Abstract
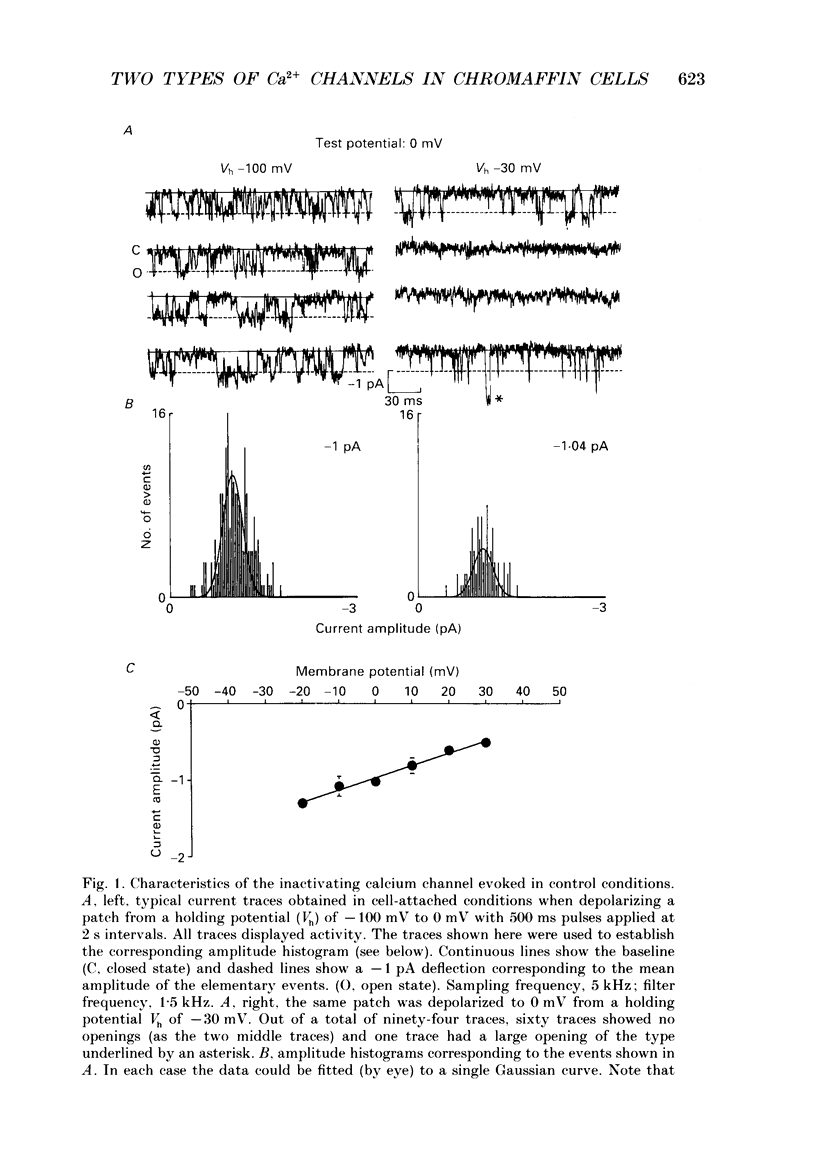
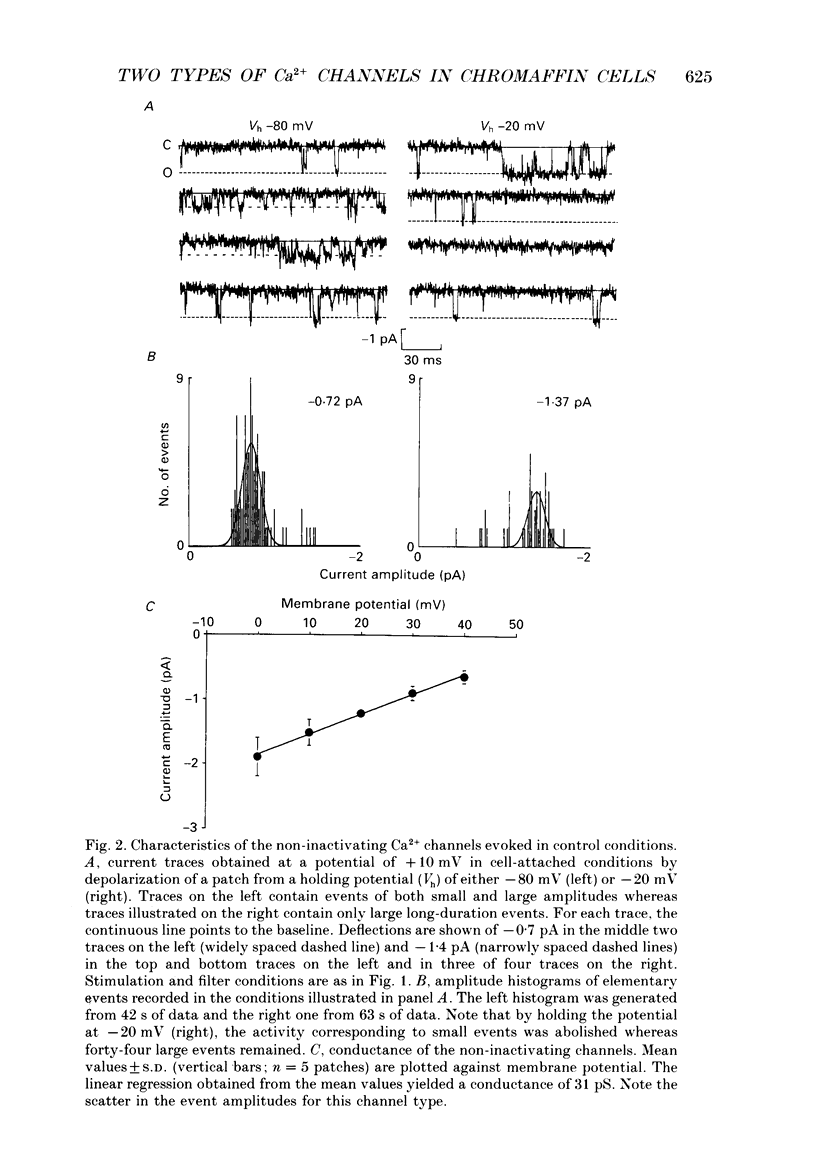
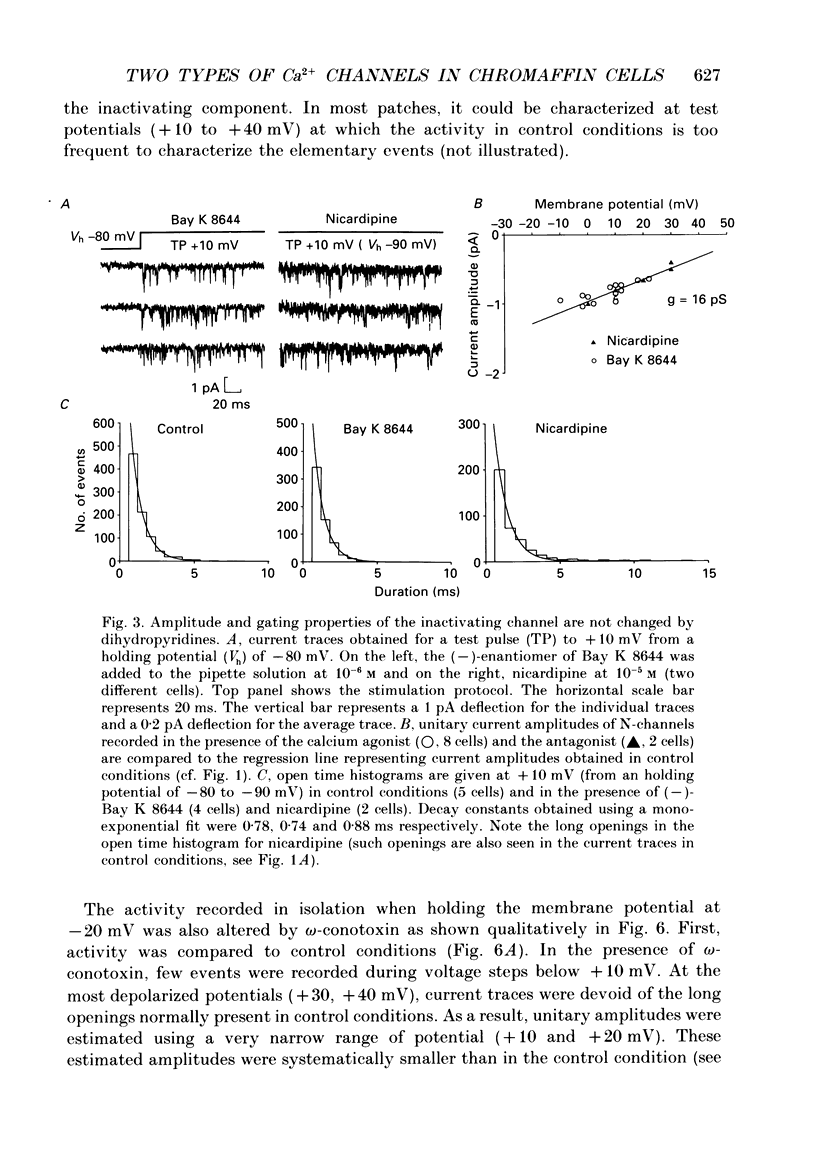
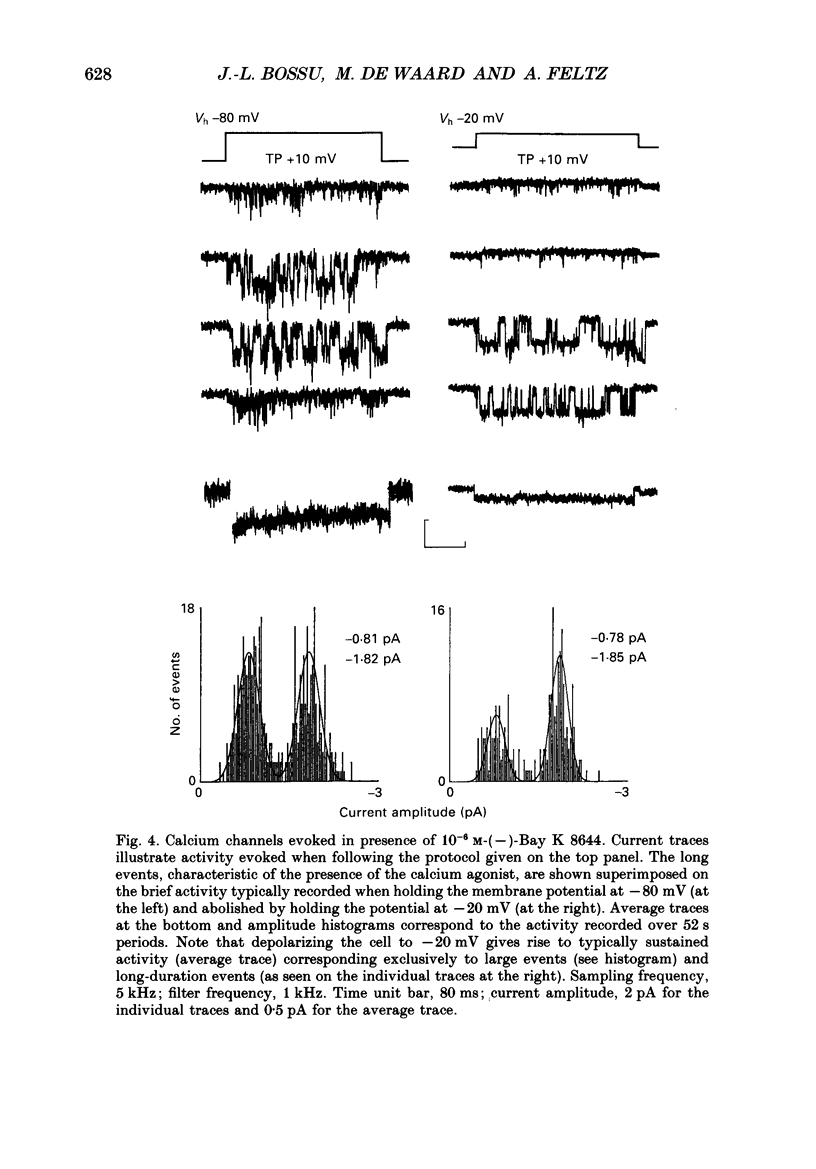
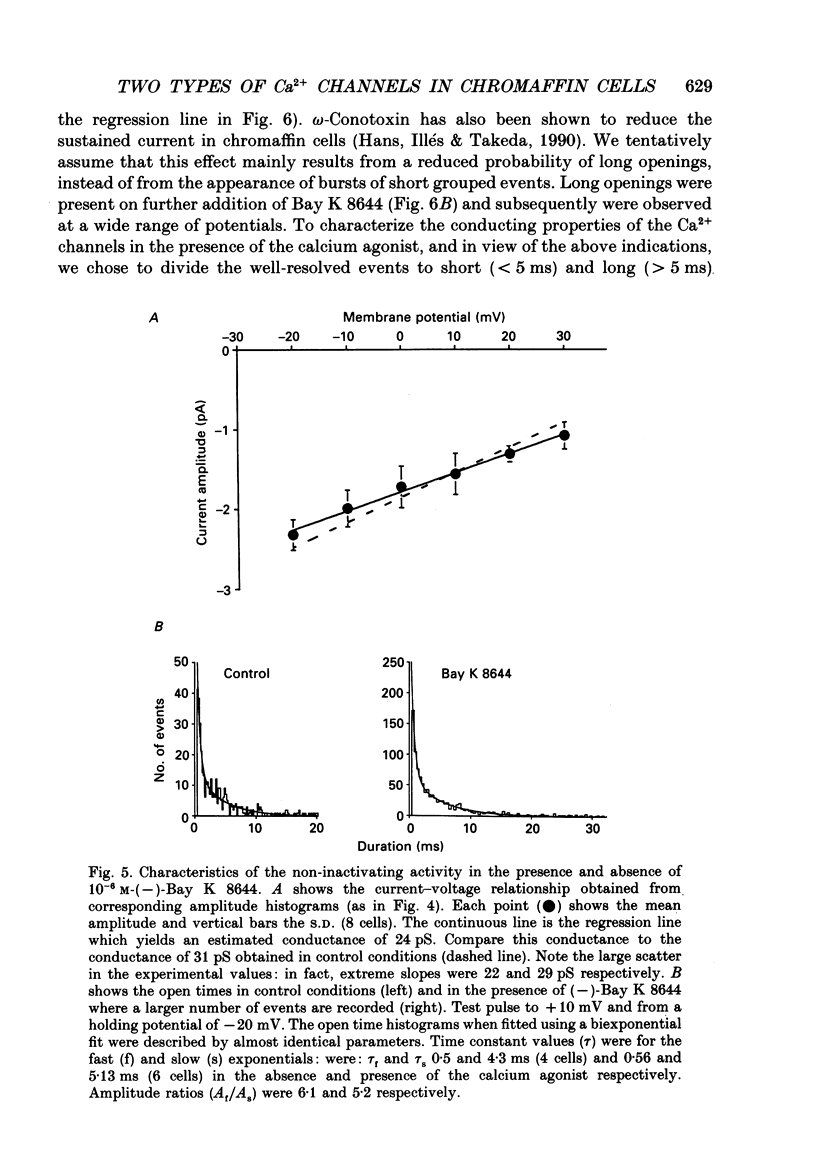
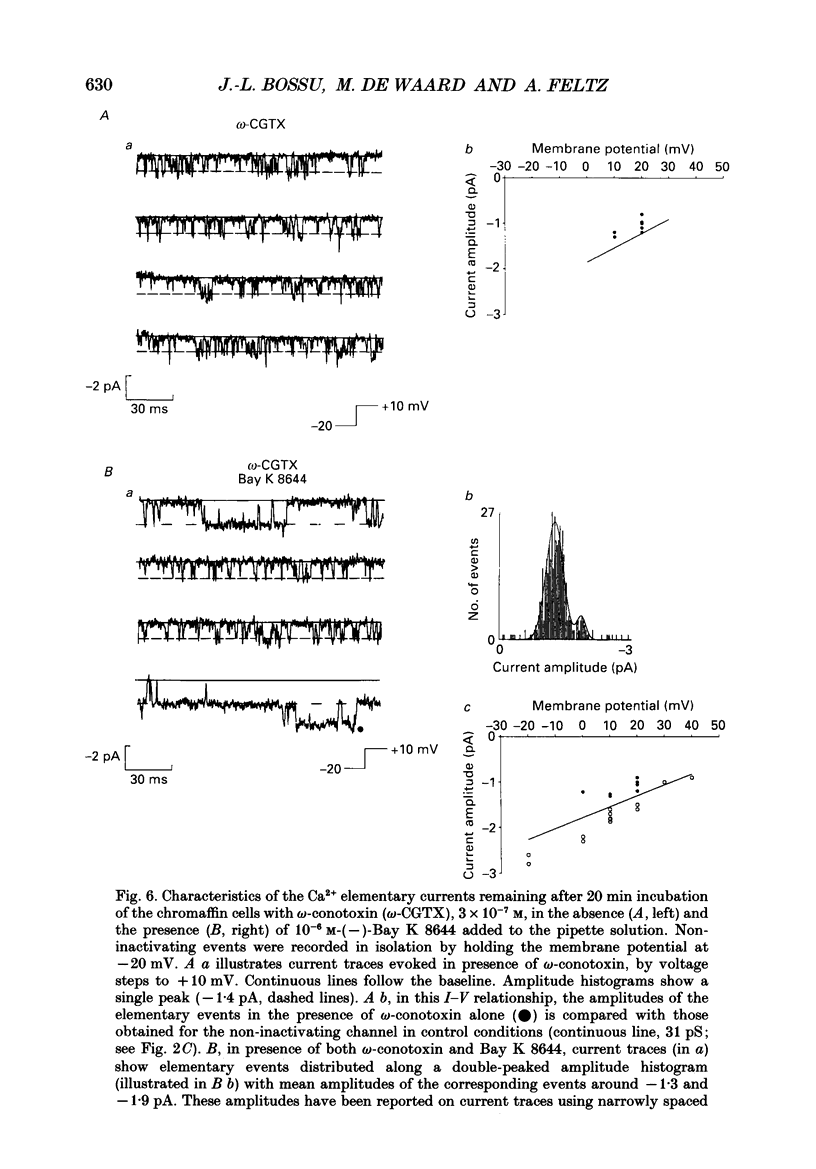
1. Calcium channel activity was recorded in chromaffin cells in the cell-attached condition, using 110 mM-Ba2+ as the permeant ion. 2. One type of calcium channel had a conductance of 16 pS, was completely inactivated at a holding potential of -20 mV and was insensitive to dihydropyridine agonists and antagonists. These characteristics correspond to a calcium channel of the N-type. 3. A second type of calcium channel was active at holding potentials of -30 mV and above, had a channel conductance of 31 pS, and was sensitive to the dihydropyridine agonist, Bay K 8644. The channel opened along two dominant modes with characteristic time constants of 0.5 and 5 ms. The main effect of Bay K 8644 was to increase the probability of both short and long openings with no change in their relative proportions (6 to 1 respectively). These characteristics correspond to a calcium channel of the L-type. 4. omega-Conotoxin affected the activity of both N- and L-type channels. It drastically reduced the number of N-type channel openings and produced complex changes in L-type channel activity. Long openings were less frequent and the conductance during short openings was slightly smaller than that measured in the presence of Bay K 8644. 5. The discussion focuses on modulation of L-type channel activity. Openings of L-type channels are rarely recorded in the cell-attached configuration under control conditions. Addition of Bay K 8644 is needed to reveal the presence of L-type channels. By contrast, L-type currents recorded in the whole-cell configuration are always observed and are insensitive to Bay K 8644. These results indicate that L-type channels are normally inoperable in chromaffin cells.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Bossu J. L., De Waard M., Feltz A. Inactivation characteristics reveal two calcium currents in adult bovine chromaffin cells. J Physiol. 1991 Jun;437:603–620. doi: 10.1113/jphysiol.1991.sp018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Single low-voltage-activated calcium channels in chick and rat sensory neurones. J Physiol. 1987 May;386:571–601. doi: 10.1113/jphysiol.1987.sp016552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R., Johnston D. Noradrenaline and beta-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature. 1987 Jun 18;327(6123):620–622. doi: 10.1038/327620a0. [DOI] [PubMed] [Google Scholar]
- Green K. A., Cottrell G. A. Actions of baclofen on components of the Ca-current in rat and mouse DRG neurones in culture. Br J Pharmacol. 1988 May;94(1):235–245. doi: 10.1111/j.1476-5381.1988.tb11520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans M., Illes P., Takeda K. The blocking effects of omega-conotoxin on Ca current in bovine chromaffin cells. Neurosci Lett. 1990 Jun 22;114(1):63–68. doi: 10.1016/0304-3940(90)90429-d. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Smith S. J. Large depolarization induces long openings of voltage-dependent calcium channels in adrenal chromaffin cells. J Neurosci. 1987 Feb;7(2):571–580. doi: 10.1523/JNEUROSCI.07-02-00571.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janigro D., Maccaferri G., Meldolesi J. Calcium channels in undifferentiated PC12 rat pheochromocytoma cells. FEBS Lett. 1989 Sep 25;255(2):398–400. doi: 10.1016/0014-5793(89)81131-7. [DOI] [PubMed] [Google Scholar]
- Kasai H., Aosaki T., Fukuda J. Presynaptic Ca-antagonist omega-conotoxin irreversibly blocks N-type Ca-channels in chick sensory neurons. Neurosci Res. 1987 Feb;4(3):228–235. doi: 10.1016/0168-0102(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Shuba YaM, Savchenko A. N. Three types of calcium channels in the membrane of mouse sensory neurons. Pflugers Arch. 1988 Jun;411(6):661–669. doi: 10.1007/BF00580863. [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Kongsamut S., Tsien R. W. Alpha-adrenergic inhibition of sympathetic neurotransmitter release mediated by modulation of N-type calcium-channel gating. Nature. 1989 Aug 24;340(6235):639–642. doi: 10.1038/340639a0. [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. Y., Tsien R. W. Spatial distribution of calcium channels and cytosolic calcium transients in growth cones and cell bodies of sympathetic neurons. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2398–2402. doi: 10.1073/pnas.85.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Stauderman K. A. Voltage-regulated calcium channels involved in the regulation of enkephalin synthesis are blocked by phorbol ester treatment. J Biol Chem. 1988 Sep 15;263(26):13173–13178. [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]


