Abstract
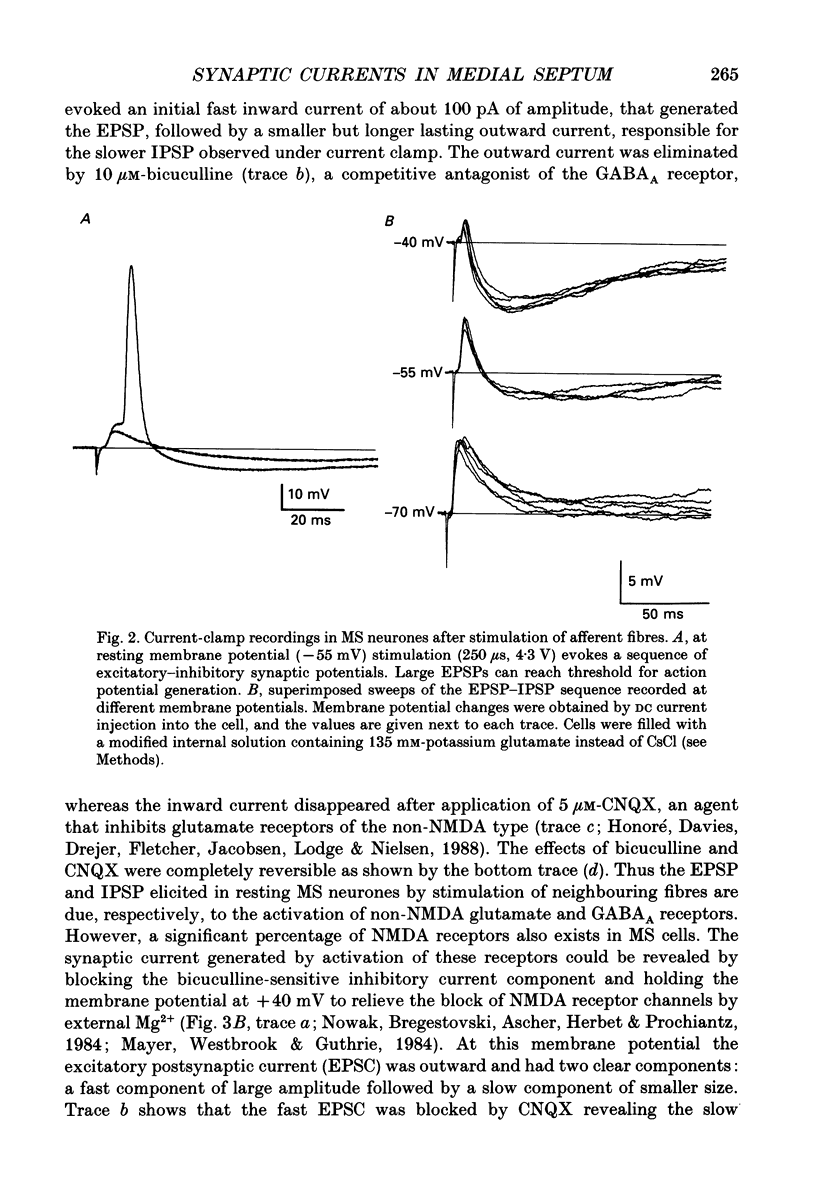
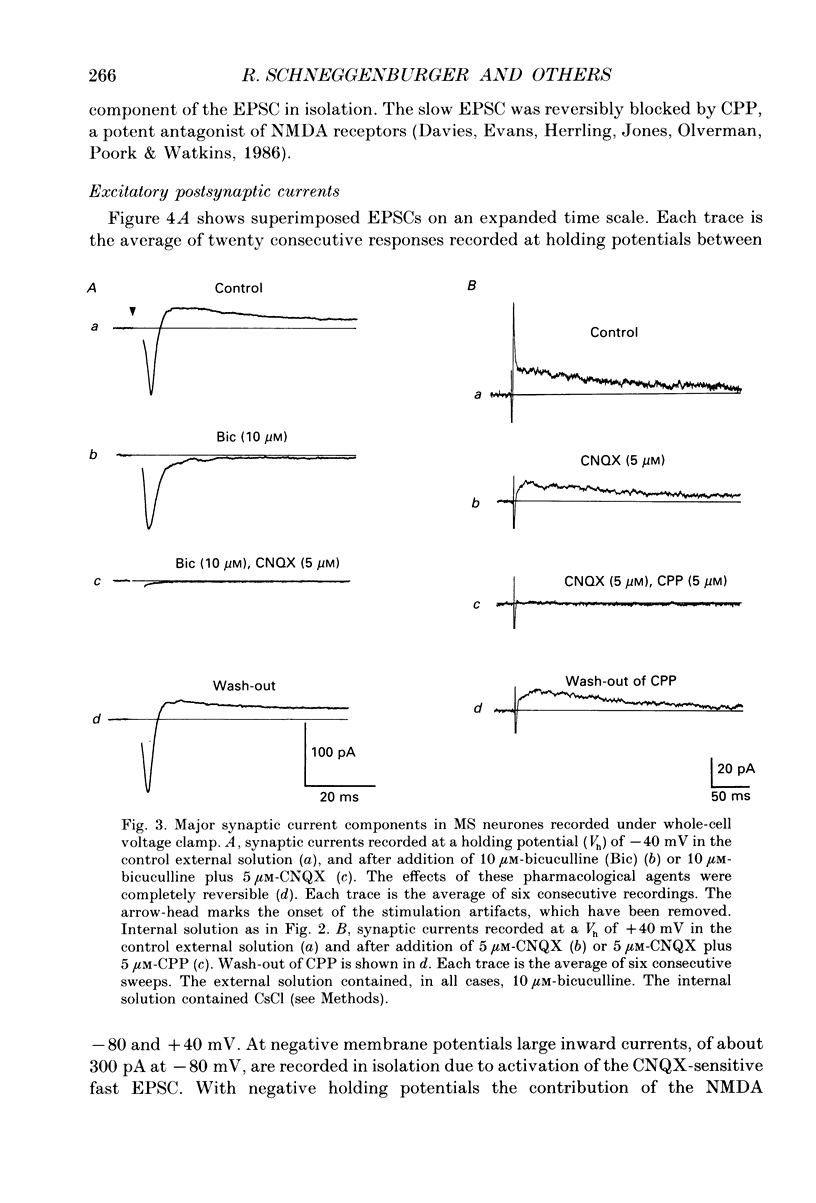
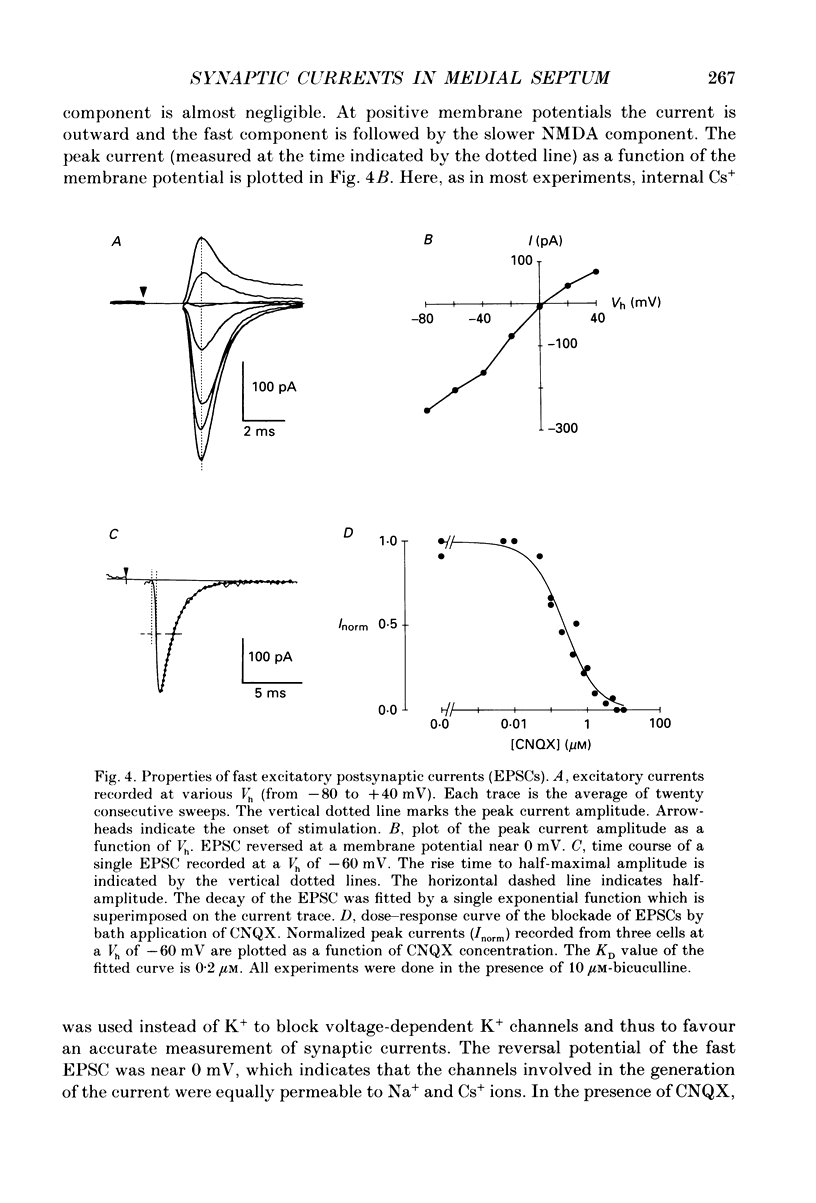
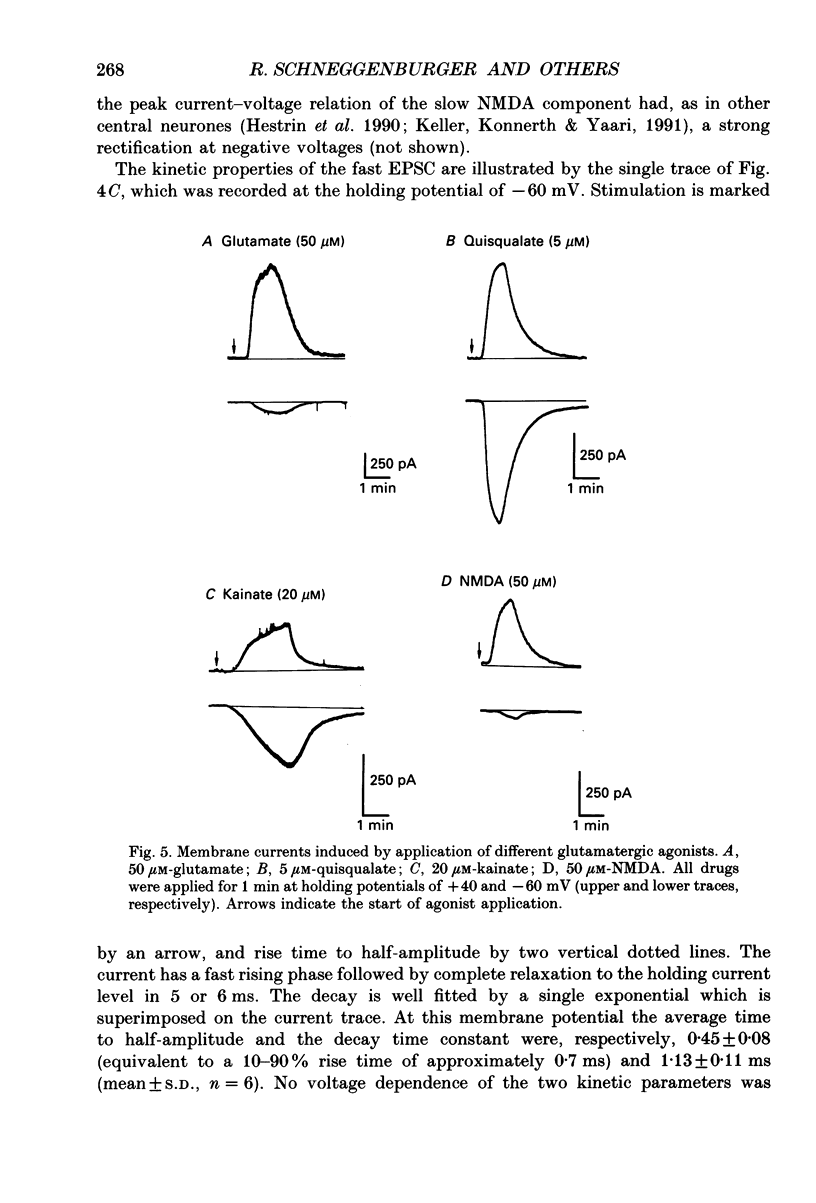
1. A thin-slice preparation was used to study the postsynaptic potentials and the underlying currents of visually identified rat medial septal (MS) neurones under tight-seal voltage- and current-clamp conditions. 2. Upon stimulation of the afferent fibres, all MS neurones exhibited a sequence of excitatory-inhibitory postsynaptic potentials (EPSP-IPSP). Under voltage clamp, with potassium glutamate as internal solution and at negative holding potentials (Vh), this synaptic pattern appeared as an initial inward current followed by a longer lasting outward current. 3. The inward postsynaptic current was completely abolished by 5 microM-6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) whereas the outward current disappeared in the presence of 10 microM-bicuculline. Thus the major excitatory and inhibitory synaptic inputs were identified as being due to activation of quisqualate/kainate glutamatergic and gamma-aminobutyric acid (GABAA) receptors, respectively. 4. At positive Vh a CNQX-resistant component of the excitatory postsynaptic current (EPSC) was revealed. This component was slower than the one mediated by the quisqualate receptor and was abolished by 3-3(2-carboxypiperazine-4-yl)propyl-1-phosphonate (CPP), indicating that N-methyl-D-aspartate (NMDA) receptors are involved in excitatory synaptic transmission in MS cells. The existence of the two main subtypes (NMDA and non-NMDA) of glutamatergic receptors in MS neurones was also confirmed by the responses of the neurones to bath application of the different agonists (glutamate, quisqualate, kainate and NMDA). 5. The CNQX-sensitive EPSC had a reversal potential near 0 mV. The fast rise time (approximately 0.7 ms) indicates a somatic location of the excitatory synapses. The relaxation kinetics of the fast EPSC were fitted by a single exponential function with a time constant of 1.13 +/- 0.1 ms. This parameter was independent of Vh. Fast EPSCs were blocked by CNQX in a dose-dependent manner (dissociation constant, KD = 0.2 microM). 6. Inhibitory postsynaptic currents (IPSCs) were studied in symmetrical chloride solutions after blockade of the excitatory receptors. The current-voltage relation was linear and reversed at 0 mV. The IPSCs had a fast rise time and their decay was best fitted by the sum of two exponentials with time constant of approximately 20 and 50 ms (Vh = -60 mV). The IPSCs were abolished by bicuculline (KD = 1 microM), a selective antagonist of GABAA receptors. As expected, bath application of GABA produced large whole-cell currents. 7. In many cells, in addition to the usual EPSP-IPSP sequence, failures of either the EPSP or the IPSP were frequently observed during the experimental protocol.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Images in this article
Selected References
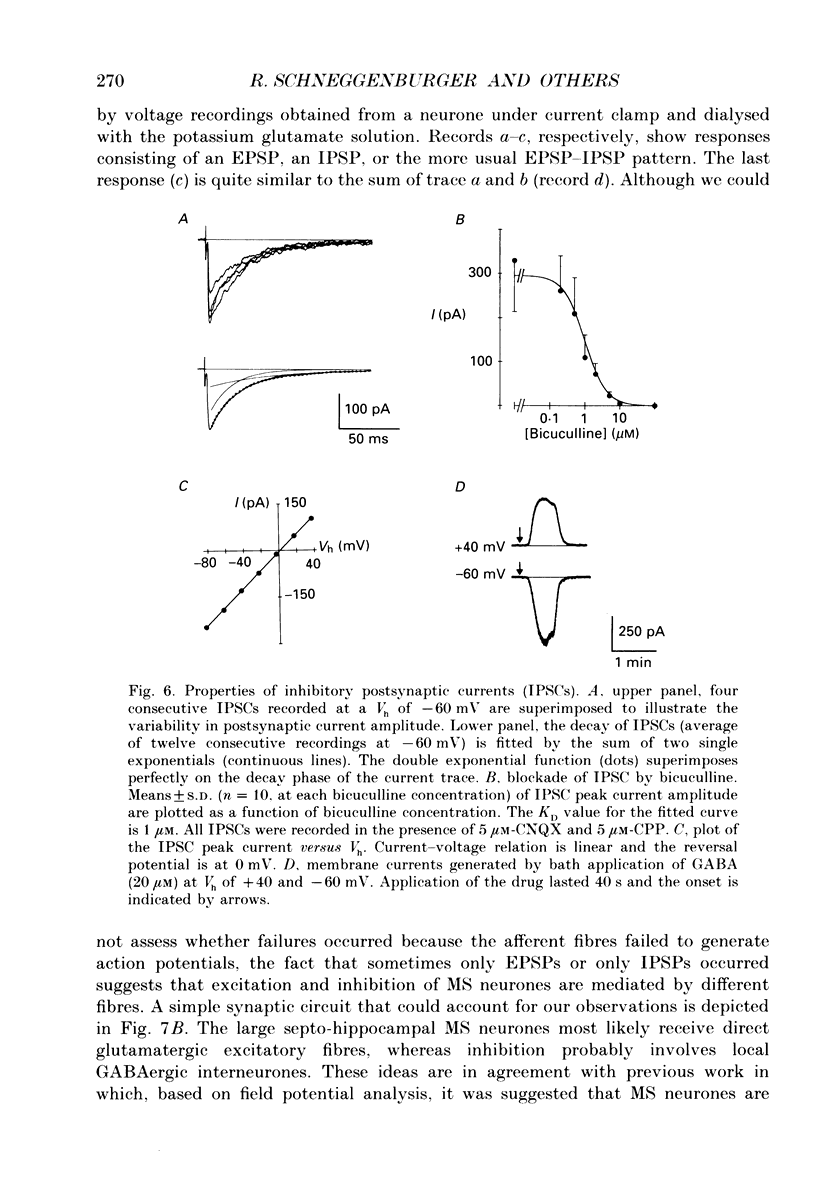
These references are in PubMed. This may not be the complete list of references from this article.
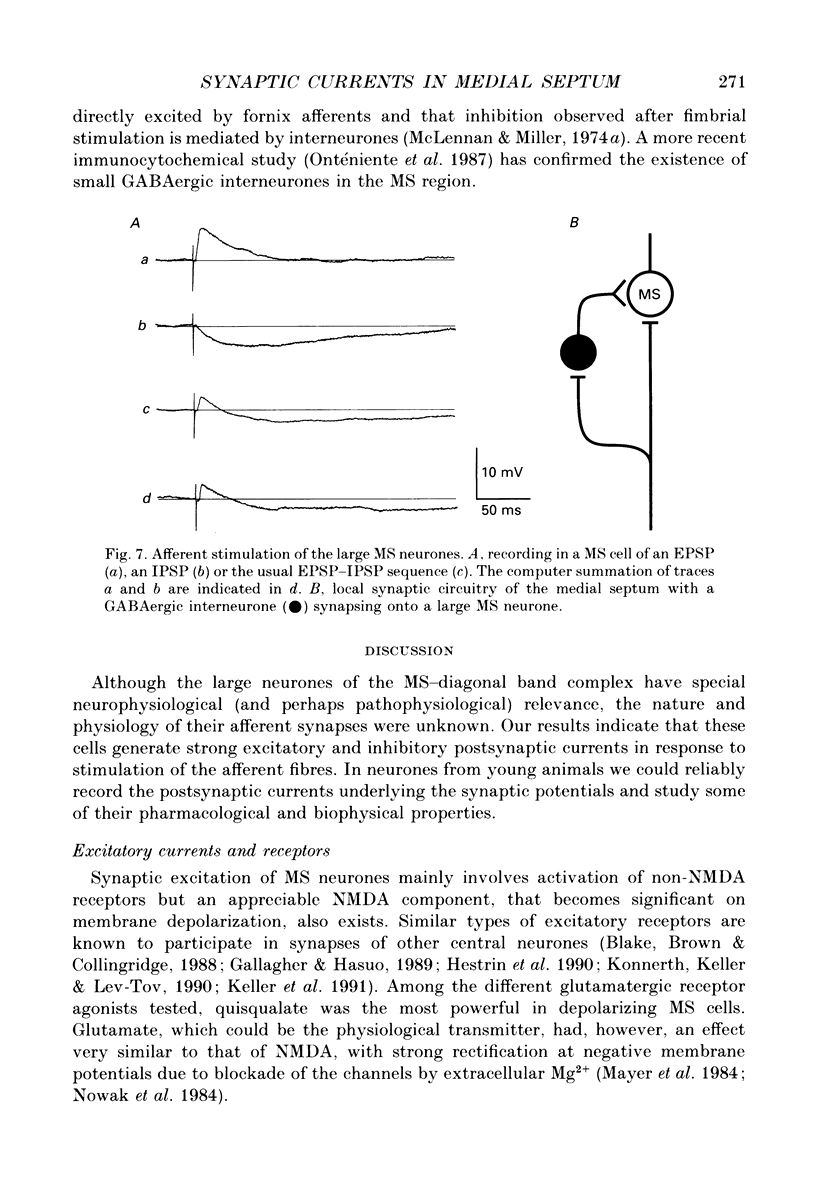
- Alvarez de Toledo G., López-Barneo J. Ionic basis of the differential neuronal activity of guinea-pig septal nucleus studied in vitro. J Physiol. 1988 Feb;396:399–415. doi: 10.1113/jphysiol.1988.sp016969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol G., Creutzfeldt O. D. Crosscorrelation between the activity of septal units and hippocampal EEG during arousal. Brain Res. 1974 Feb 15;67(1):65–75. doi: 10.1016/0006-8993(74)90298-4. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Bruce G., Hersh L. B., Gage F. H. Development of cholinergic neurons in the septal/diagonal band complex of the rat. Brain Res. 1987 Dec 1;433(2):249–256. doi: 10.1016/0165-3806(87)90028-9. [DOI] [PubMed] [Google Scholar]
- Blake J. F., Brown M. W., Collingridge G. L. CNQX blocks acidic amino acid induced depolarizations and synaptic components mediated by non-NMDA receptors in rat hippocampal slices. Neurosci Lett. 1988 Jun 29;89(2):182–186. doi: 10.1016/0304-3940(88)90378-3. [DOI] [PubMed] [Google Scholar]
- Bland S. K., Bland B. H. Medial septal modulation of hippocampal theta cell discharges. Brain Res. 1986 Jun 4;375(1):102–116. doi: 10.1016/0006-8993(86)90963-7. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Gage P. W., Robertson B. Inhibitory post-synaptic currents in rat hippocampal CA1 neurones. J Physiol. 1984 Nov;356:551–564. doi: 10.1113/jphysiol.1984.sp015482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Whole-cell current noise produced by excitatory and inhibitory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:533–553. doi: 10.1113/jphysiol.1989.sp017735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Evans R. H., Herrling P. L., Jones A. W., Olverman H. J., Pook P., Watkins J. C. CPP, a new potent and selective NMDA antagonist. Depression of central neuron responses, affinity for [3H]D-AP5 binding sites on brain membranes and anticonvulsant activity. Brain Res. 1986 Sep 10;382(1):169–173. doi: 10.1016/0006-8993(86)90127-7. [DOI] [PubMed] [Google Scholar]
- Dutar P., Lamour Y., Jobert A. Septohippocampal neurons in the rat: an in vivo intracellular study. Brain Res. 1985 Aug 5;340(1):135–142. doi: 10.1016/0006-8993(85)90782-6. [DOI] [PubMed] [Google Scholar]
- Dutar P., Rascol O., Lamour Y. Rhythmical bursting activity and GABAergic mechanisms in the medial septum of normal and pertussis toxin-pretreated rats. Exp Brain Res. 1989;77(2):374–380. doi: 10.1007/BF00274994. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. J. The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J Physiol. 1983 Sep;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN J. D., ARDUINI A. A. Hippocampal electrical activity in arousal. J Neurophysiol. 1954 Nov;17(6):533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Hasuo H. Excitatory amino acid-receptor-mediated EPSPs in rat dorsolateral septal nucleus neurones in vitro. J Physiol. 1989 Nov;418:353–365. doi: 10.1113/jphysiol.1989.sp017845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. A., McNaughton N. Comparison between the behavioural effects of septal and hippocampal lesions: a review. Neurosci Biobehav Rev. 1983 Summer;7(2):119–188. doi: 10.1016/0149-7634(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Nicoll R. A., Perkel D. J., Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990 Mar;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Johnston D., Brown T. H. Interpretation of voltage-clamp measurements in hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):464–486. doi: 10.1152/jn.1983.50.2.464. [DOI] [PubMed] [Google Scholar]
- Keller B. U., Konnerth A., Yaari Y. Patch clamp analysis of excitatory synaptic currents in granule cells of rat hippocampus. J Physiol. 1991 Apr;435:275–293. doi: 10.1113/jphysiol.1991.sp018510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A., Keller B. U., Lev-Tov A. Patch clamp analysis of excitatory synapses in mammalian spinal cord slices. Pflugers Arch. 1990 Nov;417(3):285–290. doi: 10.1007/BF00370994. [DOI] [PubMed] [Google Scholar]
- Lamour Y., Dutar P., Jobert A. Septo-hippocampal and other medial septum-diagonal band neurons: electrophysiological and pharmacological properties. Brain Res. 1984 Sep 10;309(2):227–239. doi: 10.1016/0006-8993(84)90588-2. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- McLennan H., Miller J. J. Gamma-aminobutyric acid and inhibition in the septal nuclei of the rat. J Physiol. 1974 Mar;237(3):625–633. doi: 10.1113/jphysiol.1974.sp010501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan H., Miller J. J. The hippocampal control of neuronal discharges in the septum of the rat. J Physiol. 1974 Mar;237(3):607–624. doi: 10.1113/jphysiol.1974.sp010500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Onténiente B., Geffard M., Campistron G., Calas A. An ultrastructural study of GABA-immunoreactive neurons and terminals in the septum of the rat. J Neurosci. 1987 Jan;7(1):48–54. doi: 10.1523/JNEUROSCI.07-01-00048.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETSCHE H., STUMPF C., GOGOLAK G. [The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells]. Electroencephalogr Clin Neurophysiol. 1962 Apr;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- Sah P., Hestrin S., Nicoll R. A. Properties of excitatory postsynaptic currents recorded in vitro from rat hippocampal interneurones. J Physiol. 1990 Nov;430:605–616. doi: 10.1113/jphysiol.1990.sp018310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M., Schneider H. H., Stephens D. N. Treatment strategies for senile dementia: antagonist beta-carbolines. Trends Neurosci. 1988 Jan;11(1):13–17. doi: 10.1016/0166-2236(88)90042-2. [DOI] [PubMed] [Google Scholar]
- Segal M. Properties of rat medial septal neurones recorded in vitro. J Physiol. 1986 Oct;379:309–330. doi: 10.1113/jphysiol.1986.sp016255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M., Fox S. E. Do septal neurons pace the hippocampal theta rhythm? Trends Neurosci. 1990 May;13(5):163–168. doi: 10.1016/0166-2236(90)90040-h. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Fischbach G. D. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989 Aug;3(2):209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Draguhn A., Ymer S., Seeburg P. H., Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990 Jun;4(6):919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Price D. L., Struble R. G., Clark A. W., Coyle J. T., Delon M. R. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982 Mar 5;215(4537):1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]



