Abstract
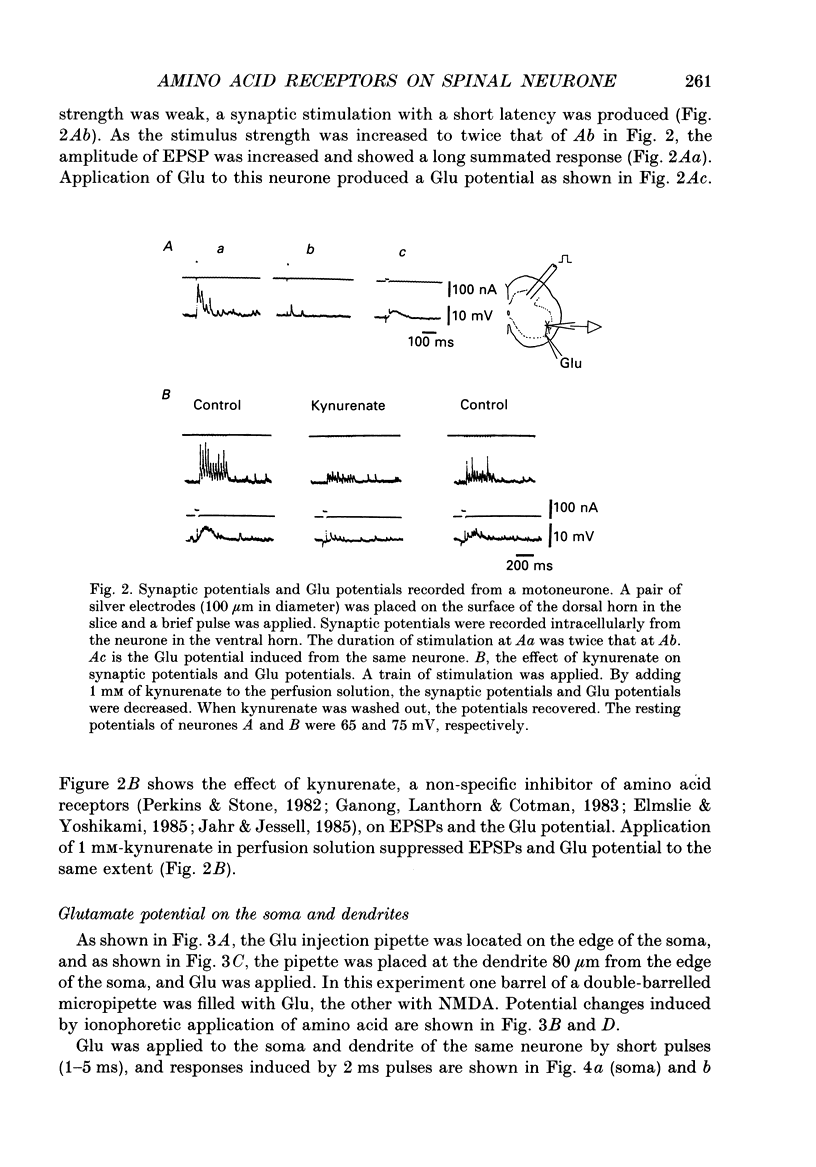
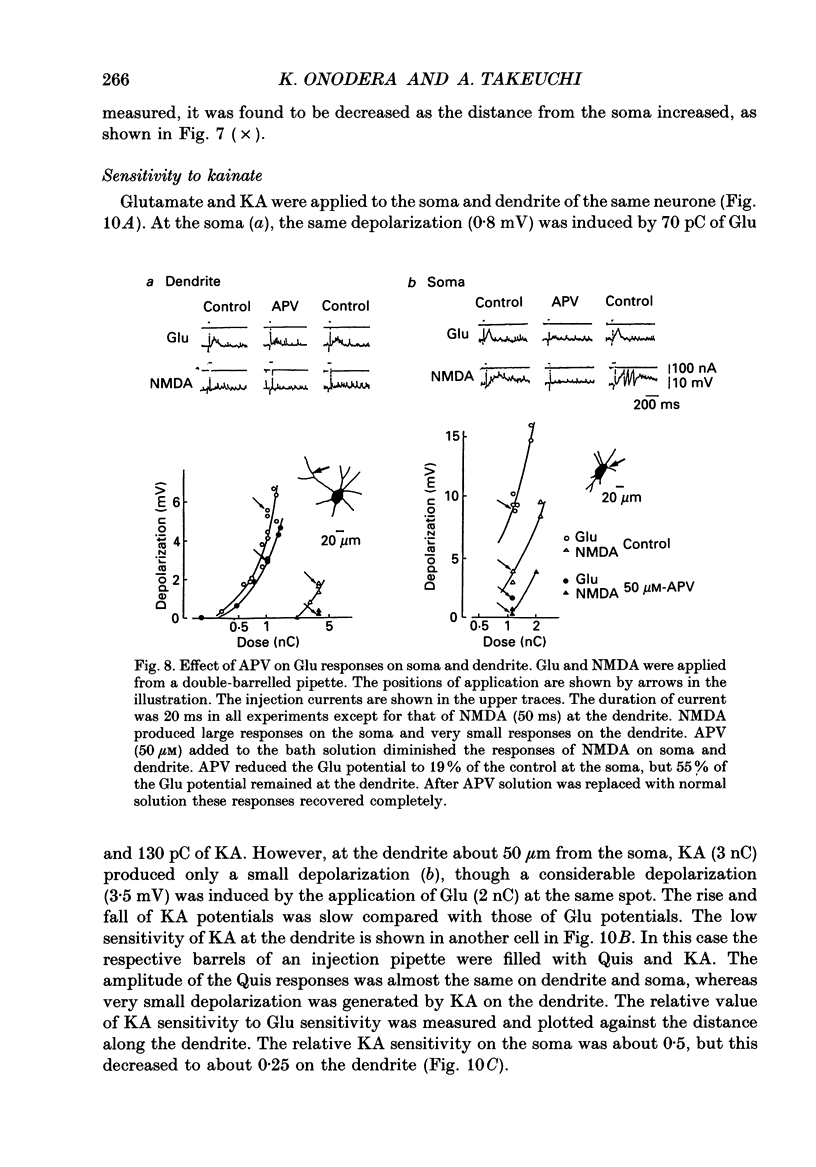
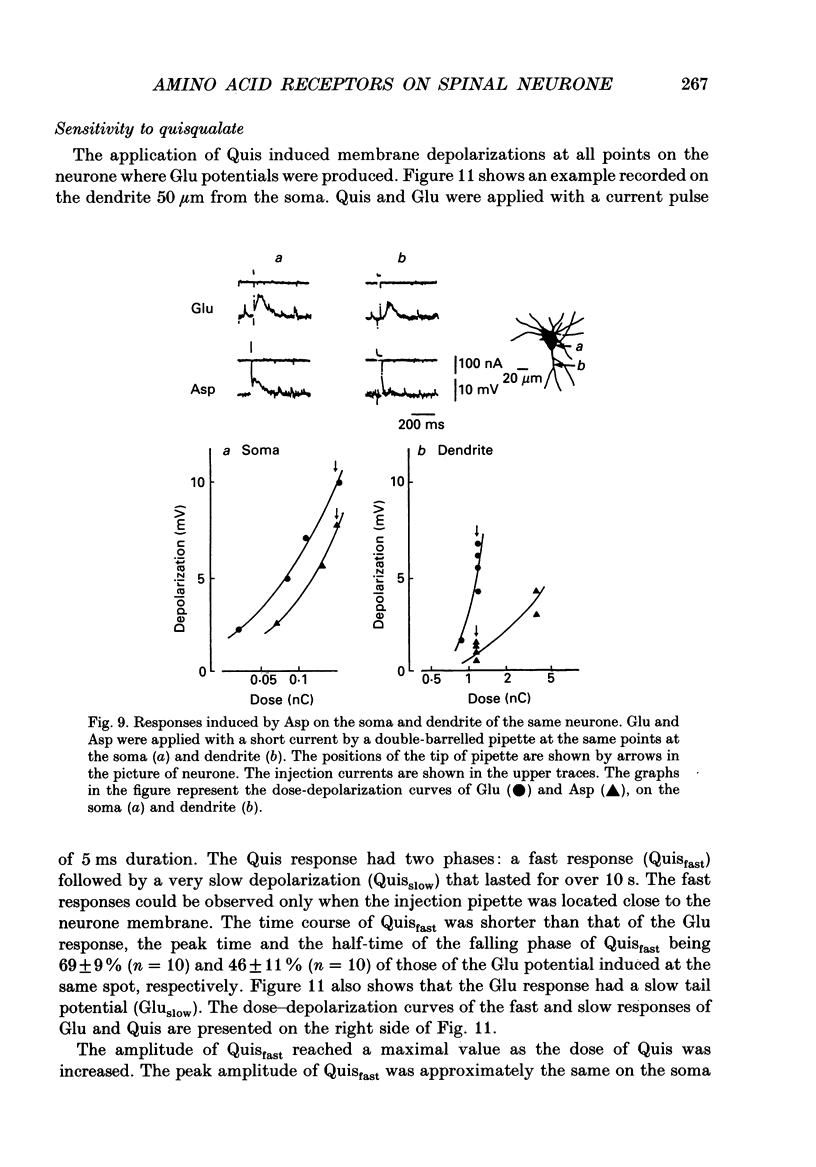
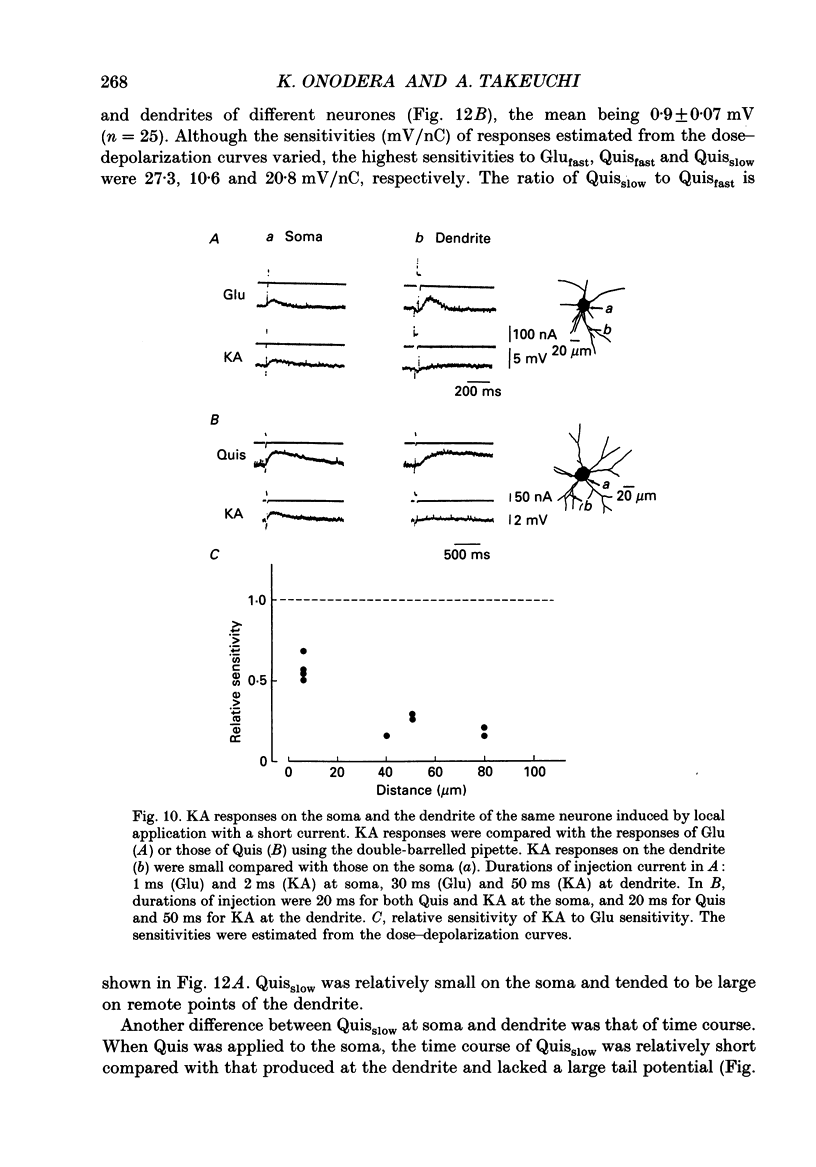
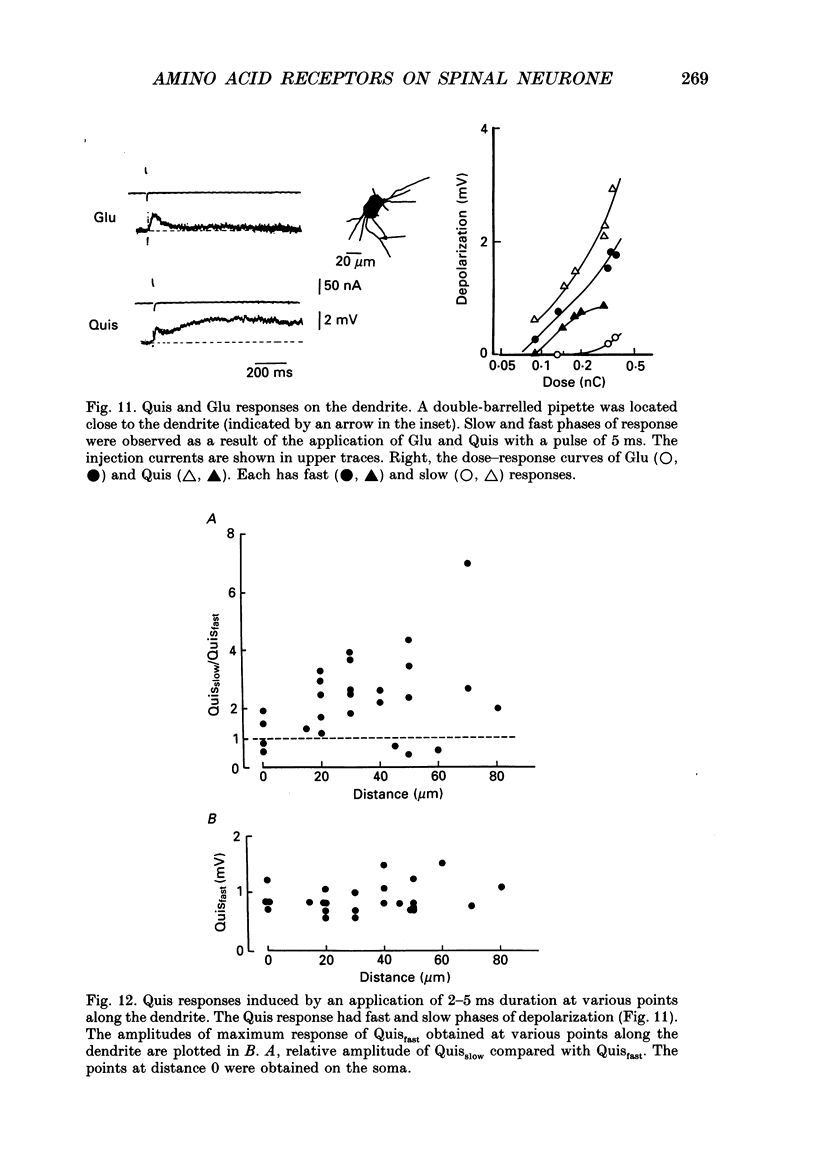
1. The distribution of excitatory amino acid receptors on ventral horn neurones was investigated using slices of newborn rat spinal cord. 2. The neurone and the tip of the pipette used to inject amino acids were visualized using Lucifer Yellow under a fluorescent microscope. The pipette was precisely located on the soma and dendrite of the neurone under visual control, and L-glutamate (Glu), L-aspartate (Asp), N-methyl-D-aspartate (NMDA), kainate (KA) and quisqualate (Quis) were ionophoretically applied with a short pulse. The potential changes were intracellularly recorded from the soma. 3. Sensitivity to Glu as tested with short pulses (1-2 ms) was almost the same at the soma and along dendrites. 4. The amplitude of the responses to NMDA produced at the soma and the proximal part of the dendrite was about the same as that of Glu, but smaller than that of Glu at the distal part of the dendrite. Suppression of the Glu potential by an NMDA receptor antagonist, 2-amino-5-phosphonovaleric acid (APV), was greater at the soma than at the dendrite, suggesting that the contribution of NMDA receptors to the Glu potential was greater at the soma. 5. Sensitivity to Asp was about one-half that to Glu sensitivity on the soma and even less on the dendrite. Sensitivity to KA was high at the soma and low at the dendrite. However, Quis responses were produced throughout the neurone. 6. The Quis response induced by the application of a short pulse showed two phases: a fast response followed by a very slow depolarization that lasted more than 10 s. 7. The fast Quis response was easily desensitized and insensitive to APV. The time course of the fast Quis potential was shorter than that of Glu. 8. The slow Quis response was more pronounced at the dendrites than at the soma and was reduced by the intracellular injection of EGTA, suggesting the contribution of Ca2+ in the cell, possibly mediated by a second messenger system. 9. Experimental results suggest that the distribution of excitatory amino acid receptors differs between the soma and the dendrites of spinal neurones.
Full text
PDF







Images in this article
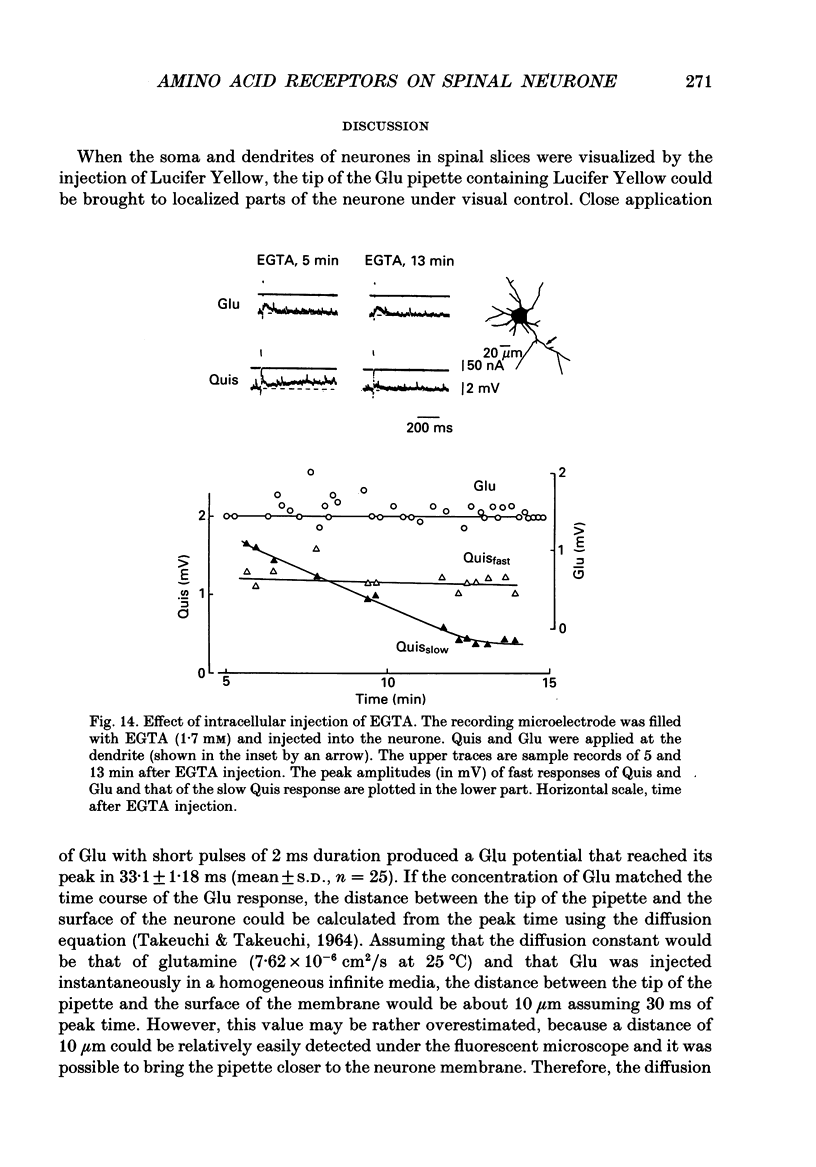
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Bregestovski P., Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988 May;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. Quisqualate- and kainate-activated channels in mouse central neurones in culture. J Physiol. 1988 May;399:227–245. doi: 10.1113/jphysiol.1988.sp017077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989 Sep 21;341(6239):230–233. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Redman S. J. Cable properties of cat spinal motoneurones measured by combining voltage clamp, current clamp and intracellular staining. J Physiol. 1989 Feb;409:63–87. doi: 10.1113/jphysiol.1989.sp017485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Howe J. R., Ogden D. C. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. J Physiol. 1988 Jun;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. On the multiple-conductance single channels activated by excitatory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:555–582. doi: 10.1113/jphysiol.1989.sp017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
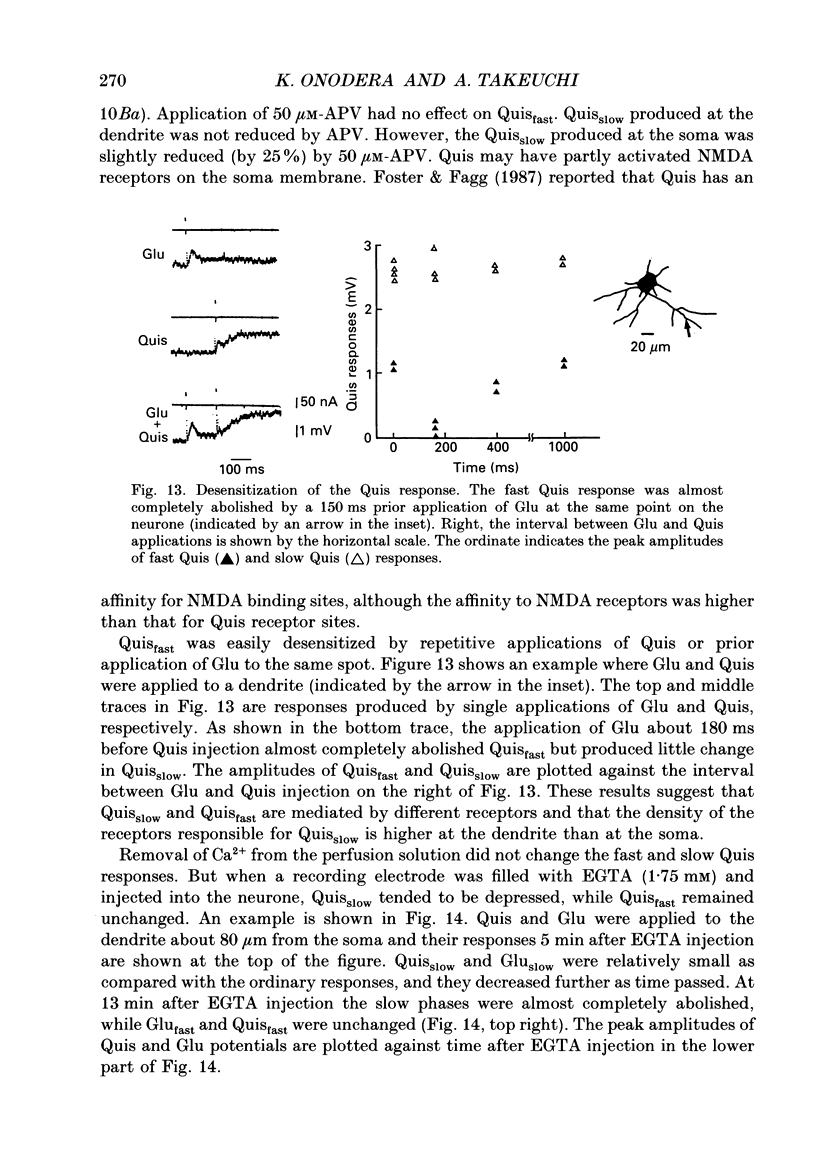
- Cull-Candy S. G., Usowicz M. M. Whole-cell current noise produced by excitatory and inhibitory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:533–553. doi: 10.1113/jphysiol.1989.sp017735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N., Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985 Jun;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Actions of D and L forms of 2-amino-5-phosphonovalerate and 2-amino-4-phosphonobutyrate in the cat spinal cord. Brain Res. 1982 Mar 11;235(2):378–386. doi: 10.1016/0006-8993(82)91017-4. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Role of excitatory amino acid receptors in mono- and polysynaptic excitation in the cat spinal cord. Exp Brain Res. 1983;49(2):280–290. doi: 10.1007/BF00238587. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Johnston G. A. Glutamate and related amino acids in cat spinal roots, dorsal root ganglia and peripheral nerves. J Neurochem. 1970 Aug;17(8):1205–1208. doi: 10.1111/j.1471-4159.1970.tb03369.x. [DOI] [PubMed] [Google Scholar]
- Elmslie K. S., Yoshikami D. Effects of kynurenate on root potentials evoked by synaptic activity and amino acids in the frog spinal cord. Brain Res. 1985 Mar 25;330(2):265–272. doi: 10.1016/0006-8993(85)90685-7. [DOI] [PubMed] [Google Scholar]
- Erdö S. L., Wolff J. R. Transient increase in ligand binding to quisqualate and kainate sites in cerebral cortex of immature rats. Neurosci Lett. 1989 Sep 25;104(1-2):161–166. doi: 10.1016/0304-3940(89)90348-0. [DOI] [PubMed] [Google Scholar]
- Fagg G. E., Jordan C. C., Webster R. A. The release of endogenous amino acids from the cat spinal cord in vivo [proceedings]. Br J Pharmacol. 1976 Nov;58(3):440P–441P. [PMC free article] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. J. The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J Physiol. 1983 Sep;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman J. A., Lambert J. D., Engberg I. The variation in action of excitatory amino acids in relation to distance of iontophoretic application to spinal motoneurones. Acta Physiol Scand. 1985 Jul;124(3):421–427. doi: 10.1111/j.1748-1716.1985.tb07678.x. [DOI] [PubMed] [Google Scholar]
- Fleshman J. W., Segev I., Burke R. B. Electrotonic architecture of type-identified alpha-motoneurons in the cat spinal cord. J Neurophysiol. 1988 Jul;60(1):60–85. doi: 10.1152/jn.1988.60.1.60. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Comparison of L-[3H]glutamate, D-[3H]aspartate, DL-[3H]AP5 and [3H]NMDA as ligands for NMDA receptors in crude postsynaptic densities from rat brain. Eur J Pharmacol. 1987 Jan 20;133(3):291–300. doi: 10.1016/0014-2999(87)90025-2. [DOI] [PubMed] [Google Scholar]
- Furuya S., Ohmori H., Shigemoto T., Sugiyama H. Intracellular calcium mobilization triggered by a glutamate receptor in rat cultured hippocampal cells. J Physiol. 1989 Jul;414:539–548. doi: 10.1113/jphysiol.1989.sp017702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganong A. H., Lanthorn T. H., Cotman C. W. Kynurenic acid inhibits synaptic and acidic amino acid-induced responses in the rat hippocampus and spinal cord. Brain Res. 1983 Aug 22;273(1):170–174. doi: 10.1016/0006-8993(83)91108-3. [DOI] [PubMed] [Google Scholar]
- Hamon B., Heinemann U. Developmental changes in neuronal sensitivity to excitatory amino acids in area CA1 of the rat hippocampus. Brain Res. 1988 Feb 1;466(2):286–290. doi: 10.1016/0165-3806(88)90054-5. [DOI] [PubMed] [Google Scholar]
- Horne A. L., Simmonds M. A. The pharmacology of quisqualate and AMPA in the cerebral cortex of the rat in vitro. Neuropharmacology. 1989 Oct;28(10):1113–1118. doi: 10.1016/0028-3908(89)90125-1. [DOI] [PubMed] [Google Scholar]
- Insel T. R., Miller L. P., Gelhard R. E. The ontogeny of excitatory amino acid receptors in rat forebrain--I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35(1):31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Jessell T. M. Synaptic transmission between dorsal root ganglion and dorsal horn neurons in culture: antagonism of monosynaptic excitatory postsynaptic potentials and glutamate excitation by kynurenate. J Neurosci. 1985 Aug;5(8):2281–2289. doi: 10.1523/JNEUROSCI.05-08-02281.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Yoshioka K., Jahr C. E. Amino acid receptor-mediated transmission at primary afferent synapses in rat spinal cord. J Exp Biol. 1986 Sep;124:239–258. doi: 10.1242/jeb.124.1.239. [DOI] [PubMed] [Google Scholar]
- Joels M., Yool A. J., Gruol D. L. Unique properties of non-N-methyl-D-aspartate excitatory responses in cultured purkinje neurons. Proc Natl Acad Sci U S A. 1989 May;86(9):3404–3408. doi: 10.1073/pnas.86.9.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. A., Lodge D., Bornstein J. C., Curtis D. R. Potentiation of L-glutamate and L-aspartate excitation of cat spinal neurones by the stereoisomers of threo-3-hydroxyaspartate. J Neurochem. 1980 Jan;34(1):241–243. doi: 10.1111/j.1471-4159.1980.tb04650.x. [DOI] [PubMed] [Google Scholar]
- Jones I. M., Jordan C. C., Morton I. K., Stagg C. J., Webster R. A. The effect of chronic dorsal root section on the concentration of free amino acids in the rabbit spinal cord. J Neurochem. 1974 Dec;23(6):1239–1244. doi: 10.1111/j.1471-4159.1974.tb12223.x. [DOI] [PubMed] [Google Scholar]
- Kiskin N. I., Krishtal O. A., Tsyndrenko AYa Excitatory amino acid receptors in hippocampal neurons: kainate fails to desensitize them. Neurosci Lett. 1986 Jan 30;63(3):225–230. doi: 10.1016/0304-3940(86)90360-5. [DOI] [PubMed] [Google Scholar]
- Lerma J., Kushner L., Zukin R. S., Bennett M. V. N-methyl-D-aspartate activates different channels than do kainate and quisqualate. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2083–2087. doi: 10.1073/pnas.86.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge D., Curtis D. R., Johnston G. A., Bornstein J. C. In vivo inactivation of quisqualate: studies in the cat spinal cord. Brain Res. 1980 Jan 27;182(2):491–495. doi: 10.1016/0006-8993(80)91211-1. [DOI] [PubMed] [Google Scholar]
- Luhmann H. J., Prince D. A. Transient expression of polysynaptic NMDA receptor-mediated activity during neocortical development. Neurosci Lett. 1990 Mar 26;111(1-2):109–115. doi: 10.1016/0304-3940(90)90353-b. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr Concanavalin A selectively reduces desensitization of mammalian neuronal quisqualate receptors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1411–1415. doi: 10.1073/pnas.86.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Mixed-agonist action of excitatory amino acids on mouse spinal cord neurones under voltage clamp. J Physiol. 1984 Sep;354:29–53. doi: 10.1113/jphysiol.1984.sp015360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Murphy S. N., Miller R. J. A glutamate receptor regulates Ca2+ mobilization in hippocampal neurons. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8737–8741. doi: 10.1073/pnas.85.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G., Pun R. Y., Westbrook G. L. Synaptic excitation in cultures of mouse spinal cord neurones: receptor pharmacology and behaviour of synaptic currents. J Physiol. 1986 Mar;372:169–190. doi: 10.1113/jphysiol.1986.sp016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Iadarola M. J., Wroblewski J. T., Costa E. Excitatory amino acid recognition sites coupled with inositol phospholipid metabolism: developmental changes and interaction with alpha 1-adrenoceptors. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1931–1935. doi: 10.1073/pnas.83.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. J., Fischbach G. D. Excitatory synaptic transmission between interneurons and motoneurons in chick spinal cord cell cultures. J Neurosci. 1986 Nov;6(11):3284–3289. doi: 10.1523/JNEUROSCI.06-11-03284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. J., Fischbach G. D. Modulation of embryonic chick motoneuron glutamate sensitivity by interneurons and agonists. J Neurosci. 1986 Nov;6(11):3290–3296. doi: 10.1523/JNEUROSCI.06-11-03290.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M. N., Stone T. W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982 Sep 9;247(1):184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Perouansky M., Grantyn R. Separation of quisqualate- and kainate-selective glutamate receptors in cultured neurons from the rat superior colliculus. J Neurosci. 1989 Jan;9(1):70–80. doi: 10.1523/JNEUROSCI.09-01-00070.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassendren F. A., Lory P., Pin J. P., Bockaert J., Nargeot J. A specific quisqualate agonist inhibits kainate responses induced in Xenopus oocytes injected with rat brain RNA. Neurosci Lett. 1989 May 8;99(3):333–339. doi: 10.1016/0304-3940(89)90469-2. [DOI] [PubMed] [Google Scholar]
- Roberts P. J., Keen P., Mitchell J. F. The distribution and axonal transport of free amino acids and related compounds in the dorsal sensory neuron of the rat, as determined by the dansyl reaction. J Neurochem. 1973 Jul;21(1):199–209. doi: 10.1111/j.1471-4159.1973.tb04239.x. [DOI] [PubMed] [Google Scholar]
- Salt T. E., Hill R. G. Neurotransmitter candidates of somatosensory primary afferent fibres. Neuroscience. 1983 Dec;10(4):1083–1103. doi: 10.1016/0306-4522(83)90101-x. [DOI] [PubMed] [Google Scholar]
- Sawada S., Higashima M., Yamamoto C. Inhibitors of high-affinity uptake augment depolarizations of hippocampal neurons induced by glutamate, kainate and related compounds. Exp Brain Res. 1985;60(2):323–329. doi: 10.1007/BF00235927. [DOI] [PubMed] [Google Scholar]
- Silverstein F. S., Chen R., Johnston M. V. The glutamate analogue quisqualic acid is neurotoxic in striatum and hippocampus of immature rat brain. Neurosci Lett. 1986 Oct 30;71(1):13–18. doi: 10.1016/0304-3940(86)90249-1. [DOI] [PubMed] [Google Scholar]
- Sladeczek F., Récasens M., Bockaert J. A new mechanism for glutamate receptor action: phosphoinositide hydrolysis. Trends Neurosci. 1988 Dec;11(12):545–549. doi: 10.1016/0166-2236(88)90183-x. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
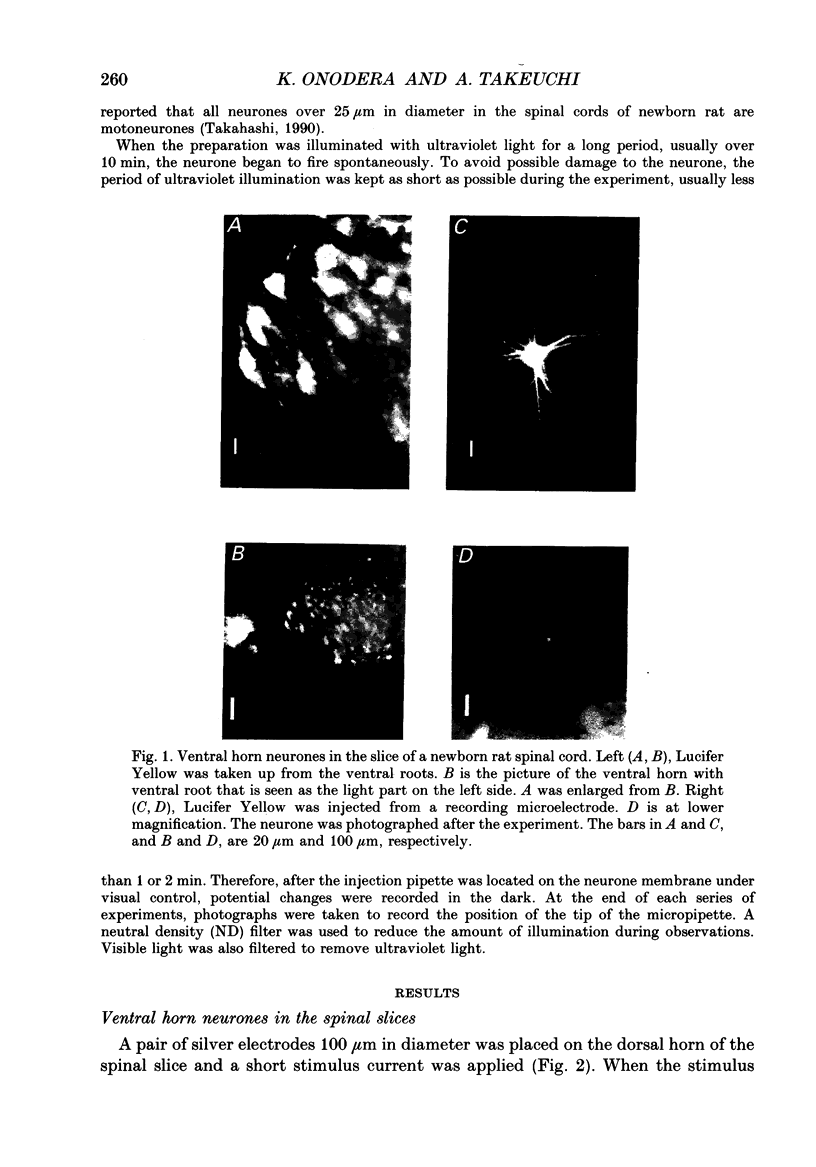
- Takahashi T. Intracellular recording from visually identified motoneurons in rat spinal cord slices. Proc R Soc Lond B Biol Sci. 1978 Jul 26;202(1148):417–421. doi: 10.1098/rspb.1978.0076. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. J Physiol. 1990 Apr;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Tsuruhara H. Slow depolarizing potentials recorded from glial cells in the rat superficial dorsal horn. J Physiol. 1987 Jul;388:597–610. doi: 10.1113/jphysiol.1987.sp016633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A. The transmitter role of glutamate in nervous systems. Jpn J Physiol. 1987;37(4):559–572. doi: 10.2170/jjphysiol.37.559. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Science. 1989 Mar 17;243(4897):1474–1477. doi: 10.1126/science.2467378. [DOI] [PubMed] [Google Scholar]
- Tremblay E., Roisin M. P., Represa A., Charriaut-Marlangue C., Ben-Ari Y. Transient increased density of NMDA binding sites in the developing rat hippocampus. Brain Res. 1988 Oct 4;461(2):393–396. doi: 10.1016/0006-8993(88)90275-2. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Thio L. L., Zorumski C. F., Fischbach G. D. Rapid desensitization of glutamate receptors in vertebrate central neurons. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4562–4566. doi: 10.1073/pnas.85.12.4562-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Weiss S. Two distinct quisqualate receptor systems are present on striatal neurons. Brain Res. 1989 Jul 3;491(1):189–193. doi: 10.1016/0006-8993(89)90104-2. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Champagnat J. Cat spinal motoneurones exhibit topographic sensitivity to glutamate and glycine. Brain Res. 1979 Jan 5;160(1):95–104. doi: 10.1016/0006-8993(79)90603-6. [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L. NMDA receptors mediate poly- and monosynaptic potentials in motoneurons of rat embryos. J Neurosci. 1990 Jan;10(1):125–135. doi: 10.1523/JNEUROSCI.10-01-00125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski C. F., Yang J. AMPA, kainate, and quisqualate activate a common receptor-channel complex on embryonic chick motoneurons. J Neurosci. 1988 Nov;8(11):4277–4286. doi: 10.1523/JNEUROSCI.08-11-04277.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]