Abstract
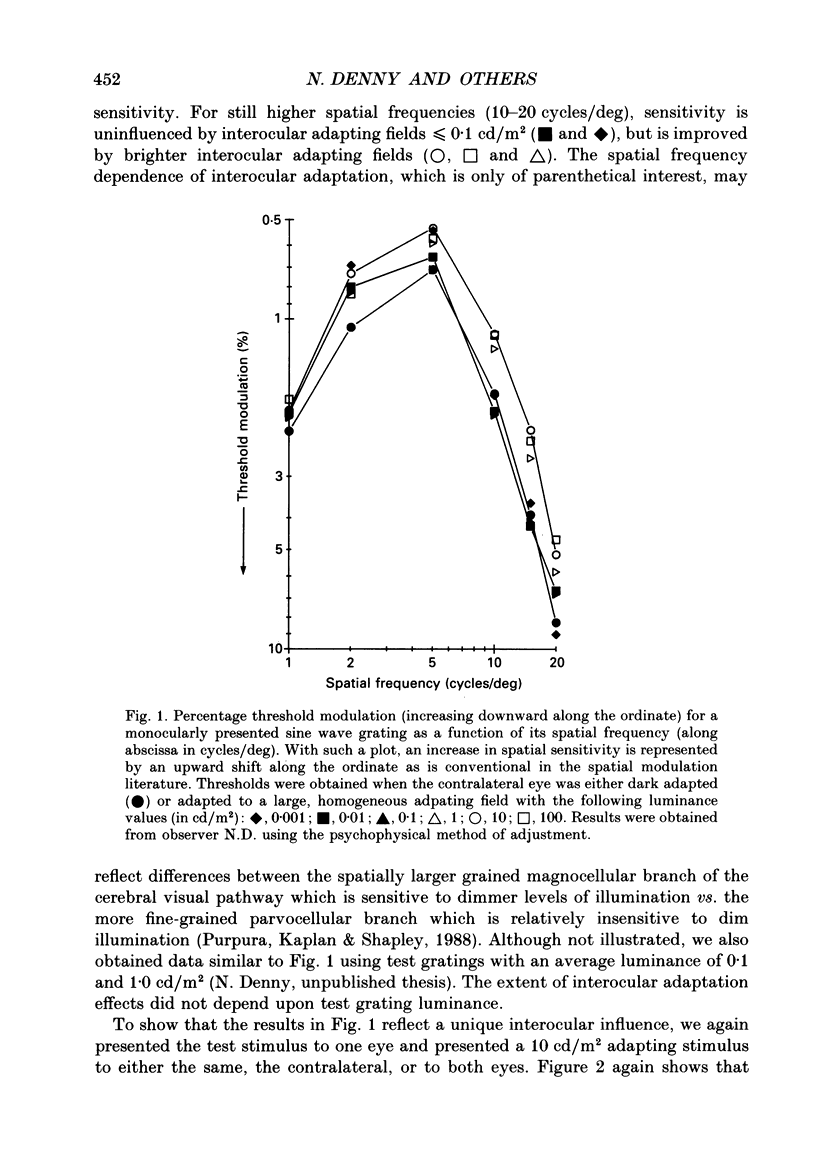
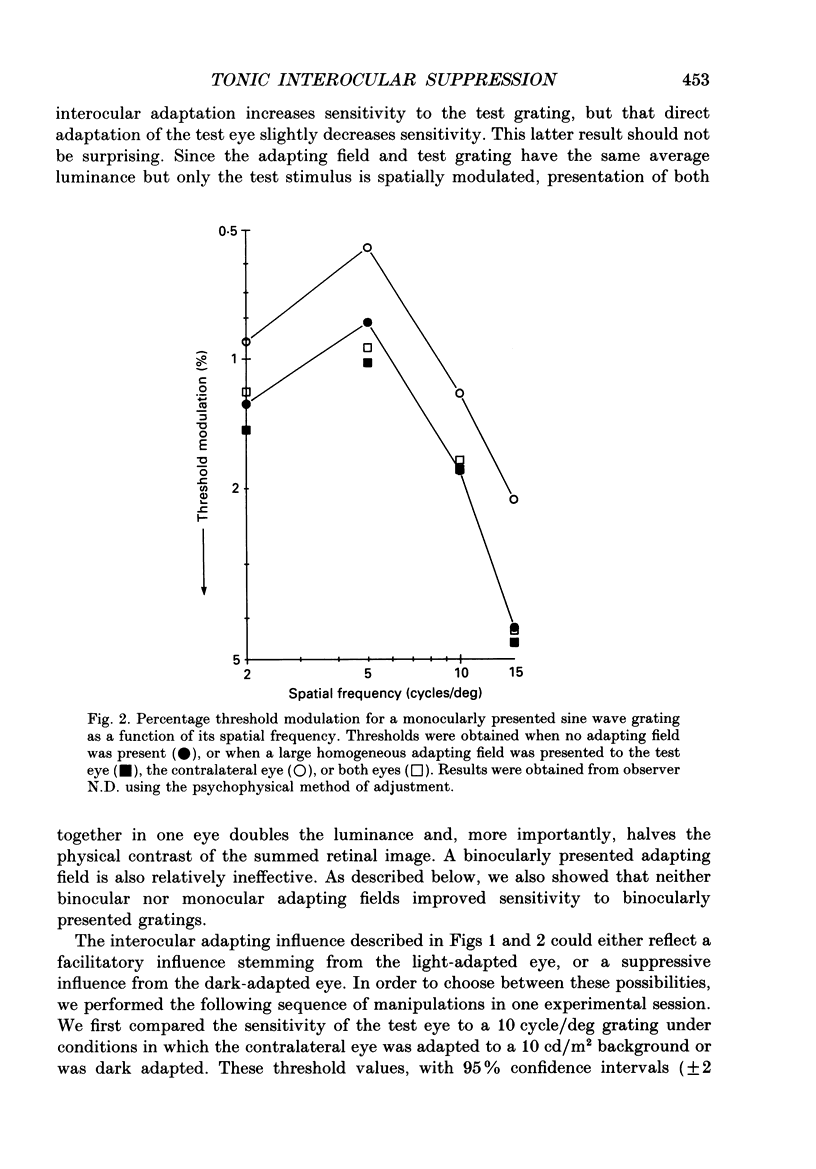
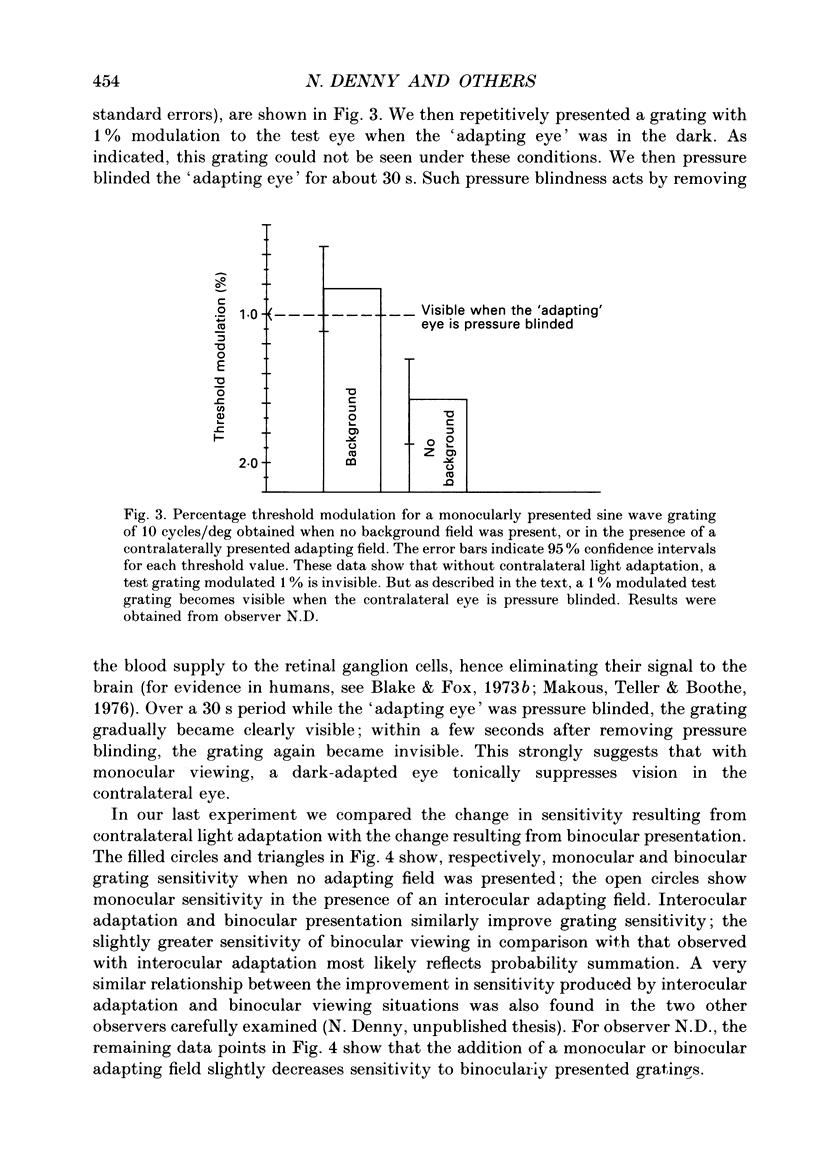
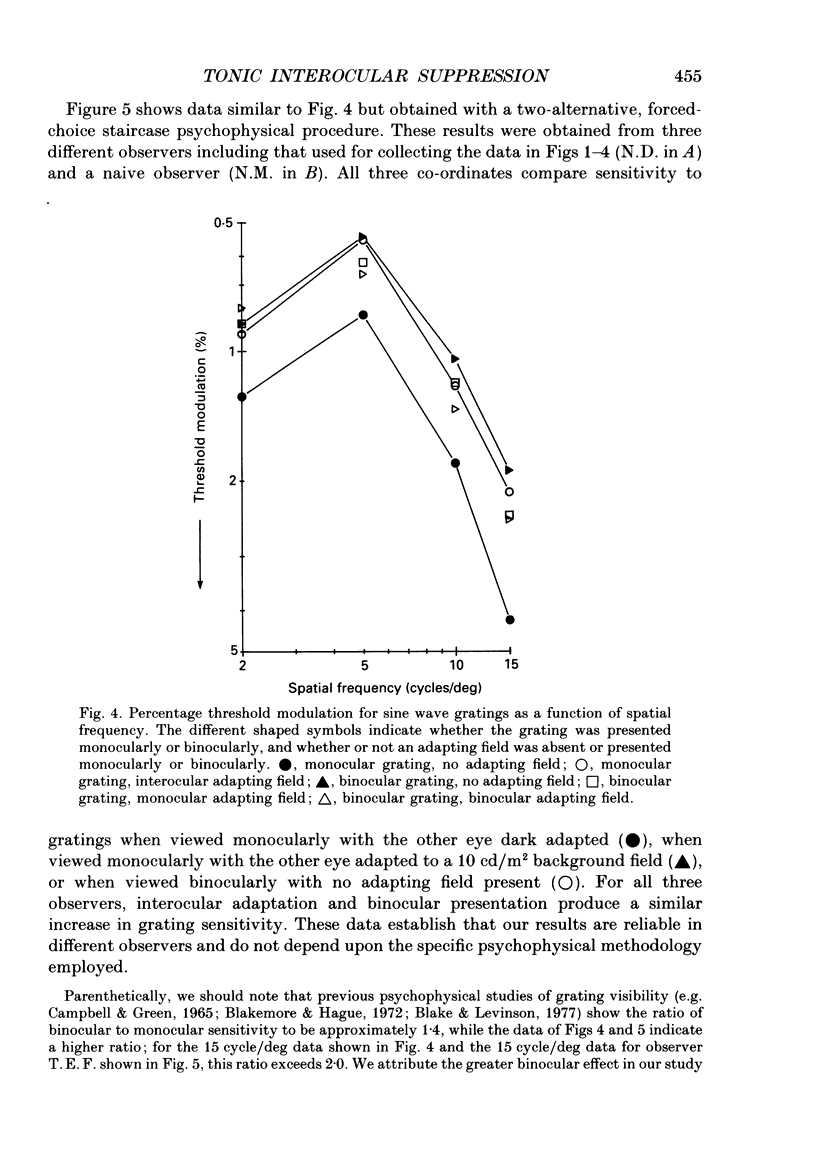
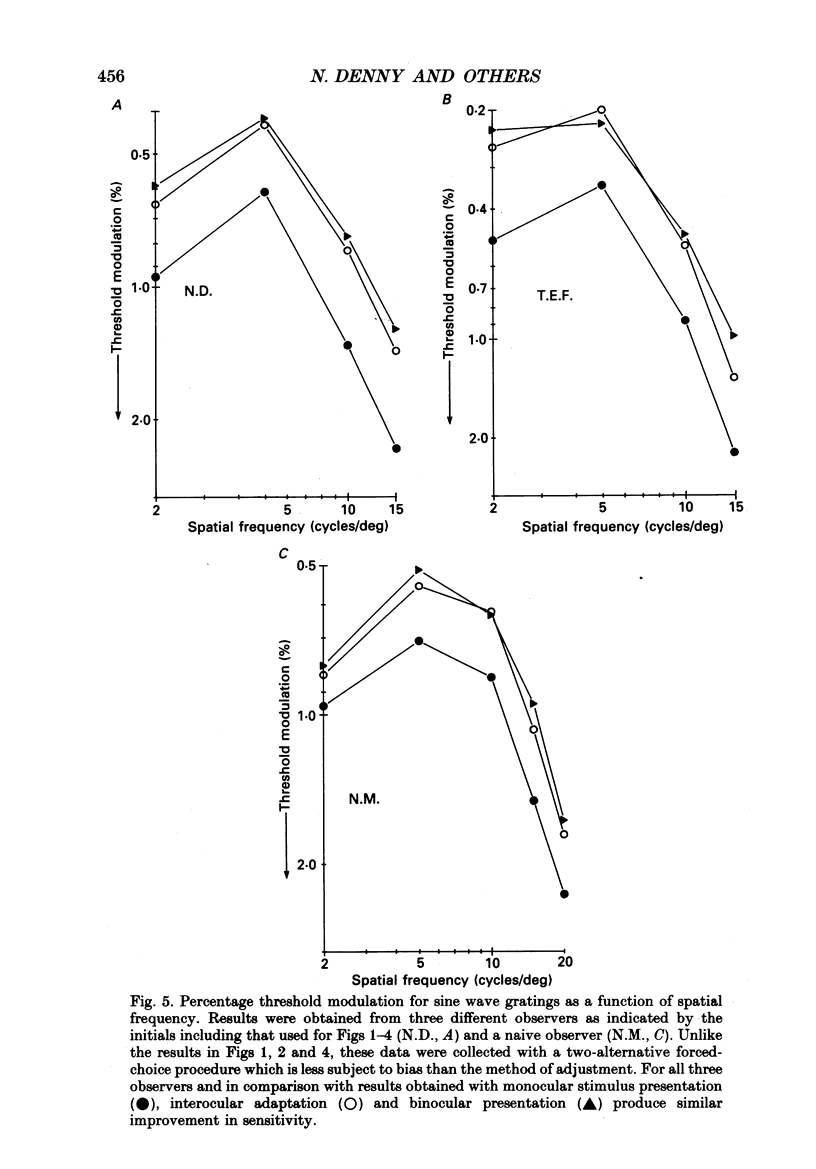
1. Spatial sensitivity of human foveal vision was examined using sinusoidally modulated gratings. Our primary concern was the influence of interocular light adaptation upon monocular visibility. 2. Interocular adapting influences depend upon spatial frequency and adapting luminance. Interocular adaptation has a negligible influence upon the sensitivity to 1 cycle/deg gratings. Any visible interocular adapting field improves the sensitivity to intermediate spatial frequencies (2-5 cycles/deg). 3. Brighter interocular backgrounds (greater than 0.1 cd/m2) improve sensitivity to higher spatial frequencies (10-20 cycles/deg). 4. The interocular adapting influences summarized in (2) and (3) above cannot be duplicated by monocular or binocular adaptation. Similarly, monocular or binocular adaptation have negligible influences upon binocular visibility. 5. The interocular adapting effect summarized in (3) above can be duplicated by pressure blinding the contralateral eye. We conclude that monocular spatial sensitivity is subject to a tonic interocular suppression (TIS) from the dark-adapted eye. 6. The spatial sensitivity resulting from binocular viewing is nearly identical to that observed by combining monocular viewing with interocular light adaptation. We suggest that the improvement in sensitivity resulting from two-eyed viewing may be attributable to the removal of TIS instead of to binocular physiological summation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach E., Peachey N. S. Interocular transfer and dark adaptation to long-wave test lights. Vision Res. 1984;24(9):1043–1048. doi: 10.1016/0042-6989(84)90081-6. [DOI] [PubMed] [Google Scholar]
- Belgum J. H., Dvorak D. R., McReynolds J. S., Miyachi E. Push-pull effect of surround illumination on excitatory and inhibitory inputs to mudpuppy retinal ganglion cells. J Physiol. 1987 Jul;388:233–243. doi: 10.1113/jphysiol.1987.sp016612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R., Levinson E. Spatial properties of binocular neurones in the human visual system. Exp Brain Res. 1977 Feb 16;27(2):221–232. doi: 10.1007/BF00237700. [DOI] [PubMed] [Google Scholar]
- Blake R., Sloane M., Fox R. Further developments in binocular summation. Percept Psychophys. 1981 Sep;30(3):266–276. doi: 10.3758/bf03214282. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Hague B. Evidence for disparity detecting neurones in the human visual system. J Physiol. 1972 Sep;225(2):437–455. doi: 10.1113/jphysiol.1972.sp009948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Green D. G. Monocular versus binocular visual acuity. Nature. 1965 Oct 9;208(5006):191–192. doi: 10.1038/208191a0. [DOI] [PubMed] [Google Scholar]
- Fox R., Blake R., Bourne J. R. Visual evoked cortical potentials during pressure-blinding. Vision Res. 1973 Feb;13(2):501–503. doi: 10.1016/0042-6989(73)90132-6. [DOI] [PubMed] [Google Scholar]
- Frumkes T. E., Eysteinsson T. The cellular basis for suppressive rod-cone interaction. Vis Neurosci. 1988;1(3):263–273. doi: 10.1017/s0952523800001929. [DOI] [PubMed] [Google Scholar]
- Frumkes T. E., Miller R. F., Slaughter M., Dacheux R. F. Physiological and pharmacological basis of GABA and glycine action on neurons of mudpuppy retina. III. Amacrine-mediated inhibitory influences on ganglion cell receptive-field organization: a model. J Neurophysiol. 1981 Apr;45(4):783–804. doi: 10.1152/jn.1981.45.4.783. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz K. E., Spear P. D. Effects of visual deprivation and alterations in binocular competition on responses of striate cortex neurons in the cat. J Comp Neurol. 1976 Nov 15;170(2):141–151. doi: 10.1002/cne.901700202. [DOI] [PubMed] [Google Scholar]
- Lansford T. G., Baker H. D. Dark adaptation: an interocular light-adaptation effect. Science. 1969 Jun 13;164(3885):1307–1309. doi: 10.1126/science.164.3885.1307. [DOI] [PubMed] [Google Scholar]
- Makous W., Teller D., Boothe R. Binocular interaction in the dark. Vision Res. 1976;16(5):473–476. doi: 10.1016/0042-6989(76)90024-9. [DOI] [PubMed] [Google Scholar]
- PERRIN F. H. A study in binocular flicker. J Opt Soc Am. 1954 Jan;44(1):60–69. doi: 10.1364/josa.44.000060. [DOI] [PubMed] [Google Scholar]
- Purpura K., Kaplan E., Shapley R. M. Background light and the contrast gain of primate P and M retinal ganglion cells. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4534–4537. doi: 10.1073/pnas.85.12.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves A., Peachey N. S., Auerbach E. Interocular sensitization to a rod-detected test. Vision Res. 1986;26(7):1119–1127. doi: 10.1016/0042-6989(86)90046-5. [DOI] [PubMed] [Google Scholar]
- Schmeisser E. T., Dawson W. W. Dichoptic interaction of harmonically related spatial and temporal frequencies. Doc Ophthalmol. 1982 May 14;53(1):37–50. doi: 10.1007/BF00143808. [DOI] [PubMed] [Google Scholar]
- Smith D. C., Spear P. D., Kratz K. E. Role of visual experience in postcritical-period reversal of effects of monocular deprivation in cat striate cortex. J Comp Neurol. 1978 Mar 15;178(2):313–328. doi: 10.1002/cne.901780207. [DOI] [PubMed] [Google Scholar]
- Trick G. L., Dawson W. W., Compton J. R. Interocular luminance differences and the binocular pattern-reversal visual-evoked response. Invest Ophthalmol Vis Sci. 1982 Mar;22(3):394–401. [PubMed] [Google Scholar]
- Tyler C. W., Apkarian P., Nakayama K. Multiple spatial-frequency tuning of electrical responses from human visual cortex. Exp Brain Res. 1978 Nov 15;33(3-4):535–550. doi: 10.1007/BF00235573. [DOI] [PubMed] [Google Scholar]
- van Sluyters R. C. Reversal of the physiological effects of brief periods of monocular deprivation in the kitten. J Physiol. 1978 Nov;284:1–17. doi: 10.1113/jphysiol.1978.sp012524. [DOI] [PMC free article] [PubMed] [Google Scholar]


