Abstract
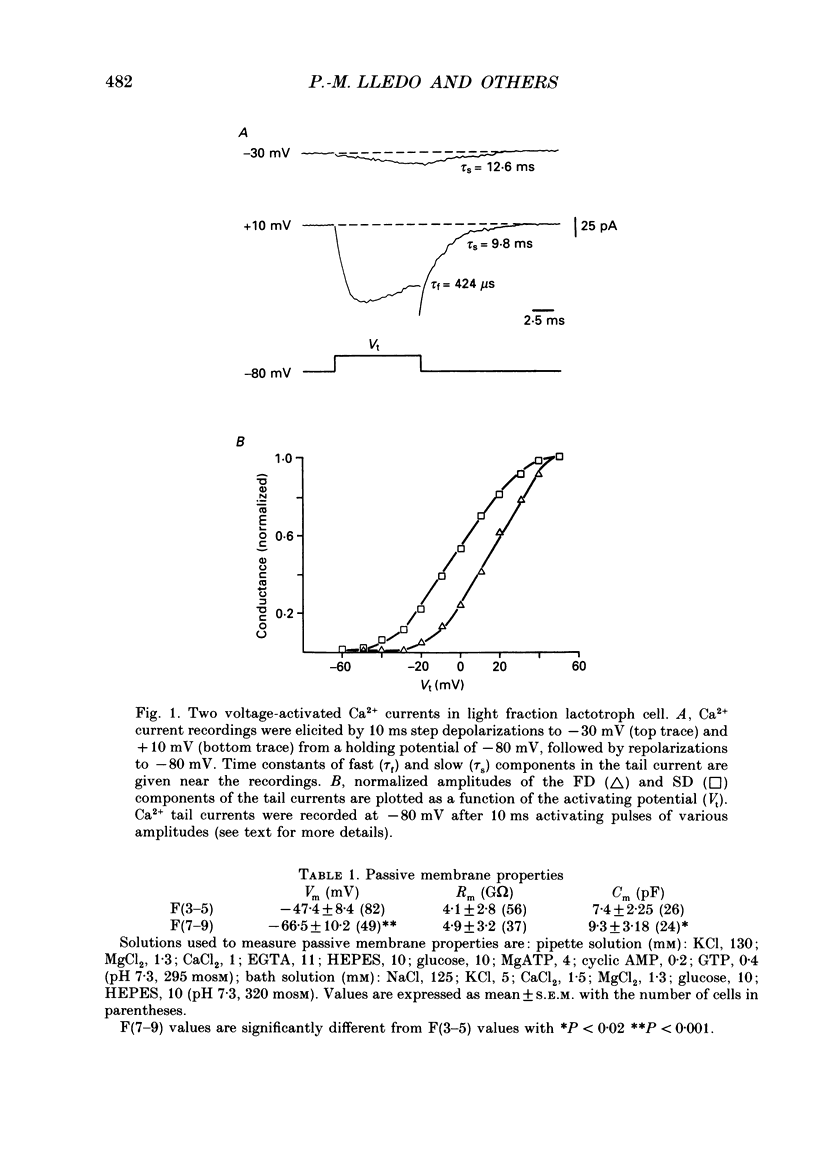
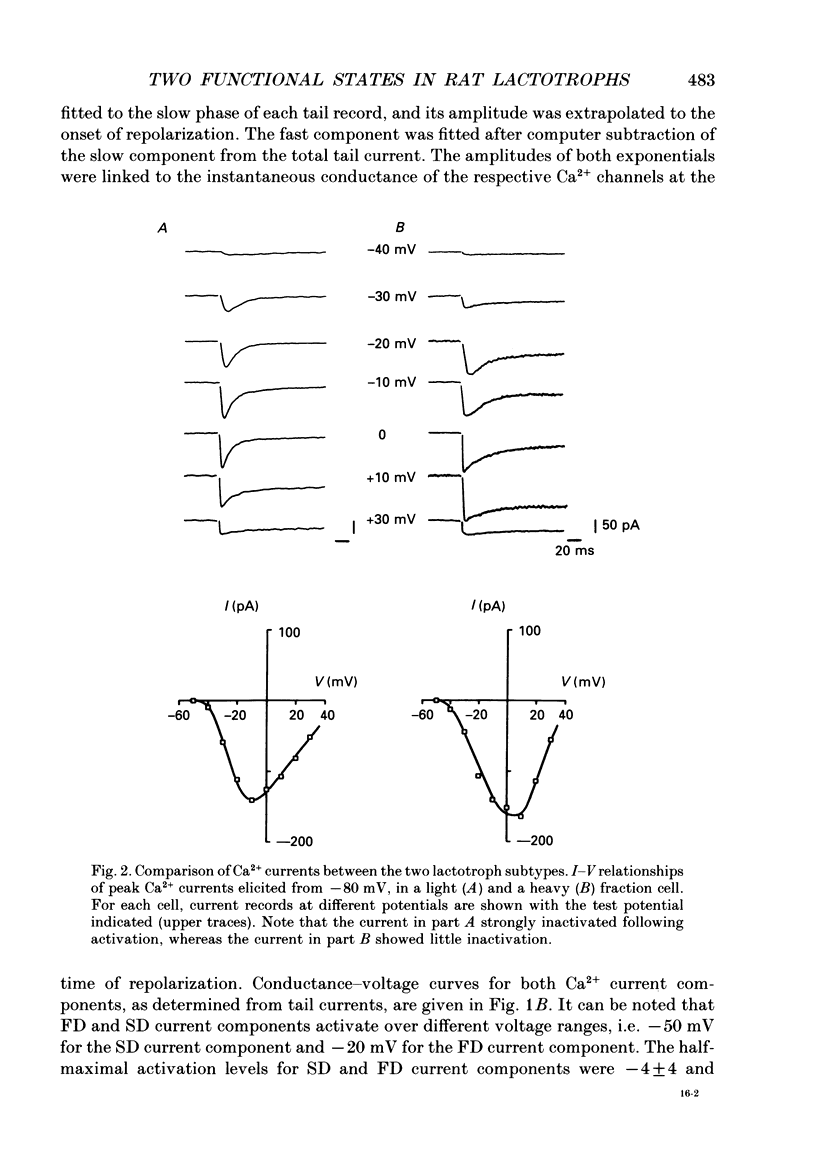
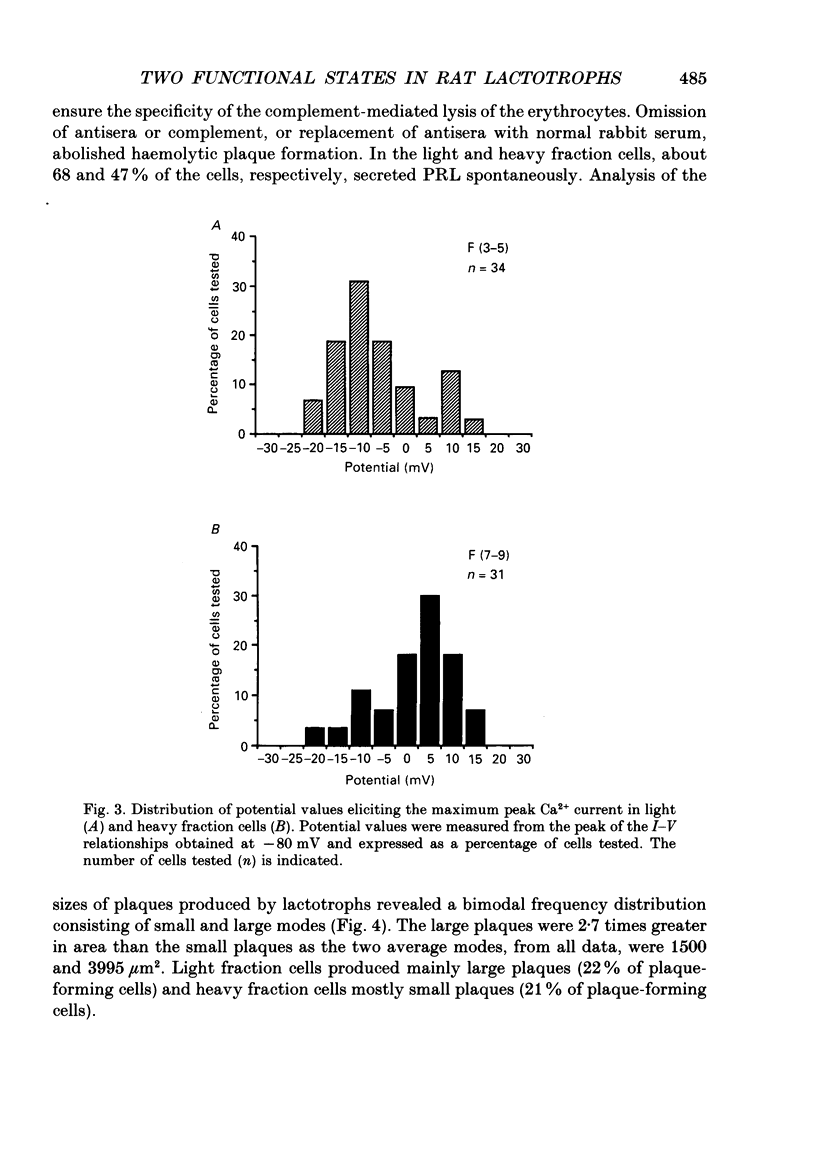
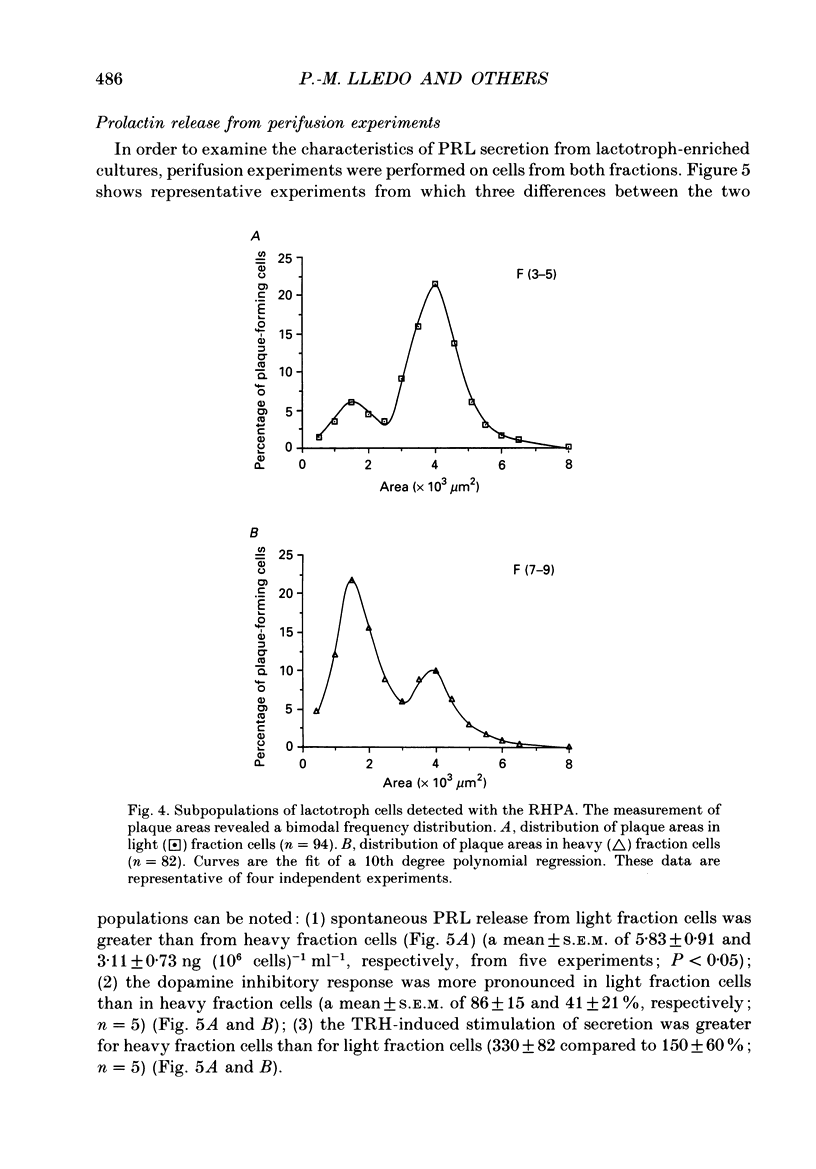
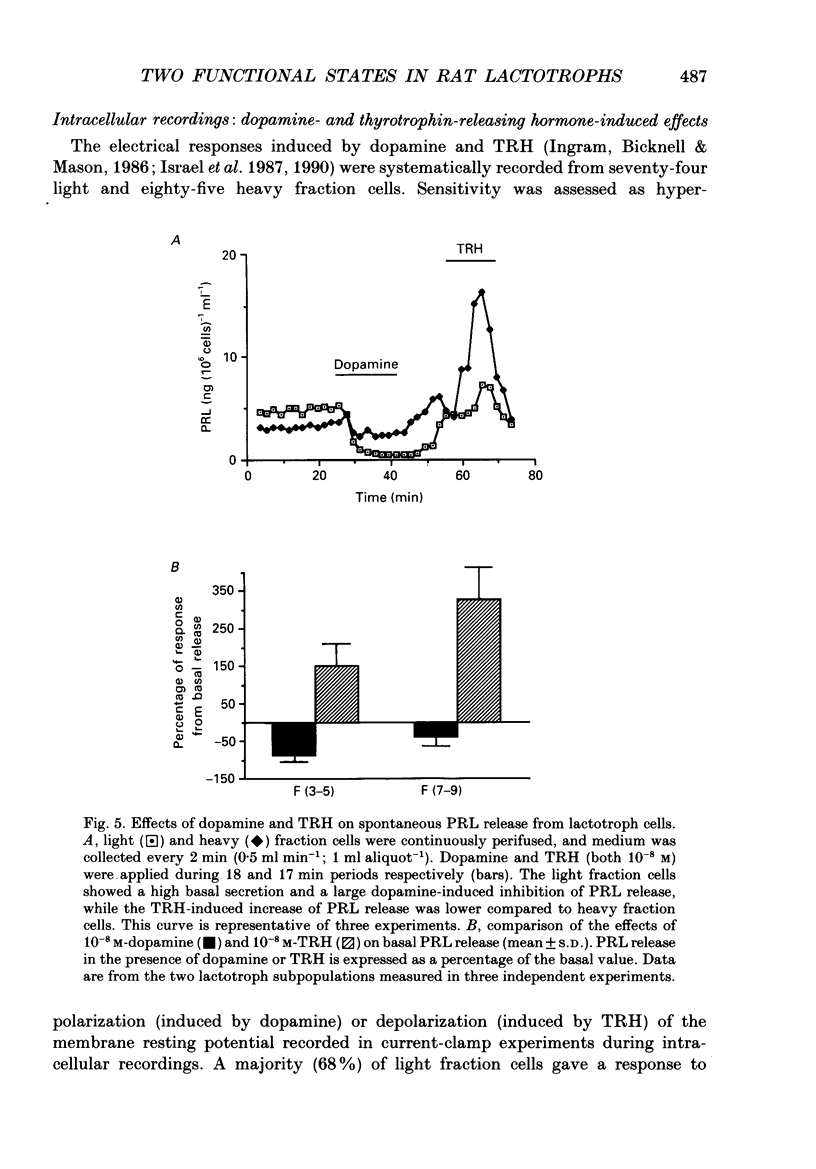
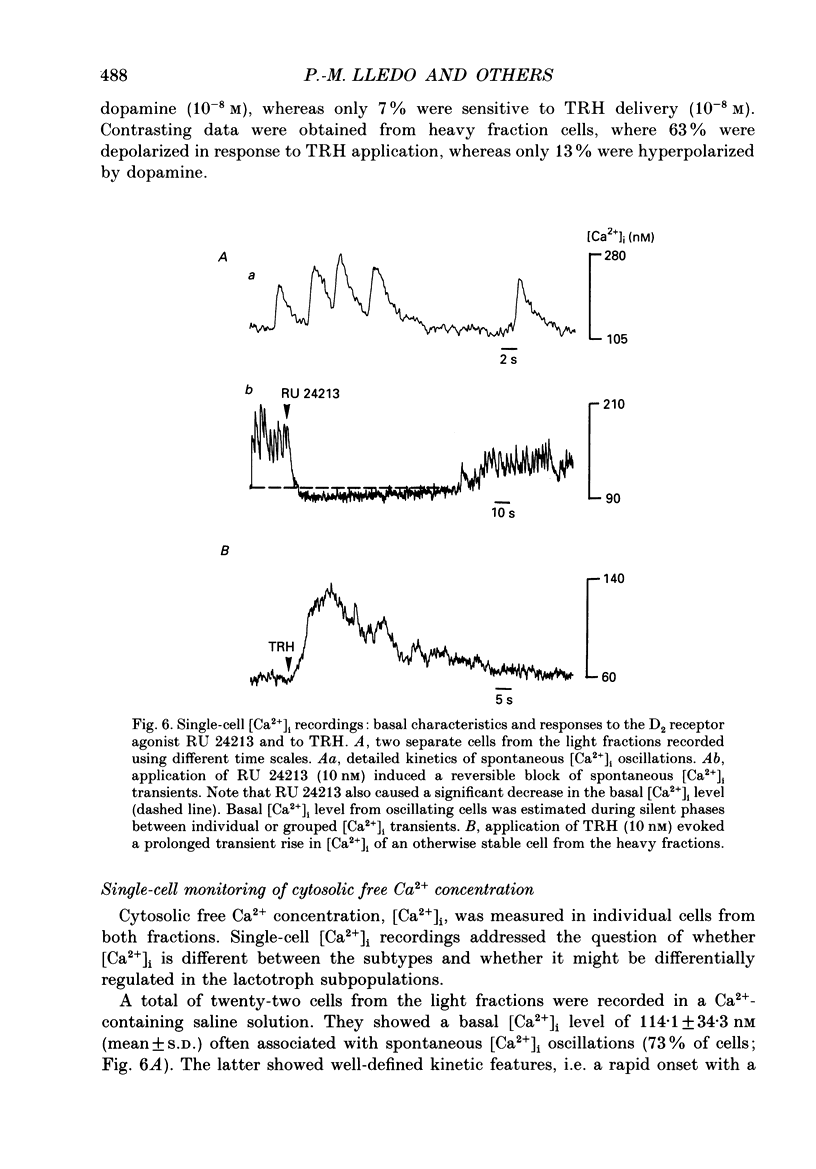
1. Lactotroph cells from lactating female rat pituitary glands were dissociated, separated and enriched on a continuous gradient of bovine serum albumin at unit gravity. Two lactotroph subpopulations were observed in the light (F(3-5)) and the heavy (F(7-9)) fractions of the gradient. Both populations were maintained for at least 6 days in culture before experiments were performed. 2. Patch-clamp recordings, in the whole-cell mode, were performed on both lactotroph subpopulations in order to measure passive membrane properties and Ca2+ currents. Resting membrane potential as well as membrane capacitance values were found to be lower in light fraction cells. The two components of Ca2+ currents, called fast and slow deactivating (FD and SD) currents, were present with different proportions in each subpopulation; the ratio of current amplitudes, SD/FD, was 2.42 +/- 0.41 (n = 18) in light fraction cells and 1.17 +/- 0.27 (n = 17) in heavy fraction cells (P less than 0.02). 3. Reverse haemolytic plaque assay showed that in the light and heavy fractions, 68 and 47% of the lactotroph cells, respectively, were secreting. Population analysis of the plaque areas revealed a bimodal frequency distribution of plaque sizes consisting of small (1500 microns 2) and large plaques (3995 microns 2). A majority of light fraction cells produced large plaques whereas most of the heavy fraction cells produced small plaques. 4. Perifusion experiments performed on enriched prolactin cells showed that (1) basal prolactin (PRL) release was higher in light fraction than in heavy fraction cells, (2) the dopamine (10(-8)M)-induced inhibition of PRL release was greater in light fraction cells (86 +/- 15%) than in heavy fraction cells (41 +/- 21%), and (3) the thyrotrophin-releasing hormone (TRH, 10(-8)M)-induced increase of PRL release was 150 +/- 60% in light fraction versus 330 +/- 82% in heavy fraction cells. 5. Current-clamp recordings were performed using the intracellular technique. Lactotrophs were categorized according to their electrophysiological response following application of dopamine or TRH (both 10(-8)M). In the light fractions, the majority of the cells tested were hyperpolarized by dopamine (68%), whereas only 7% were depolarized by TRH application. In the heavy fractions, most of the cells (63%) responded to TRH application, while only 13% were dopamine sensitive. 6. Cytosolic free Ca2+ concentration ([Ca2+]i) measurements with the fluorescent probe Indo-1 revealed two lactotroph subtypes. Most cells in the light fractions (sixteen of twenty-two tested cells) exhibited an unstable level of [Ca2+]i with values fluctuating between 114.1 +/- 34.3 and 221 +/- 50 nM (mean +/- S.D.). Application of dopamine or of the D2 receptor agonist RU 24213 (10(-8)M) resulted in the disappearance of these fluctuations and in an accompanying decrease in basal [Ca2+]i level.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Matteson D. R. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science. 1985 Jan 4;227(4682):65–67. doi: 10.1126/science.2578071. [DOI] [PubMed] [Google Scholar]
- Boockfor F. R., Frawley L. S. Functional variations among prolactin cells from different pituitary regions. Endocrinology. 1987 Mar;120(3):874–879. doi: 10.1210/endo-120-3-874. [DOI] [PubMed] [Google Scholar]
- Boockfor F. R., Hoeffler J. P., Frawley L. S. Analysis by plaque assays of GH and prolactin release from individual cells in cultures of male pituitaries. Evidence for functional heterogeneity within rat mammotrope and somatotrope populations. Neuroendocrinology. 1986;42(1):64–70. doi: 10.1159/000124250. [DOI] [PubMed] [Google Scholar]
- Cobbett P., Ingram C. D., Mason W. T. Voltage-activated currents through calcium channels in normal bovine lactotrophs. Neuroscience. 1987 Nov;23(2):661–677. doi: 10.1016/0306-4522(87)90084-4. [DOI] [PubMed] [Google Scholar]
- Cohen C. J., McCarthy R. T. Nimodipine block of calcium channels in rat anterior pituitary cells. J Physiol. 1987 Jun;387:195–225. doi: 10.1113/jphysiol.1987.sp016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota G. Calcium channel currents in pars intermedia cells of the rat pituitary gland. Kinetic properties and washout during intracellular dialysis. J Gen Physiol. 1986 Jul;88(1):83–105. doi: 10.1085/jgp.88.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Aspects of the calcium hypothesis of stimulus-secretion coupling: electrical activity in adenohypophyseal cells, and membrane retrieval after exocytosis. Methods Cell Biol. 1981;23:483–501. doi: 10.1016/s0091-679x(08)61515-0. [DOI] [PubMed] [Google Scholar]
- Frawley L. S., Clark C. L. Ovine prolactin (PRL) and dopamine preferentially inhibit PRL release from the same subpopulation of rat mammotropes. Endocrinology. 1986 Oct;119(4):1462–1466. doi: 10.1210/endo-119-4-1462. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Holl R. W., Thorner M. O., Mandell G. L., Sullivan J. A., Sinha Y. N., Leong D. A. Spontaneous oscillations of intracellular calcium and growth hormone secretion. J Biol Chem. 1988 Jul 15;263(20):9682–9685. [PubMed] [Google Scholar]
- Ingram C. D., Bicknell R. J., Mason W. T. Intracellular recordings from bovine anterior pituitary cells: modulation of spontaneous activity by regulators of prolactin secretion. Endocrinology. 1986 Dec;119(6):2508–2518. doi: 10.1210/endo-119-6-2508. [DOI] [PubMed] [Google Scholar]
- Israel J. M., Kirk C., Vincent J. D. Electrophysiological responses to dopamine of rat hypophysial cells in lactotroph-enriched primary cultures. J Physiol. 1987 Sep;390:1–22. doi: 10.1113/jphysiol.1987.sp016682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel J. M., Kukstas L. A., Vincent J. D. Plateau potentials recorded from lactating rat enriched lactotroph cells are triggered by thyrotropin releasing hormone and shortened by dopamine. Neuroendocrinology. 1990 Feb;51(2):113–122. doi: 10.1159/000125326. [DOI] [PubMed] [Google Scholar]
- Kukstas L. A., Verrier D., Zhang J., Chen C., Israel J. M., Vincent J. D. Evidence for a relationship between lactotroph heterogeneity and physiological context. Neurosci Lett. 1990 Nov 27;120(1):84–86. doi: 10.1016/0304-3940(90)90173-7. [DOI] [PubMed] [Google Scholar]
- Lewis D. L., Goodman M. B., St John P. A., Barker J. L. Calcium currents and fura-2 signals in fluorescence-activated cell sorted lactotrophs and somatotrophs of rat anterior pituitary. Endocrinology. 1988 Jul;123(1):611–621. doi: 10.1210/endo-123-1-611. [DOI] [PubMed] [Google Scholar]
- Lim N. F., Nowycky M. C., Bookman R. J. Direct measurement of exocytosis and calcium currents in single vertebrate nerve terminals. Nature. 1990 Mar 29;344(6265):449–451. doi: 10.1038/344449a0. [DOI] [PubMed] [Google Scholar]
- Lingle C. J., Sombati S., Freeman M. E. Membrane currents in identified lactotrophs of rat anterior pituitary. J Neurosci. 1986 Oct;6(10):2995–3005. doi: 10.1523/JNEUROSCI.06-10-02995.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo P. M., Legendre P., Israel J. M., Vincent J. D. Dopamine inhibits two characterized voltage-dependent calcium currents in identified rat lactotroph cells. Endocrinology. 1990 Sep;127(3):990–1001. doi: 10.1210/endo-127-3-990. [DOI] [PubMed] [Google Scholar]
- Lledo P. M., Legendre P., Zhang J., Israel J. M., Vincent J. D. Effects of dopamine on voltage-dependent potassium currents in identified rat lactotroph cells. Neuroendocrinology. 1990 Dec;52(6):545–555. doi: 10.1159/000125650. [DOI] [PubMed] [Google Scholar]
- Luque E. H., Munoz de Toro M., Smith P. F., Neill J. D. Subpopulations of lactotropes detected with the reverse hemolytic plaque assay show differential responsiveness to dopamine. Endocrinology. 1986 May;118(5):2120–2124. doi: 10.1210/endo-118-5-2120. [DOI] [PubMed] [Google Scholar]
- Malgaroli A., Vallar L., Elahi F. R., Pozzan T., Spada A., Meldolesi J. Dopamine inhibits cytosolic Ca2+ increases in rat lactotroph cells. Evidence of a dual mechanism of action. J Biol Chem. 1987 Oct 15;262(29):13920–13927. [PubMed] [Google Scholar]
- Mollard P., Guerineau N., Audin J., Dufy B. Measurement of CA2+ transients using simultaneous dual-emission microspectrofluorimetry and electrophysiology in individual pituitary cells. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1045–1052. doi: 10.1016/0006-291x(89)91775-0. [DOI] [PubMed] [Google Scholar]
- Morin A., Rosenbaum E., Tixier-Vidal A. Effects of thyrotropin-releasing hormone on prolactin compartments in normal rat pituitary cells in primary culture. Endocrinology. 1984 Dec;115(6):2278–2284. doi: 10.1210/endo-115-6-2278. [DOI] [PubMed] [Google Scholar]
- Neill J. D., Frawley L. S. Detection of hormone release from individual cells in mixed populations using a reverse hemolytic plaque assay. Endocrinology. 1983 Mar;112(3):1135–1137. doi: 10.1210/endo-112-3-1135. [DOI] [PubMed] [Google Scholar]
- Ronning S. A., Martin T. F. Characterization of Ca2+-stimulated secretion in permeable GH3 pituitary cells. J Biol Chem. 1986 Jun 15;261(17):7834–7839. [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Snyder J., Wilfinger W., Hymer W. C. Maintenance of separated rat pituitary mammotrophs in cell culture. Endocrinology. 1976 Jan;98(1):25–32. doi: 10.1210/endo-98-1-25. [DOI] [PubMed] [Google Scholar]
- St John P. A., Dufy-Barbe L., Barker J. L. Anti-prolactin cell-surface immunoreactivity identifies a subpopulation of lactotrophs from the rat anterior pituitary. Endocrinology. 1986 Dec;119(6):2783–2795. doi: 10.1210/endo-119-6-2783. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Velkeniers B., Hooghe-Peters E. L., Hooghe R., Belayew A., Smets G., Claeys A., Robberecht P., Vanhaelst L. Prolactin cell subpopulations separated on discontinuous Percoll gradient: an immunocytochemical, biochemical, and physiological characterization. Endocrinology. 1988 Sep;123(3):1619–1630. doi: 10.1210/endo-123-3-1619. [DOI] [PubMed] [Google Scholar]
- Walker A. M., Farquhar M. G. Preferential release of newly synthesized prolactin granules is the result of functional heterogeneity among mammotrophs. Endocrinology. 1980 Oct;107(4):1095–1104. doi: 10.1210/endo-107-4-1095. [DOI] [PubMed] [Google Scholar]
- Winiger B. P., Wuarin F., Zahnd G. R., Wollheim C. B., Schlegel W. Single cell monitoring of cytosolic calcium reveals subtypes of rat lactotrophs with distinct responses to dopamine and thyrotropin-releasing hormone. Endocrinology. 1987 Dec;121(6):2222–2228. doi: 10.1210/endo-121-6-2222. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chen C., Kukstas L. A., Verrier D., Vincent J. D., Israel J. M. In vitro Effects of 17Beta-Estradiol on Thyrotropin-Releasing Hormone-Induced and Dopamine-lnhibited Prolactin Release from Adult Male Rat Lactotrophs in Primary Culture. J Neuroendocrinol. 1990 Jun 1;2(3):277–284. doi: 10.1111/j.1365-2826.1990.tb00405.x. [DOI] [PubMed] [Google Scholar]


