Abstract
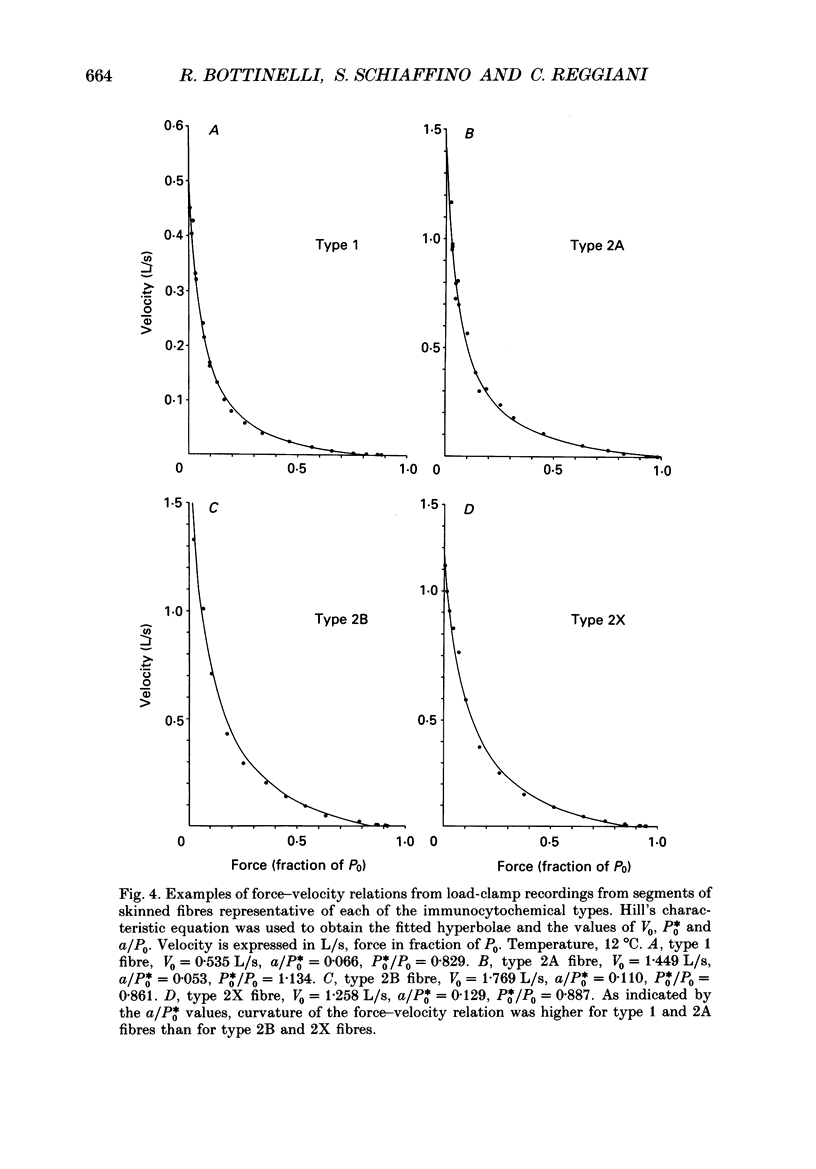
1. This study was performed to assess whether muscle contractile properties are related to the presence of specific myosin heavy chain (MHC) isoforms. 2. Force-velocity relations and MHC isoform composition were determined in seventy-four single skinned muscle fibres from rat soleus, extensor digitorum longus and plantaris muscles. 3. Four groups of fibres were identified according to their MHC isoform composition determined by monoclonal antibodies: type 1 (slow), and types 2A, 2B and 2X (fast). 4. With respect to maximum velocity of shortening (V0), the fibres formed a continuum between 0.35 and 2.84 L/s (muscle lengths per second) at 12 degrees C. V0 in type 1 fibres (slow fibres) was between 0.35 and 0.95 L/s (0.639 +/- 0.038 L/s; mean +/- S.E. of mean). V0 in type 2 fibres (fast fibres) was consistently higher than 0.91 L/s. Ranges of V0 in the three fast fibre types mostly overlapped. Type 2A and 2X fibres had similar mean V0 values (1.396 +/- 0.084 and 1.451 +/- 0.066 L/s respectively); type 2B fibres showed a higher mean V0 value (1.800 +/- 0.109 L/s) than type 2A and 2X fibres. 5. Mean values of a/P0, an index of the curvature of force-velocity relations, allowed us to identify two groups of fibres: a high curvature group comprised of type 1 (mean a/P0, 0.066 +/- 0.007) and 2A (0.066 +/- 0.024) fibres and a low curvature group comprised of type 2B (0.113 +/- 0.013) and 2X (0.132 +/- 0.008) fibres. 6. Maximal power output was lower in slow fibres than in fast fibres, and among fast fibres it was lower in type 2A fibres than in type 2X and 2B. 7. Force per unit cross-sectional area was less in slow fibres than in fast fibres. There was no relation between fibre type and cross-sectional area. 8. The results suggest that MHC composition is just one of the determinants of shortening velocity and of other muscle contractile properties.
Full text
PDF


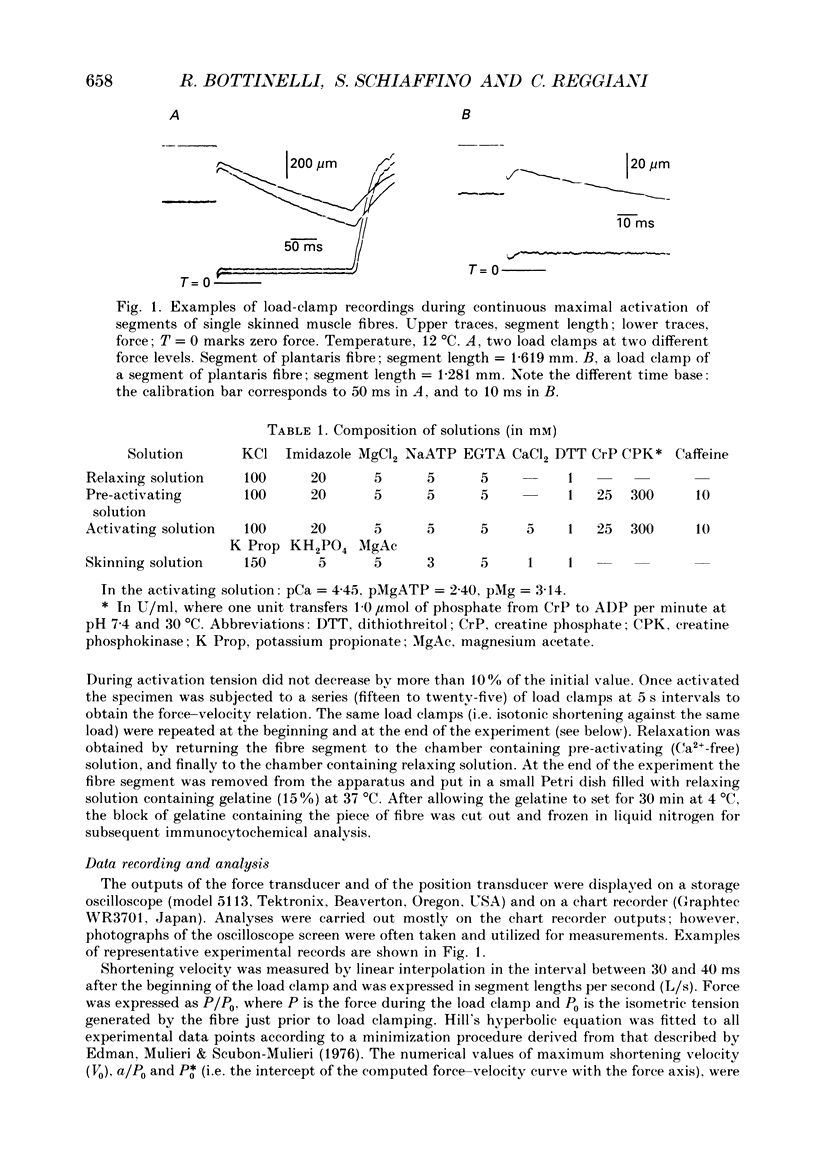
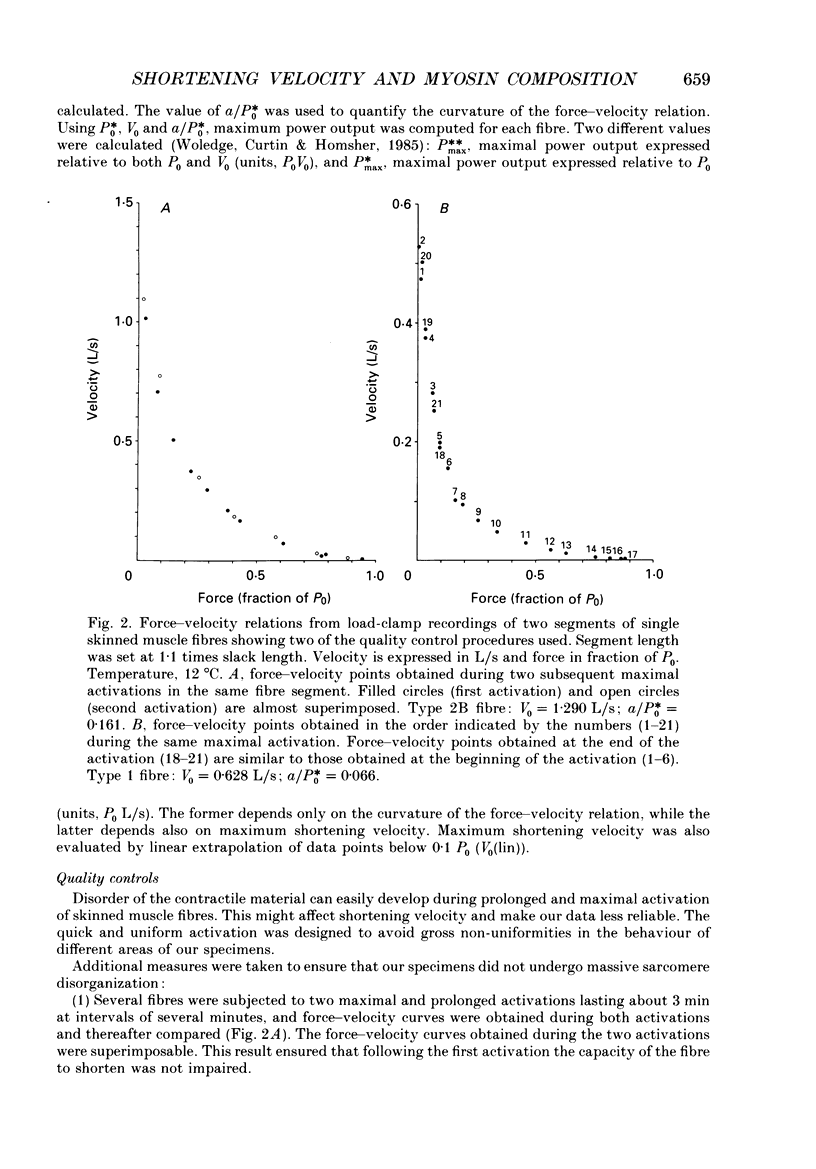

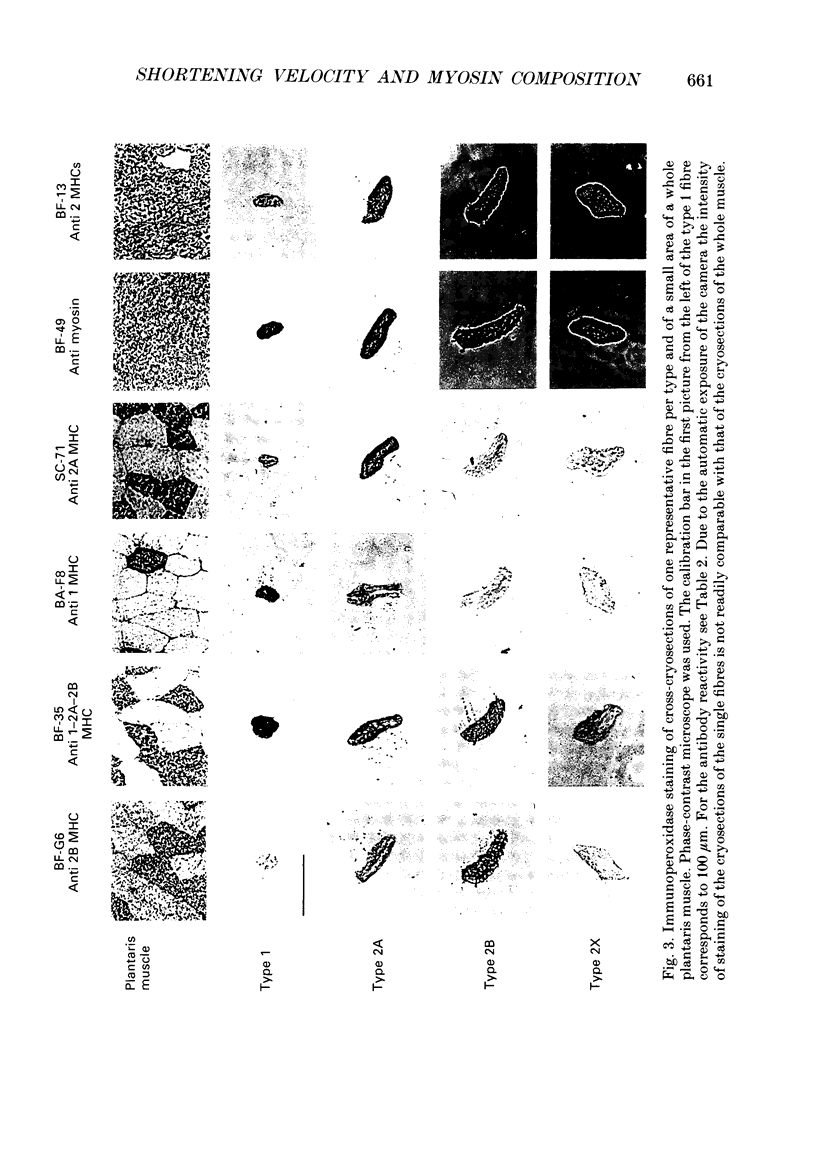

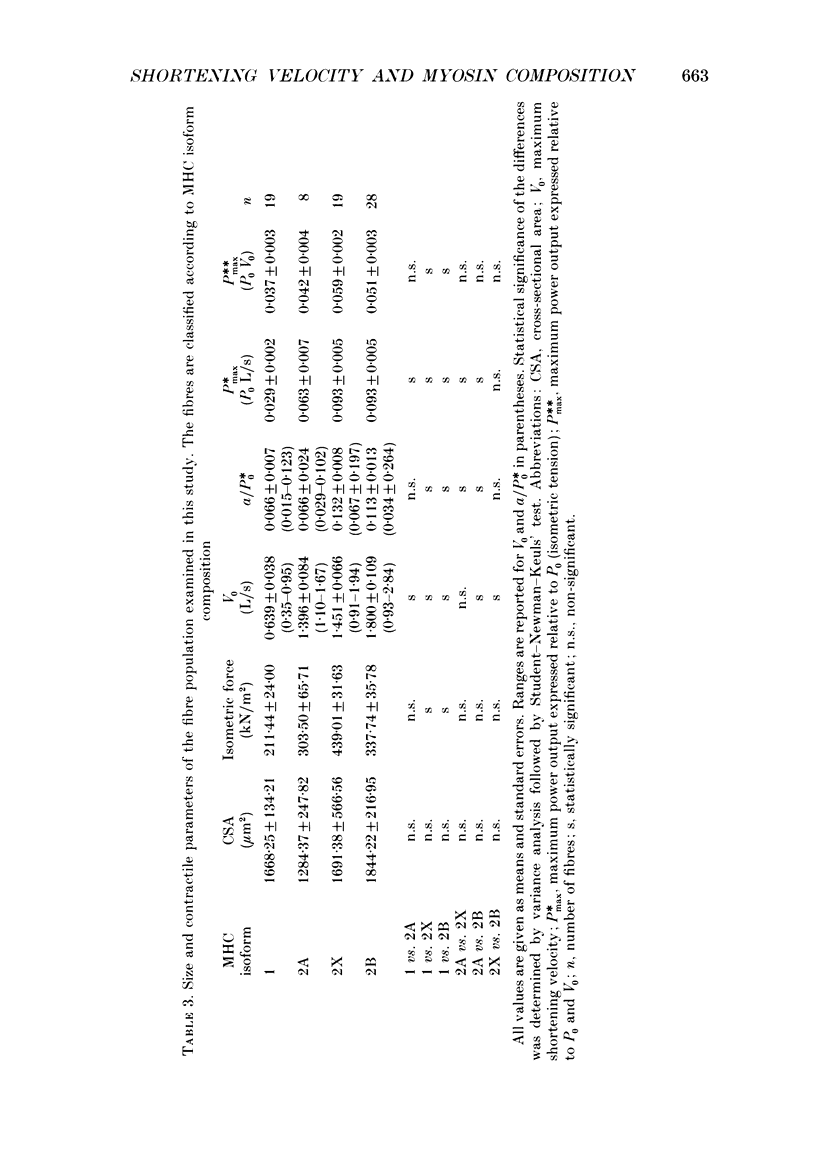
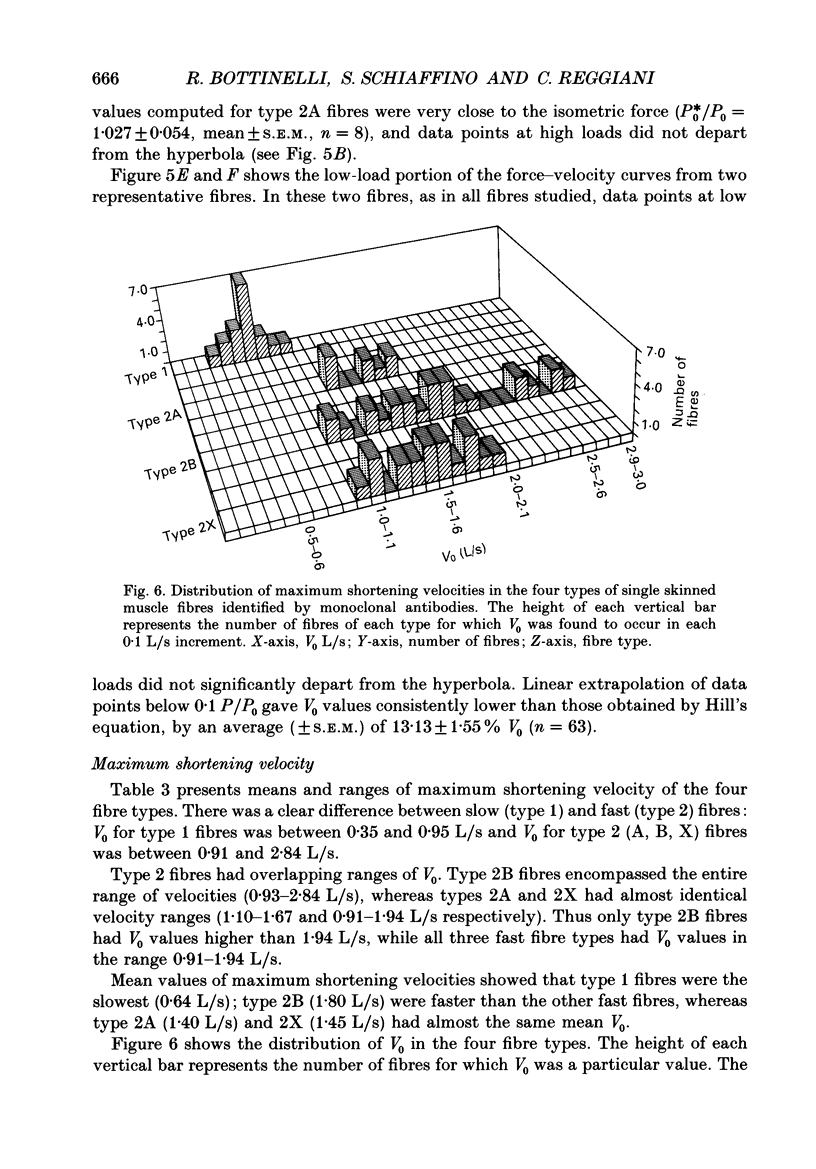






Images in this article
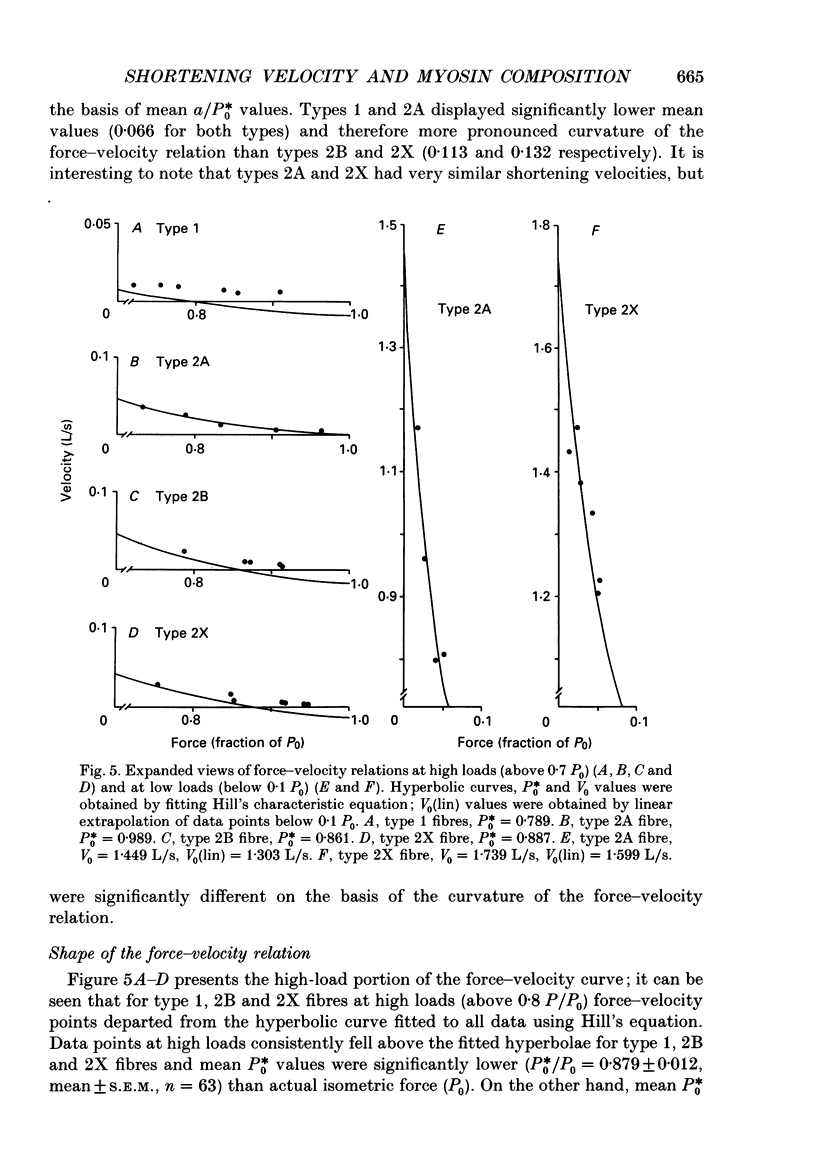
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner B. Technique for stabilizing the striation pattern in maximally calcium-activated skinned rabbit psoas fibers. Biophys J. 1983 Jan;41(1):99–102. doi: 10.1016/S0006-3495(83)84411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. The necessity of using two parameters to describe isotonic shortening velocity of muscle tissues: the effect of various interventions upon initial shortening velocity (vi) and curvature (b). Basic Res Cardiol. 1986 Jan-Feb;81(1):54–69. doi: 10.1007/BF01907427. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Three "myosin adenosine triphosphatase" systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970 Sep;18(9):670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Bär A., Pette D. Three fast myosin heavy chains in adult rat skeletal muscle. FEBS Lett. 1988 Aug 1;235(1-2):153–155. doi: 10.1016/0014-5793(88)81253-5. [DOI] [PubMed] [Google Scholar]
- Close R. Dynamic properties of fast and slow skeletal muscles of the rat after nerve cross-union. J Physiol. 1969 Oct;204(2):331–346. doi: 10.1113/jphysiol.1969.sp008916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danieli Betto D., Zerbato E., Betto R. Type 1, 2A, and 2B myosin heavy chain electrophoretic analysis of rat muscle fibers. Biochem Biophys Res Commun. 1986 Jul 31;138(2):981–987. doi: 10.1016/s0006-291x(86)80592-7. [DOI] [PubMed] [Google Scholar]
- Eddinger T. J., Cassens R. G., Moss R. L. Mechanical and histochemical characterization of skeletal muscles from senescent rats. Am J Physiol. 1986 Sep;251(3 Pt 1):C421–C430. doi: 10.1152/ajpcell.1986.251.3.C421. [DOI] [PubMed] [Google Scholar]
- Eddinger T. J., Moss R. L. Mechanical properties of skinned single fibers of identified types from rat diaphragm. Am J Physiol. 1987 Aug;253(2 Pt 1):C210–C218. doi: 10.1152/ajpcell.1987.253.2.C210. [DOI] [PubMed] [Google Scholar]
- Edman K. A. Double-hyperbolic force-velocity relation in frog muscle fibres. J Physiol. 1988 Oct;404:301–321. doi: 10.1113/jphysiol.1988.sp017291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mulieri L. A., Scubon-Mulieri B. Non-hyperbolic force-velocity relationship in single muscle fibres. Acta Physiol Scand. 1976 Oct;98(2):143–156. doi: 10.1111/j.1748-1716.1976.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C., Schiaffino S., te Kronnie G. Maximum velocity of shortening related to myosin isoform composition in frog skeletal muscle fibres. J Physiol. 1988 Jan;395:679–694. doi: 10.1113/jphysiol.1988.sp016941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C., te Kronnie G. Differences in maximum velocity of shortening along single muscle fibres of the frog. J Physiol. 1985 Aug;365:147–163. doi: 10.1113/jphysiol.1985.sp015764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Ferenczi M. A., Goldman Y. E., Simmons R. M. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. J Physiol. 1984 May;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Maughan D. W. Swelling of skinned muscle fibers of the frog. Experimental observations. Biophys J. 1977 Aug;19(2):103–116. doi: 10.1016/S0006-3495(77)85573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaser M. L., Moss R. L., Reiser P. J. Variations in contractile properties of rabbit single muscle fibres in relation to troponin T isoforms and myosin light chains. J Physiol. 1988 Dec;406:85–98. doi: 10.1113/jphysiol.1988.sp017370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol. 1986 Jan;370:181–199. doi: 10.1113/jphysiol.1986.sp015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell L. C., Faulkner J. A., Lieberman D. A. Histochemical manifestations of age and endurance training in skeletal muscle fibers. Am J Physiol. 1973 Feb;224(2):356–361. doi: 10.1152/ajplegacy.1973.224.2.356. [DOI] [PubMed] [Google Scholar]
- Mercadier J. J., Lompré A. M., Wisnewsky C., Samuel J. L., Bercovici J., Swynghedauw B., Schwartz K. Myosin isoenzyme changes in several models of rat cardiac hypertrophy. Circ Res. 1981 Aug;49(2):525–532. doi: 10.1161/01.res.49.2.525. [DOI] [PubMed] [Google Scholar]
- Ranatunga K. W. Temperature-dependence of shortening velocity and rate of isometric tension development in rat skeletal muscle. J Physiol. 1982 Aug;329:465–483. doi: 10.1113/jphysiol.1982.sp014314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser P. J., Greaser M. L., Moss R. L. Myosin heavy chain composition of single cells from avian slow skeletal muscle is strongly correlated with velocity of shortening during development. Dev Biol. 1988 Oct;129(2):400–407. doi: 10.1016/0012-1606(88)90387-9. [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Kasper C. E., Greaser M. L., Moss R. L. Functional significance of myosin transitions in single fibers of developing soleus muscle. Am J Physiol. 1988 May;254(5 Pt 1):C605–C613. doi: 10.1152/ajpcell.1988.254.5.C605. [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Moss R. L., Giulian G. G., Greaser M. L. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985 Aug 5;260(16):9077–9080. [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Sartore S., Saggin L., Ausoni S., Vianello M., Gundersen K., Lømo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989 Jun;10(3):197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Staron R. S., Pette D. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in rabbit muscle fibers. Histochemistry. 1986;86(1):19–23. doi: 10.1007/BF00492341. [DOI] [PubMed] [Google Scholar]
- Staron R. S., Pette D. The multiplicity of combinations of myosin light chains and heavy chains in histochemically typed single fibres. Rabbit soleus muscle. Biochem J. 1987 May 1;243(3):687–693. doi: 10.1042/bj2430687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staron R. S., Pette D. The multiplicity of combinations of myosin light chains and heavy chains in histochemically typed single fibres. Rabbit tibialis anterior muscle. Biochem J. 1987 May 1;243(3):695–699. doi: 10.1042/bj2430695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Sréter F. A., Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J Biol Chem. 1988 Jun 25;263(18):9034–9039. [PubMed] [Google Scholar]
- Termin A., Staron R. S., Pette D. Changes in myosin heavy chain isoforms during chronic low-frequency stimulation of rat fast hindlimb muscles. A single-fiber study. Eur J Biochem. 1989 Dec 22;186(3):749–754. doi: 10.1111/j.1432-1033.1989.tb15269.x. [DOI] [PubMed] [Google Scholar]
- Woledge R. C. The energetics of tortoise muscle. J Physiol. 1968 Aug;197(3):685–707. doi: 10.1113/jphysiol.1968.sp008582. [DOI] [PMC free article] [PubMed] [Google Scholar]



