Abstract
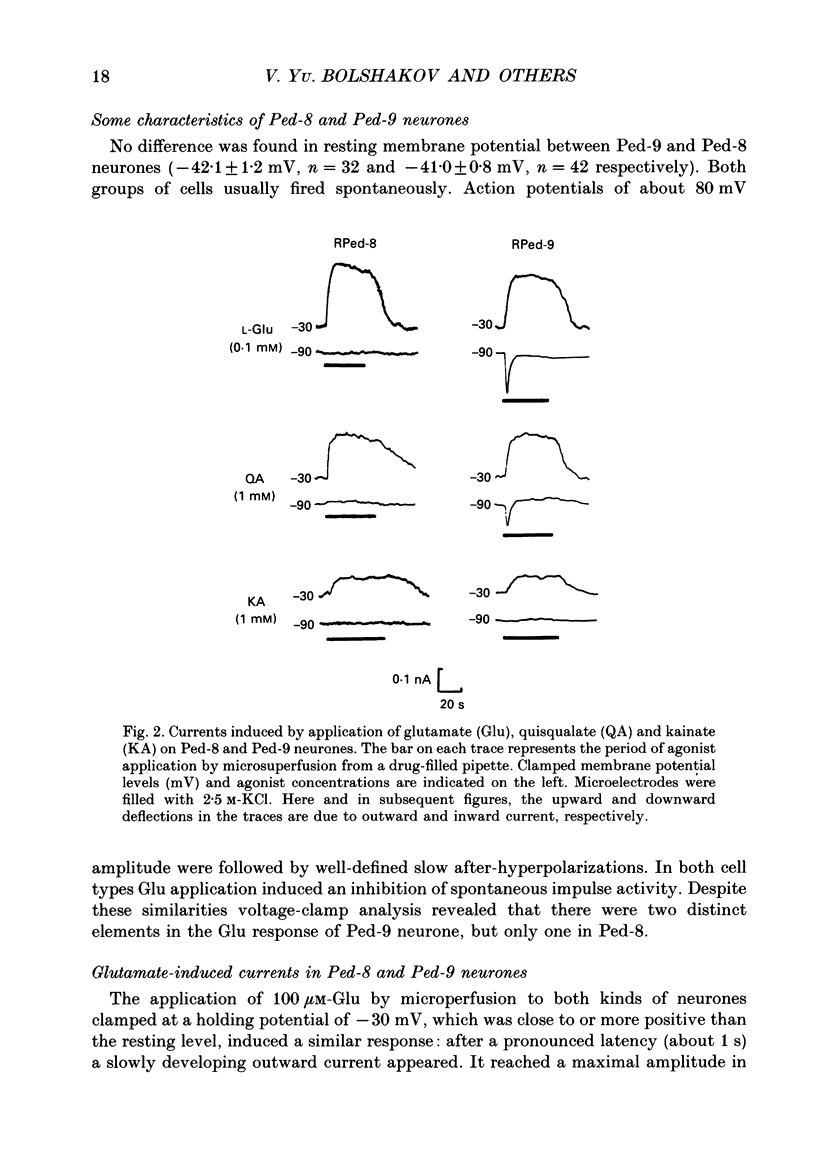
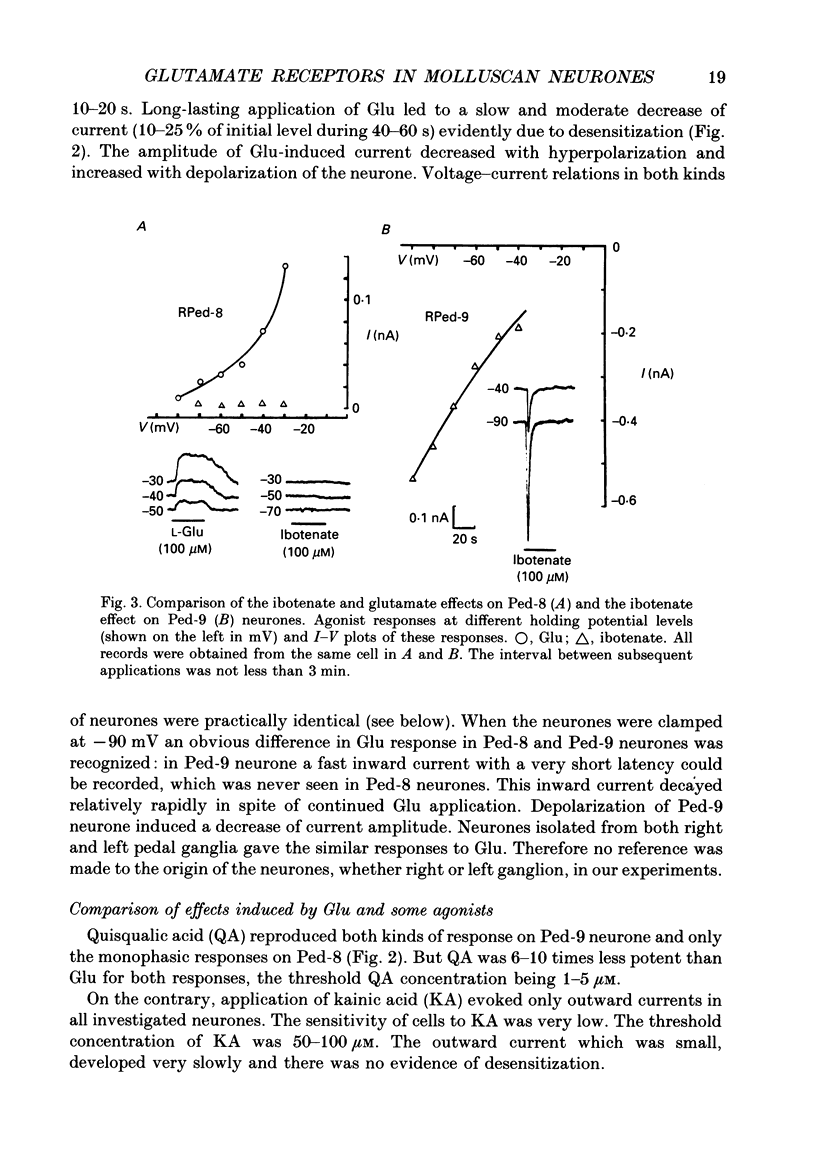
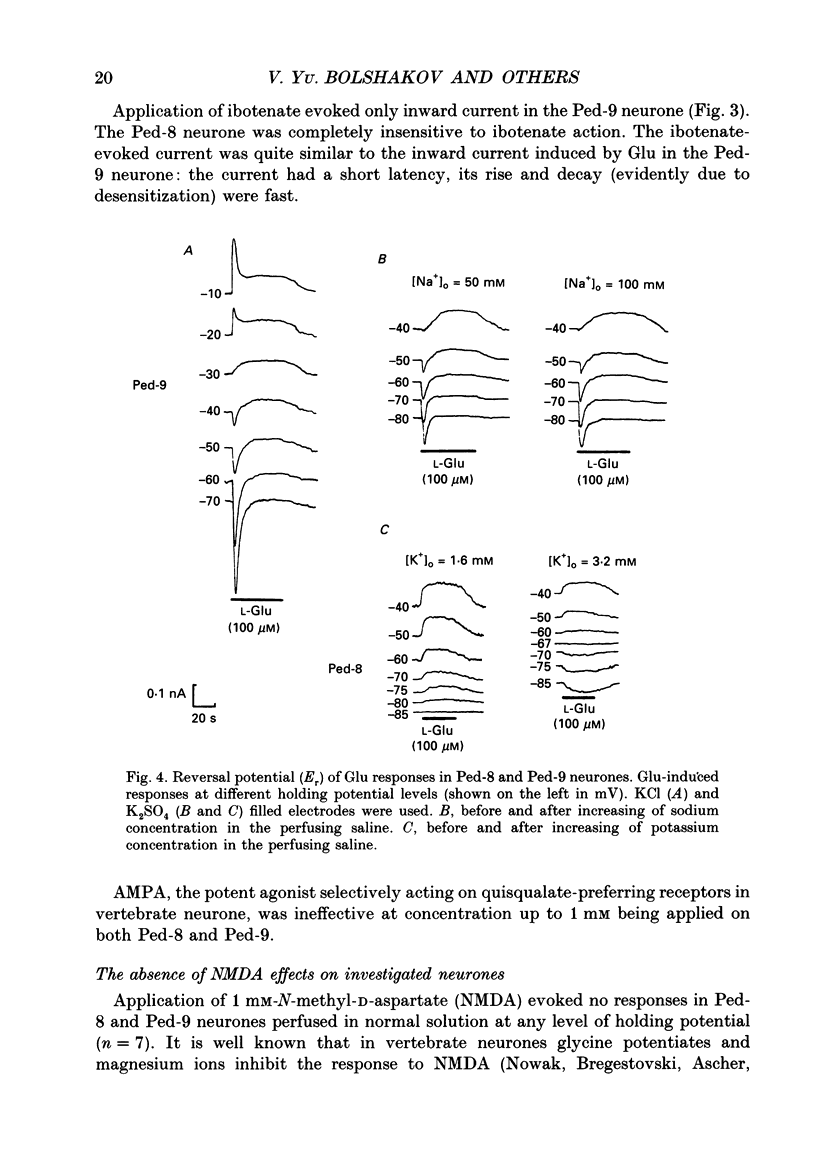
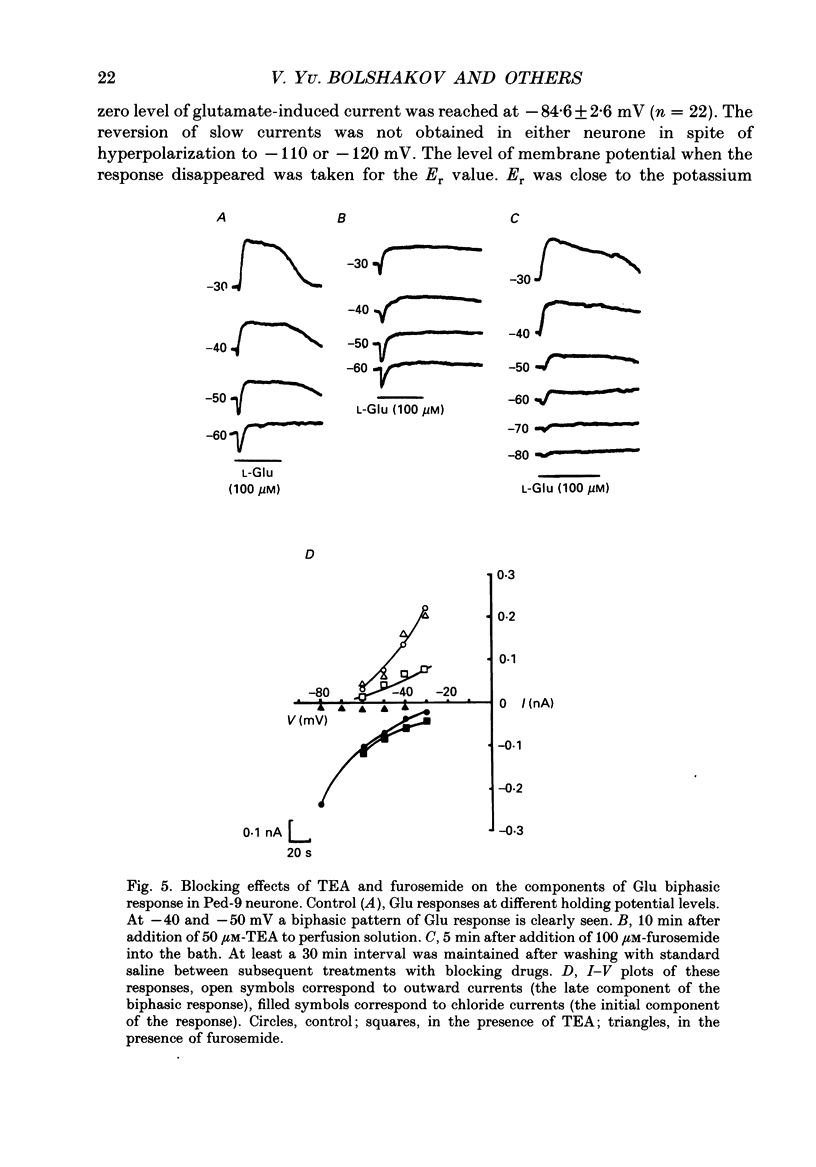
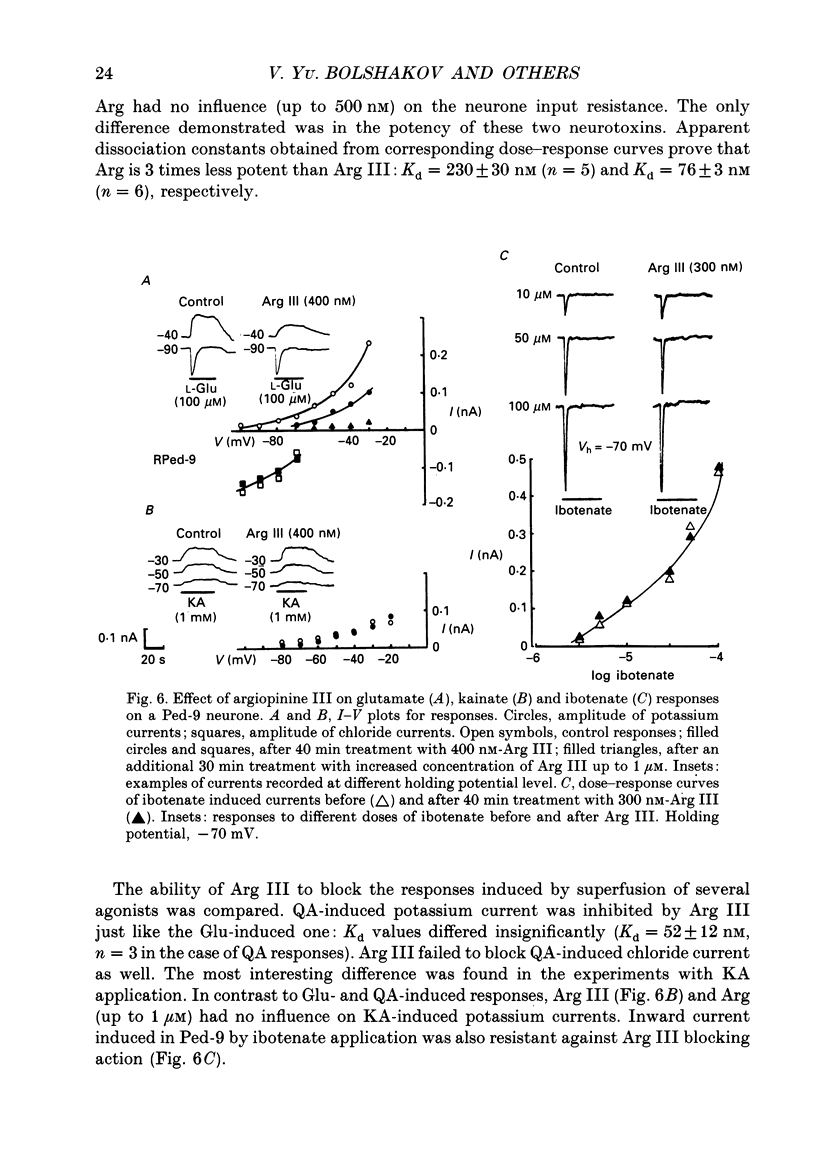
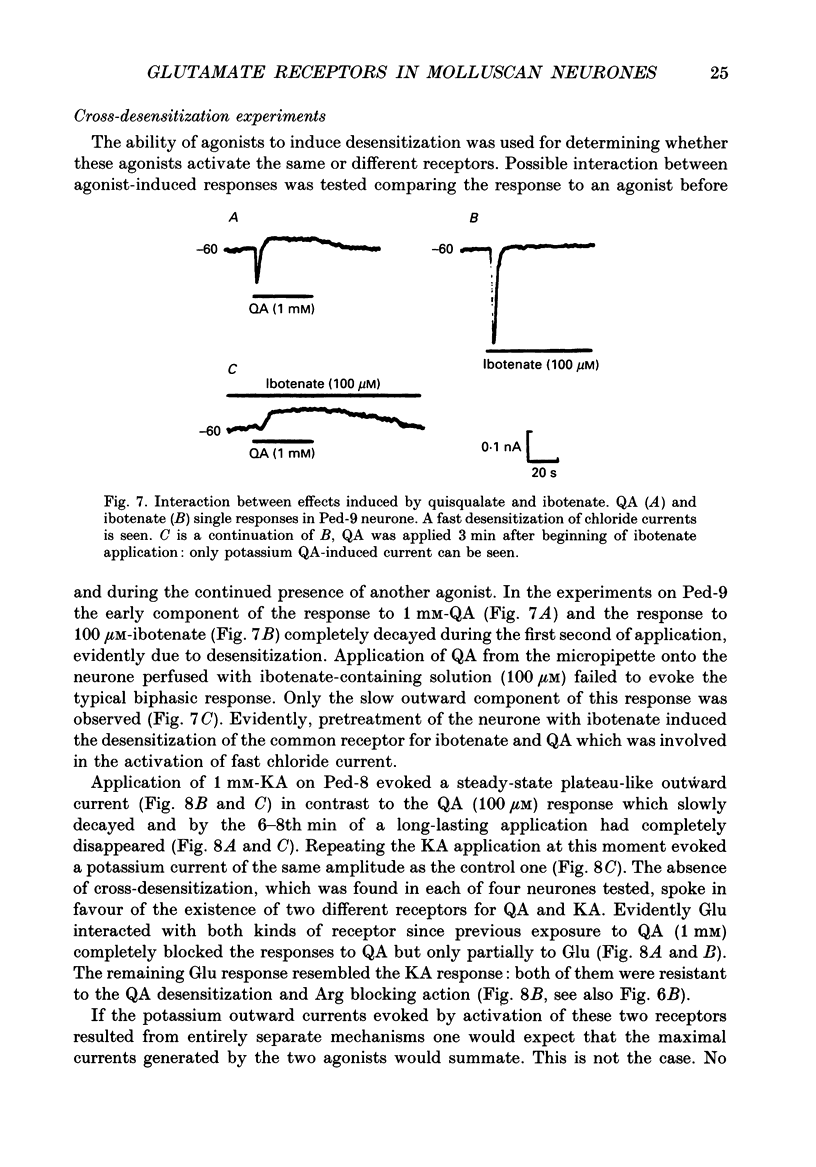
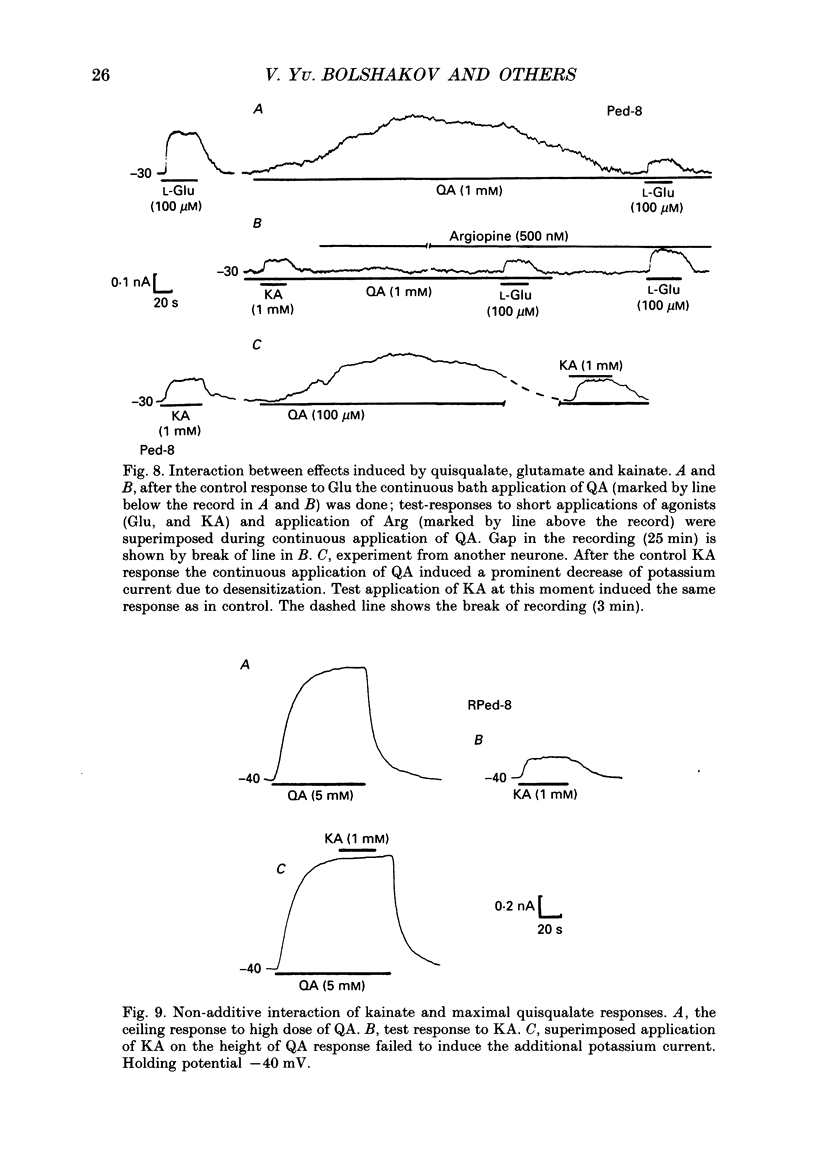
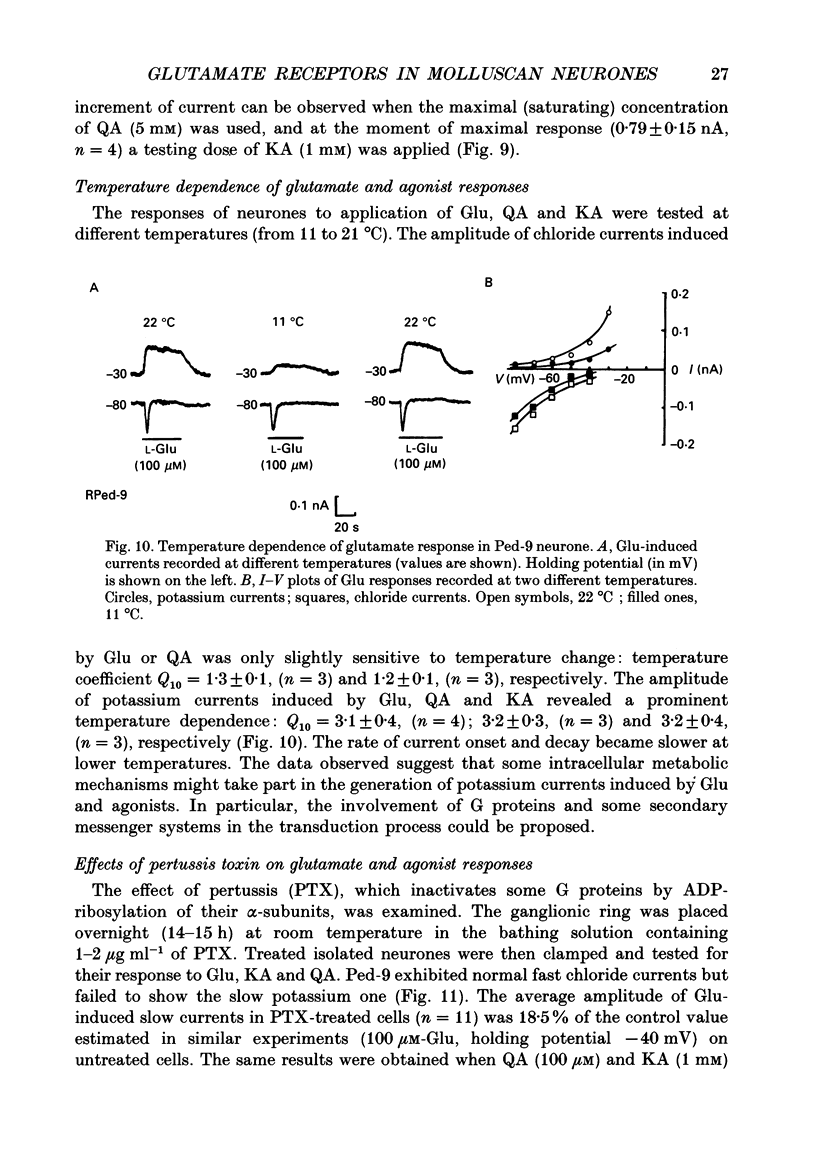
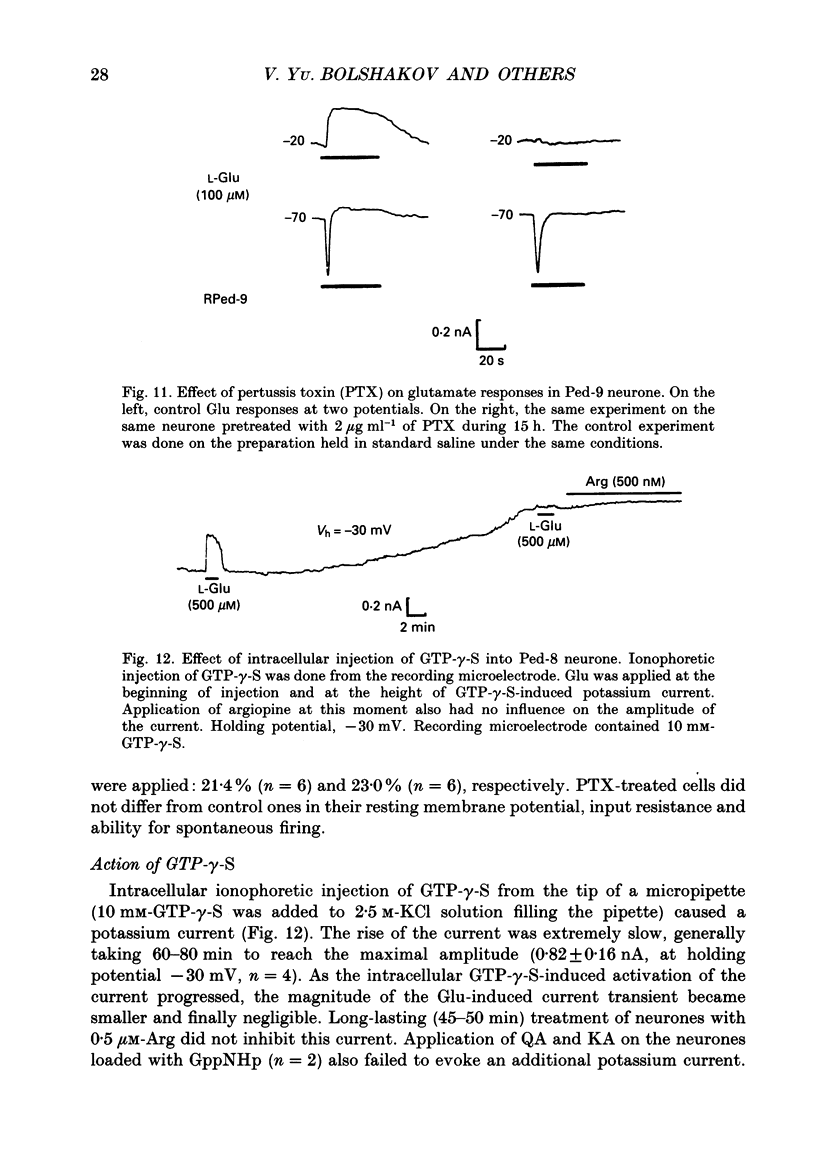
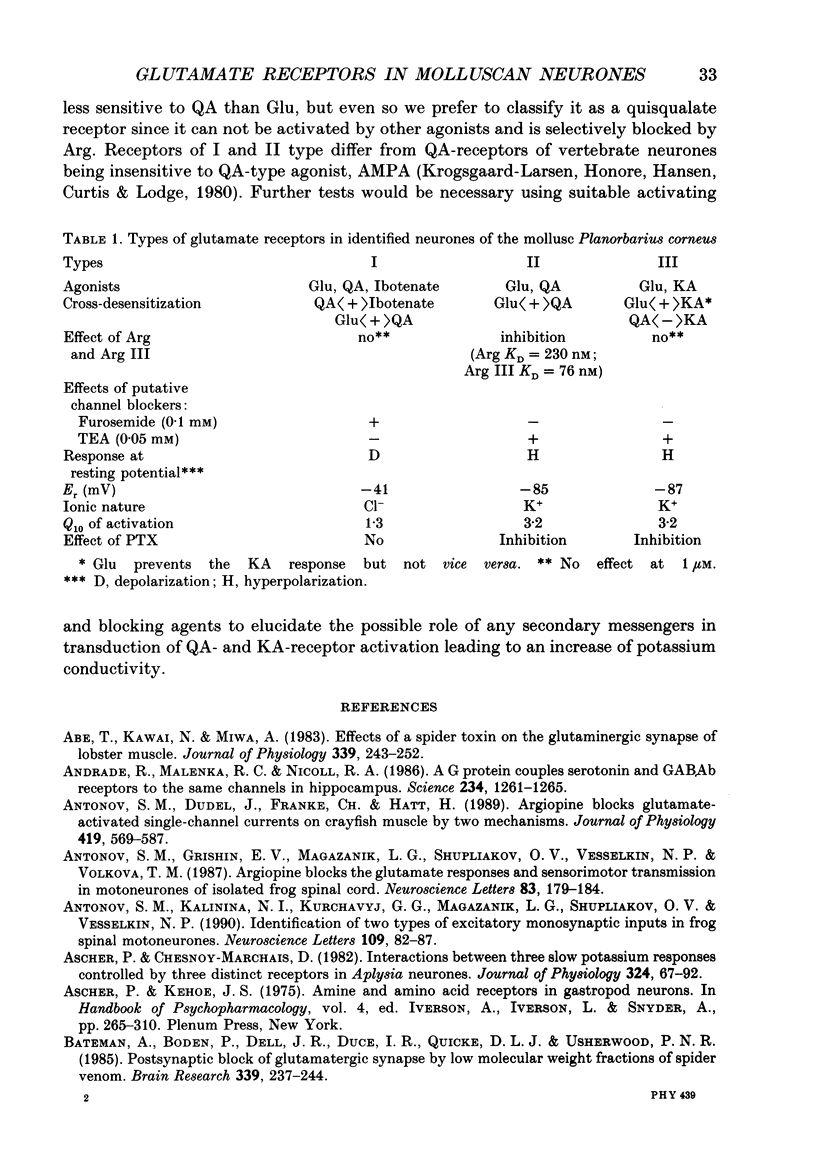
1. The membrane currents evoked by glutamate agonists on isolated and identified neurones of molluscan pedal ganglia were investigated using the voltage clamp technique. 2. The fast chloride current (Er (reversal potential) = -41 mV) evoked in a Ped-9 neurone by application of glutamate, quisqualate and ibotenic acid could be blocked by furosemide (0.1 mM). The slow potassium current (Er = -85 mV) evoked in Ped-8 and Ped-9 neurones by glutamate, quisqualate and kainate could be blocked by tetraethylammonium (50 microM). 3. N-Methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methyl-4-isoxazole-proprionic acid (AMPA) failed to induce a response in neurones studies. 4. The spider venoms argiopine and argiopinine III (50-500 nM) selectively inhibited quisqualate-induced potassium current, but had no influence on glutamate-, ibotenate- or quisqualate-induced chloride and kainate-induced potassium currents. Glutamate-induced potassium current was partially inhibited by argiopine and argiopinine III. 5. The existence of several types of distinct glutamate receptors was confirmed in cross-desensitization experiments, and a lack of interaction was observed between quisqualate and kainate. 6. Potassium currents induced both by quisqualate and kainate strongly depended on temperature and could be blocked by pertussis toxin. Intracellular injection of the calcium chelator, EGTA, did not affect quisqualate and kainate responses. 7. In neurones loaded with non-hydrolysable GTP analogues, GTP-gamma-S (guanosine-5'-O-(3-thio)triphosphate) or GppNHp (5'-guanylylimidodiphosphate), the potassium current was gradually induced in the absence of agonists. As this current progressed, the magnitude of the glutamate- or kainate-evoked current transients became smaller and finally negligible. The GTP-gamma-S-induced current was not inhibited by argiopine. 8. These data indicate that in the molluscan neurones studied there are at least three pharmacologically distinct glutamate receptors: (1) a receptor of quisqualate-ibotenate type which directly controls chloride channel; (2) quisqualate and (3) kainate receptors which control in calcium-independent manner the common potassium channel by activation of GTP-binding protein.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Kawai N., Miwa A. Effects of a spider toxin on the glutaminergic synapse of lobster muscle. J Physiol. 1983 Jun;339:243–252. doi: 10.1113/jphysiol.1983.sp014714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R., Malenka R. C., Nicoll R. A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986 Dec 5;234(4781):1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Antonov S. M., Dudel J., Franke C., Hatt H. Argiopine blocks glutamate-activated single-channel currents on crayfish muscle by two mechanisms. J Physiol. 1989 Dec;419:569–587. doi: 10.1113/jphysiol.1989.sp017887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov S. M., Grishin E. V., Magazanik L. G., Shupliakov O. V., Vesselkin N. P., Volkova T. M. Argiopin blocks the glutamate responses and sensorimotor transmission in motoneurones of isolated frog spinal cord. Neurosci Lett. 1987 Dec 16;83(1-2):179–184. doi: 10.1016/0304-3940(87)90237-0. [DOI] [PubMed] [Google Scholar]
- Antonov S. M., Kalinina N. I., Kurchavyj G. G., Magazanik L. G., Shupliakov O. V., Vesselkin N. P. Identification of two types of excitatory monosynaptic inputs in frog spinal motoneurones. Neurosci Lett. 1990 Feb 5;109(1-2):82–87. doi: 10.1016/0304-3940(90)90541-g. [DOI] [PubMed] [Google Scholar]
- Ascher P., Chesnoy-Marchais D. Interactions between three slow potassium responses controlled by three distinct receptors in Aplysia neurones. J Physiol. 1982 Mar;324:67–92. doi: 10.1113/jphysiol.1982.sp014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A., Boden P., Dell A., Duce I. R., Quicke D. L., Usherwood P. N. Postsynaptic block of a glutamatergic synapse by low molecular weight fractions of spider venom. Brain Res. 1985 Jul 29;339(2):237–244. doi: 10.1016/0006-8993(85)90088-5. [DOI] [PubMed] [Google Scholar]
- Brezina V. Guanosine 5'-triphosphate analogue activates potassium current modulated by neurotransmitters in Aplysia neurones. J Physiol. 1988 Dec;407:15–40. doi: 10.1113/jphysiol.1988.sp017401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Franke C., Hatt H., Usherwood P. N. Chloride channels gated by extrajunctional glutamate receptors (H-receptors) on locust leg muscle. Brain Res. 1989 Mar 6;481(2):215–220. doi: 10.1016/0006-8993(89)90796-8. [DOI] [PubMed] [Google Scholar]
- GERSCHENFELD H. M., LASANSKY A. ACTION OF GLUTAMIC ACID AND OTHER NATURALLY OCCURRING AMINO-ACIDS ON SNAIL CENTRAL NEURONS. Int J Neuropharmacol. 1964 Jul;3:301–314. doi: 10.1016/0028-3908(64)90022-x. [DOI] [PubMed] [Google Scholar]
- Geck P., Heinz E. The Na-K-2Cl cotransport system. J Membr Biol. 1986;91(2):97–105. doi: 10.1007/BF01925787. [DOI] [PubMed] [Google Scholar]
- Ger B. A., Katchman A. N., Zeimal E. V. Biphasic acetylcholine responses of molluscan neurones: their dependence on acetylcholine concentration. Brain Res. 1979 May 11;167(2):426–430. doi: 10.1016/0006-8993(79)90840-0. [DOI] [PubMed] [Google Scholar]
- Ger B. A., Zeimal E. V. Pharmacological study of two kinds of cholinoreceptors on the membrane of identified completely isolated neurones of Planorbarius corneus. Brain Res. 1977 Jan 31;121(1):131–139. doi: 10.1016/0006-8993(77)90443-7. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Ikemoto Y., Akaike N. The glutamate-induced chloride current in Aplysia neurones lacks pharmacological properties seen for excitatory responses to glutamate. Eur J Pharmacol. 1988 Jun 10;150(3):313–318. doi: 10.1016/0014-2999(88)90012-x. [DOI] [PubMed] [Google Scholar]
- Jackson H., Usherwood P. N. Spider toxins as tools for dissecting elements of excitatory amino acid transmission. Trends Neurosci. 1988 Jun;11(6):278–283. doi: 10.1016/0166-2236(88)90112-9. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Jones P. G., Rosser S. J., Bulloch A. G. Glutamate suppression of feeding and the underlying output of effector neurons in Helisoma. Brain Res. 1987 Dec 22;437(1):56–68. doi: 10.1016/0006-8993(87)91526-5. [DOI] [PubMed] [Google Scholar]
- KERKUT G. A., WALKER R. J. The specific chemical sensitivity of Helix nerve cells. Comp Biochem Physiol. 1962 Dec;7:277–288. doi: 10.1016/0010-406x(62)90172-x. [DOI] [PubMed] [Google Scholar]
- Katchman A. N., Zeimal E. V. Ionic mechanisms of the rapid (nicotinic) phase of acetylcholine response in identified Planobarius corneus neurones. Brain Res. 1982 Jun 3;241(1):95–103. doi: 10.1016/0006-8993(82)91232-x. [DOI] [PubMed] [Google Scholar]
- Kato M., Oomura Y., Maruhashi J., Shimizu N. Chemical characteristics of the L-glutamate receptor on the Onchidium neuron. J Neurosci. 1983 Mar;3(3):549–556. doi: 10.1523/JNEUROSCI.03-03-00549.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Synaptic block of a calcium-activated potassium conductance in Aplysia neurones. J Physiol. 1985 Dec;369:439–474. doi: 10.1113/jphysiol.1985.sp015910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Transformation by concanavalin A of the response of molluscan neurones to L-glutamate. Nature. 1978 Aug 31;274(5674):866–869. doi: 10.1038/274866a0. [DOI] [PubMed] [Google Scholar]
- Kerkut G. A., Horn N., Walker R. J. Long-lasting synaptic inhibition and its transmitter in the snail Helix aspersa. Comp Biochem Physiol. 1969 Sep 15;30(6):1061–1074. doi: 10.1016/0010-406x(69)91044-5. [DOI] [PubMed] [Google Scholar]
- Kerry C. J., Ramsey R. L., Sansom M. S., Usherwood P. N. Single channel studies of non-competitive antagonism of a quisqualate-sensitive glutamate receptor by argiotoxin636--a fraction isolated from orb-web spider venom. Brain Res. 1988 Sep 6;459(2):312–327. doi: 10.1016/0006-8993(88)90647-6. [DOI] [PubMed] [Google Scholar]
- Kiskin N. I., Krishtal O. A., Tsyndrenko AYa Excitatory amino acid receptors in hippocampal neurons: kainate fails to desensitize them. Neurosci Lett. 1986 Jan 30;63(3):225–230. doi: 10.1016/0304-3940(86)90360-5. [DOI] [PubMed] [Google Scholar]
- Kostenko M. A., Geletyuk V. I., Veprintsev B. N. Completely isolated neurons in the mollusc, Lymnaea stagnalis. A new objective for nerve cell biology investigation. Comp Biochem Physiol A Comp Physiol. 1974 Sep 1;49(1A):89–100. doi: 10.1016/0300-9629(74)90544-1. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P., Honoré T., Hansen J. J., Curtis D. R., Lodge D. New class of glutamate agonist structurally related to ibotenic acid. Nature. 1980 Mar 6;284(5751):64–66. doi: 10.1038/284064a0. [DOI] [PubMed] [Google Scholar]
- McCreery M. J., Carpenter D. O. Modulation of neuronal responses to L-glutamate in Aplysia. Cell Mol Neurobiol. 1984 Mar;4(1):91–95. doi: 10.1007/BF00710945. [DOI] [PubMed] [Google Scholar]
- Miwa A., Kawai N., Saito M., Pan-Hou H., Yoshioka M. Effect of a spider toxin (JSTX) on excitatory postsynaptic current at neuromuscular synapse of spiny lobster. J Neurophysiol. 1987 Aug;58(2):319–326. doi: 10.1152/jn.1987.58.2.319. [DOI] [PubMed] [Google Scholar]
- Miwa A., Kawai N., Ui M. Pertussis toxin blocks presynaptic glutamate receptors--a novel 'glutamateB' receptor in the lobster neuromuscular synapse. Brain Res. 1987 Jul 21;416(1):162–165. doi: 10.1016/0006-8993(87)91510-1. [DOI] [PubMed] [Google Scholar]
- Neild T. O., Thomas R. C. Intracellular chloride activity and the effects of acetylcholine in snail neurones. J Physiol. 1974 Oct;242(2):453–470. doi: 10.1113/jphysiol.1974.sp010717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A. The blockade of GABA mediated responses in the frog spinal cord by ammonium ions and furosemide. J Physiol. 1978 Oct;283:121–132. doi: 10.1113/jphysiol.1978.sp012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Oomura Y., Ooyama H., Sawada M. Analysis of hyperpolarizations induced by glutamate and acetylcholine on Onchidium neurones. J Physiol. 1974 Dec;243(2):321–341. doi: 10.1113/jphysiol.1974.sp010756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott S. M., Kerkut G. A., Walker R. J. Structure-activity studies on glutamate receptor sites of three identifiable neurones in the sub-oesophageal ganglia of Helix aspersa. Comp Biochem Physiol C. 1975 Jun 1;51(1):91–100. doi: 10.1016/0306-4492(75)90044-1. [DOI] [PubMed] [Google Scholar]
- Saito M., Kawai N., Miwa A., Pan-Hou H., Yoshioka M. Spider toxin (JSTX) blocks glutamate synapse in hippocampal pyramidal neurons. Brain Res. 1985 Nov 4;346(2):397–399. doi: 10.1016/0006-8993(85)90878-9. [DOI] [PubMed] [Google Scholar]
- Smart T. G. Excitatory amino acids: the involvement of second messengers in the signal transduction process. Cell Mol Neurobiol. 1989 Jun;9(2):193–206. doi: 10.1007/BF00713028. [DOI] [PubMed] [Google Scholar]
- Strange P. G. The structure and mechanism of neurotransmitter receptors. Implications for the structure and function of the central nervous system. Biochem J. 1988 Jan 15;249(2):309–318. doi: 10.1042/bj2490309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Szczepaniak A. C., Cottrell G. A. Biphasic action of glutamic acid and synpatic inhibition in an identified serotonin-containing neurone. Nat New Biol. 1973 Jan 10;241(106):62–64. doi: 10.1038/newbio241062a0. [DOI] [PubMed] [Google Scholar]
- Walker R. J. The action of kainic acid and quisqualic acid on the glutamate receptors of three identifiable neurones from the brain of the snail, Helix aspersa. Comp Biochem Physiol C. 1976;55(1):61–67. doi: 10.1016/0306-4492(76)90013-7. [DOI] [PubMed] [Google Scholar]
- Walker R. J., Woodruff G. N., Kerkut G. A. The effect of ibotenic acid and muscimol on single neurons of the snail, Helix aspersa. Comp Gen Pharmacol. 1971 Jun;2(6):168–174. doi: 10.1016/0010-4035(71)90007-3. [DOI] [PubMed] [Google Scholar]


