Abstract
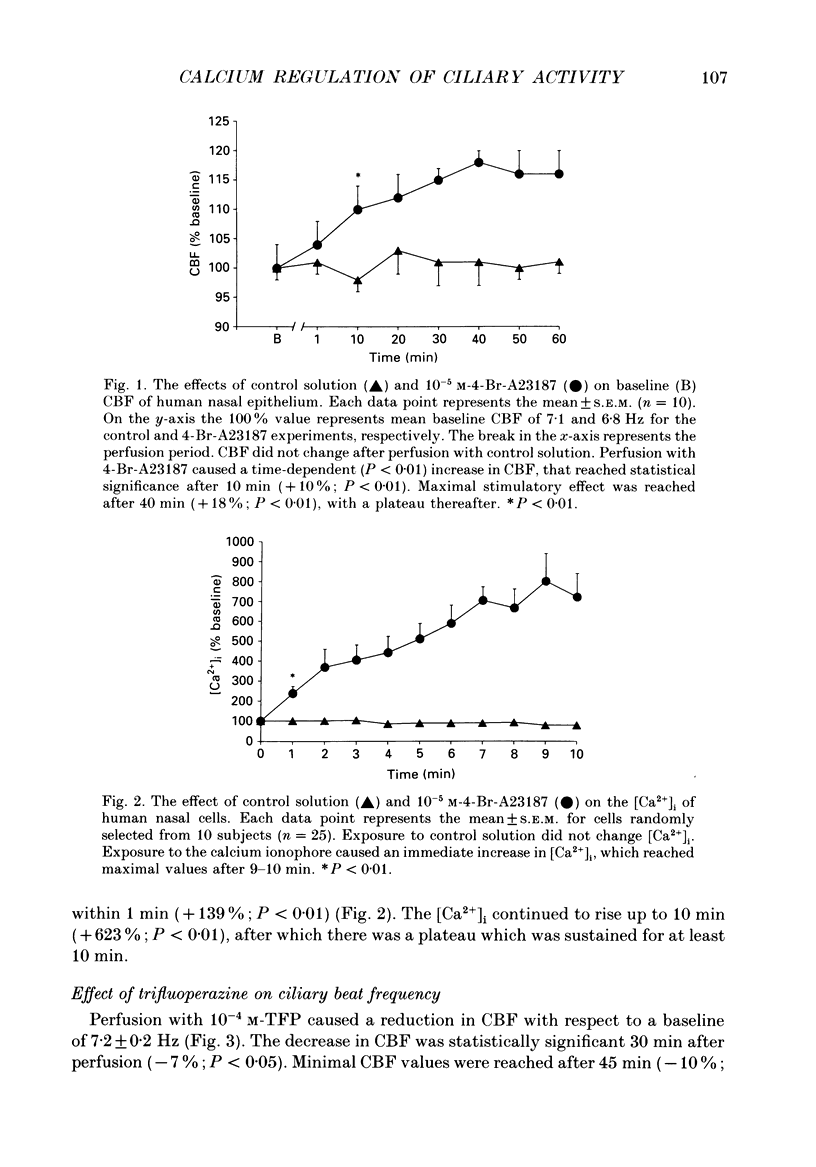
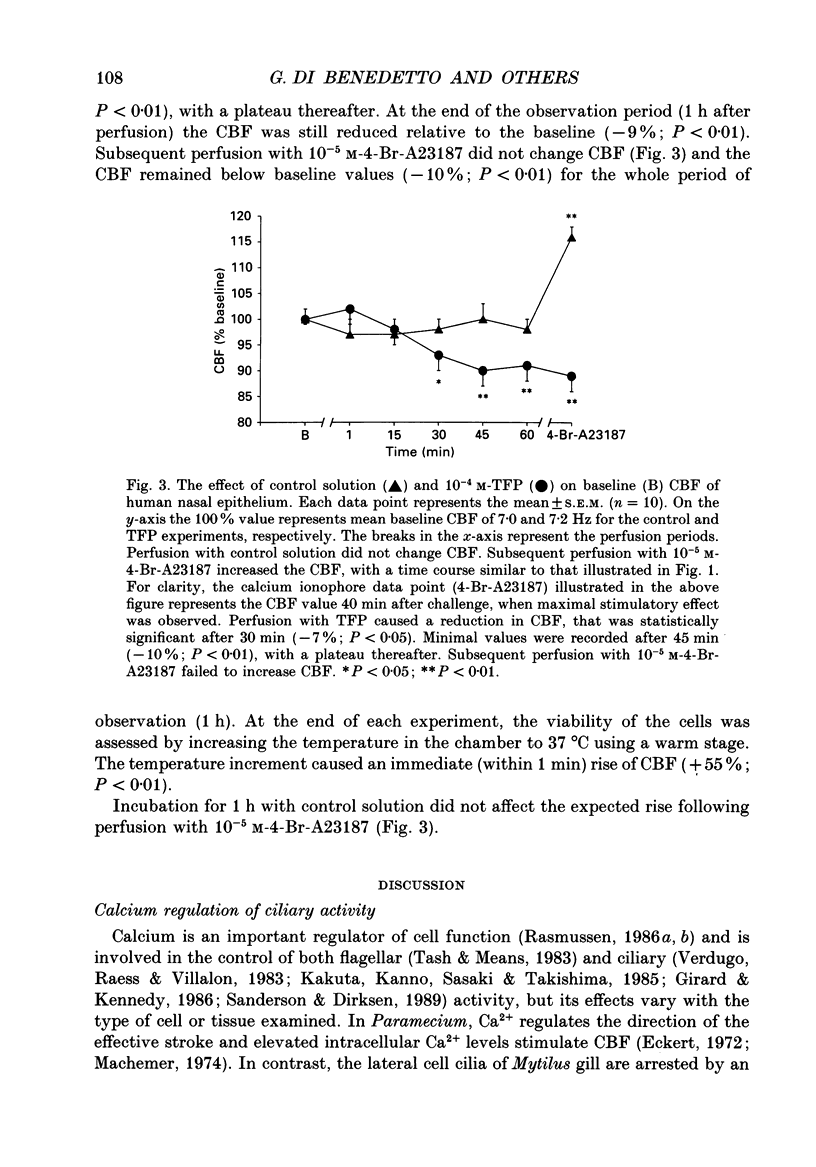
1. The changes in ciliary beat frequency (CBF) of human nasal respiratory epithelial cells were measured in vitro with a photometric technique following exposure to either 4-bromo-calcium ionophore A23187 (4-Br-A23187) or trifluoperazine (TFP), an inhibitor of calmodulin-sensitive calcium-dependent protein kinases. Changes in intracellular free calcium concentrations in response to 4-Br-A23187 were studied using a fluorescent dye (Fura-2). 2. Addition of 10(-5) M-4-Br-A23187 caused a time-dependent (P less than 0.01) rise in CBF. The increment in CBF was statistically significant 10 min after challenge (+10%; P less than 0.01) and was sustained for at least 1 h, with maximal stimulation after 40 min (+ 18%; P less than 0.01). 3. Exposure to 10(-5) M-4-Br-A23187 caused an immediate increase in intracellular free calcium concentration, which preceded the rise in CBF. 4. TFP (10(-4) M) caused a reduction of baseline CBF (-10%; P less than 0.01) and prevented the expected rise when the cells were subsequently exposed to 10(-5) M-4-Br-A23187. 5. We conclude that: (1) calcium ionophore stimulates the CBF of human respiratory cells; (2) this effect is mediated through a calmodulin-sensitive system, since it is abolished in the presence of TFP; (3) the same pathway appears to control the basal CBF of these cells, since TFP also decreases CBF.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Babcock D. F., First N. L., Lardy H. A. Action of ionophore A23187 at the cellular level. Separation of effects at the plasma and mitochondrial membranes. J Biol Chem. 1976 Jul 10;251(13):3881–3886. [PubMed] [Google Scholar]
- Colbran R. J., Schworer C. M., Hashimoto Y., Fong Y. L., Rich D. P., Smith M. K., Soderling T. R. Calcium/calmodulin-dependent protein kinase II. Biochem J. 1989 Mar 1;258(2):313–325. doi: 10.1042/bj2580313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALHAMN T., RYLANDER R. Frequency of ciliary beat measured with a photo-sensitive cell. Nature. 1962 Nov 10;196:592–593. doi: 10.1038/196592a0. [DOI] [PubMed] [Google Scholar]
- Deber C. M., Tom-Kun J., Mack E., Grinstein S. Bromo-A23187: a nonfluorescent calcium ionophore for use with fluorescent probes. Anal Biochem. 1985 May 1;146(2):349–352. doi: 10.1016/0003-2697(85)90550-0. [DOI] [PubMed] [Google Scholar]
- Debono M., Molloy R. M., Dorman D. E., Paschal J. W., Babcock D. F., Deber C. M., Pfeiffer D. R. Synthesis and characterization of halogenated derivatives of the ionophore A23187: enhanced calcium ion transport specificity by the 4-bromo derivative. Biochemistry. 1981 Nov 24;20(24):6865–6872. doi: 10.1021/bi00527a019. [DOI] [PubMed] [Google Scholar]
- Di Benedetto G., Gill J., Lopez-Vidriero M. T., Clarke S. W. The effect of cryopreservation on ciliary beat frequency of human respiratory epithelium. Cryobiology. 1989 Aug;26(4):328–332. doi: 10.1016/0011-2240(89)90056-4. [DOI] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Girard P. R., Kennedy J. R. Calcium regulation of ciliary activity in rabbit tracheal epithelial explants and outgrowth. Eur J Cell Biol. 1986 Apr;40(2):203–209. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Kakuta Y., Kanno T., Sasaki H., Takishima T. Effect of Ca2+ on the ciliary beat frequency of skinned dog tracheal epithelium. Respir Physiol. 1985 Apr;60(1):9–19. doi: 10.1016/0034-5687(85)90036-2. [DOI] [PubMed] [Google Scholar]
- Kennedy J. R., Duckett K. E. The study of ciliary frequencies with an optical spectrum analysis system. Exp Cell Res. 1981 Sep;135(1):147–156. doi: 10.1016/0014-4827(81)90307-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Tamaoki J., Sakai N., Chiyotani A., Takizawa T. Inhibition of ciliary activity by phorbol esters in rabbit tracheal epithelial cells. Lung. 1989;167(5):277–284. doi: 10.1007/BF02714957. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Mechanism by which psychotropic drugs inhibit adenosine cyclic 3',5'-monophosphate phosphodiesterase of brain. Mol Pharmacol. 1976 Jul;12(4):581–589. [PubMed] [Google Scholar]
- Luk C. K., Dulfano M. J. Effect of pH, viscosity and ionic-strength changes on ciliary beating frequency of human bronchial explants. Clin Sci (Lond) 1983 Apr;64(4):449–451. doi: 10.1042/cs0640449. [DOI] [PubMed] [Google Scholar]
- Murphy E., Cheng E., Yankaskas J., Stutts M. J., Boucher R. C. Cell calcium levels of normal and cystic fibrosis nasal epithelium. Pediatr Res. 1988 Jul;24(1):79–84. doi: 10.1203/00006450-198807000-00019. [DOI] [PubMed] [Google Scholar]
- Prince W. T., Rasmussen H., Berridge M. J. The role of calcium in fly salivary gland secretion analyzed with the ionophore A-23187. Biochim Biophys Acta. 1973 Nov 2;329(1):98–107. doi: 10.1016/0304-4165(73)90012-3. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. The calcium messenger system (1). N Engl J Med. 1986 Apr 24;314(17):1094–1101. doi: 10.1056/NEJM198604243141707. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. The calcium messenger system (2). N Engl J Med. 1986 May 1;314(18):1164–1170. doi: 10.1056/NEJM198605013141807. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Ross S. M., Corrsin S. Results of an analytical model of mucociliary pumping. J Appl Physiol. 1974 Sep;37(3):333–340. doi: 10.1152/jappl.1974.37.3.333. [DOI] [PubMed] [Google Scholar]
- Rutland J., Cole P. J. Non-invasive sampling of nasal cilia for measurement of beat frequency and study of ultrastructure. Lancet. 1980 Sep 13;2(8194):564–565. doi: 10.1016/s0140-6736(80)91995-9. [DOI] [PubMed] [Google Scholar]
- Sanderson M. J., Dirksen E. R. Mechanosensitive and beta-adrenergic control of the ciliary beat frequency of mammalian respiratory tract cells in culture. Am Rev Respir Dis. 1989 Feb;139(2):432–440. doi: 10.1164/ajrccm/139.2.432. [DOI] [PubMed] [Google Scholar]
- Schatzman R. C., Wise B. C., Kuo J. F. Phospholipid-sensitive calcium-dependent protein kinase: inhibition by antipsychotic drugs. Biochem Biophys Res Commun. 1981 Feb 12;98(3):669–676. doi: 10.1016/0006-291x(81)91166-9. [DOI] [PubMed] [Google Scholar]
- Seybold Z. V., Mariassy A. T., Stroh D., Kim C. S., Gazeroglu H., Wanner A. Mucociliary interaction in vitro: effects of physiological and inflammatory stimuli. J Appl Physiol (1985) 1990 Apr;68(4):1421–1426. doi: 10.1152/jappl.1990.68.4.1421. [DOI] [PubMed] [Google Scholar]
- Sleigh M. A., Blake J. R., Liron N. The propulsion of mucus by cilia. Am Rev Respir Dis. 1988 Mar;137(3):726–741. doi: 10.1164/ajrccm/137.3.726. [DOI] [PubMed] [Google Scholar]
- Stolze H., Schulz I. Effect of atropine, ouabain, antimycin A, and A23187 on "trigger Ca2+ pool" in exocrine pancreas. Am J Physiol. 1980 Apr;238(4):G338–G348. doi: 10.1152/ajpgi.1980.238.4.G338. [DOI] [PubMed] [Google Scholar]
- Tash J. S., Means A. R. Cyclic adenosine 3',5' monophosphate, calcium and protein phosphorylation in flagellar motility. Biol Reprod. 1983 Feb;28(1):75–104. doi: 10.1095/biolreprod28.1.75. [DOI] [PubMed] [Google Scholar]
- Verdugo P., Raess B. V., Villalon M. The role of calmodulin in the regulation of ciliary movement in mammalian epithelial cilia. J Submicrosc Cytol. 1983 Jan;15(1):95–96. [PubMed] [Google Scholar]
- Wanner A. Clinical aspects of mucociliary transport. Am Rev Respir Dis. 1977 Jul;116(1):73–125. doi: 10.1164/arrd.1977.116.1.73. [DOI] [PubMed] [Google Scholar]


