Abstract
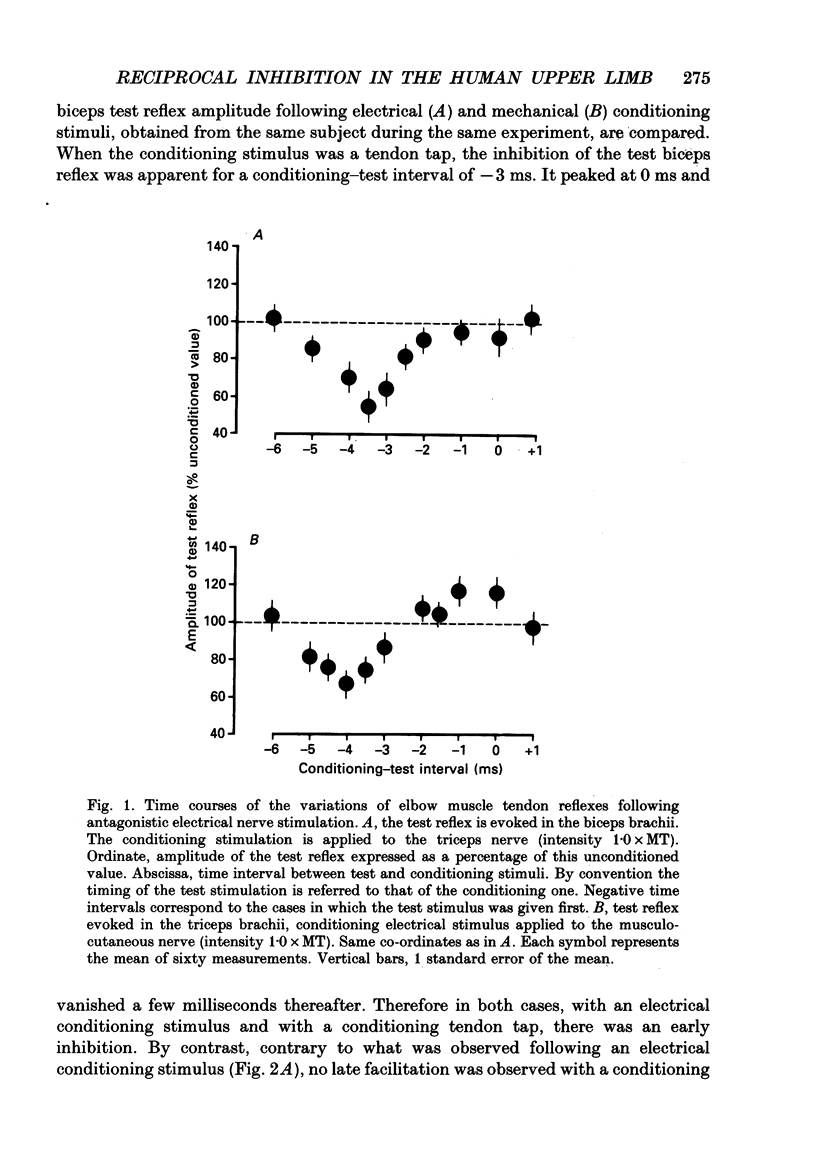
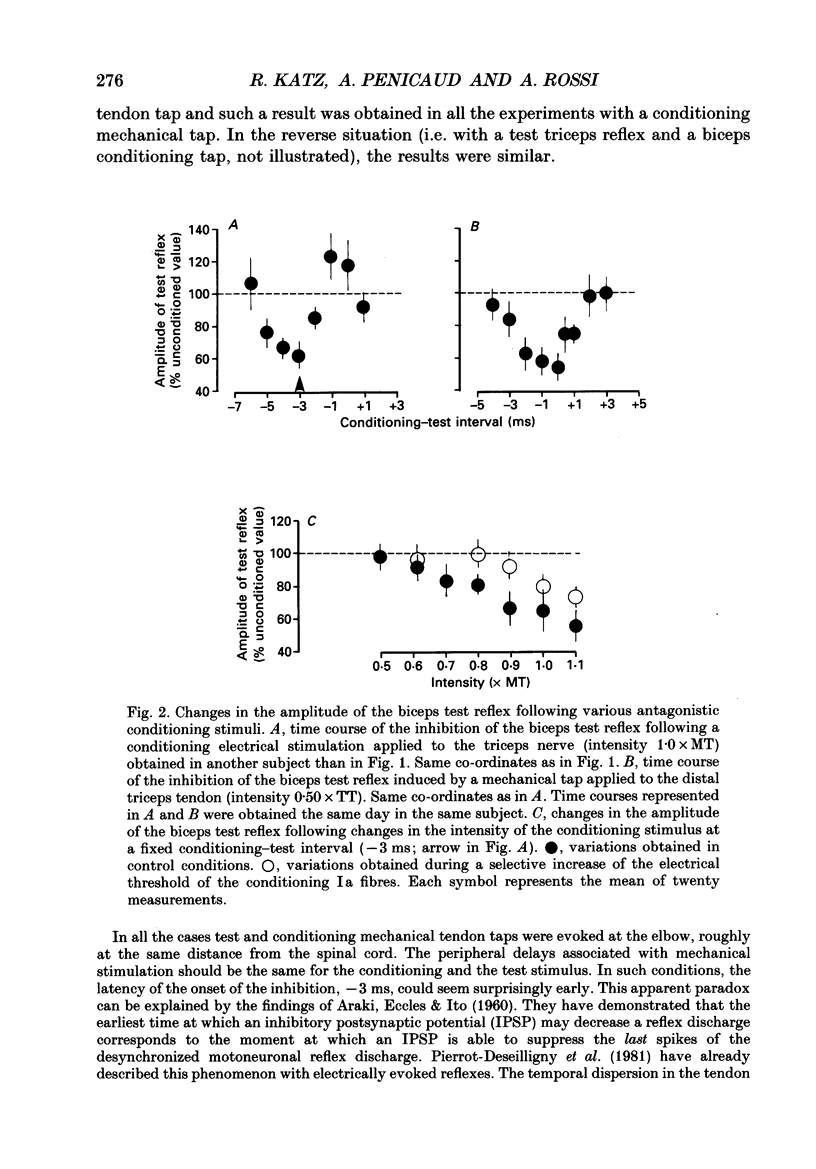
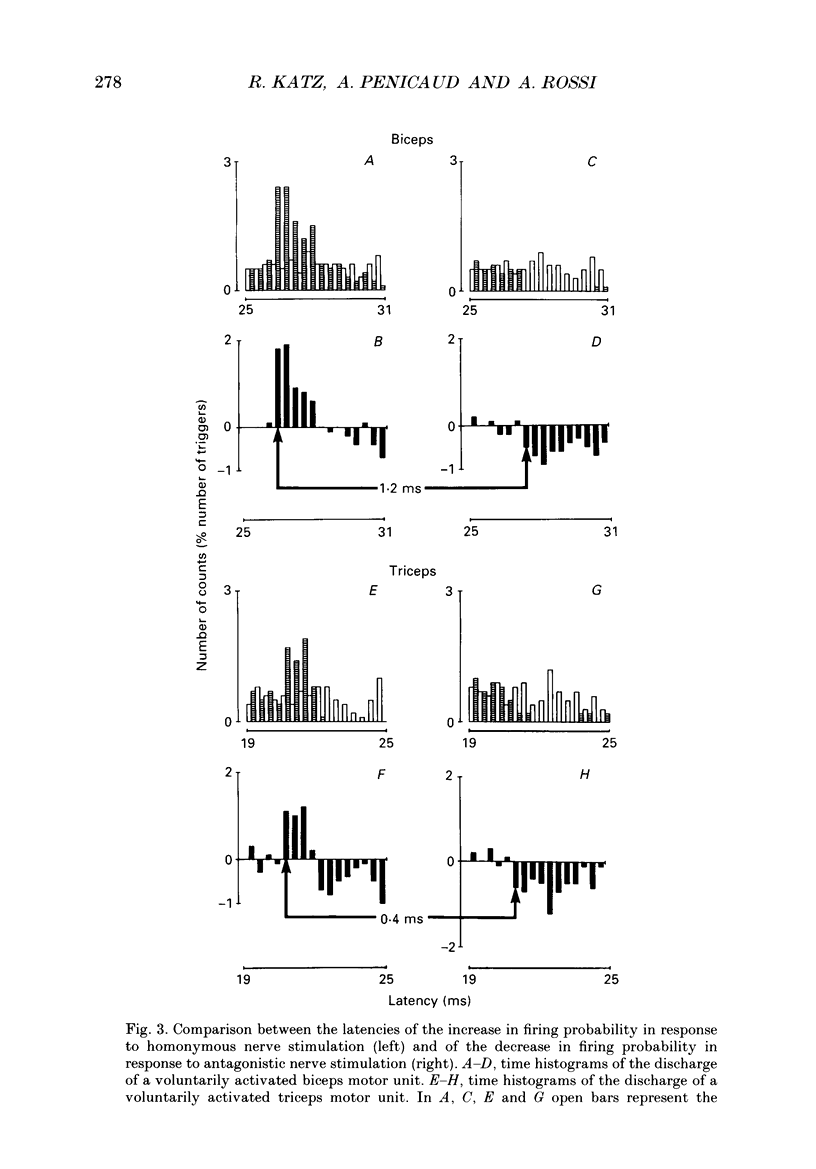
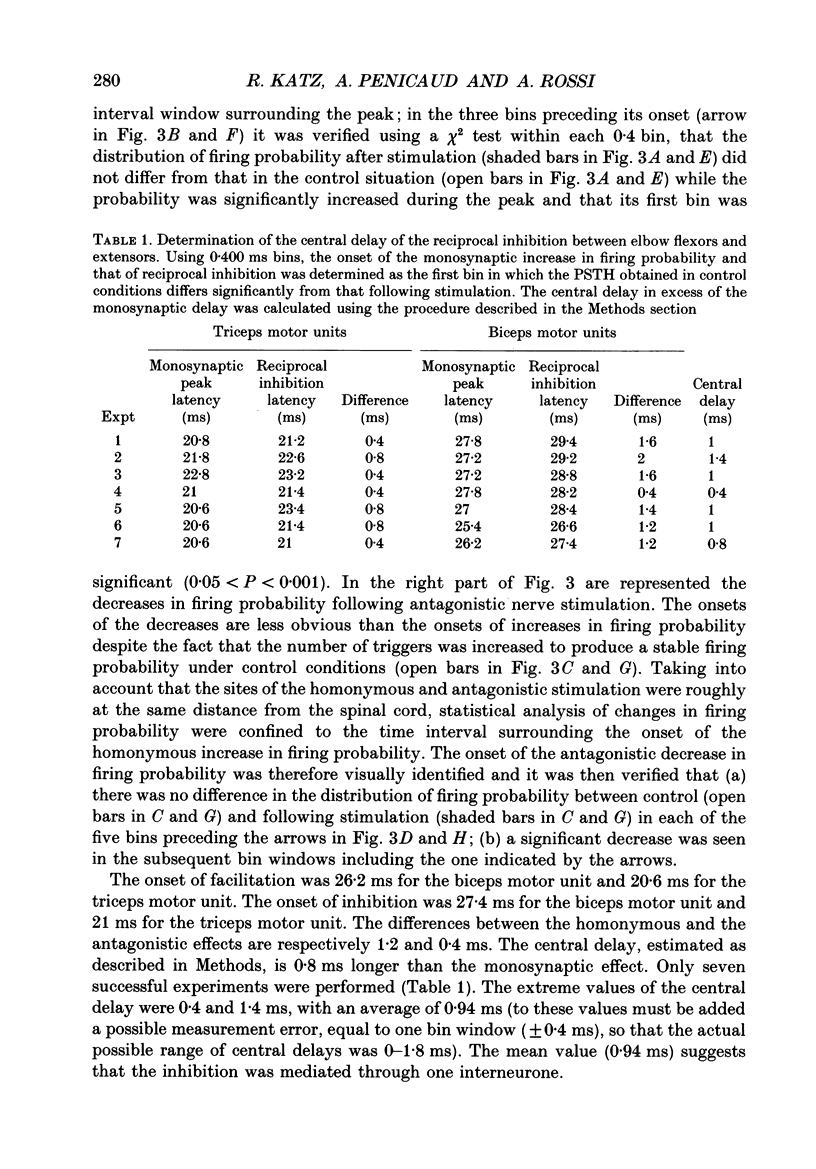
1. Reciprocal inhibition between elbow flexor and extensor muscles (biceps and triceps brachii) has been investigated in nine healthy subjects. Two techniques were used to assess changes in motoneurone excitability after stimulation of antagonist muscle afferents: (1) monosynaptic reflexes elicited by a mechanical stimulation of the distal muscle tendon (tendon tap); (2) post-stimulus time histograms (PSTH) of voluntarily activated motor units. 2. Electrical stimulation of the antagonist muscle nerve produced a short-latency and short-lasting inhibition of the flexor and extensor motoneurones. The amount of this inhibition was found to be similar in both motor nuclei. 3. The inhibition could be evoked with conditioning electrical stimuli as low as 0.7 x motor threshold (MT) or by very weak tendon taps applied to the antagonist tendon. In the former case the threshold of this inhibition was found to be consistently increased after raising the threshold of Ia afferent fibres by a long-lasting muscle vibration. Since a contribution from cutaneous afferent fibres was ruled out, it is concluded that this inhibition was Ia in origin. 4. Post-stimulus time histograms of voluntarily activated triceps and biceps motor units were made following electrical stimulation of homonymous and antagonist muscle afferents. This enabled an estimate of the central synaptic delay of the inhibitory process. An average central delay of 0.94 ms in excess of that of monosynaptic facilitation was found, thus suggesting that the inhibitory process could be mediated by only one interneurone. 5. A conditioning reflex discharge elicited in the antagonist muscle by a tendon tap depressed or suppressed this inhibition. This depression was maximal when the reflex discharge was elicited 10-20 ms before the conditioning stimulus for the inhibition and never lasted more than 30 ms. It is argued that the only mechanism compatible with such a depression is the inhibitory activity of Renshaw cells acting on the pathway mediating reciprocal inhibition. 6. We conclude that group Ia afferent fibres from elbow extensor and flexor muscles project monosynaptically onto Ia inhibitory interneurones to mediate disynaptic reciprocal inhibition of antagonist motoneurones.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., EOCLES J. C., ITO M. Correlation of the inhibitory post-synaptic potential of motoneurones with the latency and time course of inhibition of monosynaptic reflexes. J Physiol. 1960 Dec;154:354–377. doi: 10.1113/jphysiol.1960.sp006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F., Campadelli P., Cavallari P. Inhibition from radial group I afferents of H-reflex in wrist flexors. Electromyogr Clin Neurophysiol. 1983 Mar-Apr;23(3):187–193. [PubMed] [Google Scholar]
- Bratzlavsky M., Isch F., Eecken H vander Study of evoked responses in proximal muscles of the superior and inferior limbs. Eur Neurol. 1971;5(3):171–185. doi: 10.1159/000114069. [DOI] [PubMed] [Google Scholar]
- Burke D., Gandevia S. C., McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol. 1984 Sep;52(3):435–448. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Bussel B., Pierrot-Deseilligny E. Inhibition of human motoneurons, probably of Renshaw origin, elicited by an orthodromic motor discharge. J Physiol. 1977 Jul;269(2):319–339. doi: 10.1113/jphysiol.1977.sp011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari P., Katz R. Pattern of projections of group I afferents from forearm muscles to motoneurones supplying biceps and triceps muscles in man. Exp Brain Res. 1989;78(3):465–478. doi: 10.1007/BF00230235. [DOI] [PubMed] [Google Scholar]
- Crone C., Hultborn H., Jespersen B., Nielsen J. Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol. 1987 Aug;389:163–185. doi: 10.1113/jphysiol.1987.sp016652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C., Hultborn H., Mazières L., Morin C., Nielsen J., Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res. 1990;81(1):35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Day B. L., Marsden C. D., Obeso J. A., Rothwell J. C. Reciprocal inhibition between the muscles of the human forearm. J Physiol. 1984 Apr;349:519–534. doi: 10.1113/jphysiol.1984.sp015171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E., Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983 Aug;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E., Jankowska E., Johannisson T., Lipski J. Autogenetic inhibition of motoneurones by impulses in group Ia muscle spindle afferents. J Physiol. 1979 Aug;293:173–195. doi: 10.1113/jphysiol.1979.sp012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier E., Meunier S., Pierrot-Deseilligny E., Shindo M. Evidence for interneuronally mediated Ia excitatory effects to human quadriceps motoneurones. J Physiol. 1986 Aug;377:143–169. doi: 10.1113/jphysiol.1986.sp016179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahne M., Illert M., Wietelmann D. Recurrent inhibition in the cat distal forelimb. Brain Res. 1988 Jul 19;456(1):188–192. doi: 10.1016/0006-8993(88)90362-9. [DOI] [PubMed] [Google Scholar]
- Heckman C. J., Condon S. M., Hutton R. S., Enoka R. M. Can Ib axons be selectively activated by electrical stimuli in human subjects? Exp Neurol. 1984 Dec;86(3):576–582. doi: 10.1016/0014-4886(84)90090-6. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol. 1971 Jul;215(3):591–612. doi: 10.1113/jphysiol.1971.sp009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Pierrot-Deseilligny E., Wigström H. Recurrent inhibition and afterhyperpolarization following motoneuronal discharge in the cat. J Physiol. 1979 Dec;297(0):253–266. doi: 10.1113/jphysiol.1979.sp013038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles J. F. Reciprocal inhibition during agonist and antagonist contraction. Exp Brain Res. 1986;62(1):212–214. doi: 10.1007/BF00237419. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Padel Y., Tanaka R. Disynaptic inhibition of spinal motoneurones from the motor cortex in the monkey. J Physiol. 1976 Jun;258(2):467–487. doi: 10.1113/jphysiol.1976.sp011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R., Morin C., Pierrot-Deseilligny E., Hibino R. Conditioning of H reflex by a preceding subthreshold tendon reflex stimulus. J Neurol Neurosurg Psychiatry. 1977 Jun;40(6):575–580. doi: 10.1136/jnnp.40.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG A., WINSBURY G. Selective adequate activation of large afferents from muscle spindles and Golgi tendon organs. Acta Physiol Scand. 1960 Jul 15;49:155–164. doi: 10.1111/j.1748-1716.1960.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Meunier S., Penicaud A., Pierrot-Deseilligny E., Rossi A. Monosynaptic Ia excitation and recurrent inhibition from quadriceps to ankle flexors and extensors in man. J Physiol. 1990 Apr;423:661–675. doi: 10.1113/jphysiol.1990.sp018046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y., Tanaka R., Yanagisawa N. Reciprocal group I inhibition on triceps surae motoneurons in man. J Neurophysiol. 1971 Nov;34(6):1010–1017. doi: 10.1152/jn.1971.34.6.1010. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Morin C., Bergego C., Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res. 1981;42(3-4):337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Rossi A., Mazzocchio R., Scarpini C. Changes in Ia reciprocal inhibition from the peroneal nerve to the soleus alpha-motoneurons with different static body positions in man. Neurosci Lett. 1988 Feb 3;84(3):283–286. doi: 10.1016/0304-3940(88)90521-6. [DOI] [PubMed] [Google Scholar]
- Stephens J. A., Usherwood T. P., Garnett R. Technique for studying synaptic connections of single motoneurones in man. Nature. 1976 Sep 23;263(5575):343–344. doi: 10.1038/263343a0. [DOI] [PubMed] [Google Scholar]
- Tanaka R. Reciprocal Ia inhibition during voluntary movements in man. Exp Brain Res. 1974;21(5):529–540. doi: 10.1007/BF00237171. [DOI] [PubMed] [Google Scholar]


