Abstract
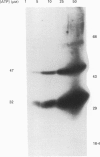
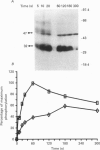
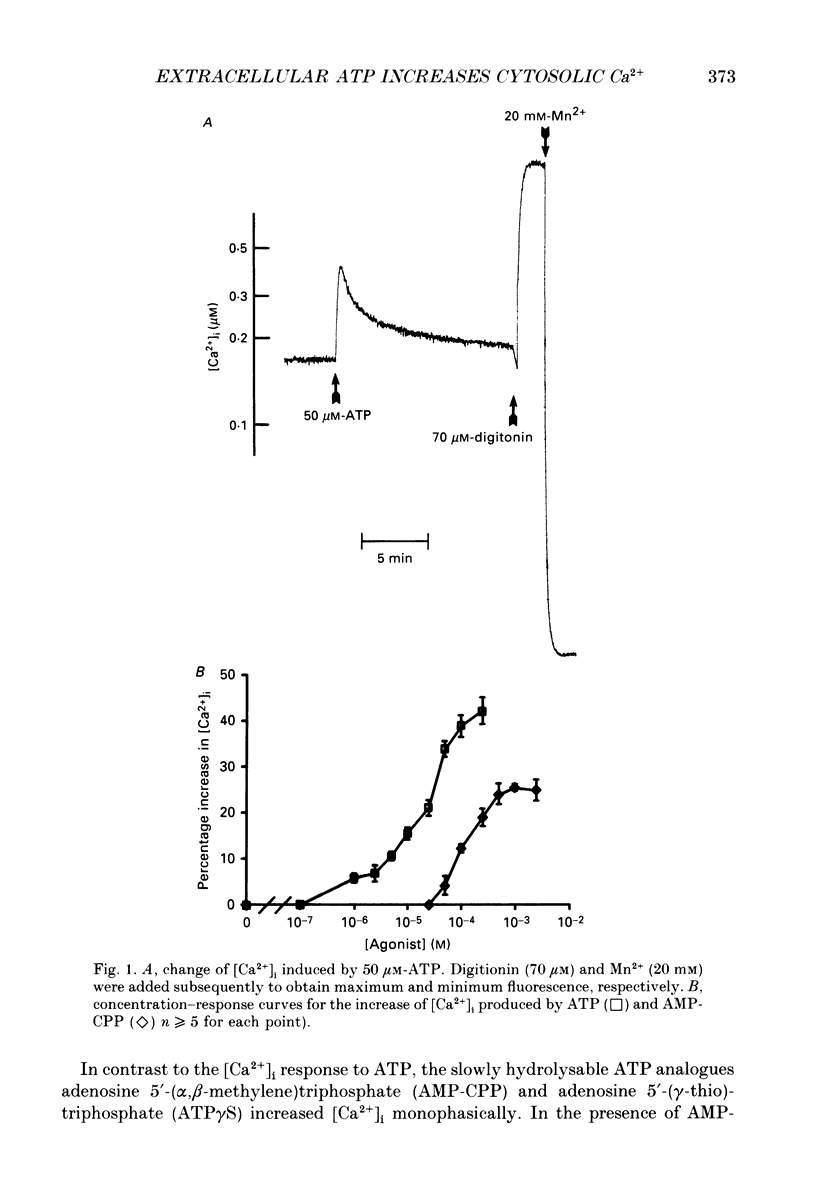
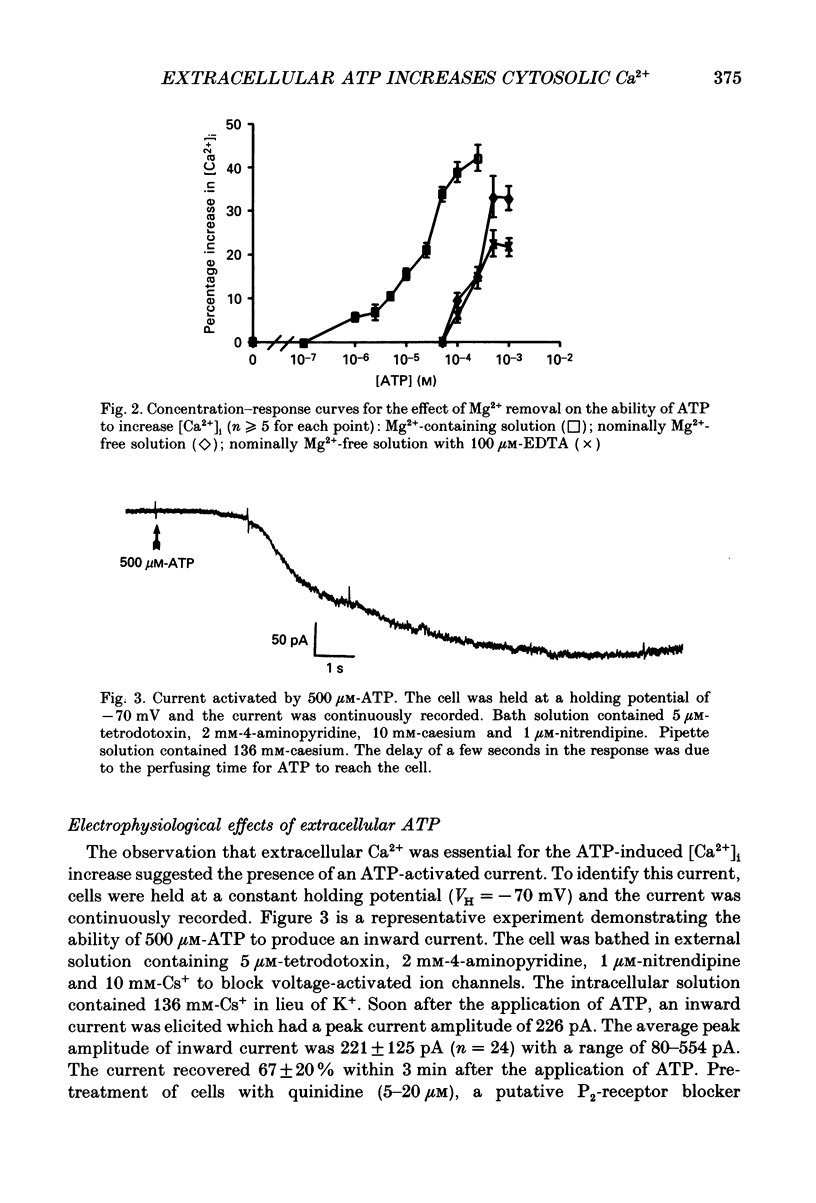
1. Changes in the cytosolic Ca2+ concentration ([Ca2+]i) of isolated rat ventricular myocytes in suspension were measured in response to extracellular ATP using the fluorescent Ca2+ indicators Quin-2 and Fura-2. 2. ATP produced a concentration-, time- and Mg(2+)-dependent, biphasic increase of [Ca2+]i whereas slowly hydrolysable ATP analogues produced a slow, monophasic increase of [Ca2+]i and the non-hydrolysable ATP analogues were without effect. 3. Extracellular Ca2+ was required for the ATP-induced increase of [Ca2+]i and pre-treatment of the cells with caffeine, ryanodine, verapamil or nimodipine partially inhibited the [Ca2+]i increase. 4. Whole-cell patch-clamp experiments revealed that ATP activated an ionic current that had a linear current-voltage relationship with a reversal potential near O mV. Quinidine, a putative P2 purinergic receptor blocker, abolished the ATP-activated current. The ATP-activated current was Mg2+ dependent. 5. Associated with the ATP-activated current was cellular depolarization. In a physiological solution, ATP depolarized cells to the threshold for the firing of action potentials. In the presence of the voltage-activated ion channel blockers tetrodotoxin, 4-aminopyridine, caesium and nitrendipine, ATP depolarized cells to -44 +/- 6 mV from a resting potential of -66 +/- 4 mV (n = 11). 6. Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis and autoradiography demonstrated that extracellular ATP stimulated the phosphorylation of several extracellular membrane-bound proteins. The phosphorylation of these proteins was concentration, time and Mg2+ dependent. Pre-treatment of cells with the slowly hydrolysable ATP analogues inhibited the ATP-induced phosphorylation. Adenosine 5'-O-3-thiotriphosphate (ATP gamma S) thiophosphorylated proteins with the same apparent molecular weight as the proteins phosphorylated by ATP. 7. These results suggest that the ATP-induced increase of [Ca2+]i is a result of the activation, possibly by protein phosphorylation, of a novel ion channel carrying inward current. The ATP-activated channel may be permeable to Na+ and Ca2+ and causes [Ca2+]i to rise. More importantly, this inward current depolarizes the cell to the threshold of inducing spontaneous firing of action potentials. The firing of action potentials results in the influx of Ca2+ through L-type Ca2+ channels which would trigger Ca2+ release from the sarcoplasmic reticulum and lead to the increase in [Ca2+]i.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J Neurosci. 1990 Jan;10(1):1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Williams C. A., Ceelen P. W. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990 Jan;10(1):11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D. ATP-activated channels gate calcium entry in single smooth muscle cells dissociated from rabbit ear artery. J Physiol. 1989 Dec;419:689–701. doi: 10.1113/jphysiol.1989.sp017893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Byrne N. G., Large W. A. Action of externally applied adenosine triphosphate on single smooth muscle cells dispersed from rabbit ear artery. J Physiol. 1987 Jun;387:473–488. doi: 10.1113/jphysiol.1987.sp016585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Björnsson O. G., Monck J. R., Williamson J. R. Identification of P2Y purinoceptors associated with voltage-activated cation channels in cardiac ventricular myocytes of the rat. Eur J Biochem. 1989 Dec 8;186(1-2):395–404. doi: 10.1111/j.1432-1033.1989.tb15222.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Cobbold P. H., Rink T. J. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem J. 1987 Dec 1;248(2):313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger R. S., Raffaeli S., Moreno-Sanchez R., Sakai M., Capogrossi M. C., Spurgeon H. A., Hansford R. G., Lakatta E. G. Extracellular ATP has a potent effect to enhance cytosolic calcium and contractility in single ventricular myocytes. Cell Calcium. 1988 Aug;9(4):193–199. doi: 10.1016/0143-4160(88)90023-1. [DOI] [PubMed] [Google Scholar]
- De Young M. B., Scarpa A. ATP receptor-induced Ca2+ transients in cardiac myocytes: sources of mobilized Ca2+. Am J Physiol. 1989 Oct;257(4 Pt 1):C750–C758. doi: 10.1152/ajpcell.1989.257.4.C750. [DOI] [PubMed] [Google Scholar]
- De Young M. B., Scarpa A. Extracellular ATP induces Ca2+ transients in cardiac myocytes which are potentiated by norepinephrine. FEBS Lett. 1987 Oct 19;223(1):53–58. doi: 10.1016/0014-5793(87)80508-2. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Friel D. D. An ATP-sensitive conductance in single smooth muscle cells from the rat vas deferens. J Physiol. 1988 Jul;401:361–380. doi: 10.1113/jphysiol.1988.sp017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D., Bean B. P. Two ATP-activated conductances in bullfrog atrial cells. J Gen Physiol. 1988 Jan;91(1):1–27. doi: 10.1085/jgp.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirano Y., Abe S., Sawanobori T., Hiraoka M. External ATP-induced changes in [Ca2+]i and membrane currents in mammalian atrial myocytes. Am J Physiol. 1991 Apr;260(4 Pt 1):C673–C680. doi: 10.1152/ajpcell.1991.260.4.C673. [DOI] [PubMed] [Google Scholar]
- Hume R. I., Honig M. G. Excitatory action of ATP on embryonic chick muscle. J Neurosci. 1986 Mar;6(3):681–690. doi: 10.1523/JNEUROSCI.06-03-00681.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. G., Jr Accumulation of biological amines into chromaffin granules: a model for hormone and neurotransmitter transport. Physiol Rev. 1988 Jan;68(1):232–307. doi: 10.1152/physrev.1988.68.1.232. [DOI] [PubMed] [Google Scholar]
- Kolb H. A., Wakelam M. J. Transmitter-like action of ATP on patched membranes of cultured myoblasts and myotubes. Nature. 1983 Jun 16;303(5918):621–623. doi: 10.1038/303621a0. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Pidoplichko V. I. Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci Lett. 1983 Jan 31;35(1):41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Legssyer A., Poggioli J., Renard D., Vassort G. ATP and other adenine compounds increase mechanical activity and inositol trisphosphate production in rat heart. J Physiol. 1988 Jul;401:185–199. doi: 10.1113/jphysiol.1988.sp017157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Increased free calcium in endothelial cells under stimulation with adenine nucleotides. J Cell Physiol. 1986 Mar;126(3):414–420. doi: 10.1002/jcp.1041260312. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Lechleiter J. D., Cantley L. C., Talamo B. R. Extracellular ATP increases free cytosolic calcium in rat parotid acinar cells. Differences from phospholipase C-linked receptor agonists. Biochem J. 1988 Oct 1;255(1):291–300. [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Matsuki N. Adenosine triphosphate-activated inward current in isolated smooth muscle cells from rat vas deferens. Pflugers Arch. 1987 Aug;409(6):644–646. doi: 10.1007/BF00584668. [DOI] [PubMed] [Google Scholar]
- Niedergerke R., Page S. Two physiological agents that appear to facilitate calcium discharge from the sarcoplasmic reticulum in frog heart cells: adrenalin an ATP. Proc R Soc Lond B Biol Sci. 1981 Nov 13;213(1192):325–344. doi: 10.1098/rspb.1981.0069. [DOI] [PubMed] [Google Scholar]
- Phaneuf S., Berta P., Casanova J., Cavadore J. C. ATP stimulates inositol phosphates accumulation and calcium mobilization in a primary culture of rat aortic myocytes. Biochem Biophys Res Commun. 1987 Mar 13;143(2):454–460. doi: 10.1016/0006-291x(87)91375-1. [DOI] [PubMed] [Google Scholar]
- Powell T., Twist V. W. A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Commun. 1976 Sep 7;72(1):327–333. doi: 10.1016/0006-291x(76)90997-9. [DOI] [PubMed] [Google Scholar]
- Scamps F., Vassort G. Mechanism of extracellular ATP-induced depolarization in rat isolated ventricular cardiomyocytes. Pflugers Arch. 1990 Nov;417(3):309–316. doi: 10.1007/BF00370997. [DOI] [PubMed] [Google Scholar]
- Scubon-Mulieri B., Parsons R. L. Desensitization and recovery at the frog neuromuscular junction. J Gen Physiol. 1977 Apr;69(4):431–447. doi: 10.1085/jgp.69.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu S. S., Sharma V. K., Banerjee S. P. Measurement of cytosolic free calcium concentration in isolated rat ventricular myocytes with quin 2. Circ Res. 1984 Dec;55(6):830–834. doi: 10.1161/01.res.55.6.830. [DOI] [PubMed] [Google Scholar]
- Sheu S. S., Sharma V. K., Uglesity A. Na+-Ca2+ exchange contributes to increase of cytosolic Ca2+ concentration during depolarization in heart muscle. Am J Physiol. 1986 Apr;250(4 Pt 1):C651–C656. doi: 10.1152/ajpcell.1986.250.4.C651. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975 May;247(1):145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tawada Y., Furukawa K., Shigekawa M. ATP-induced calcium transient in cultured rat aortic smooth muscle cells. J Biochem. 1987 Dec;102(6):1499–1509. doi: 10.1093/oxfordjournals.jbchem.a122197. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford L. A., Cusack N. J., Hourani S. M. The structure-activity relationships of ectonucleotidases and of excitatory P2-purinoceptors: evidence that dephosphorylation of ATP analogues reduces pharmacological potency. Eur J Pharmacol. 1987 Sep 2;141(1):123–130. doi: 10.1016/0014-2999(87)90418-3. [DOI] [PubMed] [Google Scholar]