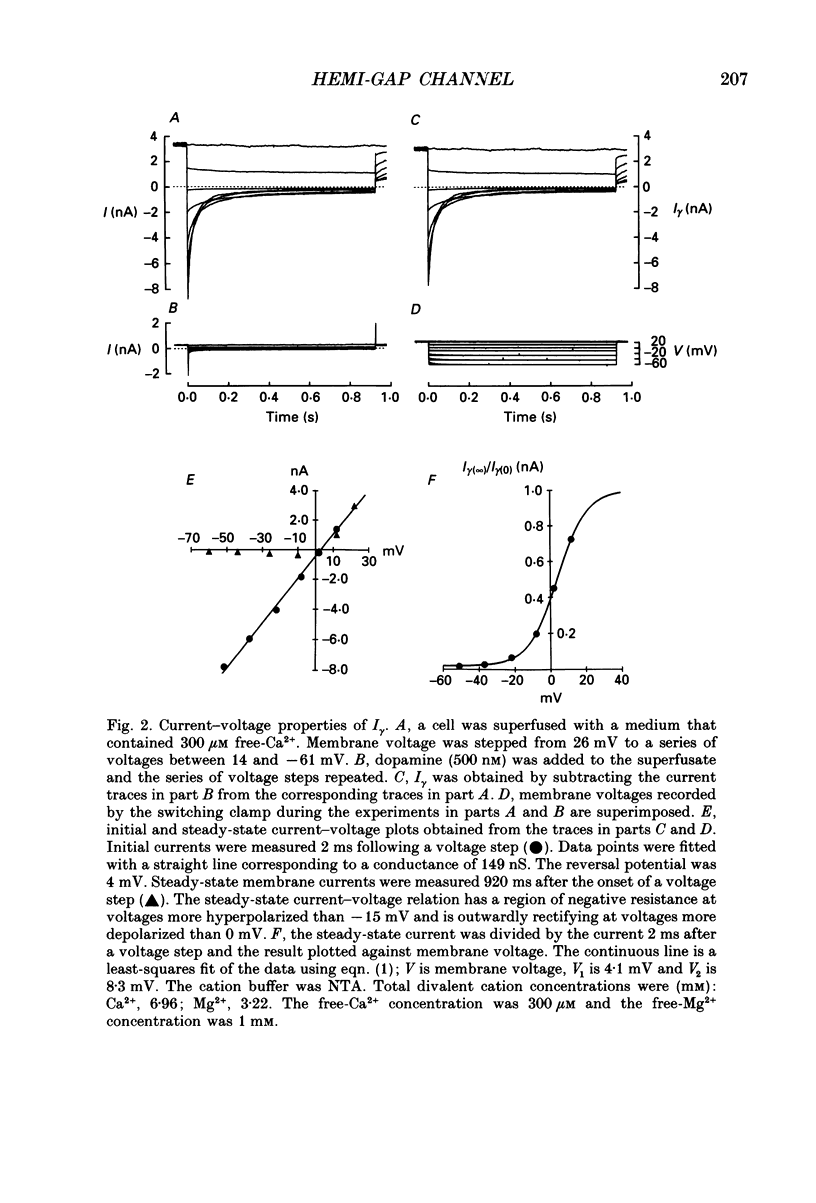
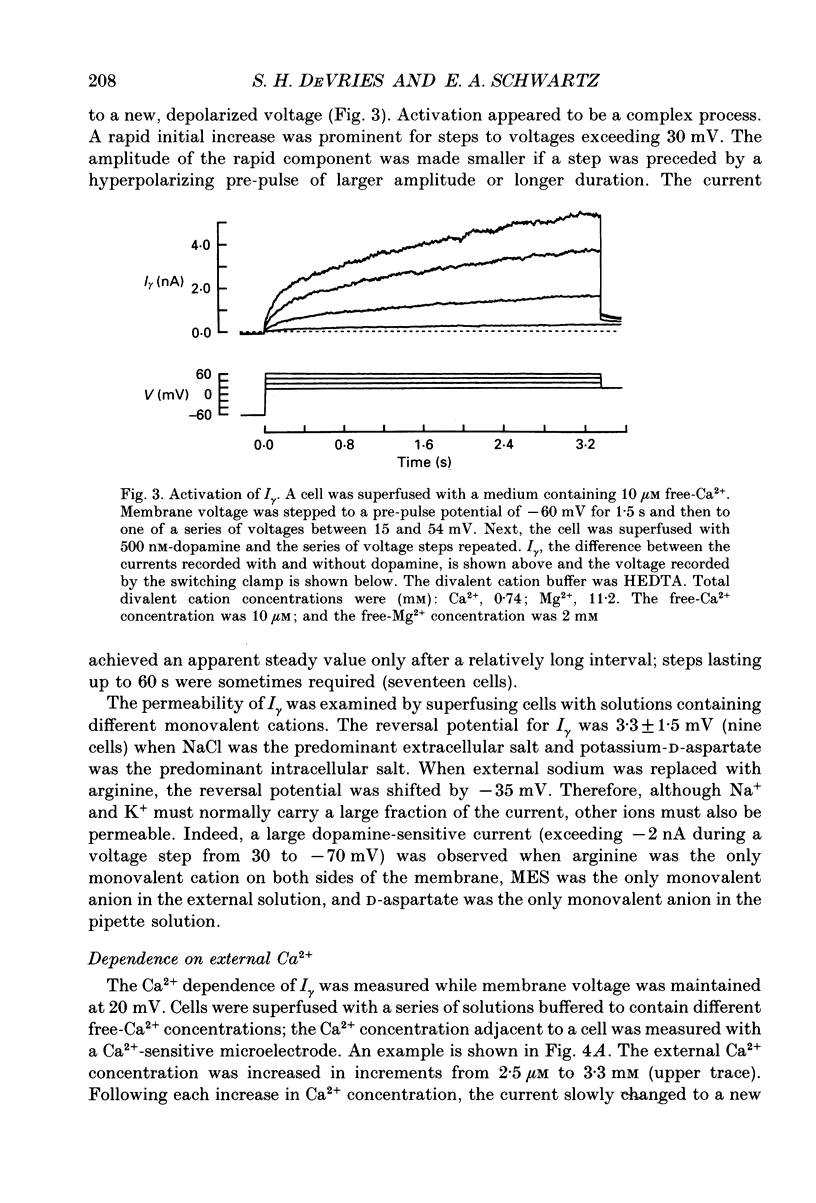
Abstract
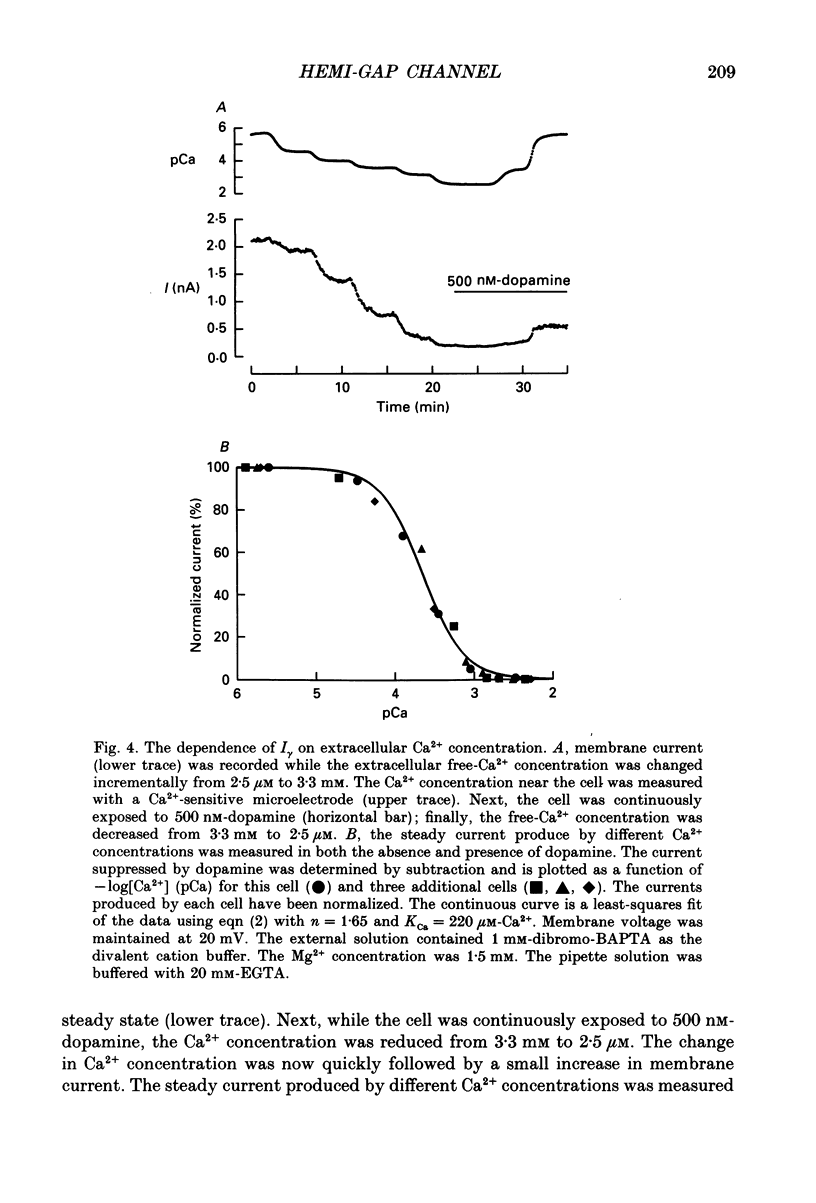
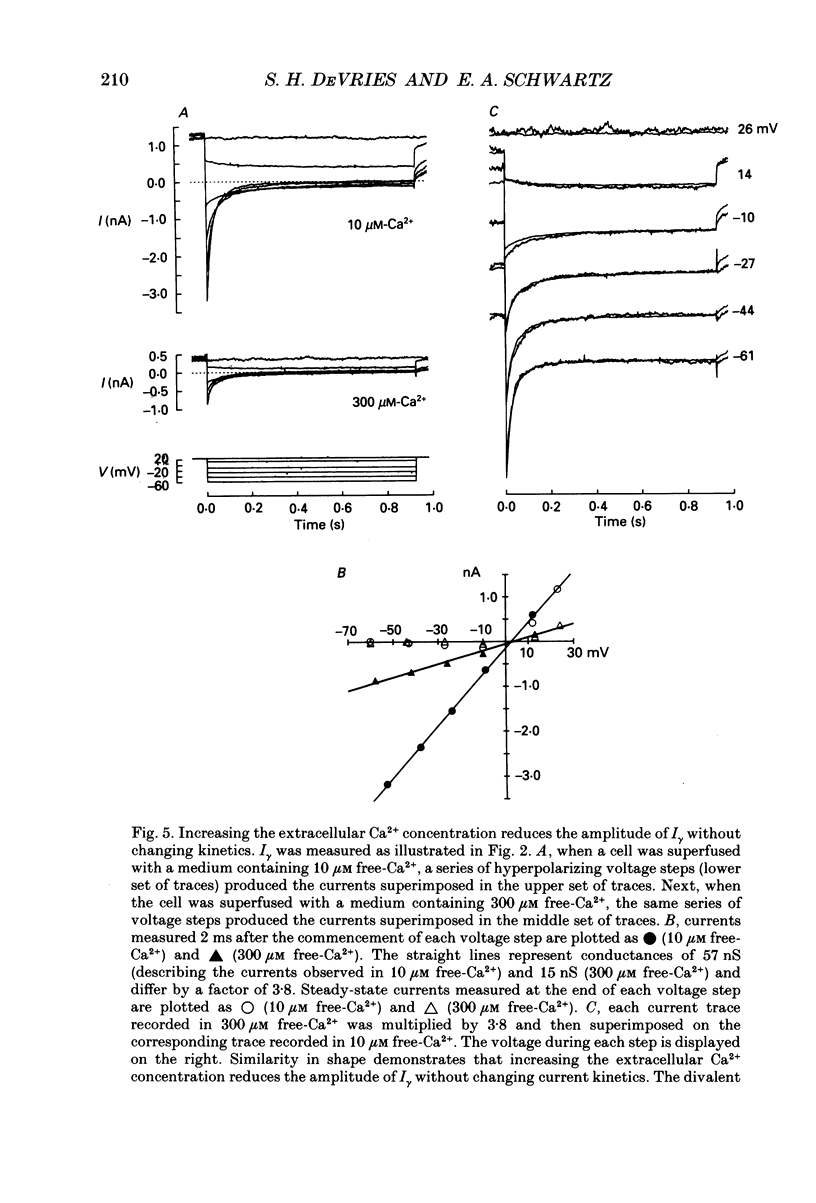
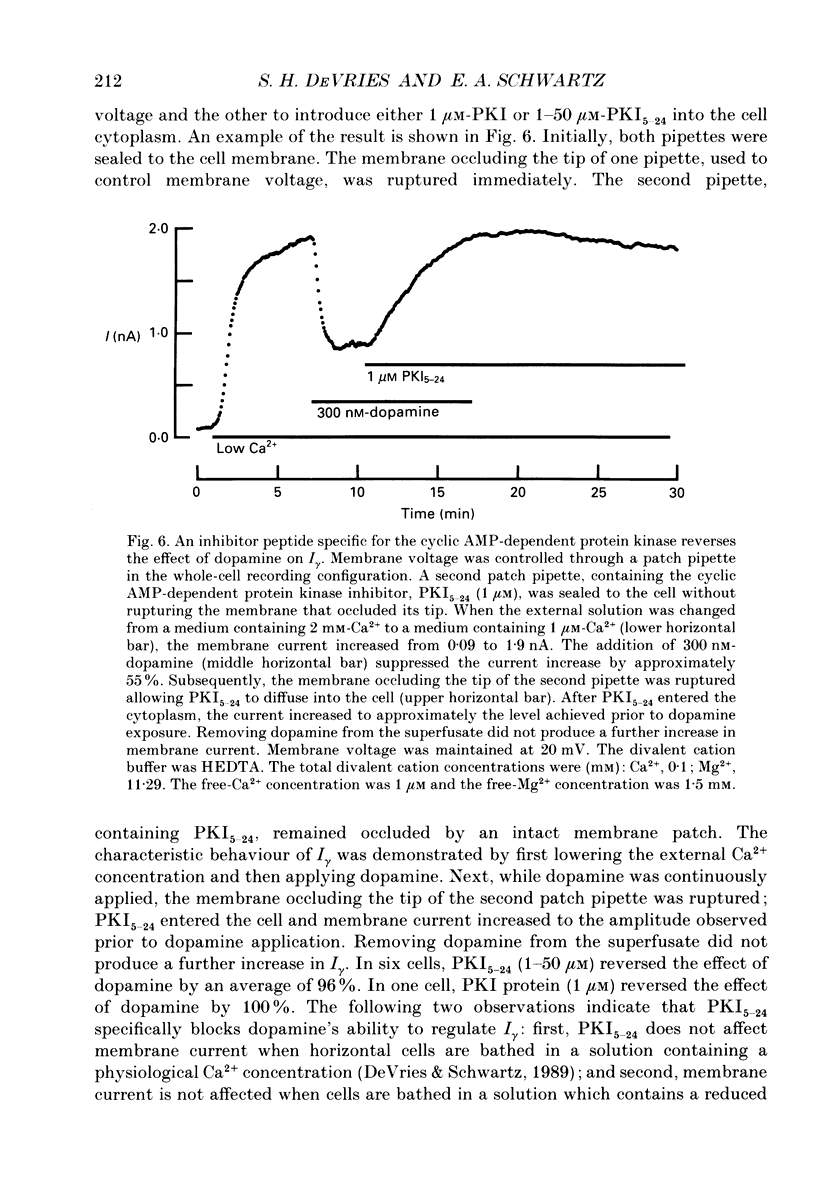
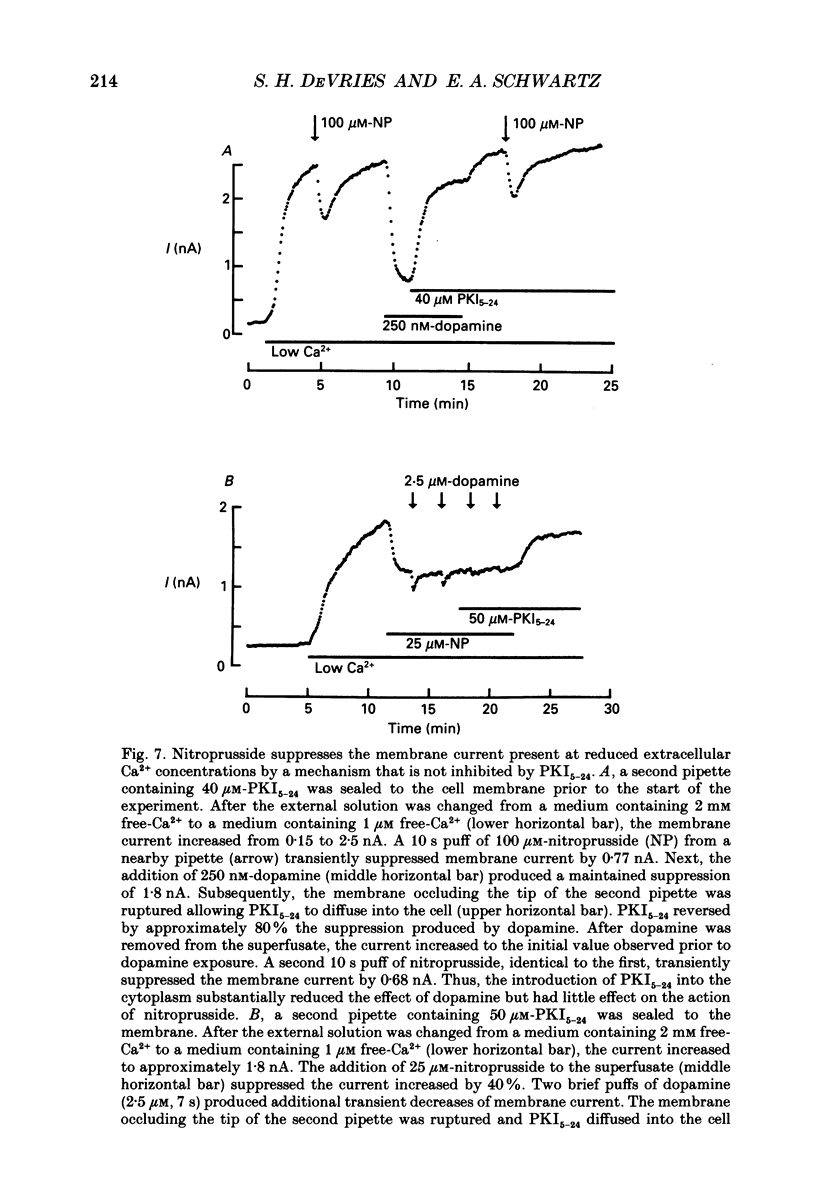
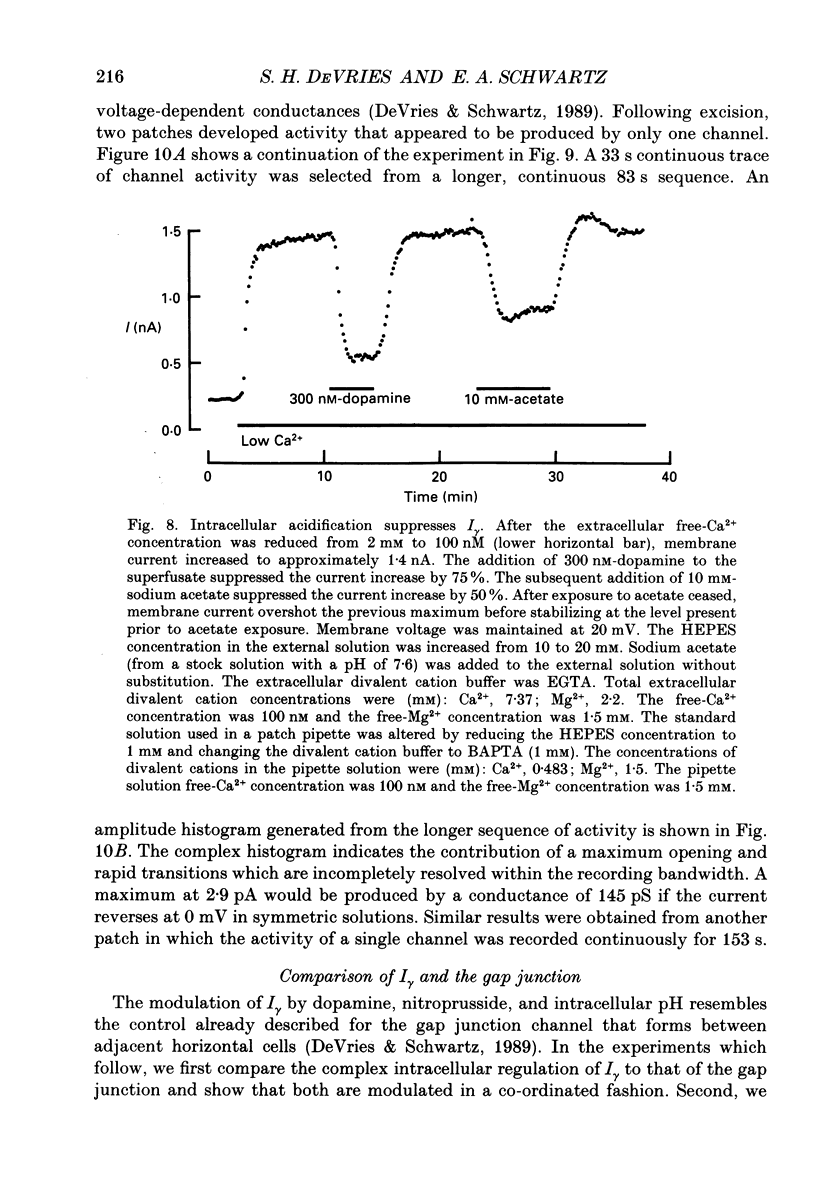
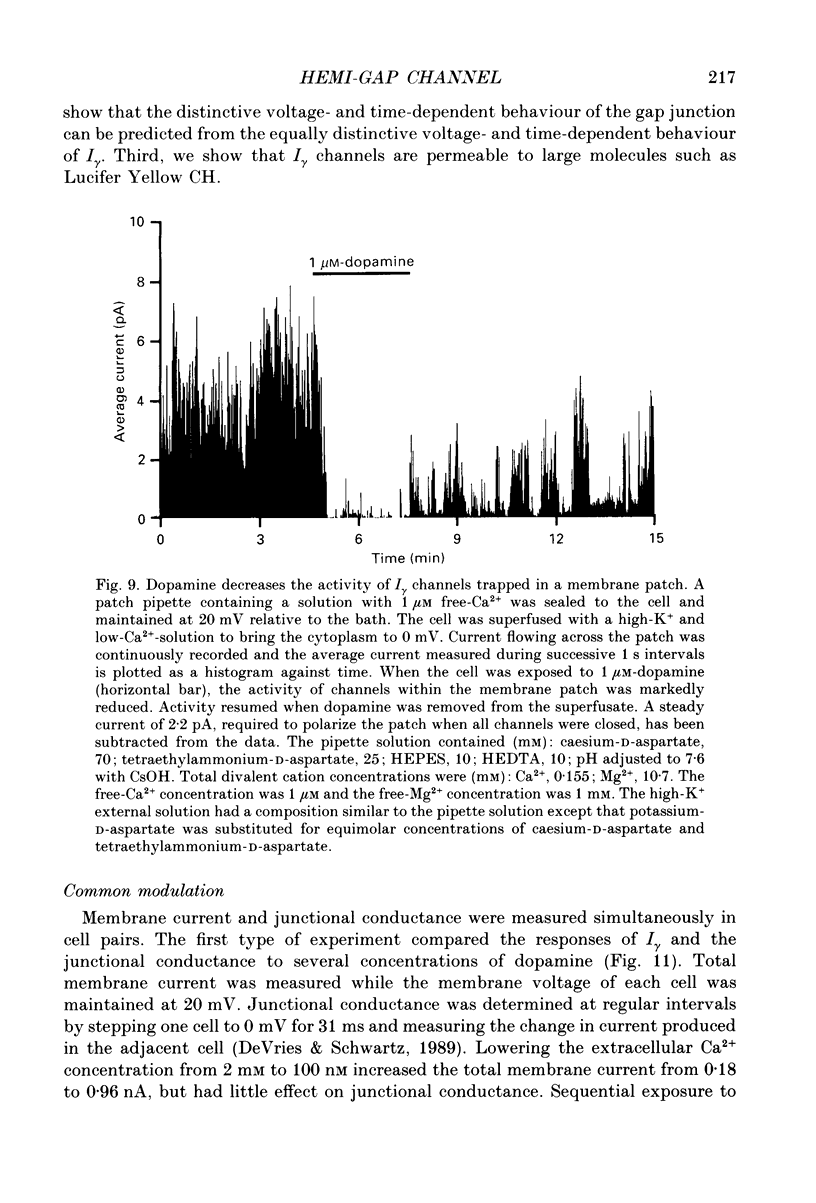
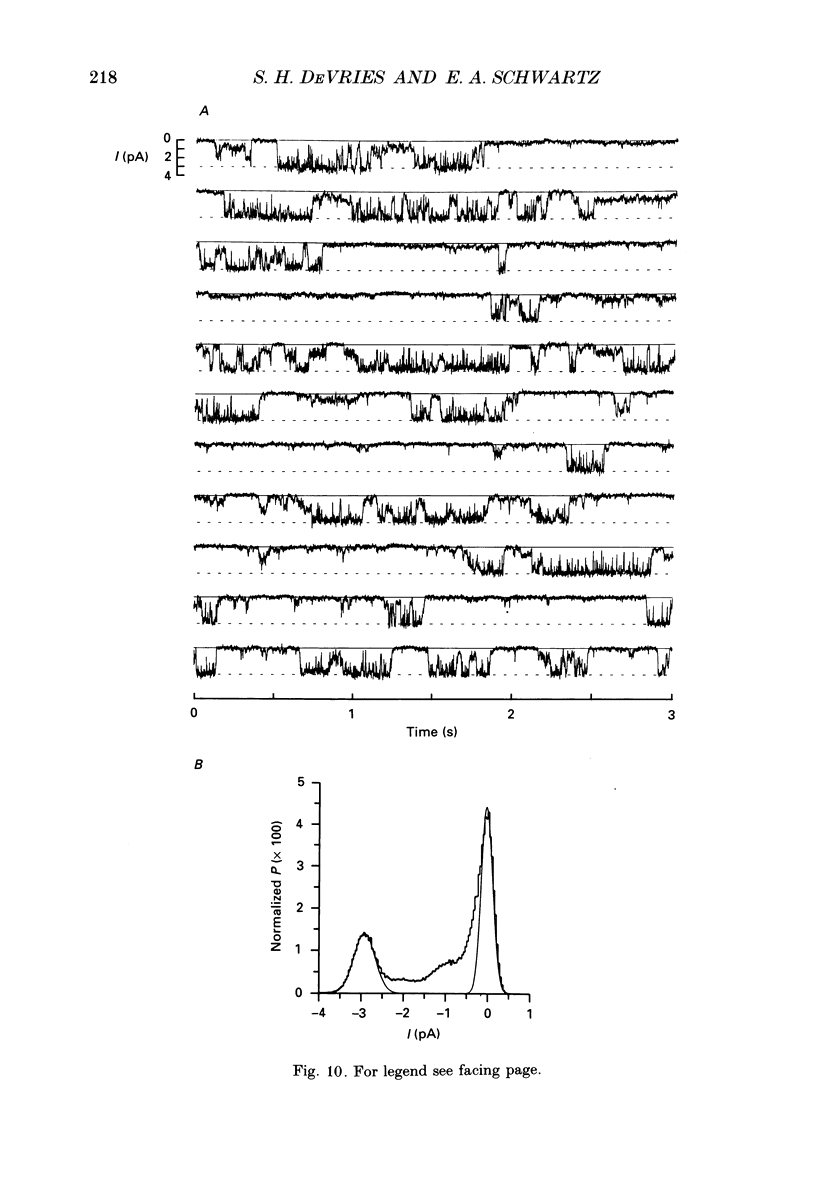
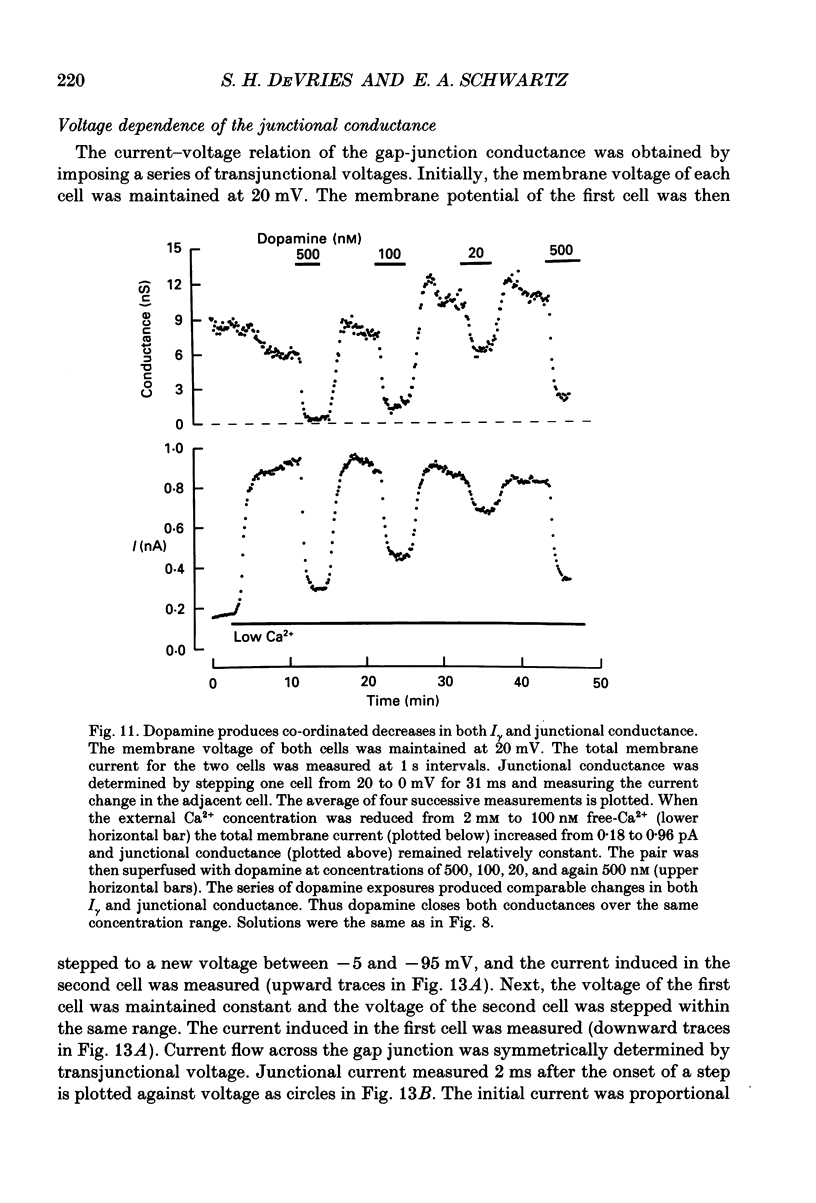
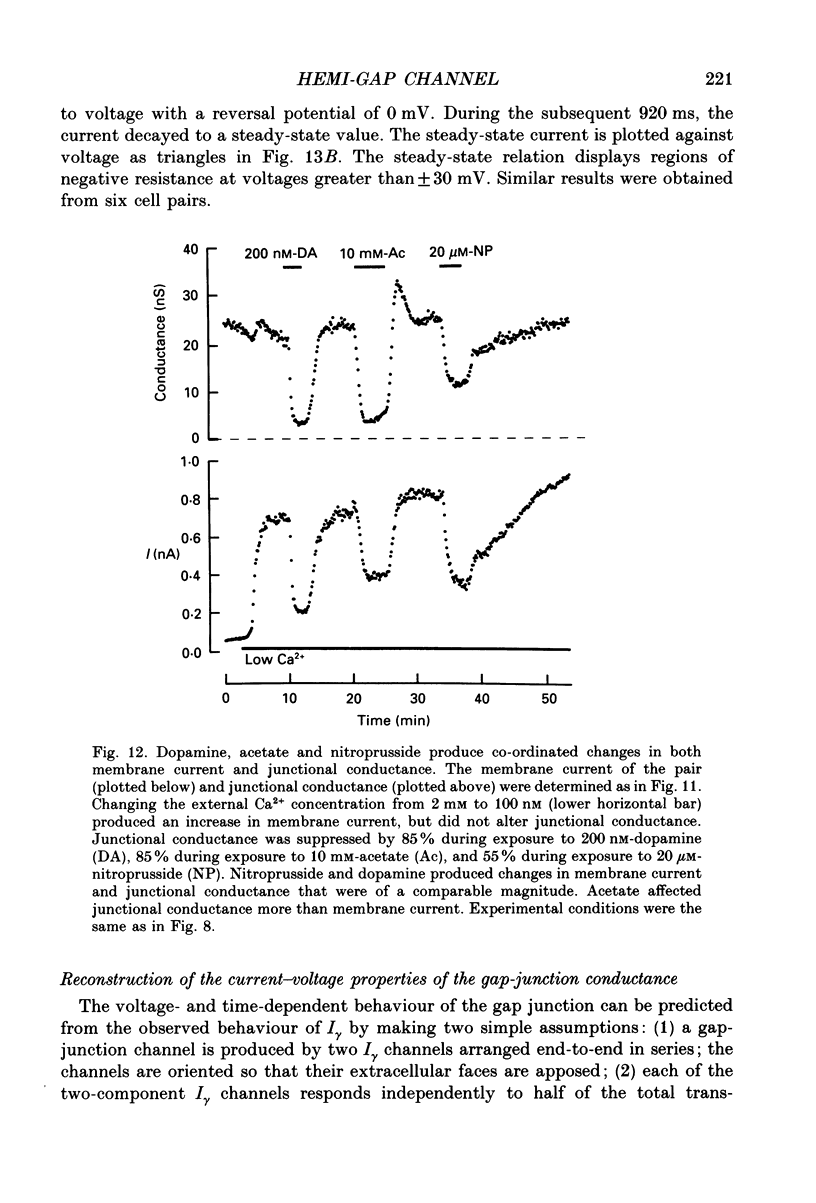
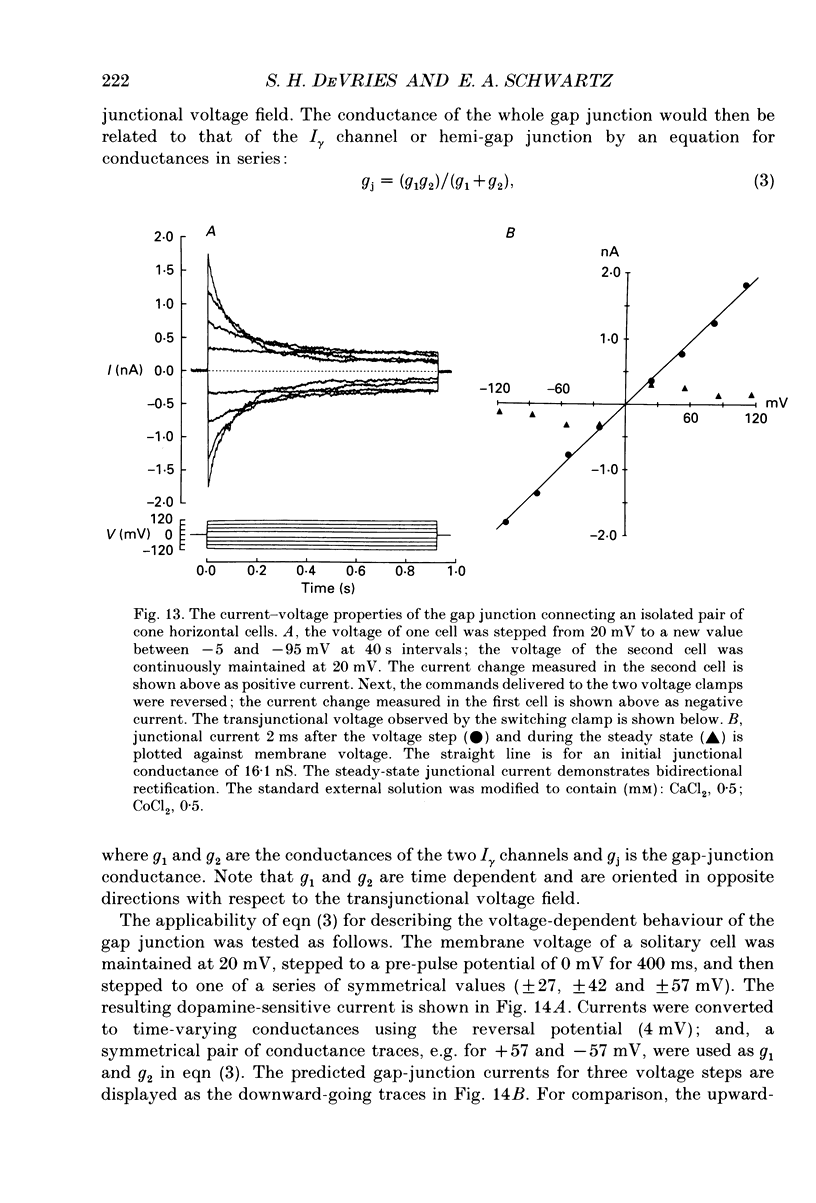
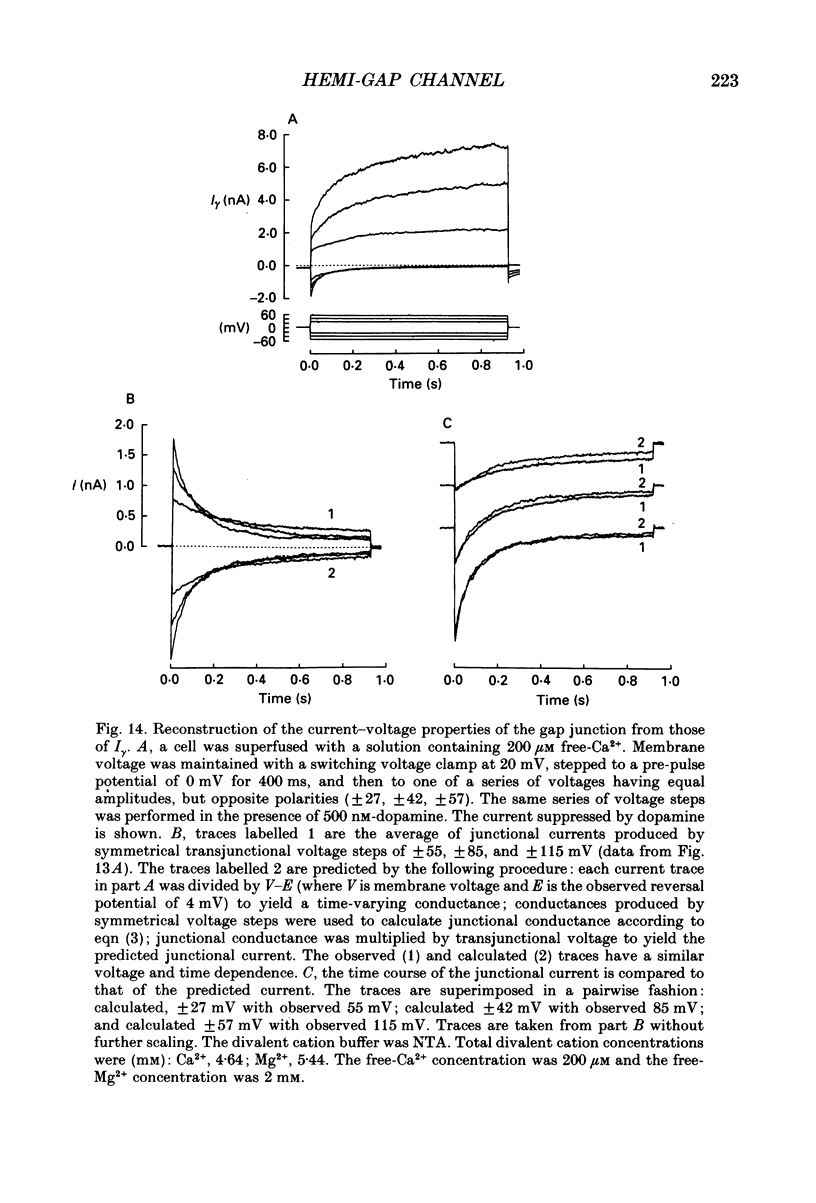
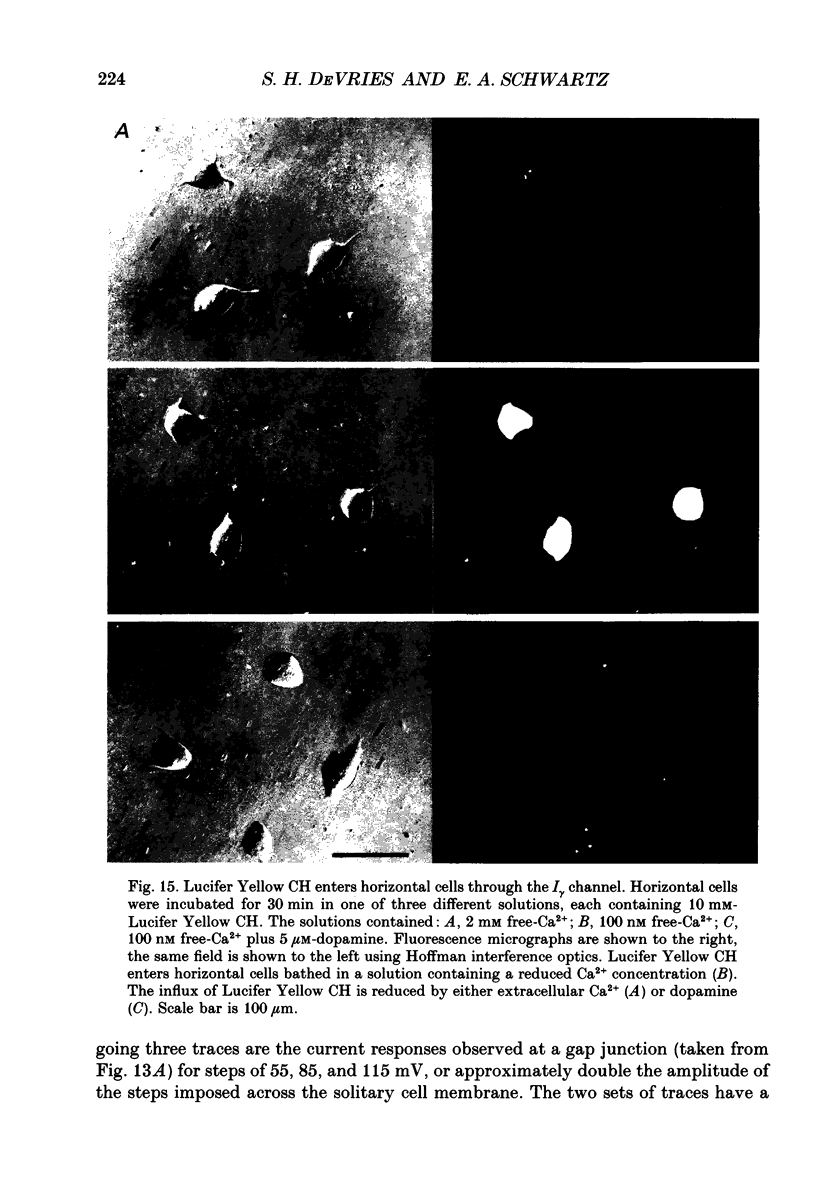
1. Solitary horizontal cells were isolated from catfish retinas and their membrane current was recorded with a whole-cell voltage clamp. Reducing the extracellular Ca2+ concentration produced a current that could be suppressed by dopamine. This Ca(2+)- and dopamine-sensitive current is hereafter termed I gamma. The voltage dependence, cytoplasmic regulation, and permeability of the I gamma channel suggest that it is half of a gap-junction channel. 2. I gamma was voltage and time dependent. In the steady state, the current-voltage relation displayed outward rectification at voltages more depolarized than 0 mV and a negative resistance region at voltages more hyperpolarized than -15 mV. The reversal potential was 3.3 +/- 1.5 mV when NaCl was the predominant extracellular salt and potassium-D-aspartate was the predominant intracellular salt. 3. The size of I gamma depended on the extracellular Ca2+ concentration. I gamma was maximal at external Ca2+ concentrations below 10 microM, half-maximal at 220 microM-Ca2+, and reduced to less than 4% of its maximum amplitude at external Ca2+ concentrations above 1 mM. Increasing the extracellular Ca2+ concentration reduced the amplitude of I gamma without changing the shape of the current-voltage relation or the kinetics of inactivation. Thus, rectification does not result from a voltage-dependent block by extracellular Ca2+. 4. Patches of cell membrane were voltage clamped in both the cell-attached and excised-patch configurations. In the cell-attached configuration, the addition of dopamine to the solution outside the patch pipette blocked the opening of channels within the membrane patch. Thus, dopamine closes I gamma channels by initiating an intracellular messenger cascade. In the excised-patch configuration, a maximum conductance of 145 pS was measured while Cs+ and tetraethylammonium+ (TEA+) were the only monovalent cations on both sides of the membrane. 5. The ability of dopamine to suppress I gamma was blocked by introducing an inhibitor of the cyclic AMP-dependent protein kinase, PKI5-24, into the cytoplasm. Thus, the action of dopamine is mediated by a pathway that includes the activation of a cyclic AMP-dependent kinase. 6. I gamma was suppressed by nitroprusside, an agent which activates guanylate cyclase and increases the intracellular cyclic GMP concentration. The effect of nitroprusside was not altered by the intracellular application of PKI5-24. Thus, nitroprusside suppresses I gamma through a pathway that does not include the activation of a cyclic AMP-dependent kinase.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Barrio L. C., Bargiello T. A., Spray D. C., Hertzberg E., Sáez J. C. Gap junctions: new tools, new answers, new questions. Neuron. 1991 Mar;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin family of gap junction proteins. J Membr Biol. 1990 Jul;116(3):187–194. doi: 10.1007/BF01868459. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- DeVries S. H., Schwartz E. A. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989 Jul;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Beyer E. C., Swenson K. I., Paul D. L., Goodenough D. A. Cloning and expression of a Xenopus embryonic gap junction protein. Science. 1989 Mar 3;243(4895):1194–1195. doi: 10.1126/science.2466337. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Glass D. B., Cheng H. C., Kemp B. E., Walsh D. A. Differential and common recognition of the catalytic sites of the cGMP-dependent and cAMP-dependent protein kinases by inhibitory peptides derived from the heat-stable inhibitor protein. J Biol Chem. 1986 Sep 15;261(26):12166–12171. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Spray D. C., Bennett M. V. Kinetic properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):95–117. doi: 10.1085/jgp.77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Kadowitz P. J. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu Rev Pharmacol Toxicol. 1985;25:171–191. doi: 10.1146/annurev.pa.25.040185.001131. [DOI] [PubMed] [Google Scholar]
- Kraig R. P., Chesler M. Astrocytic acidosis in hyperglycemic and complete ischemia. J Cereb Blood Flow Metab. 1990 Jan;10(1):104–114. doi: 10.1038/jcbfm.1990.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M. Retinal horizontal cell gap junctional conductance is modulated by dopamine through a cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7319–7323. doi: 10.1073/pnas.84.20.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon D. G., Knapp A. G., Dowling J. E. Horizontal cell gap junctions: single-channel conductance and modulation by dopamine. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7639–7643. doi: 10.1073/pnas.86.19.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Trautmann A. Acetylcholine modulation of the conductance of intercellular junctions between rat lacrimal cells. J Physiol. 1986 Aug;377:283–295. doi: 10.1113/jphysiol.1986.sp016187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Trautmann A. Single-channel currents of an intercellular junction. 1985 Sep 26-Oct 2Nature. 317(6035):331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Noma A., Tsuboi N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J Physiol. 1987 Jan;382:193–211. doi: 10.1113/jphysiol.1987.sp016363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984 Oct;4(10):2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez J. C., Spray D. C., Nairn A. C., Hertzberg E., Greengard P., Bennett M. V. cAMP increases junctional conductance and stimulates phosphorylation of the 27-kDa principal gap junction polypeptide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2473–2477. doi: 10.1073/pnas.83.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Ginzberg R. D., Morales E. A., Gatmaitan Z., Arias I. M. Electrophysiological properties of gap junctions between dissociated pairs of rat hepatocytes. J Cell Biol. 1986 Jul;103(1):135–144. doi: 10.1083/jcb.103.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Saez J. C., Brosius D., Bennett M. V., Hertzberg E. L. Isolated liver gap junctions: gating of transjunctional currents is similar to that in intact pairs of rat hepatocytes. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5494–5497. doi: 10.1073/pnas.83.15.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Stern J. H., Harris A. L., Bennett M. V. Gap junctional conductance: comparison of sensitivities to H and Ca ions. Proc Natl Acad Sci U S A. 1982 Jan;79(2):441–445. doi: 10.1073/pnas.79.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson K. I., Jordan J. R., Beyer E. C., Paul D. L. Formation of gap junctions by expression of connexins in Xenopus oocyte pairs. Cell. 1989 Apr 7;57(1):145–155. doi: 10.1016/0092-8674(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Sáez J. C., Connor J. A., Spray D. C., Bennett M. V. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teranishi T., Negishi K., Kato S. Regulatory effect of dopamine on spatial properties of horizontal cells in carp retina. J Neurosci. 1984 May;4(5):1271–1280. doi: 10.1523/JNEUROSCI.04-05-01271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Verselis V. K., Bennett M. V., Bargiello T. A. A voltage-dependent gap junction in Drosophila melanogaster. Biophys J. 1991 Jan;59(1):114–126. doi: 10.1016/S0006-3495(91)82204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner R., Levine E., Rabadan-Diehl C., Dahl G. Formation of hybrid cell-cell channels. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5380–5384. doi: 10.1073/pnas.86.14.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Cohn Z. A., Gilula N. B. Functional assembly of gap junction conductance in lipid bilayers: demonstration that the major 27 kd protein forms the junctional channel. Cell. 1987 Mar 13;48(5):733–743. doi: 10.1016/0092-8674(87)90071-7. [DOI] [PubMed] [Google Scholar]