Abstract
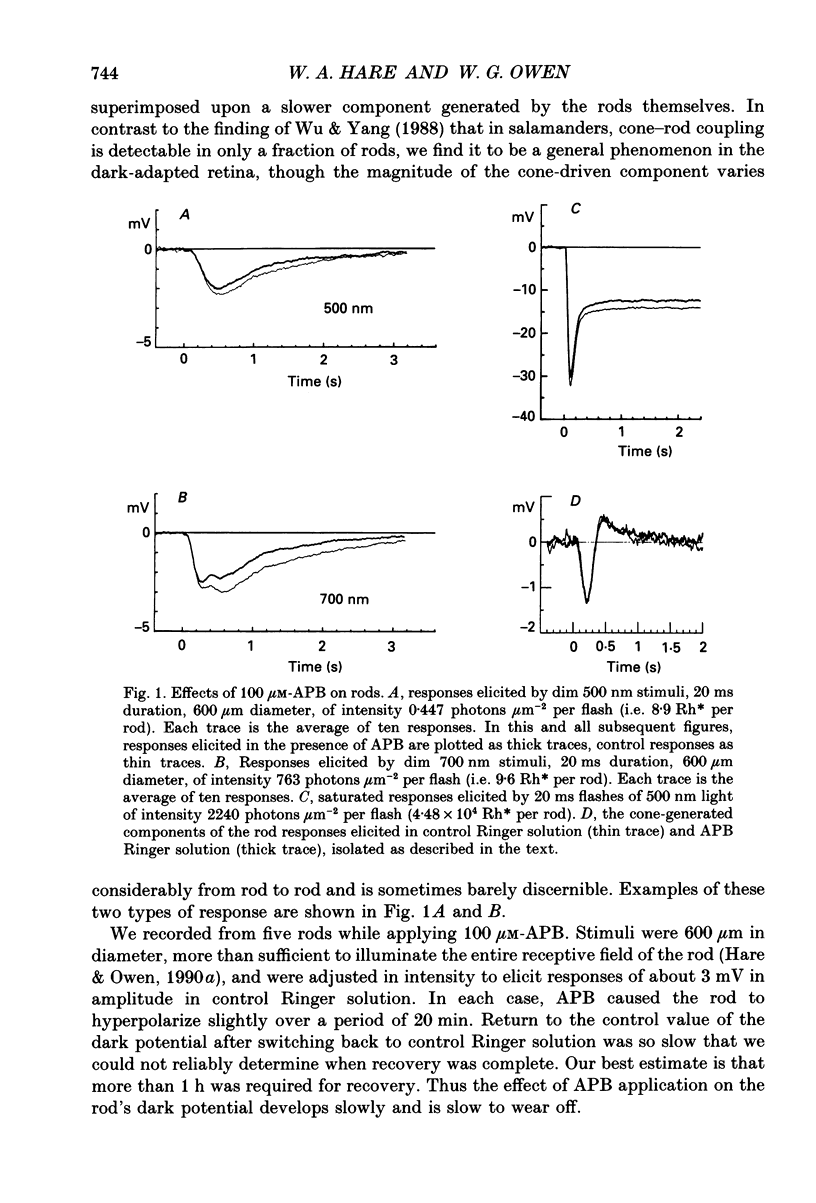
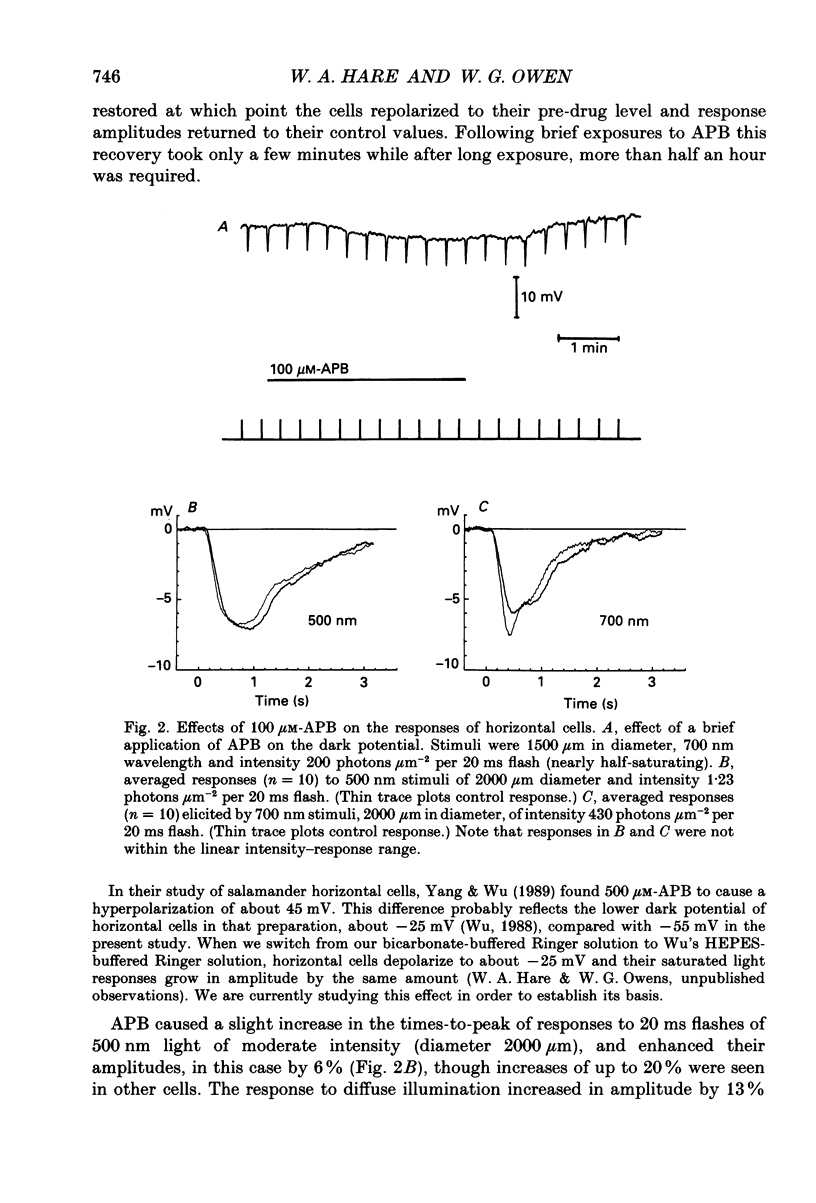
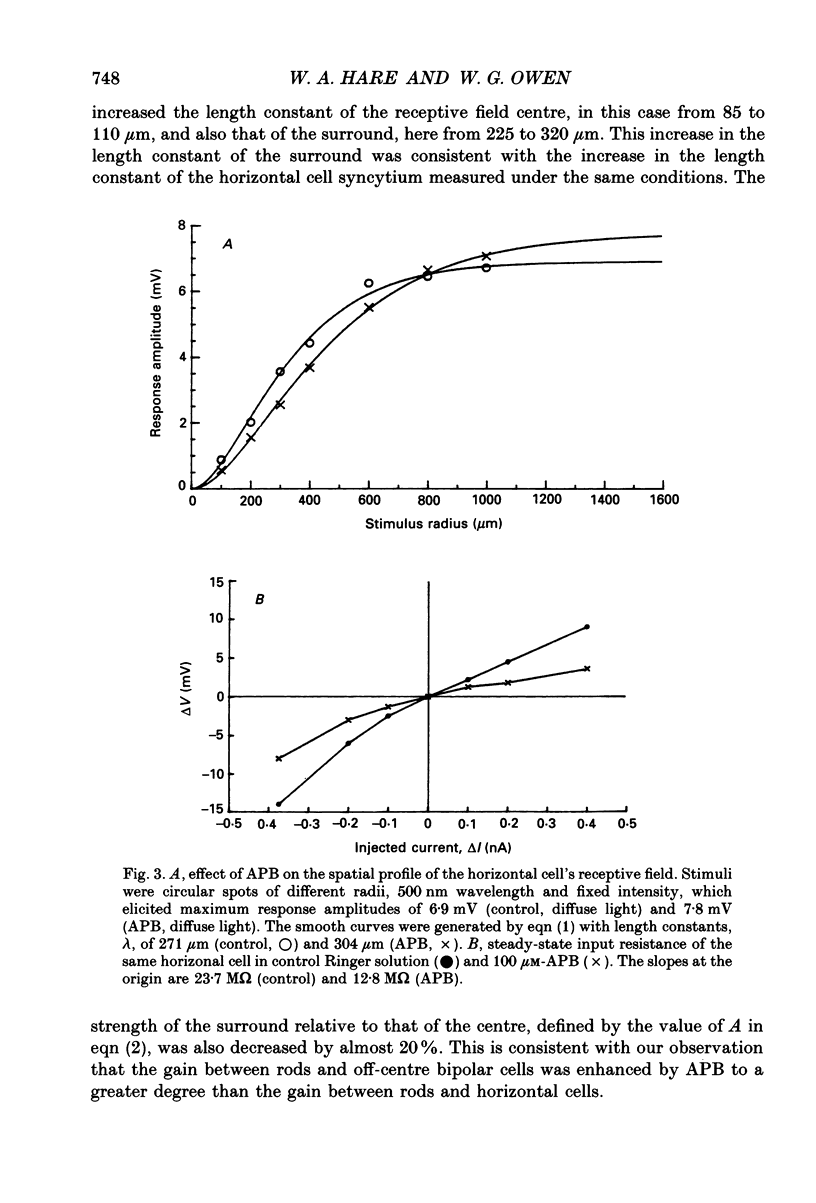
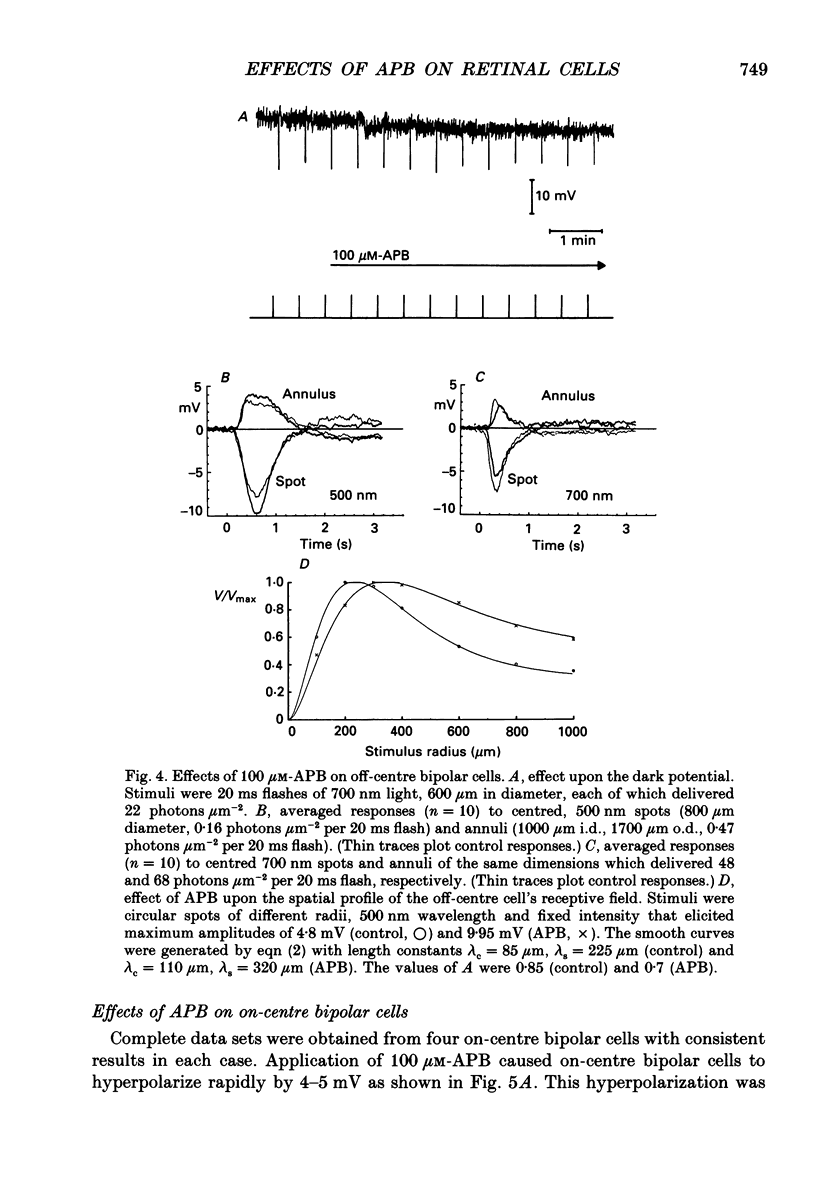
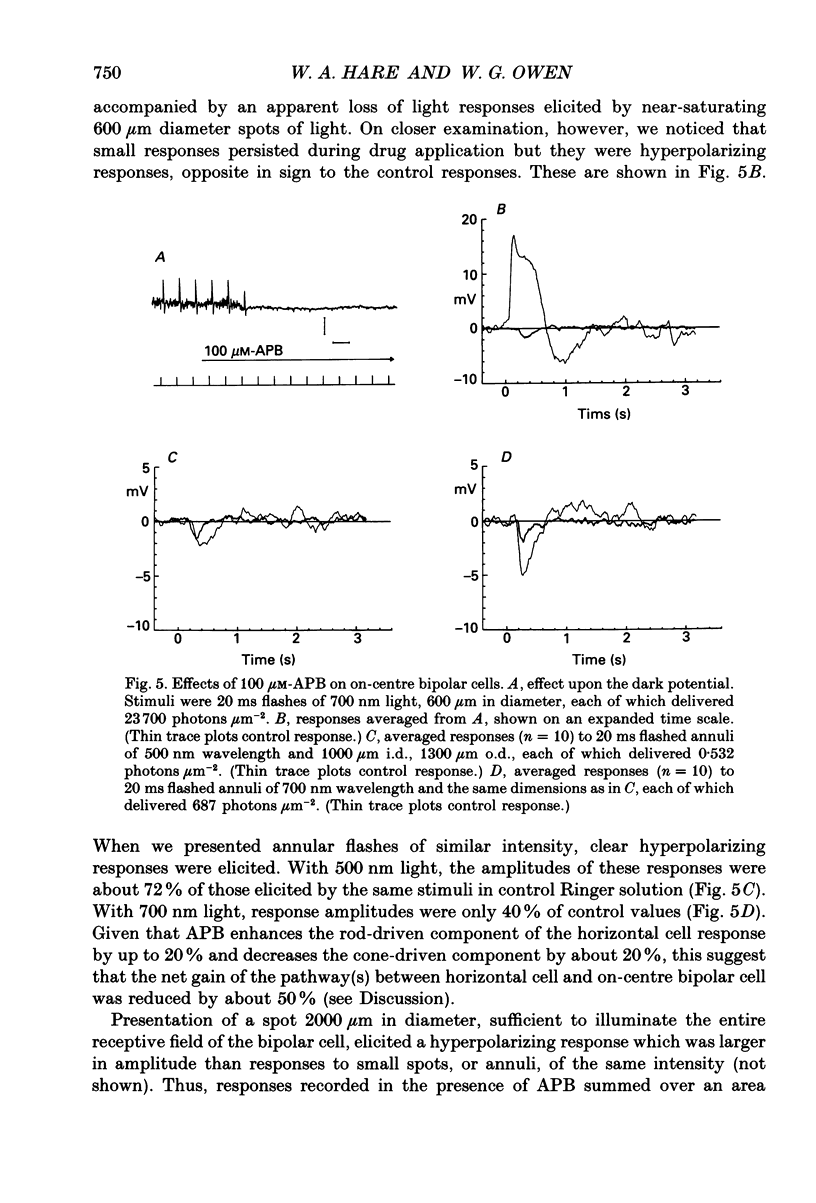
1. We studied the effects of 2-amino-4-phosphonobutyric acid (APB) on the response properties of rods, horizontal cells and bipolar cells in the isolated, perfused retina of the tiger salamander, Ambystoma tigrinum. A concentration of 100 microM was found to be sufficient to elicit maximal effects. 2. Rods hyperpolarized slightly upon exposure to 100 microM-APB and their response amplitudes were slightly reduced. The amplitude of the cone-generated component of the rod's response to 700 nm light was not significantly affected by APB. 3. Horizontal cells hyperpolarized by 2-5 mV upon exposure to 100 microM-APB. The rod-driven component of the horizontal cell response increased in amplitude while the cone-driven component decreased in amplitude. APB thus causes an increase in voltage gain between rods and horizontal cells and a decrease in cone/horizontal cell gain. These findings can be explained in terms of an APB-induced reduction in transmitter release from the cones. 4. APB at a concentration of 100 microM caused an increase in the length constant of the horizontal cell syncytium. Our analysis shows this to be due primarily to a 50% reduction in the coupling impedance between the cells of the syncytium. 5. The effects of APB on off-centre bipolar cells were qualitatively similar to those on horizontal cells. APB increased the amplitudes of rod-driven responses and reduced those of cone-driven responses. The length constants, both of the receptive field centre and of the surround, were increased and the strength of the surround relative to the centre was reduced by about 20%. 6. APB abolished the depolarizing light responses of the receptive field centres of on-centre bipolar cells. A hyperpolarizing response remained whose spatial properties were similar to those of the receptive field surround. We believe this response to reflect a direct (feedforward) input to on-centre bipolar cells from horizontal cells.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Falk G. Responses of rod bipolar cells in the dark-adapted retina of the dogfish, Scyliorhinus canicula. J Physiol. 1980 Mar;300:115–150. doi: 10.1113/jphysiol.1980.sp013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Hare W. A., Owen W. G. Voltage gain of signal transfer from retinal rods to bipolar cells in the tiger salamander. J Physiol. 1987 Oct;391:125–140. doi: 10.1113/jphysiol.1987.sp016730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries S. H., Schwartz E. A. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989 Jul;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. J., McReynolds J. S. APB increases apparent coupling between horizontal cells in mudpuppy retina. Vision Res. 1989;29(5):541–544. doi: 10.1016/0042-6989(89)90040-0. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Piccolino M. Sustained feedback effects of L-horizontal cells on turtle cones. Proc R Soc Lond B Biol Sci. 1980 Jan 17;206(1165):465–480. doi: 10.1098/rspb.1980.0008. [DOI] [PubMed] [Google Scholar]
- Hare W. A., Lowe J. S., Owen G. Morphology of physiologically identified bipolar cells in the retina of the tiger salamander, Ambystoma tigrinum. J Comp Neurol. 1986 Oct 1;252(1):130–138. doi: 10.1002/cne.902520108. [DOI] [PubMed] [Google Scholar]
- Hare W. A., Owen W. G. Spatial organization of the bipolar cell's receptive field in the retina of the tiger salamander. J Physiol. 1990 Feb;421:223–245. doi: 10.1113/jphysiol.1990.sp017942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans M., Van Dijk B. W., Spekreijse H. Interaction between the soma and the axon terminal of horizontal cells in carp retina. Vision Res. 1990;30(7):1011–1016. doi: 10.1016/0042-6989(90)90110-7. [DOI] [PubMed] [Google Scholar]
- Knapp A. G., Dowling J. E. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. 1987 Jan 29-Feb 4Nature. 325(6103):437–439. doi: 10.1038/325437a0. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., Simon E. J. The relation between intercellular coupling and electrical noise in turtle photoreceptors. J Physiol. 1976 Dec;263(2):257–286. doi: 10.1113/jphysiol.1976.sp011631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Contacts between receptors and electrophysiologically identified neurones in the retina of the larval tiger salamander. J Physiol. 1978 Dec;285:531–542. doi: 10.1113/jphysiol.1978.sp012587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Synaptic action mediating cone responses to annular illumination in the retina of the larval tiger salamander. J Physiol. 1981 Jan;310:205–214. doi: 10.1113/jphysiol.1981.sp013544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M. Retinal horizontal cell gap junctional conductance is modulated by dopamine through a cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7319–7323. doi: 10.1073/pnas.84.20.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G., Lukasiewicz P., Werblin F. Amacrine cell interactions underlying the response to change in the tiger salamander retina. J Neurosci. 1989 Feb;9(2):726–735. doi: 10.1523/JNEUROSCI.09-02-00726.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor A. V., Maksimov V. V. Passivnye élektricheskie svoistva modeli ploskoi kletki. Biofizika. 1969 Mar-Apr;14(2):328–335. [PubMed] [Google Scholar]
- Nawy S., Copenhagen D. R. Intracellular cesium separates two glutamate conductances in retinal bipolar cells of goldfish. Vision Res. 1990;30(7):967–972. doi: 10.1016/0042-6989(90)90105-t. [DOI] [PubMed] [Google Scholar]
- Nawy S., Copenhagen D. R. Multiple classes of glutamate receptor on depolarizing bipolar cells in retina. Nature. 1987 Jan 1;325(6099):56–58. doi: 10.1038/325056a0. [DOI] [PubMed] [Google Scholar]
- Nawy S., Jahr C. E. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990 Jul 19;346(6281):269–271. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- Nawy S., Sie A., Copenhagen D. R. The glutamate analog 2-amino-4-phosphonobutyrate antagonizes synaptic transmission from cones to horizontal cells in the goldfish retina. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1726–1730. doi: 10.1073/pnas.86.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryan P. M. Properties of the depolarizing synaptic potential evoked by peripheral illumination in cones of the turtle retina. J Physiol. 1973 Nov;235(1):207–223. doi: 10.1113/jphysiol.1973.sp010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen W. G., Hare W. A. Signal transfer from photoreceptors to bipolar cells in the retina of the tiger salamander. Neurosci Res Suppl. 1989;10:S77–S87. doi: 10.1016/0921-8696(89)90011-x. [DOI] [PubMed] [Google Scholar]
- Owen W. G., Torre V. High-pass filtering of small signals by retinal rods. Ionic studies. Biophys J. 1983 Mar;41(3):325–339. doi: 10.1016/S0006-3495(83)84444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984 Oct;4(10):2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantis M., Everett K., Attwell D. A presynaptic action of glutamate at the cone output synapse. Nature. 1988 Mar 31;332(6163):451–453. doi: 10.1038/332451a0. [DOI] [PubMed] [Google Scholar]
- Shiells R. A., Falk G. Glutamate receptors of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proc Biol Sci. 1990 Nov 22;242(1304):91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- Shiells R. A., Falk G., Naghshineh S. Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature. 1981 Dec 10;294(5841):592–594. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981 Jan 9;211(4478):182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Tachibana M., Kaneko A. L-glutamate-induced depolarization in solitary photoreceptors: a process that may contribute to the interaction between photoreceptors in situ. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5315–5319. doi: 10.1073/pnas.85.14.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teranishi T., Negishi K., Kato S. Dopamine modulates S-potential amplitude and dye-coupling between external horizontal cells in carp retina. Nature. 1983 Jan 20;301(5897):243–246. doi: 10.1038/301243a0. [DOI] [PubMed] [Google Scholar]
- Teranishi T., Negishi K., Kato S. Regulatory effect of dopamine on spatial properties of horizontal cells in carp retina. J Neurosci. 1984 May;4(5):1271–1280. doi: 10.1523/JNEUROSCI.04-05-01271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre V., Owen W. G. High-pass filtering of small signals by the rod network in the retina of the toad, Bufo marinus. Biophys J. 1983 Mar;41(3):305–324. doi: 10.1016/S0006-3495(83)84443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P., Owen W. G., Woodworth M. Gap junctions among the perikarya, dendrites, and axon terminals of the luminosity-type horizontal cell of the turtle retina. J Comp Neurol. 1983 Jun 1;216(4):359–368. doi: 10.1002/cne.902160402. [DOI] [PubMed] [Google Scholar]
- Wu S. M. Synaptic transmission from rods to horizontal cells in dark-adapted tiger salamander retina. Vision Res. 1988;28(1):1–8. [PubMed] [Google Scholar]
- Wu S. M., Yang X. L. Electrical coupling between rods and cones in the tiger salamander retina. Proc Natl Acad Sci U S A. 1988 Jan;85(1):275–278. doi: 10.1073/pnas.85.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. L., Wu S. M. Effects of CNQX, APB, PDA, and kynurenate on horizontal cells of the tiger salamander retina. Vis Neurosci. 1989 Sep;3(3):207–212. doi: 10.1017/s0952523800009962. [DOI] [PubMed] [Google Scholar]


