Abstract
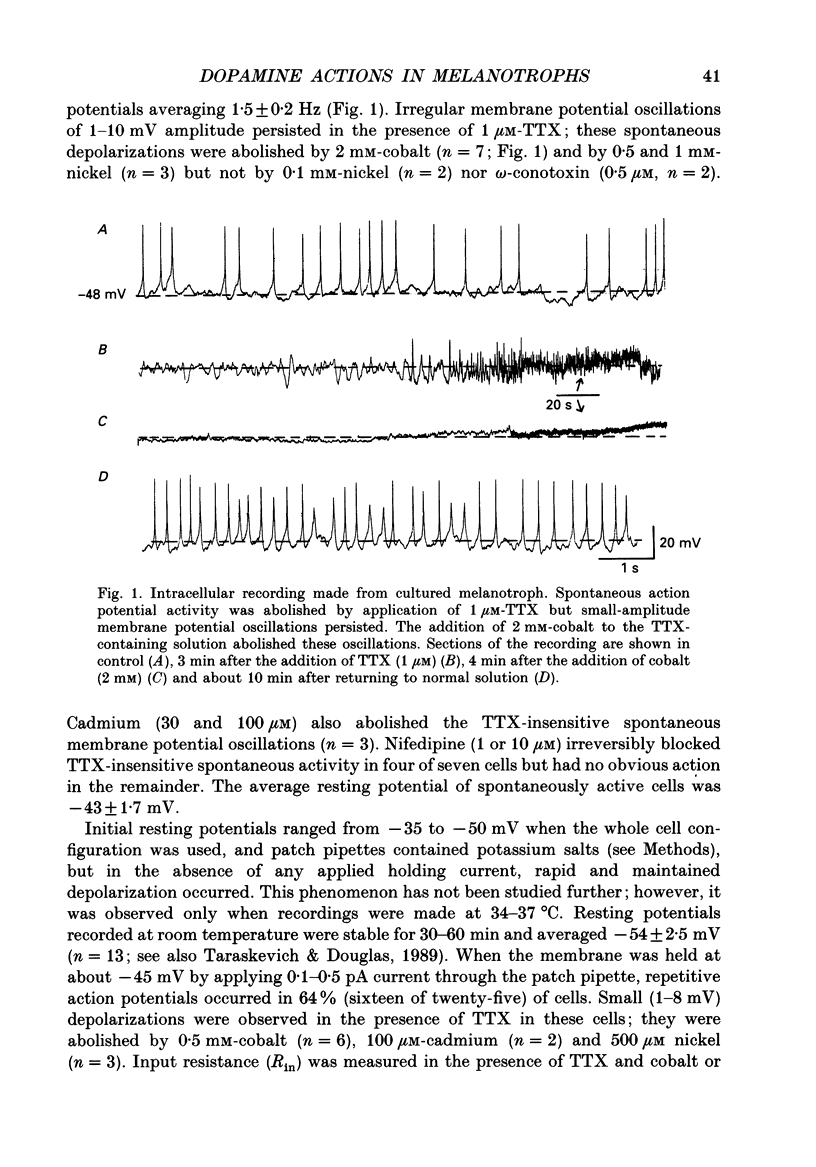
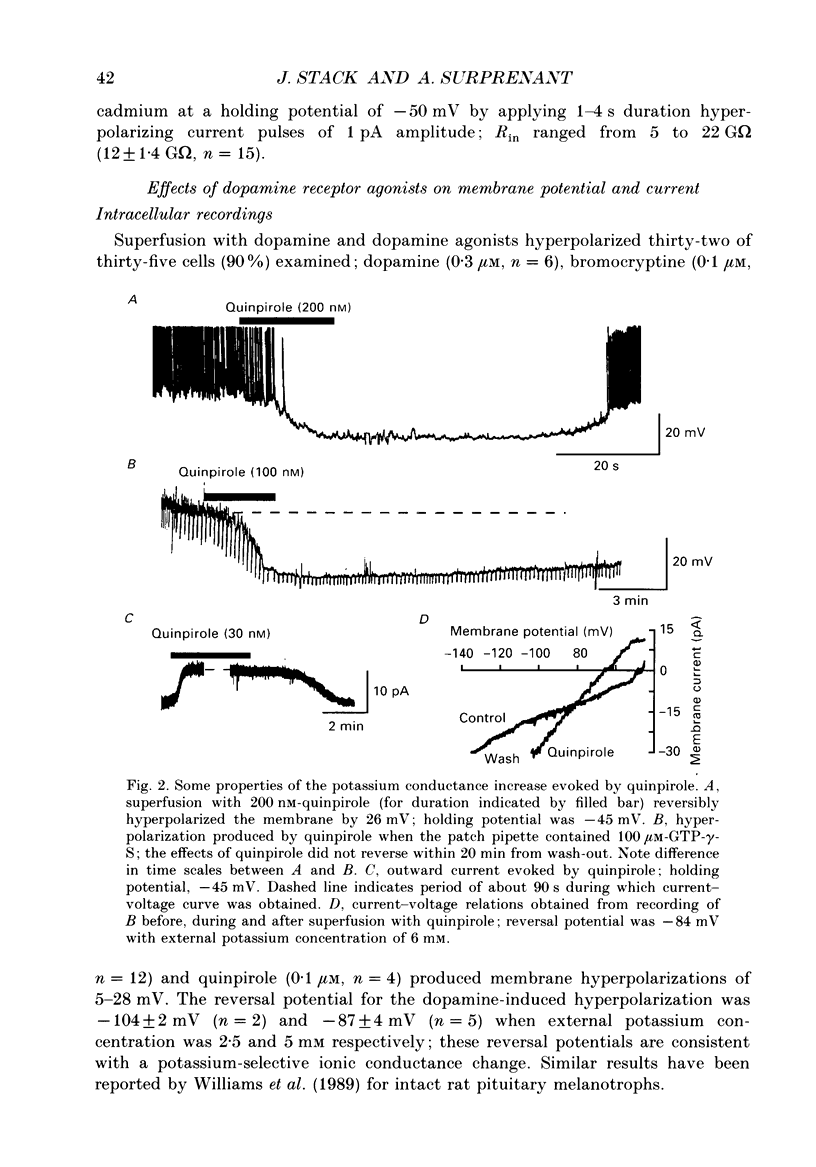
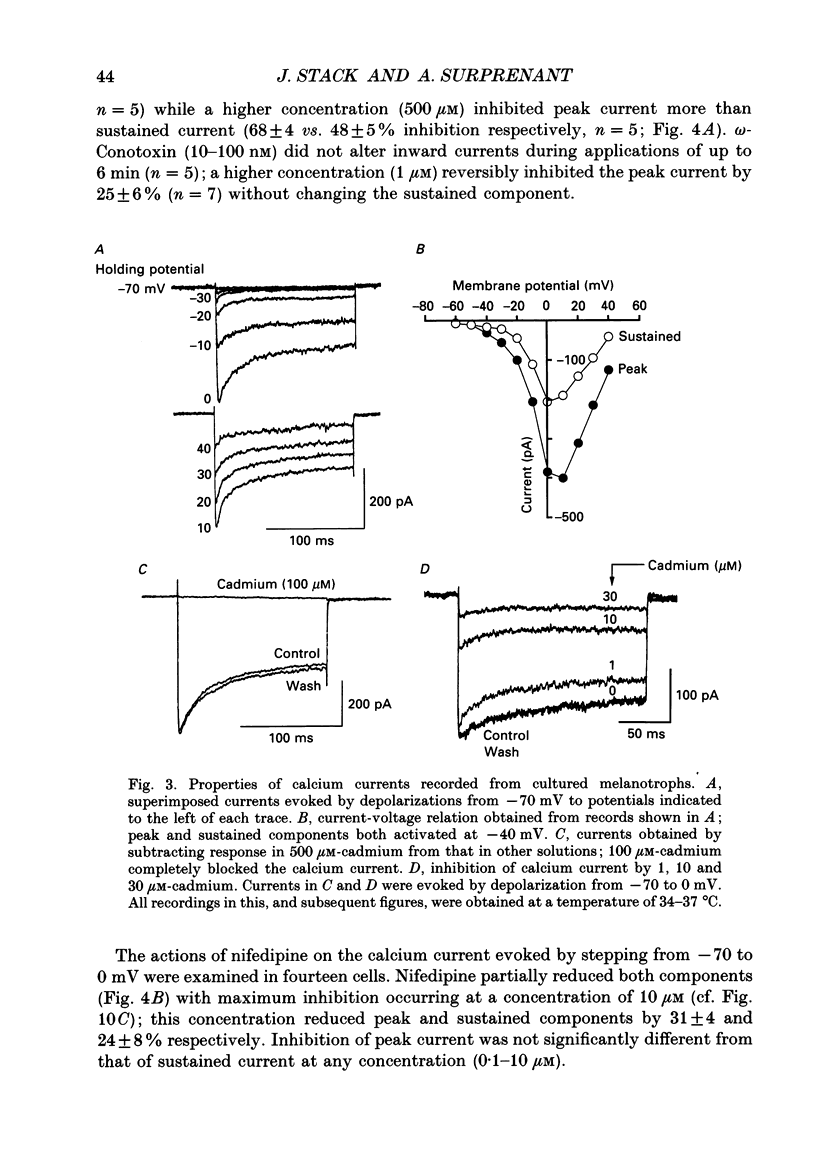
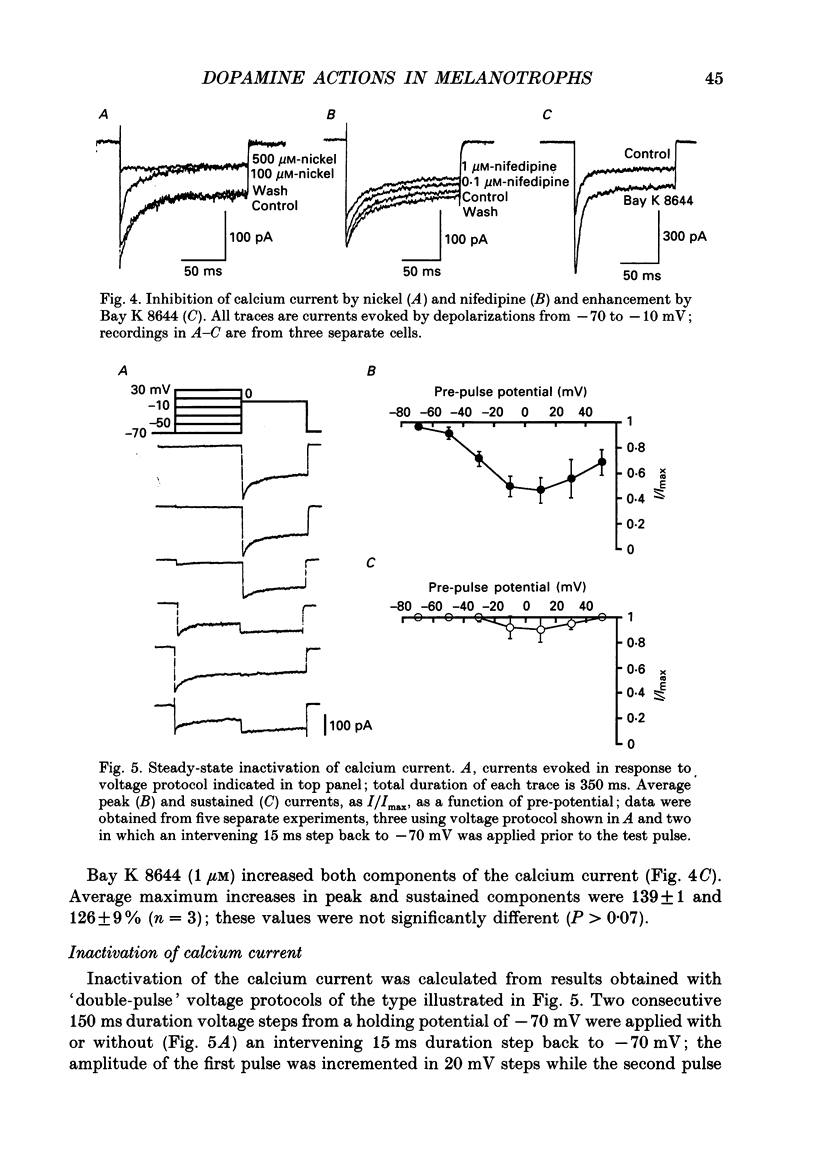
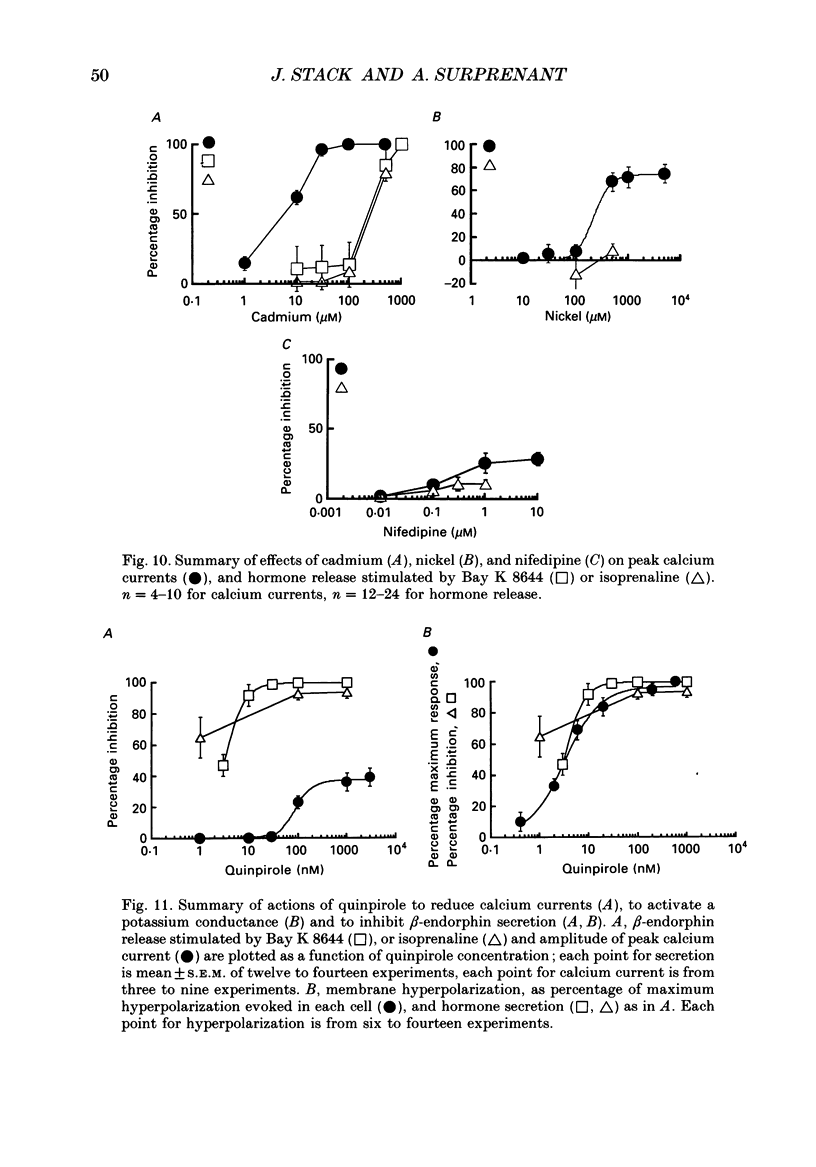
1. Intracellular and whole-cell recordings were made from primary cultures of rat intermediate pituitary cells; beta-endorphin secretion was also measured by radioimmunoassay. The effects of dopamine receptor activation on hormone secretion, calcium currents and resting potassium conductance were compared. 2. Spontaneous sodium-dependent action potentials occurred in 82% of cells recorded with intracellular microelectrodes and 64% of cells recorded with whole-cell patch electrodes; the same proportion of cells showed spontaneous calcium-dependent depolarizations in the presence of tetrodotoxin. 3. Calcium currents recorded from holding potentials of -90 or -70 mV showed transient and sustained components, both of which activated at -40 mV and had similar current-voltage relations. Bay K 8644 (1 microM) increased both components by about 130% while nifedipine (1-10 microM) decreased them by a maximum of 30%. Nickel (500 microM) inhibited transient and sustained components by 68 and 50%; cadmium (100 microM) abolished the current. omega-Conotoxin (1 microM) reversibly inhibited the transient component by 26%. 4. The dopamine D2 receptor agonist, quinpirole (0.1-10 microM) inhibited transient and sustained components in all cells by a maximum of 40 and 25% respectively. Quinpirole did not alter the time course of the current. 5. Quinpirole (1-100 nM) hyperpolarized 90% of cells from which intracellular recordings were made and 55% of cells recorded from with whole-cell patch pipettes. Maximum hyperpolarization of 16 +/- 4 mV from a resting potential of -44 +/- 5 mV was observed with 100 nM-quinpirole; concentration producing half-maximal effect was 3 nM. The hyperpolarization resulted from an increase in potassium conductance. 6. Quinpirole (1-100 nM) decreased basal beta-endorphin secretion by 55% and abolished secretion stimulated by Bay K 8644 or isoprenaline; concentrations producing half-maximal inhibitions were 5-10 nM. Tetrodotoxin (1 microM), nifedipine (1 microM), nickel (500 microM) and cadmium (100 microM) did not alter basal or stimulated secretion although higher concentrations of cadmium did inhibit stimulated hormone release. 7. Pertussis toxin pre-treatment prevented all actions of quinpirole. 8. Thus, concentrations of quinpirole that abolished stimulated hormone secretion did not alter calcium currents; conversely, concentrations of calcium channel blockers that partially or completely inhibited calcium currents did not alter basal or stimulated secretion. These results may indicate that calcium influx through the voltage-dependent calcium channels measured in these experiments does not contribute significantly to hormone release from melanotrophs.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. G., Herbert E., Hinman M., Shibuya H., Pert C. B. Coordinate control of corticotropin, beta-lipotropin, and beta-endorphin release in mouse pituitary cell cultures. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4972–4976. doi: 10.1073/pnas.75.10.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki T., Kasai H. Characterization of two kinds of high-voltage-activated Ca-channel currents in chick sensory neurons. Differential sensitivity to dihydropyridines and omega-conotoxin GVIA. Pflugers Arch. 1989 Jun;414(2):150–156. doi: 10.1007/BF00580957. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Matteson D. R. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science. 1985 Jan 4;227(4682):65–67. doi: 10.1126/science.2578071. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Beaulieu M., Felder R., Kebabian J. W. D-2 dopaminergic agonists and adenosine 3',5'-monophosphate directly regulate the synthesis of alpha-melanocyte-stimulating hormone-like peptides by cultured rat melanotrophs. Endocrinology. 1986 Mar;118(3):1032–1039. doi: 10.1210/endo-118-3-1032. [DOI] [PubMed] [Google Scholar]
- Bittner M. A., Holz R. W., Neubig R. R. Guanine nucleotide effects on catecholamine secretion from digitonin-permeabilized adrenal chromaffin cells. J Biol Chem. 1986 Aug 5;261(22):10182–10188. [PubMed] [Google Scholar]
- Carbone E., Sher E., Clementi F. Ca currents in human neuroblastoma IMR32 cells: kinetics, permeability and pharmacology. Pflugers Arch. 1990 Apr;416(1-2):170–179. doi: 10.1007/BF00370239. [DOI] [PubMed] [Google Scholar]
- Childs G. V., Marchetti C., Brown A. M. Involvement of sodium channels and two types of calcium channels in the regulation of adrenocorticotropin release. Endocrinology. 1987 May;120(5):2059–2069. doi: 10.1210/endo-120-5-2059. [DOI] [PubMed] [Google Scholar]
- Cota G., Hiriart M. Hormonal and neurotransmitter regulation of Ca channel activity in cultured adenohypophyseal cells. Soc Gen Physiol Ser. 1989;44:143–165. [PubMed] [Google Scholar]
- Cote T., Munemura M., Eskay R. L., Kebabian J. W. Biochemical identification of the beta-adrenoceptor and evidence for the involvement of an adenosine 3',5'-monophosphate system in the beta-adrenergically induced release of alph-melanocyte-stimulating hormone in the intermediate lobe of the rat pituitary gland. Endocrinology. 1980 Jul;107(1):108–116. doi: 10.1210/endo-107-1-108. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Elmslie K. S., Zhou W., Jones S. W. LHRH and GTP-gamma-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990 Jul;5(1):75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild S., Itoh Y., Kebabian J. W., Luini A., Reisine T. Forskolin enhances basal and potassium-evoked hormone release from normal and malignant pituitary tissue: the role of calcium. Endocrinology. 1986 Jan;118(1):268–279. doi: 10.1210/endo-118-1-268. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield J. M., Allen R. G., Stack J., Ronnekleiv O. Post-translational processing of pro-opiomelanocortin (POMC)-derived peptides during fetal monkey pituitary development. II. beta-Lipotropin (beta-LPH)-related peptides. Dev Biol. 1988 Mar;126(1):164–172. doi: 10.1016/0012-1606(88)90250-3. [DOI] [PubMed] [Google Scholar]
- Israel J. M., Kirk C., Vincent J. D. Electrophysiological responses to dopamine of rat hypophysial cells in lactotroph-enriched primary cultures. J Physiol. 1987 Sep;390:1–22. doi: 10.1113/jphysiol.1987.sp016682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. II. Inactivation. J Gen Physiol. 1989 Jul;94(1):169–182. doi: 10.1085/jgp.94.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M. MIF-1 and Tyr-MIF-1 antagonize morphine and opioid but not non-opioid stress-induced analgesia in the snail, Cepaea nemoralis. Peptides. 1987 Jan-Feb;8(1):1–5. doi: 10.1016/0196-9781(87)90155-0. [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Kongsamut S., Tsien R. W. Alpha-adrenergic inhibition of sympathetic neurotransmitter release mediated by modulation of N-type calcium-channel gating. Nature. 1989 Aug 24;340(6235):639–642. doi: 10.1038/340639a0. [DOI] [PubMed] [Google Scholar]
- Lledo P. M., Legendre P., Israel J. M., Vincent J. D. Dopamine inhibits two characterized voltage-dependent calcium currents in identified rat lactotroph cells. Endocrinology. 1990 Sep;127(3):990–1001. doi: 10.1210/endo-127-3-990. [DOI] [PubMed] [Google Scholar]
- Luini A., De Matteis M. A. Evidence that receptor-linked G protein inhibits exocytosis by a post-second-messenger mechanism in AtT-20 cells. J Neurochem. 1990 Jan;54(1):30–38. doi: 10.1111/j.1471-4159.1990.tb13279.x. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Synthesis and secretion of corticotropins, melanotropins, and endorphins by rat intermediate pituitary cells. J Biol Chem. 1979 Aug 25;254(16):7885–7894. [PubMed] [Google Scholar]
- Marchetti C., Carbone E., Lux H. D. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflugers Arch. 1986 Feb;406(2):104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Sikdar S. K. Characteristics of voltage-gated Ca2+ currents in ovine gonadotrophs. J Physiol. 1989 Aug;415:367–391. doi: 10.1113/jphysiol.1989.sp017726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. T., Sikdar S. K. Characterization of voltage-gated sodium channels in ovine gonadotrophs: relationship to hormone secretion. J Physiol. 1988 May;399:493–517. doi: 10.1113/jphysiol.1988.sp017093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D., Tsien R. W. Different types of calcium channels. J Exp Biol. 1986 Sep;124:177–190. doi: 10.1242/jeb.124.1.177. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Goldman M. E., Kebabian J. W. Forskolin stimulates adenylate cyclase activity, adenosine 3',5'-monophosphate production and peptide release from the intermediate lobe of the rat pituitary gland. Endocrinology. 1984 Mar;114(3):761–766. doi: 10.1210/endo-114-3-761. [DOI] [PubMed] [Google Scholar]
- Munemura M., Eskay R. L., Kebabian J. W. Release of alpha-melanocyte-stimulating hormone from dispersed cells of the intermediate lobe of the rat pituitary gland: involvement of catecholamines and adenosine 3',5'-monophosphate. Endocrinology. 1980 Jun;106(6):1795–1803. doi: 10.1210/endo-106-6-1795. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- North R. A. Twelfth Gaddum memorial lecture. Drug receptors and the inhibition of nerve cells. Br J Pharmacol. 1989 Sep;98(1):13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Rane S. G., Holz G. G., 4th, Dunlap K. Dihydropyridine inhibition of neuronal calcium current and substance P release. Pflugers Arch. 1987 Aug;409(4-5):361–366. doi: 10.1007/BF00583789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees L. H., Cook D. M., Kendall J. W., Allen C. F., Kramer R. M., Ratcliffe J. G., Knight R. A. A radioimmunoassay for rat plasma ACTH. Endocrinology. 1971 Jul;89(1):254–261. doi: 10.1210/endo-89-1-254. [DOI] [PubMed] [Google Scholar]
- Schofield G. G., Ikeda S. R. Sodium and calcium currents of acutely isolated adult rat superior cervical ganglion neurons. Pflugers Arch. 1988 May;411(5):481–490. doi: 10.1007/BF00582368. [DOI] [PubMed] [Google Scholar]
- Shen K. Z., Surprenant A. Mechanisms underlying presynaptic inhibition through alpha 2-adrenoceptors in guinea-pig submucosal neurones. J Physiol. 1990 Dec;431:609–628. doi: 10.1113/jphysiol.1990.sp018350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A., Shen K. Z., North R. A., Tatsumi H. Inhibition of calcium currents by noradrenaline, somatostatin and opioids in guinea-pig submucosal neurones. J Physiol. 1990 Dec;431:585–608. doi: 10.1113/jphysiol.1990.sp018349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Effects of BAY K 8644 on Ca-channel currents and electrical activity in mouse melanotrophs. Brain Res. 1989 Jul 3;491(1):102–108. doi: 10.1016/0006-8993(89)90091-7. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Effects of Bay K 8644 and other dihydropyridines on basal and potassium-evoked output of MSH from mouse melanotrophs in vitro. Neuroendocrinology. 1986;44(3):384–389. doi: 10.1159/000124673. [DOI] [PubMed] [Google Scholar]
- Tomiko S. A., Taraskevich P. S., Douglas W. W. Effects of veratridine, tetrodotoxin and other drugs that alter electrical behaviour on secretion of melanocyte-stimulating hormone from melanotrophs of the pituitary pars intermedia. Neuroscience. 1984 Aug;12(4):1223–1228. doi: 10.1016/0306-4522(84)90016-2. [DOI] [PubMed] [Google Scholar]
- Ullrich S., Wollheim C. B. GTP-dependent inhibition of insulin secretion by epinephrine in permeabilized RINm5F cells. Lack of correlation between insulin secretion and cyclic AMP levels. J Biol Chem. 1988 Jun 25;263(18):8615–8620. [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Williams P. J., MacVicar B. A., Pittman Q. J. A dopaminergic inhibitory postsynaptic potential mediated by an increased potassium conductance. Neuroscience. 1989;31(3):673–681. doi: 10.1016/0306-4522(89)90432-6. [DOI] [PubMed] [Google Scholar]
- Williams P. J., MacVicar B. A., Pittman Q. J. Synaptic modulation by dopamine of calcium currents in rat pars intermedia. J Neurosci. 1990 Mar;10(3):757–763. doi: 10.1523/JNEUROSCI.10-03-00757.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikami D., Bagabaldo Z., Olivera B. M. The inhibitory effects of omega-conotoxins on Ca channels and synapses. Ann N Y Acad Sci. 1989;560:230–248. doi: 10.1111/j.1749-6632.1989.tb24100.x. [DOI] [PubMed] [Google Scholar]


