Abstract
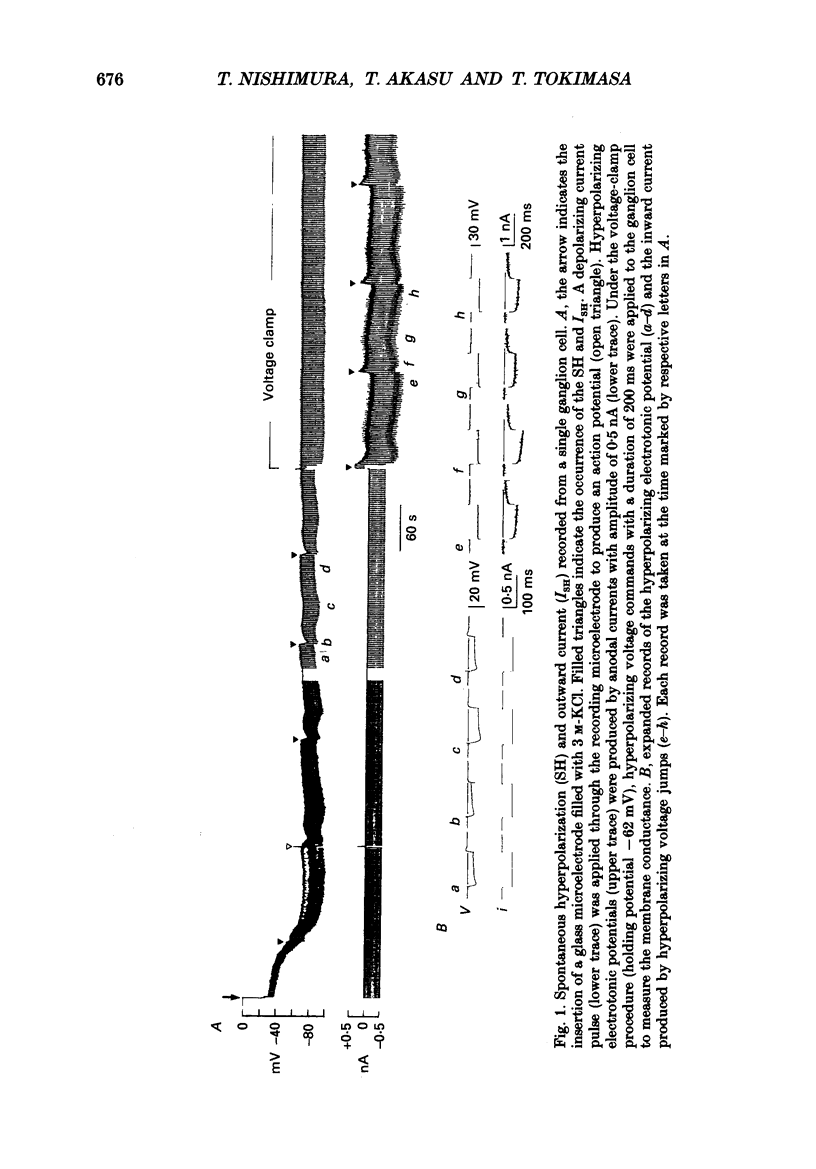
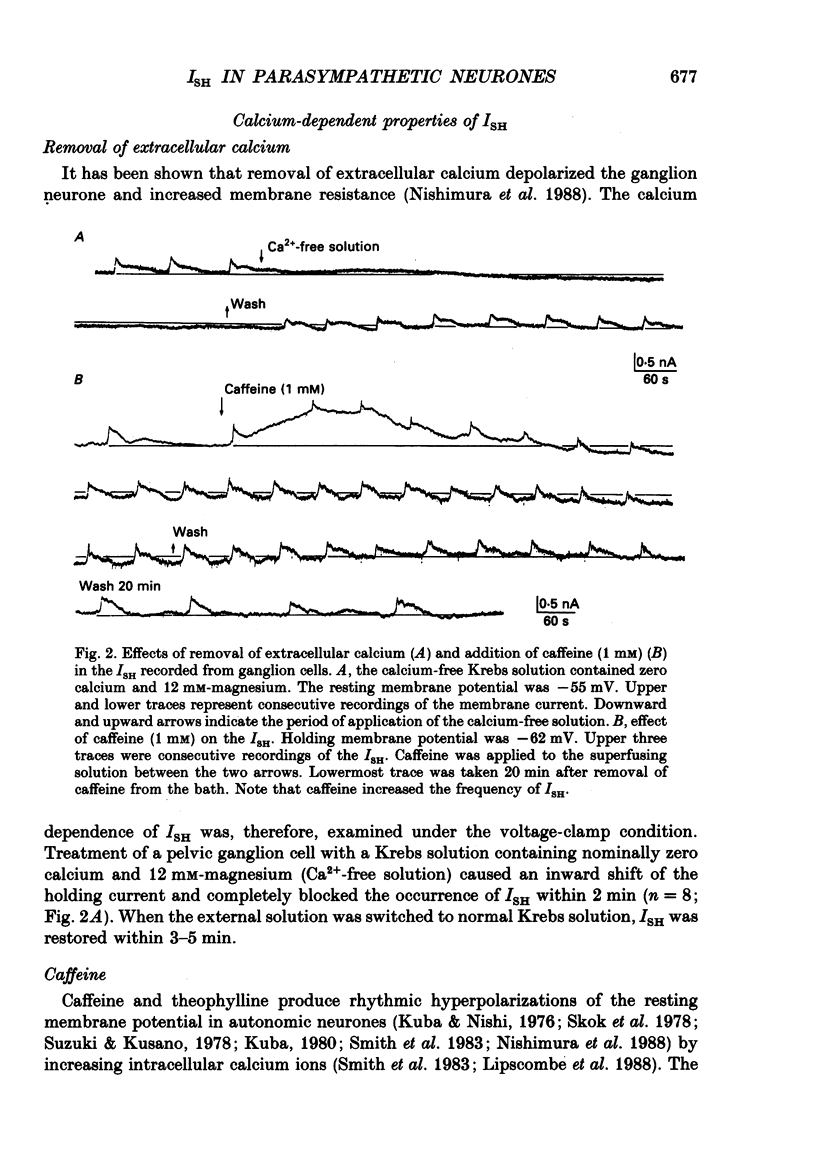
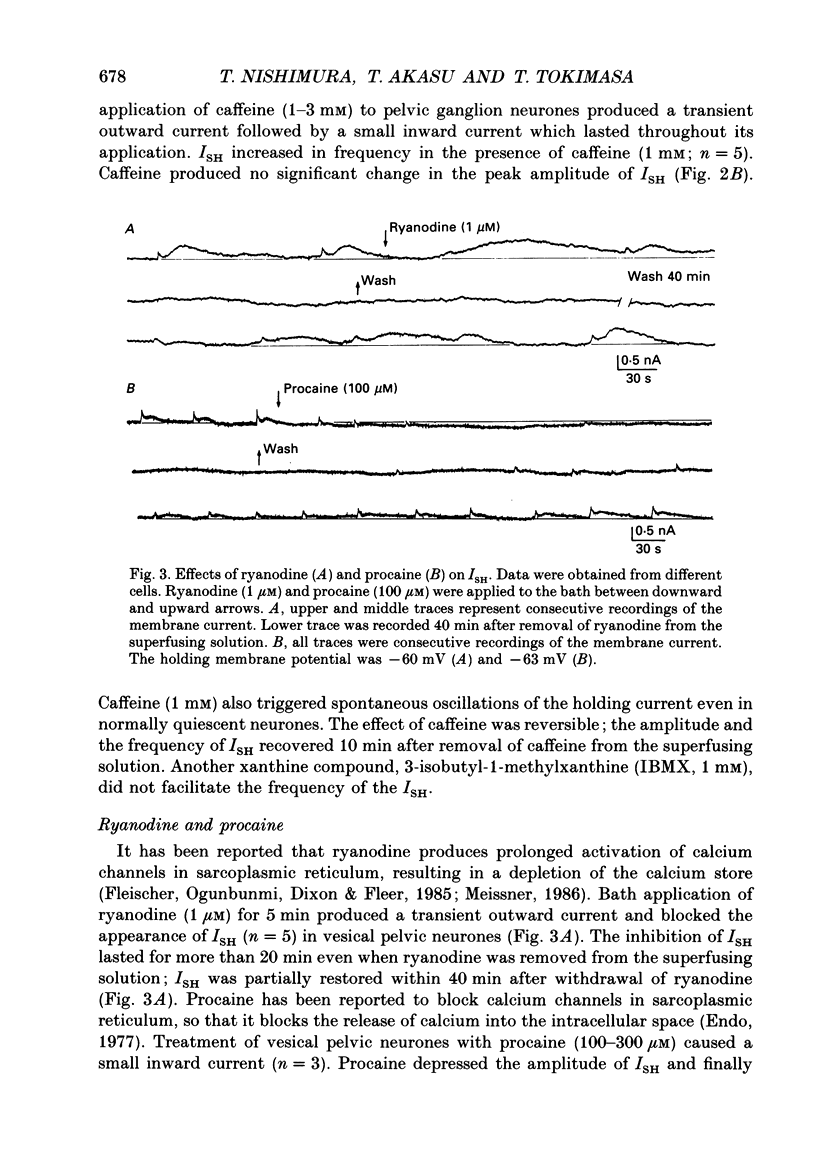
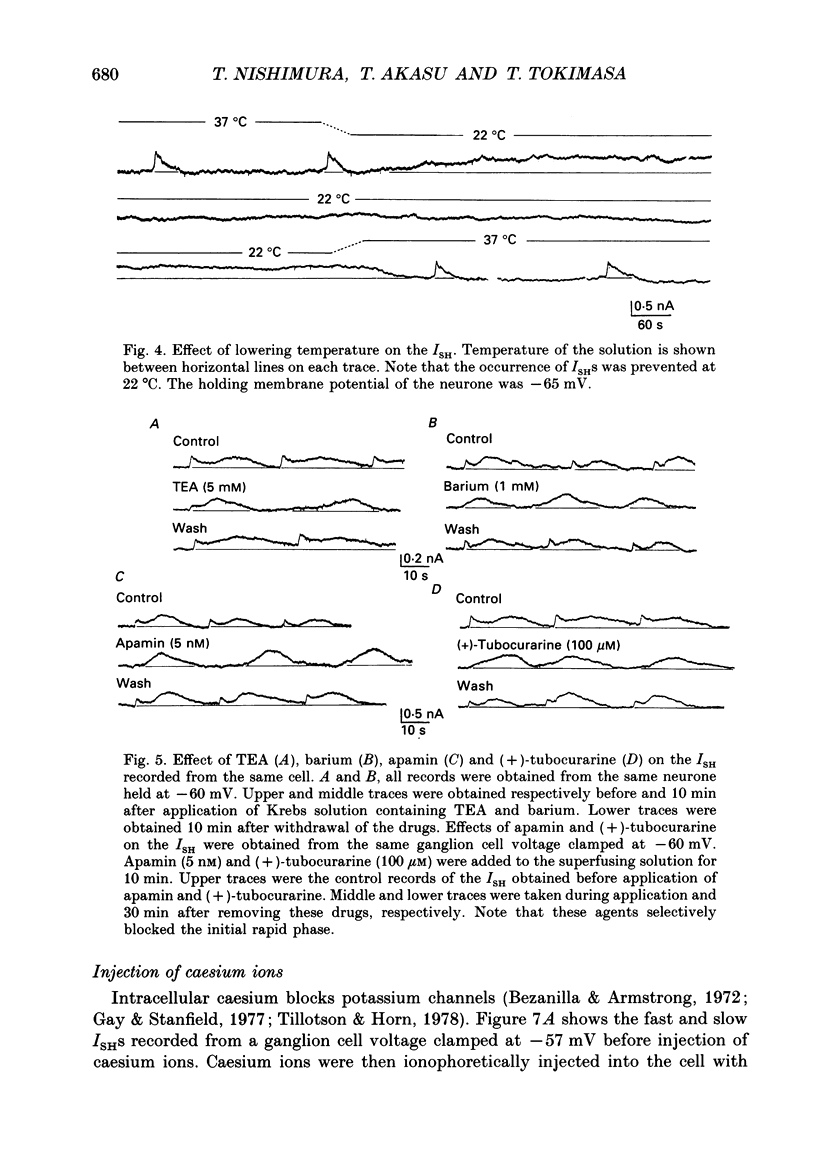
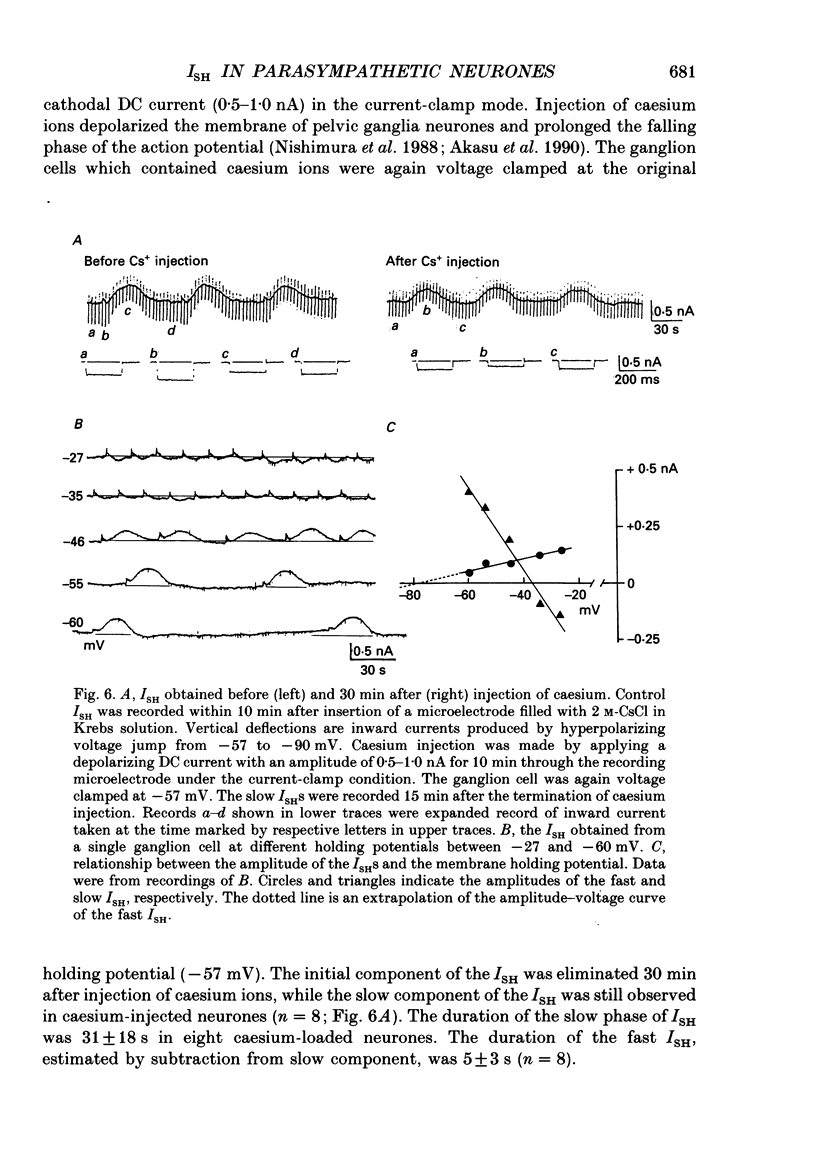
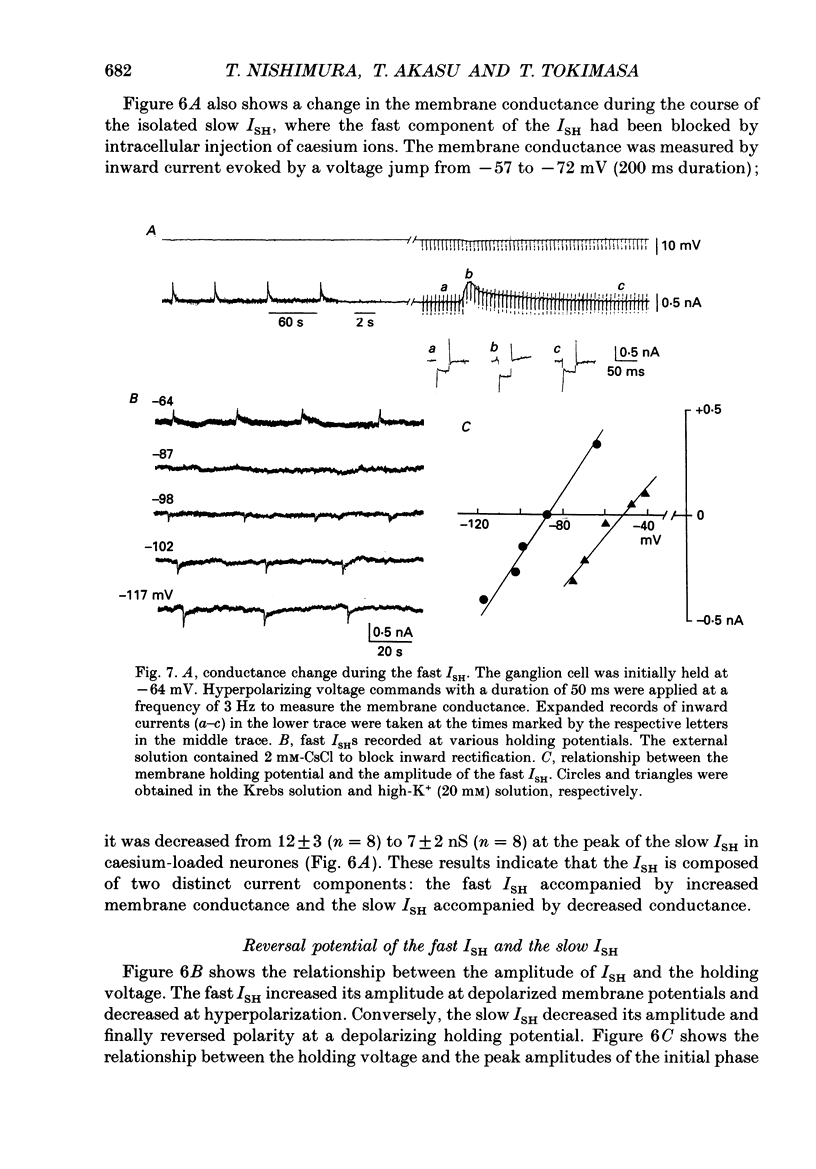
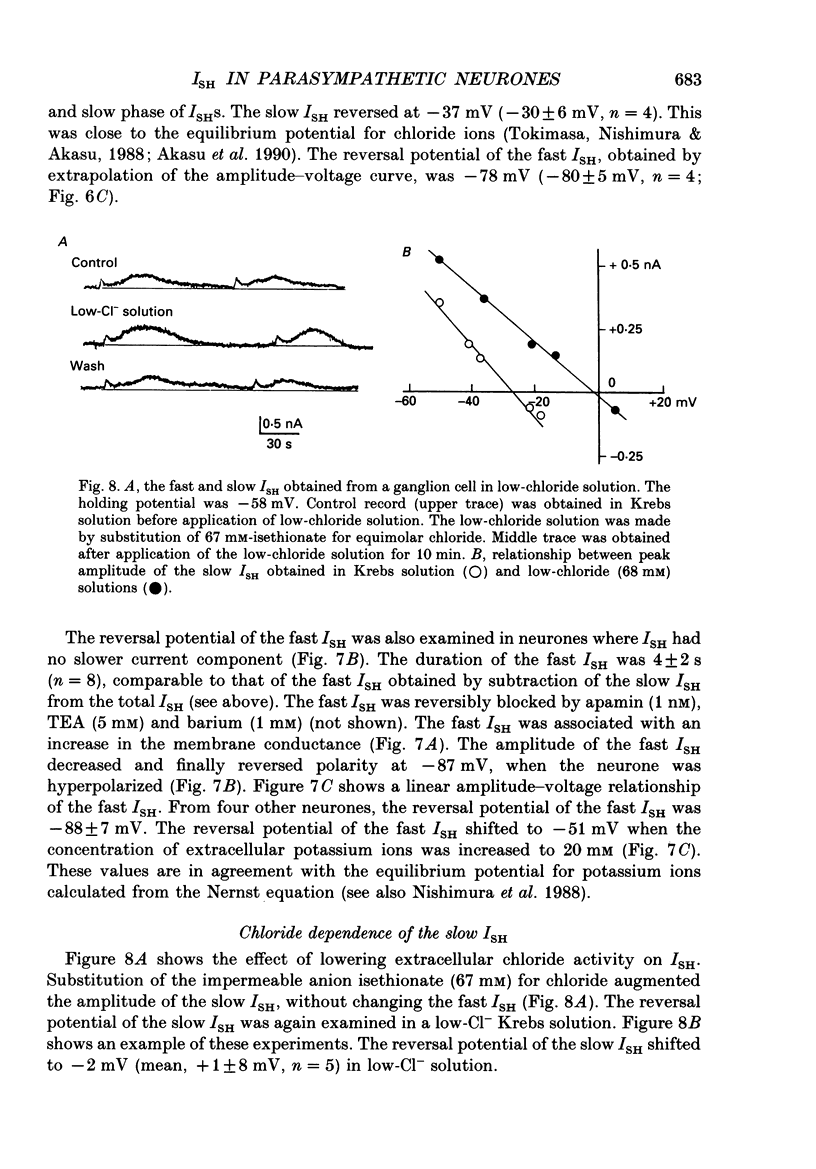
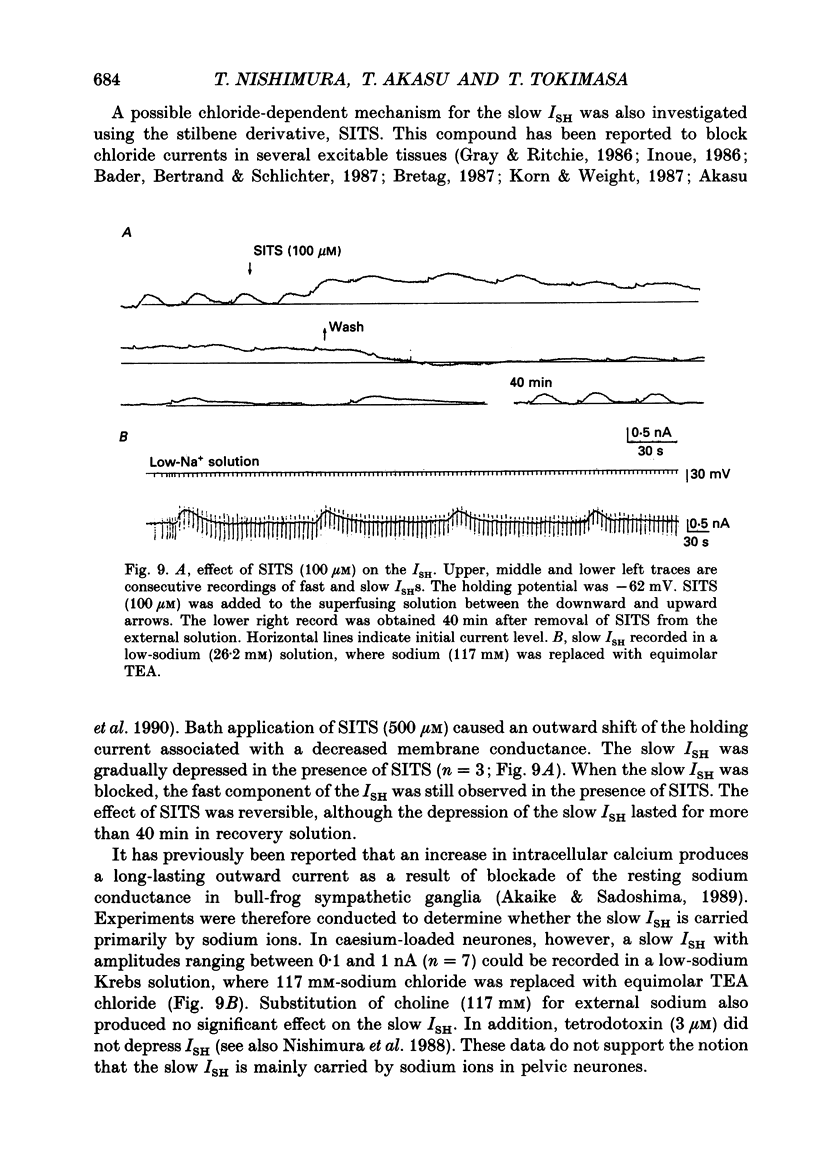
1. Voltage-clamp recordings were made from neurones of vesical pelvic ganglia isolated from the rabbit urinary bladder. A rhythmic outward current, ISH, which corresponds to the spontaneous hyperpolarization, occurred at fairly constant intervals in fifty-eight of eighty-four neurones superfused with Krebs solution. The peak amplitude of the ISH was 0.5 +/- 0.2 nA (n = 48; mean +/- S.E.M.). 2. The ISH was eliminated in a Krebs solution containing nominally zero calcium and 12 mM-magnesium. Lowering the temperature of the superfusing solution from 36 to 22 degrees C also inhibited the occurrence of the ISH. 3. Bath application of caffeine increased the frequency of ISH. In contrast, ryanodine and procaine reversibly blocked ISH. 4. In thirty-four of fifty-eight neurones, the ISH was composed of two current components, an initial fast ISH with duration of 1-10 s and a slow ISH lasting 15-60 s. In the remaining twenty-four neurones, ISH showed only the fast component. 5. The fast ISH was associated with an increased membrane conductance and the slow ISH was associated with a decreased membrane conductance. The reversal potentials of the fast and the slow ISH were -88 +/- 7 mV (n = 4) and -30 +/- 6 mV (n = 4), respectively. 6. Tetraethylammonium (5 mM) and barium (1 mM) blocked the fast ISH but not the slow ISH. Intracellular caesium injected by ionophoresis through a Cs(+)-filled microelectrode blocked the fast ISH, without affecting the slow ISH. Apamin and (+)-tubocurarine selectively suppressed the fast component of the ISH. 7. Substitution of isethionate (67 mM) for chloride increased the amplitude of the slow ISH and shifted the reversal potential of the slow ISH to +1 +/- 8 mV (n = 5). A slow ISH with amplitude of 0.1-1 nA and was still observed in a low-sodium (26.2 mM) solution. The stilbene derivative, 4-acetamido-4'-isothiocyanostilbene-2,2'-disulphonic acid (SITS), a chloride channel blocker, suppressed the slow ISH. 8. These results suggest that ISH is composed of two distinct calcium-dependent currents, a fast ISH produced by activation of potassium conductance and a slow ISH produced by inactivation of chloride conductance. 9. The after-hyperpolarization (AHP) following the action potential was also composed of apamin-sensitive and insensitive spontaneous hyperpolarizing oscillations. The apamin-insensitive component of IAHP was increased by lowering external chloride activity, while it was depressed by SITS.
Full text
PDF



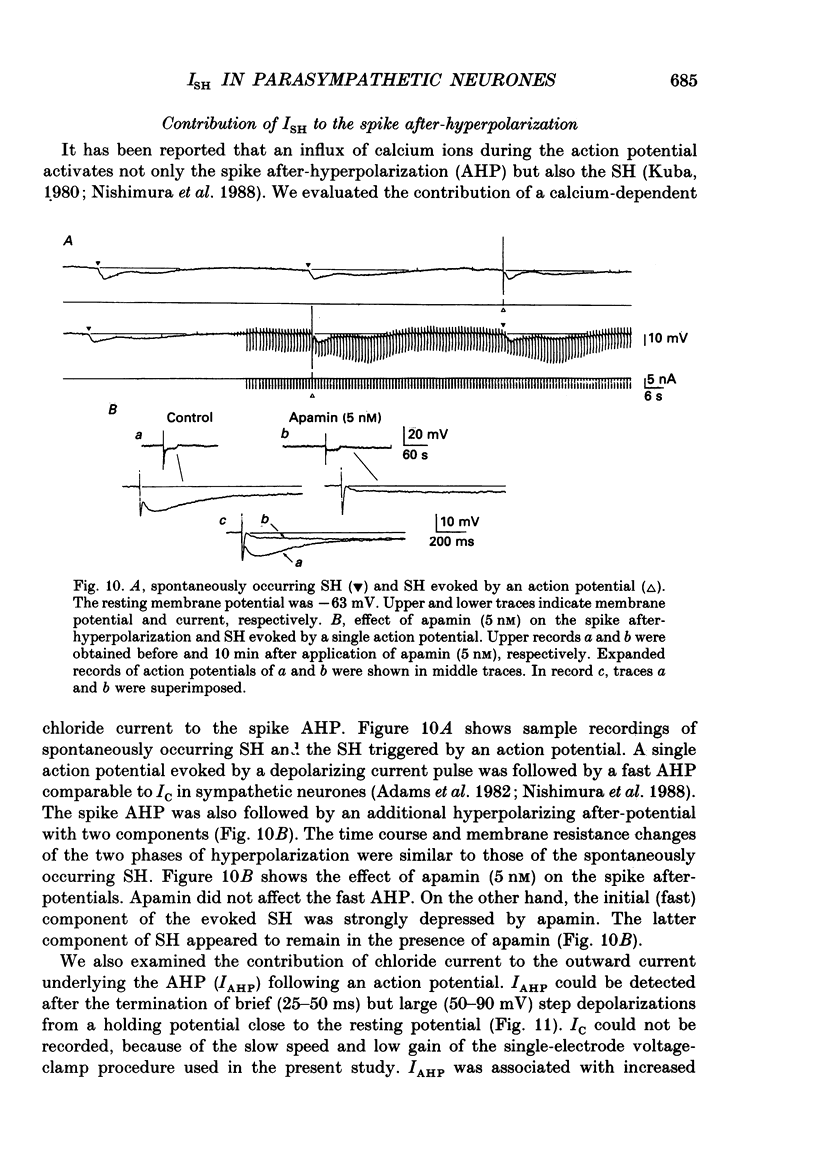
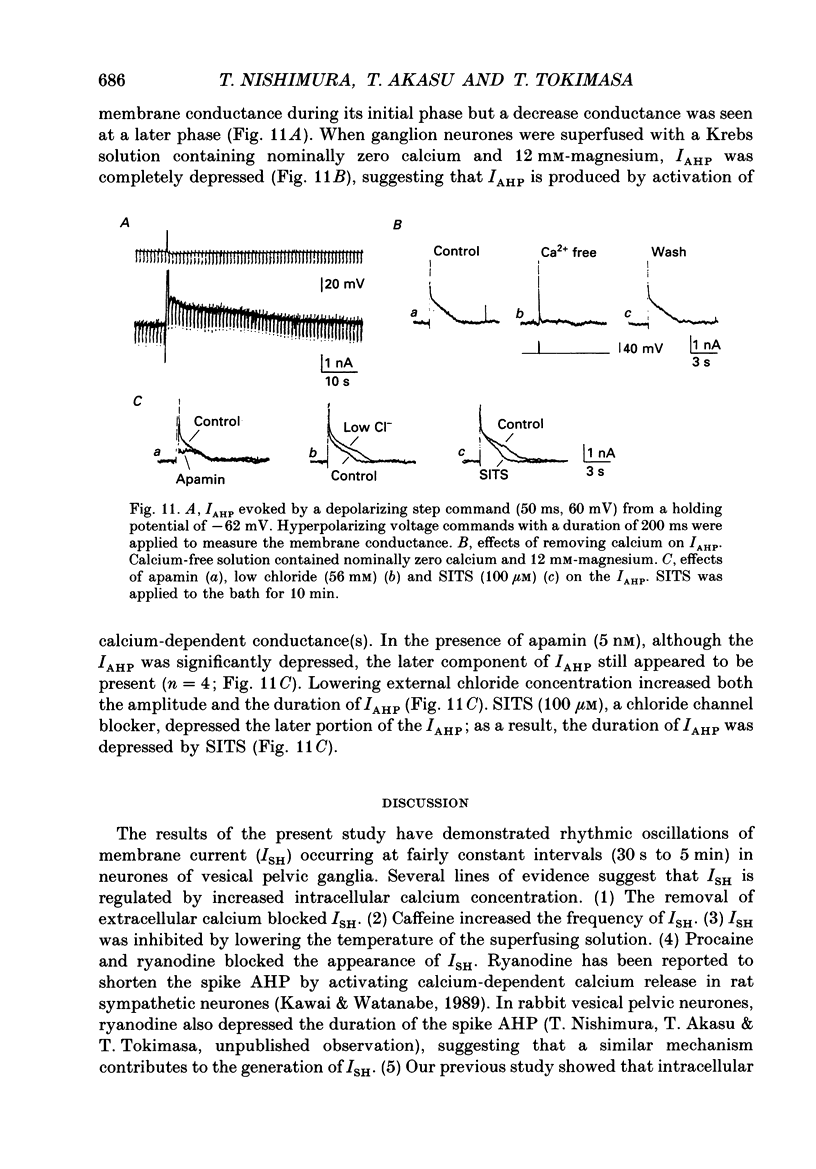




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Akaike N., Sadoshima J. Caffeine affects four different ionic currents in the bull-frog sympathetic neurone. J Physiol. 1989 May;412:221–244. doi: 10.1113/jphysiol.1989.sp017612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Nishimura T., Tokimasa T. Calcium-dependent chloride current in neurones of the rabbit pelvic parasympathetic ganglia. J Physiol. 1990 Mar;422:303–320. doi: 10.1113/jphysiol.1990.sp017985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Potassium pores of nerve and muscle membranes. Membranes. 1975;3:325–358. [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schlichter R. Calcium-activated chloride current in cultured sensory and parasympathetic quail neurones. J Physiol. 1987 Dec;394:125–148. doi: 10.1113/jphysiol.1987.sp016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J Gen Physiol. 1972 Nov;60(5):588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W., Brown D. A. Apamin and d-tubocurarine block the afterhyperpolarization of rat supraoptic neurosecretory neurons. Neurosci Lett. 1987 Nov 23;82(2):185–190. doi: 10.1016/0304-3940(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Bretag A. H. Muscle chloride channels. Physiol Rev. 1987 Apr;67(2):618–724. doi: 10.1152/physrev.1987.67.2.618. [DOI] [PubMed] [Google Scholar]
- Cassell J. F., McLachlan E. M. Two calcium-activated potassium conductances in a subpopulation of coeliac neurones of guinea-pig and rabbit. J Physiol. 1987 Dec;394:331–349. doi: 10.1113/jphysiol.1987.sp016873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Jiang Z. G., Mo N. Tubocurarine suppresses slow calcium-dependent after-hyperpolarization in guinea-pig inferior mesenteric ganglion cells. J Physiol. 1986 Jun;375:499–514. doi: 10.1113/jphysiol.1986.sp016130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay L. A., Stanfield P. R. Cs(+) causes a voltage-dependent block of inward K currents in resting skeletal muscle fibres. Nature. 1977 May 12;267(5607):169–170. doi: 10.1038/267169a0. [DOI] [PubMed] [Google Scholar]
- Grafe P., Mayer C. J., Wood J. D. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J Physiol. 1980 Aug;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. T. Oscillations of free cytosolic calcium evoked by cholinergic and catecholaminergic agonists in rat parotid acinar cells. J Physiol. 1988 Dec;406:35–53. doi: 10.1113/jphysiol.1988.sp017367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. T., Ritchie J. M. A voltage-gated chloride conductance in rat cultured astrocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):267–288. doi: 10.1098/rspb.1986.0055. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Johnson S. M., van Helden D. F. The slow calcium-dependent potassium current in a myenteric neurone of the guinea-pig ileum. J Physiol. 1985 Apr;361:315–337. doi: 10.1113/jphysiol.1985.sp015648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Leblanc N. Macroscopic K+ currents in single smooth muscle cells of the rabbit portal vein. J Physiol. 1989 Jun;413:49–73. doi: 10.1113/jphysiol.1989.sp017641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue I. Modification of K conductance of the squid axon membrane by SITS. J Gen Physiol. 1986 Oct;88(4):507–520. doi: 10.1085/jgp.88.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Oomura Y., Yakushiji T., Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986 Nov 13;324(6093):156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- Kawai T., Watanabe M. Blockade of Ca-activated K conductance by apamin in rat sympathetic neurones. Br J Pharmacol. 1986 Jan;87(1):225–232. doi: 10.1111/j.1476-5381.1986.tb10175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Watanabe M. Effects of ryanodine on the spike after-hyperpolarization in sympathetic neurones of the rat superior cervical ganglion. Pflugers Arch. 1989 Mar;413(5):470–475. doi: 10.1007/BF00594175. [DOI] [PubMed] [Google Scholar]
- Koketsu K., Akasu T., Miyagawa M. Identification of gK systems activated by [Ca2+]. Brain Res. 1982 Jul 15;243(2):369–372. doi: 10.1016/0006-8993(82)90263-3. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Weight F. F. Patch-clamp study of the calcium-dependent chloride current in AtT-20 pituitary cells. J Neurophysiol. 1987 Dec;58(6):1431–1451. doi: 10.1152/jn.1987.58.6.1431. [DOI] [PubMed] [Google Scholar]
- Kuba K., Morita K., Nohmi M. Origin of calcium ions involved in the generation of a slow afterhyperpolarization in bullfrog sympathetic neurones. Pflugers Arch. 1983 Nov;399(3):194–202. doi: 10.1007/BF00656714. [DOI] [PubMed] [Google Scholar]
- Kuba K., Nishi S. Rhythmic hyperpolarizations and depolarization of sympathetic ganglion cells induced by caffeine. J Neurophysiol. 1976 May;39(3):547–563. doi: 10.1152/jn.1976.39.3.547. [DOI] [PubMed] [Google Scholar]
- Kuba K. Release of calcium ions linked to the activation of potassium conductance in a caffeine-treated sympathetic neurone. J Physiol. 1980 Jan;298:251–269. doi: 10.1113/jphysiol.1980.sp013079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B., Pennefather P. Potassium currents evoked by brief depolarizations in bull-frog sympathetic ganglion cells. J Physiol. 1987 Jun;387:519–548. doi: 10.1113/jphysiol.1987.sp016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. W., Tsien R. Y. Imaging of cytosolic Ca2+ transients arising from Ca2+ stores and Ca2+ channels in sympathetic neurons. Neuron. 1988 Jul;1(5):355–365. doi: 10.1016/0896-6273(88)90185-7. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Weight F. F. Action potential repolarization may involve a transient, Ca2+-sensitive outward current in a vertebrate neurone. Nature. 1982 Nov 11;300(5888):185–188. doi: 10.1038/300185a0. [DOI] [PubMed] [Google Scholar]
- Magaribuchi T., Ito Y., Kuriyama H. Effects of rapid cooling on the mechanical and electrical activities of smooth muscles of guinea pig stomach and taenia coli. J Gen Physiol. 1973 Mar;61(3):323–341. doi: 10.1085/jgp.61.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985 Jul;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfee D. A., Yarowsky P. J. Calcium-dependent potentials in the mammalian sympathetic neurone. J Physiol. 1979 May;290(2):507–523. doi: 10.1113/jphysiol.1979.sp012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Morita K., Koketsu K., Kuba K. Oscillation of [Ca2+]i-linked K+ conductance in bullfrog sympathetic ganglion cell is sensitive to intracellular anions. Nature. 1980 Jan 10;283(5743):204–205. doi: 10.1038/283204a0. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A., Tokimasa T. The calcium-activated potassium conductance in guinea-pig myenteric neurones. J Physiol. 1982 Aug;329:341–354. doi: 10.1113/jphysiol.1982.sp014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Tokimasa T., Akasu T. Calcium-dependent potassium conductance in neurons of rabbit vesical pelvic ganglia. J Auton Nerv Syst. 1988 Sep;24(1-2):133–145. doi: 10.1016/0165-1838(88)90142-7. [DOI] [PubMed] [Google Scholar]
- Nohmi M., Kuba K. (+)-Tubocurarine blocks the Ca2+-dependent K+-channel of the bullfrog sympathetic ganglion cell. Brain Res. 1984 May 28;301(1):146–148. doi: 10.1016/0006-8993(84)90412-8. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Owen D. G., Segal M., Barker J. L. A Ca-dependent Cl- conductance in cultured mouse spinal neurones. Nature. 1984 Oct 11;311(5986):567–570. doi: 10.1038/311567a0. [DOI] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger P. J., Leibowitz M. D., Subers E. M., Nathanson N. M., Almers W., Hille B. Agonists that suppress M-current elicit phosphoinositide turnover and Ca2+ transients, but these events do not explain M-current suppression. Neuron. 1988 Aug;1(6):477–484. doi: 10.1016/0896-6273(88)90178-x. [DOI] [PubMed] [Google Scholar]
- Sakai T., Kurihara S., Yoshioka T. Action of manganese ions on excitation-contractions coupling of frog skeletal muscle fibres. Jpn J Physiol. 1974 Oct;24(5):513–530. doi: 10.2170/jjphysiol.24.513. [DOI] [PubMed] [Google Scholar]
- Scott R. H., McGuirk S. M., Dolphin A. C. Modulation of divalent cation-activated chloride ion currents. Br J Pharmacol. 1988 Jul;94(3):653–662. doi: 10.1111/j.1476-5381.1988.tb11572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skok V. I., Storch N. N., Nishi S. The effect of caffeine on the neurons of a mammalian sympathetic ganglion. Neuroscience. 1978;3(8):697–708. doi: 10.1016/0306-4522(78)90066-0. [DOI] [PubMed] [Google Scholar]
- Smith S. J., MacDermott A. B., Weight F. F. Detection of intracellular Ca2+ transients in sympathetic neurones using arsenazo III. 1983 Jul 28-Aug 3Nature. 304(5924):350–352. doi: 10.1038/304350a0. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Kusano K. Hyperpolarizing potentials induced by Ca-mediated K-conductance increase in hamster submandibular ganglion cells. J Neurobiol. 1978 Sep;9(5):367–392. doi: 10.1002/neu.480090504. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Kusano K. Rhythmic membrane potential changes in hamster parasympathetic neurons. J Auton Nerv Syst. 1983 Jul;8(3):213–236. doi: 10.1016/0165-1838(83)90107-8. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Minota S., Kuba K., Koyano K., Abe T. Differential effects of apamin on Ca2+-dependent K+ currents in bullfrog sympathetic ganglion cells. Neurosci Lett. 1986 Sep 12;69(3):233–238. doi: 10.1016/0304-3940(86)90485-4. [DOI] [PubMed] [Google Scholar]
- Tillotson D., Horn R. Inactivation without facilitation of calcium conductance in caesium-loaded neurones of Aplysia. Nature. 1978 May 25;273(5660):312–314. doi: 10.1038/273312a0. [DOI] [PubMed] [Google Scholar]
- Tokimasa T., Nishimura T., Akasu T. Calcium-activated chloride conductance in parasympathetic neurons of the rabbit urinary bladder. J Auton Nerv Syst. 1988 Sep;24(1-2):123–131. doi: 10.1016/0165-1838(88)90141-5. [DOI] [PubMed] [Google Scholar]


