Abstract
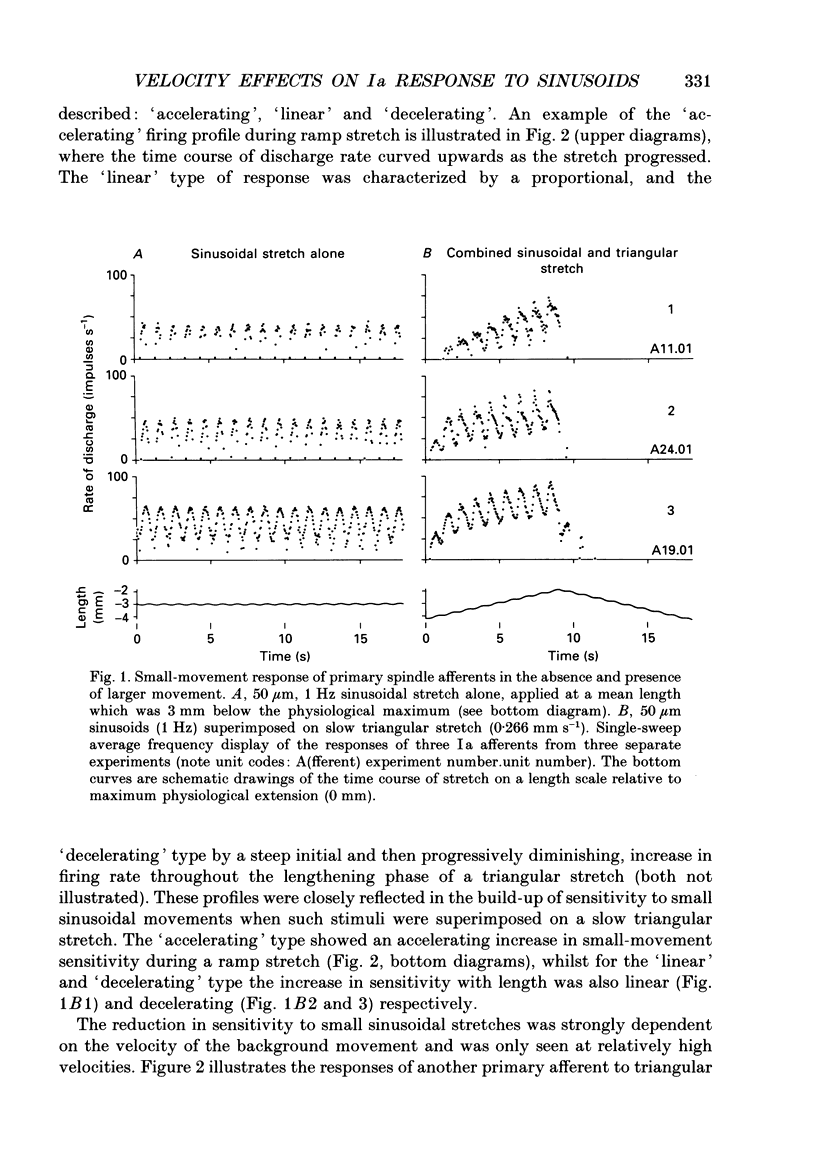
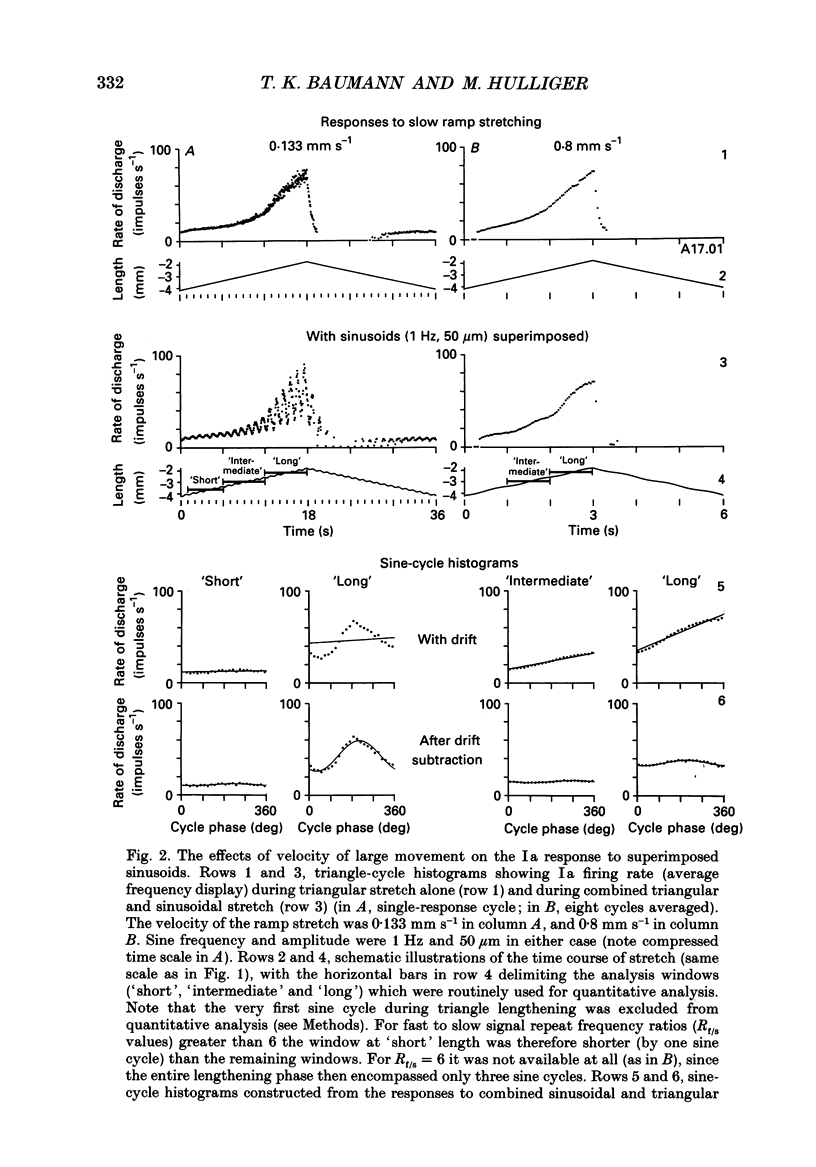
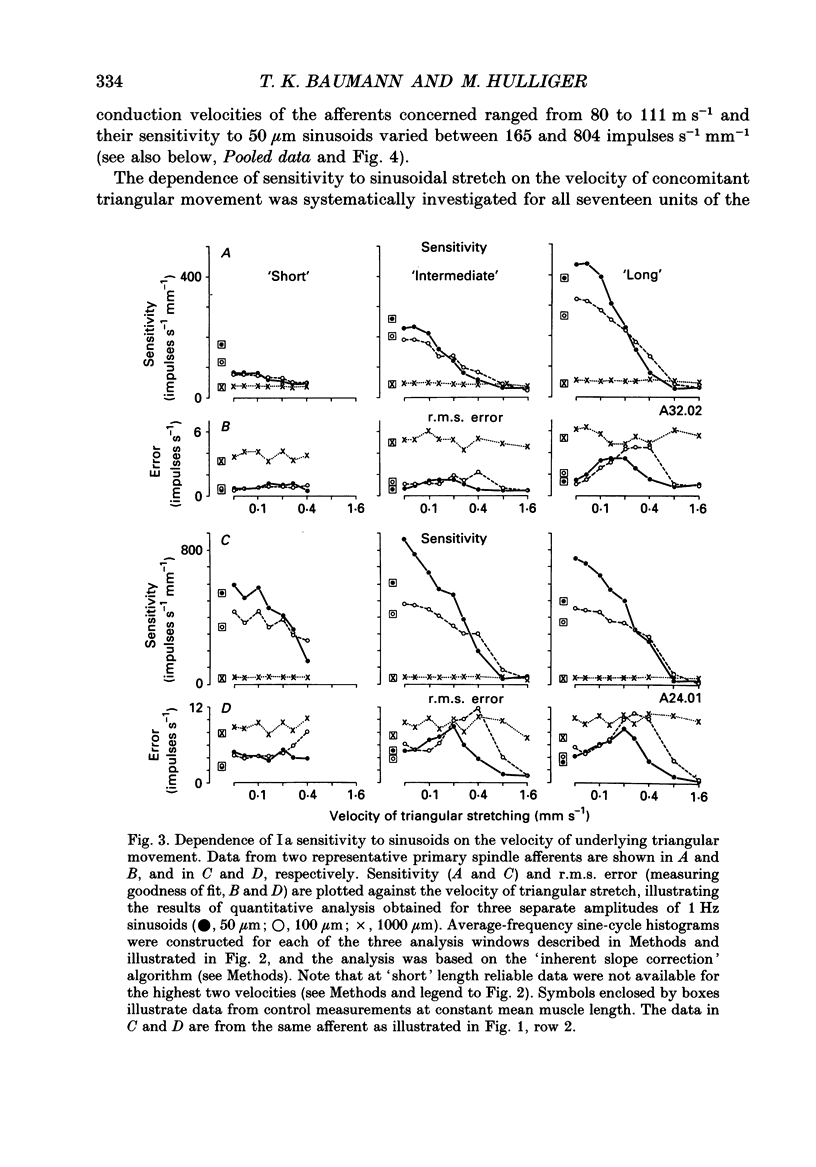
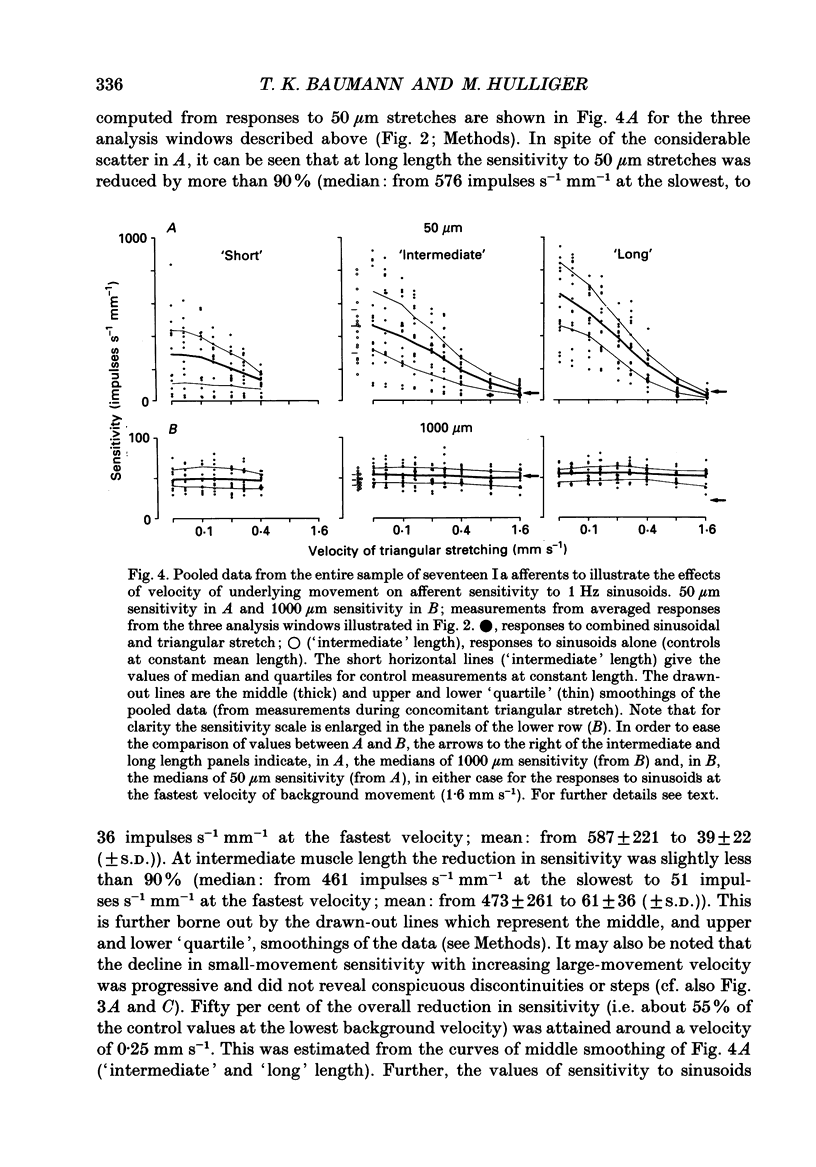
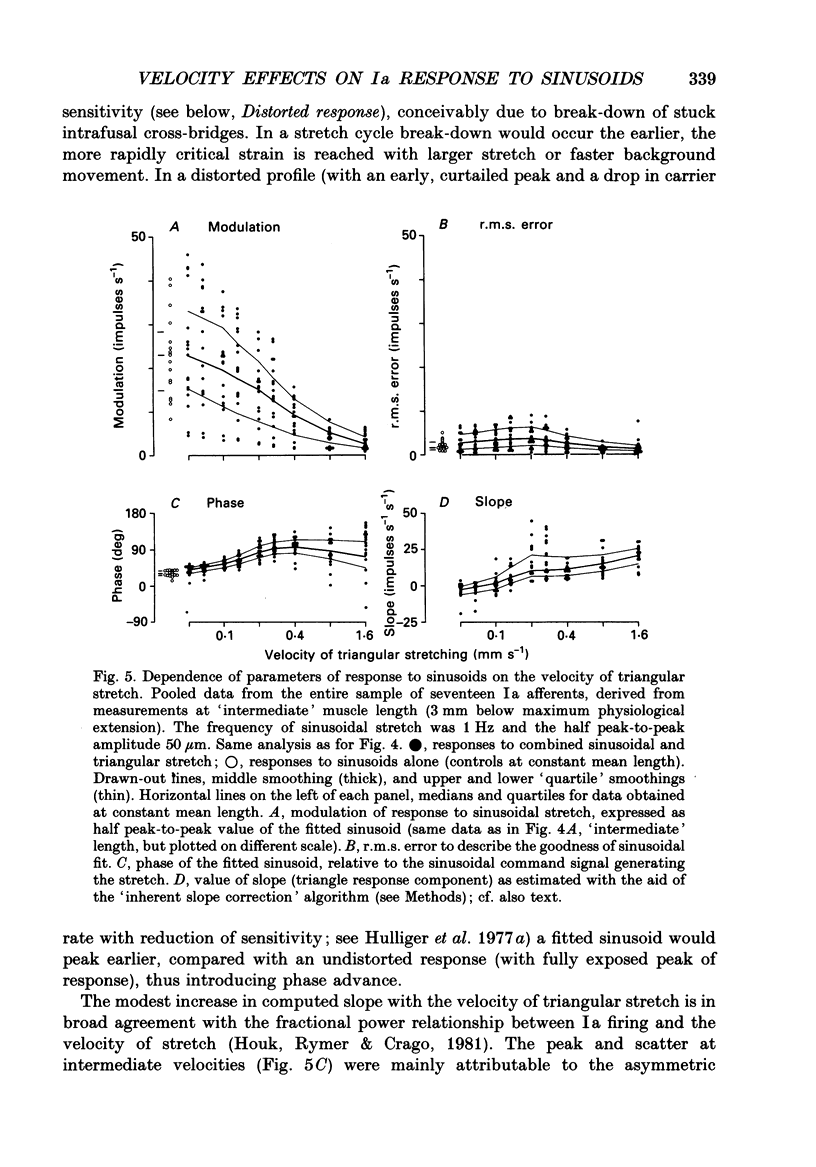
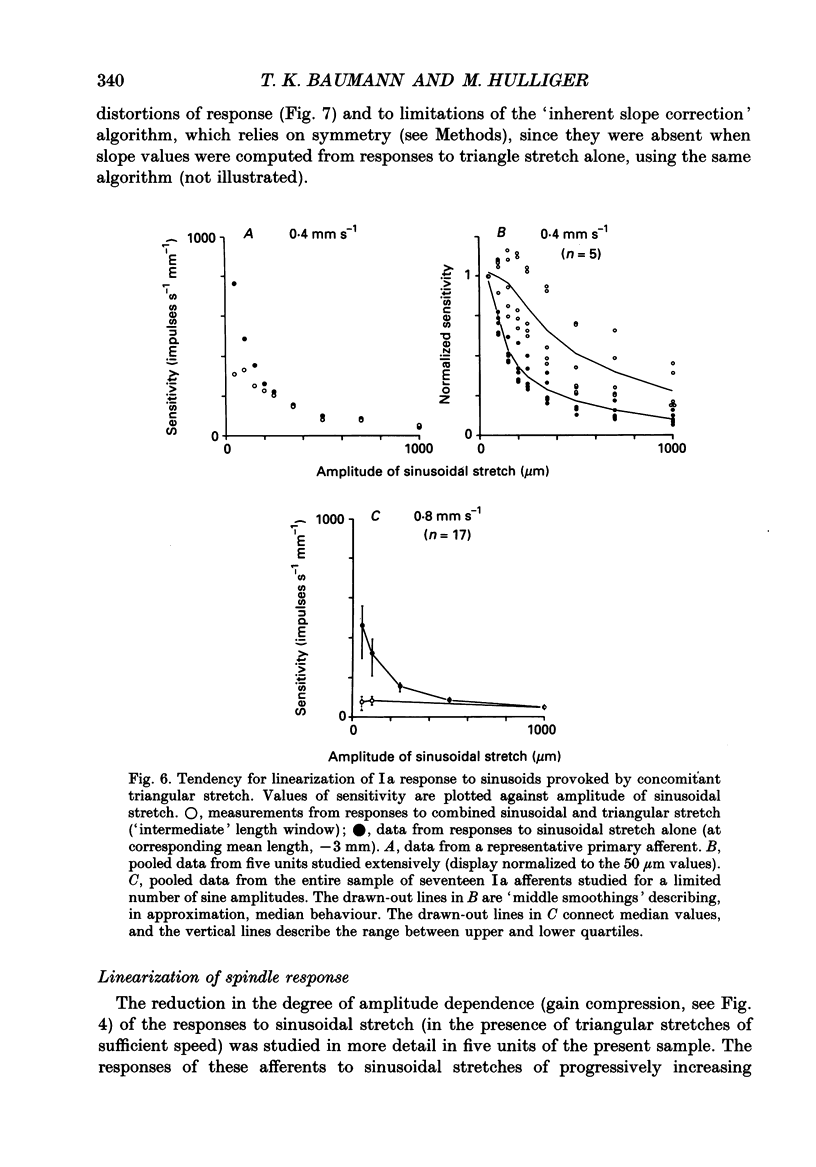
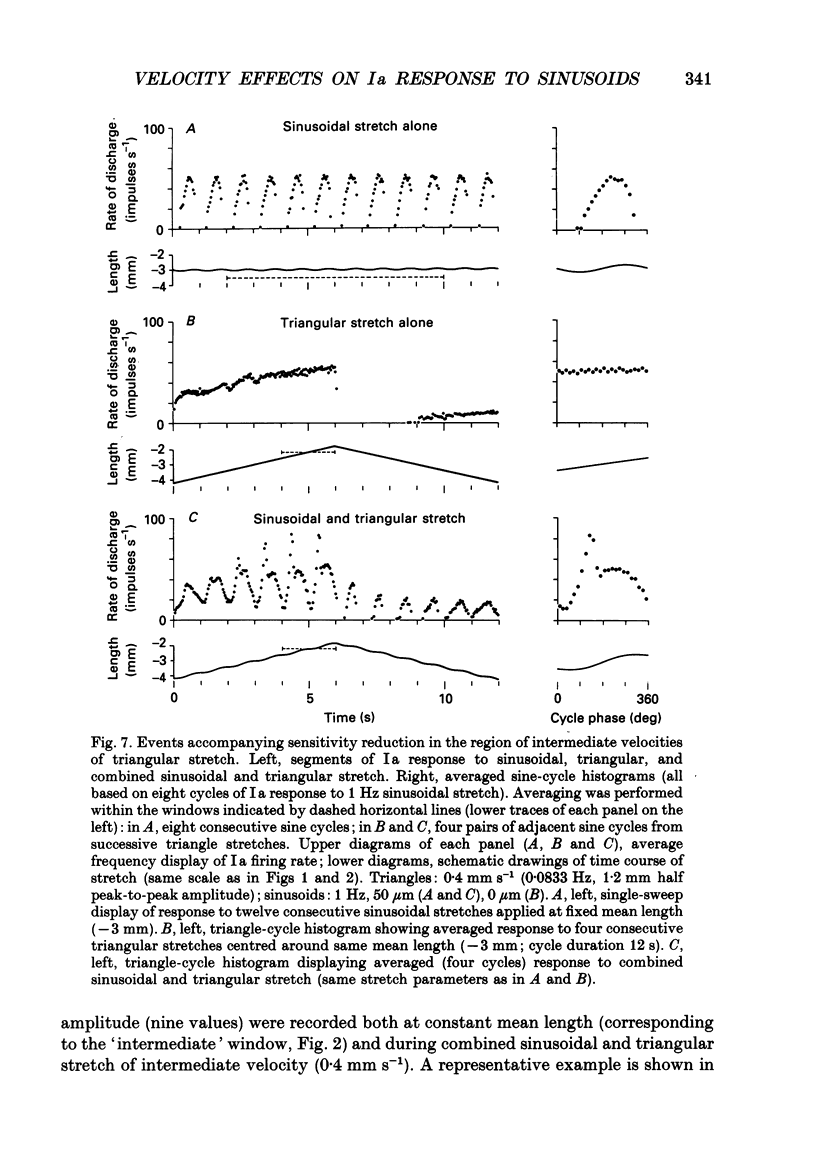
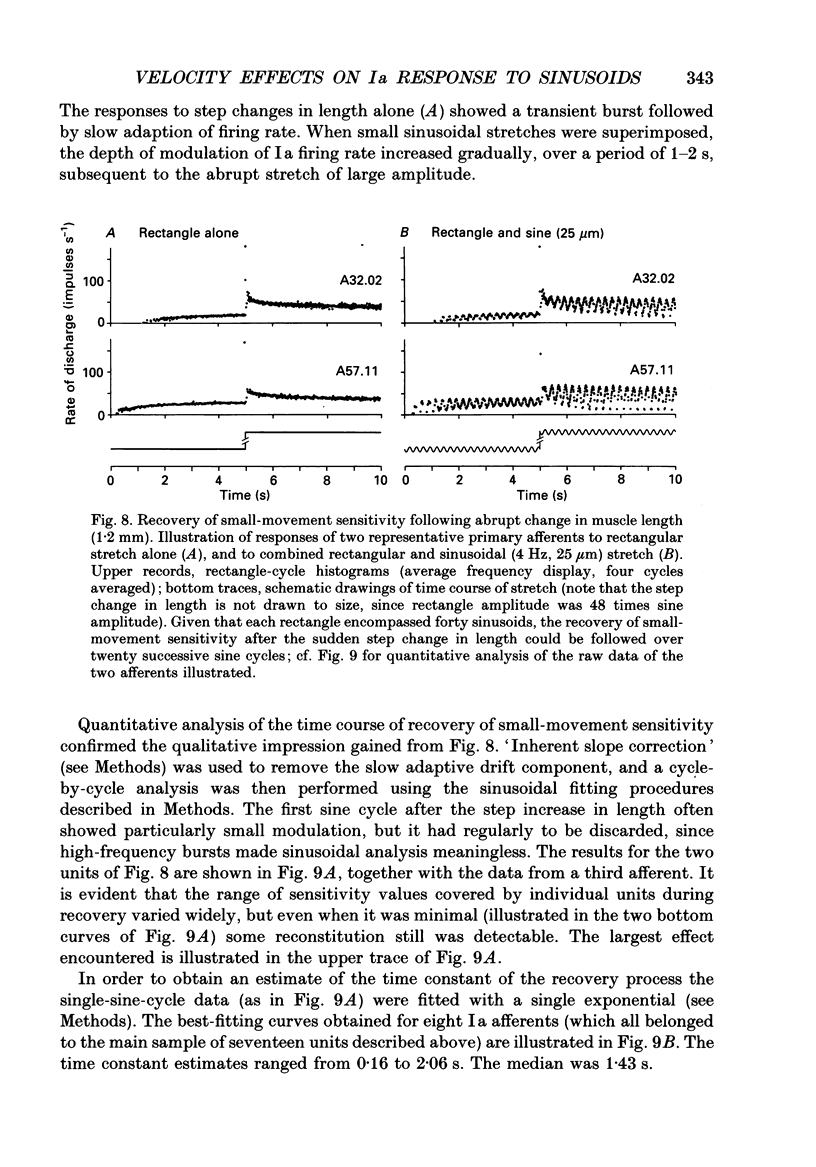
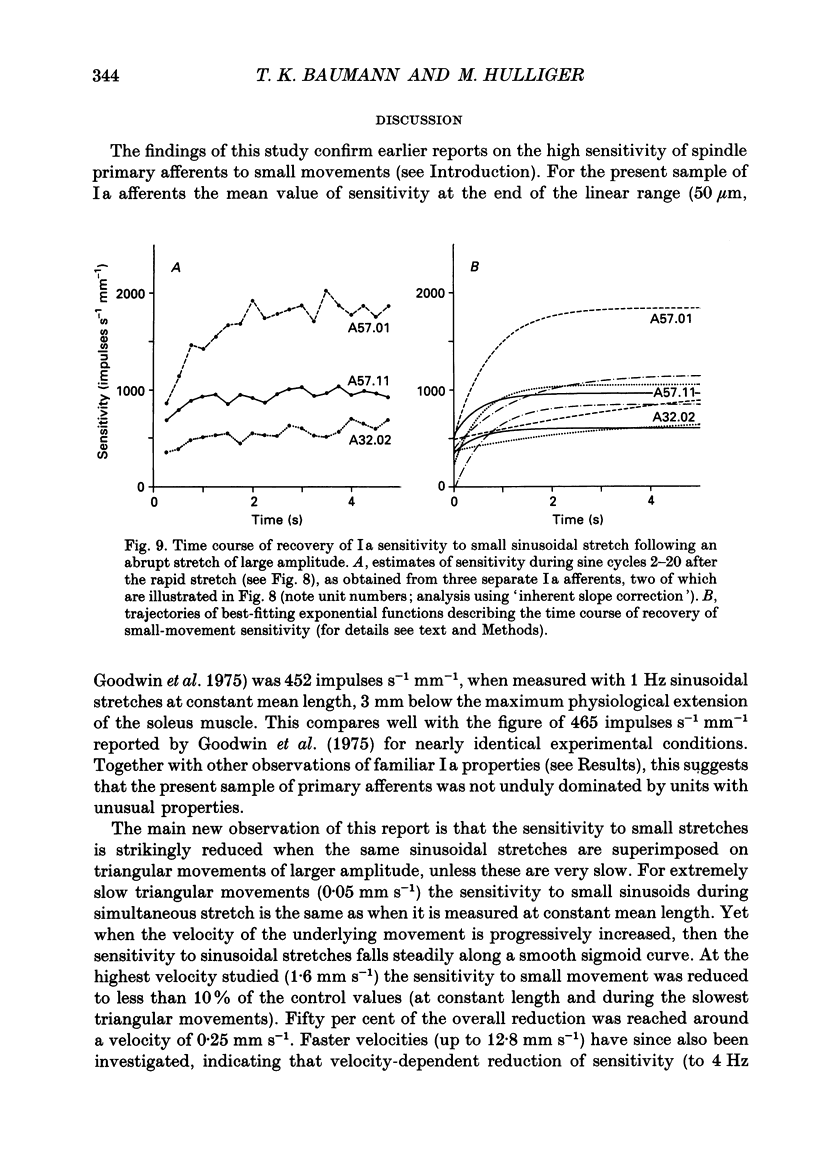
1. The responses of de-efferented soleus muscle spindle primary afferents to 1 Hz sinusoidal stretches, which were superimposed on triangular background movements of intermediate amplitude (1.2 mm, half peak-to-peak) and widely varying speed, were recorded in anaesthetized cats. 2. Compared with control responses to the same sinusoids applied at fixed mean muscle length, the sensitivity to small (50 and 100 microns, half peak-to-peak), but not to large (1000 microns), sinusoidal movements was dramatically reduced during concomitant stretching, unless the background movements were extremely slow (well below 0.005 resting lengths per second). 3. For small stretches (50 and 100 microns) the reduction of sensitivity caused by background movement depended on the speed of this movement. For the highest velocity studied (1.6 mm s-1, corresponding to about 0.03 resting lengths per second) sensitivity dropped to below 10% of the control values. 4. The functional implication is that the sensitivity of spindle Ia afferents to small irregularities of voluntary movements (of any but the slowest speeds) may well be very much lower than it has hitherto been inferred from the striking sensitivity to minute disturbances at fixed mean muscle length. The present finding clearly puts extra demands on the gain of any spinal or central reflex actions of the sensory feedback from spindle afferents. 5. The effect is interpreted in terms of the widely accepted cross-bridge hypothesis of spindle small-movement sensitivity. The result suggests that in de-efferented intrafusal muscle fibres, which are subjected to imposed stretches, connected cross-bridges (conveying a friction-like property to the contractile fibre poles) may exist not only in a state of permanent attachment, but also in a dynamic equilibrium between stretch-induced detachment and reattachment. Indirect evidence further suggests that the duration of this disruption and reattachment cycle is of the order of 1 s.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann T. K., Emonet-Dénand F., Hulliger M. After-effects of fusimotor stimulation on spindle la afferents' dynamic sensitivity, revealed during slow movements. Brain Res. 1982 Jan 28;232(2):460–465. doi: 10.1016/0006-8993(82)90289-x. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Goodwin G. M., Matthews P. B. After-effects of fusimotor stimulation on the response of muscle spindle primary afferent endings. J Physiol. 1969 Dec;205(3):677–694. doi: 10.1113/jphysiol.1969.sp008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C. The responses of frog muscle spindles and fast and slow muscle fibres to a variety of mechanical inputs. J Physiol. 1971 Oct;218(1):1–17. doi: 10.1113/jphysiol.1971.sp009601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelguen J. M. Static and dynamic fusimotor action on the response of spindle primary endings to sinusoidal stretches in the cat. Brain Res. 1979 Jun 15;169(1):45–54. doi: 10.1016/0006-8993(79)90372-x. [DOI] [PubMed] [Google Scholar]
- Emonet-Dénand F., Hunt C. C., Laporte Y. Effects of stretch on dynamic fusimotor after-effects in cat muscle spindles. J Physiol. 1985 Mar;360:201–213. doi: 10.1113/jphysiol.1985.sp015612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Dénand F., Hunt C. C., Laporte Y. Fusimotor after-effects on responses of primary endings to test dynamic stimuli in cat muscle spindles. J Physiol. 1985 Mar;360:187–200. doi: 10.1113/jphysiol.1985.sp015611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Dénand F., Laporte Y., Tristant A. Effects of slow muscle stretch on the responses of primary and secondary endings to small amplitude periodic stretches in de-efferented soleus muscle spindles. Brain Res. 1980 Jun 9;191(2):551–554. doi: 10.1016/0006-8993(80)91304-9. [DOI] [PubMed] [Google Scholar]
- Emonet-Dénand F., Laporte Y., Tristant A. Modifications du décours temporel des réponses de terminaisons fusoriales primaires à de petites variations périodiques de longueur produites par un allongement musclaire lent. C R Seances Acad Sci D. 1980 Sep 22;291(3):349–351. [PubMed] [Google Scholar]
- Goodwin G. M., Hulliger M., Matthews P. B. The effects of fusimotor stimulation during small amplitude stretching on the frequency-response of the primary ending of the mammalian muscle spindle. J Physiol. 1975 Dec;253(1):175–206. doi: 10.1113/jphysiol.1975.sp011186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan Z., Houk J. C. Analysis of response properties of deefferented mammalian spindle receptors based on frequency response. J Neurophysiol. 1975 May;38(3):663–672. doi: 10.1152/jn.1975.38.3.663. [DOI] [PubMed] [Google Scholar]
- Hasan Z., Houk J. C. Transition in sensitivity of spindle receptors that occurs when muscle is stretched more than a fraction of a millimeter. J Neurophysiol. 1975 May;38(3):673–689. doi: 10.1152/jn.1975.38.3.673. [DOI] [PubMed] [Google Scholar]
- Hill D. K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol. 1968 Dec;199(3):637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk J. C., Rymer W. Z., Crago P. E. Dependence of dynamic response of spindle receptors on muscle length and velocity. J Neurophysiol. 1981 Jul;46(1):143–166. doi: 10.1152/jn.1981.46.1.143. [DOI] [PubMed] [Google Scholar]
- Hulliger M., Matthews P. B., Noth J. Effects of combining static and dynamic fusimotor stimulation on the response of the muscle spindle primary ending to sinusoidal stretching. J Physiol. 1977 Jun;267(3):839–856. doi: 10.1113/jphysiol.1977.sp011840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M., Matthews P. B., Noth J. Static and dynamic fusimotor action on the response of Ia fibres to low frequency sinusoidal stretching of widely ranging amplitude. J Physiol. 1977 Jun;267(3):811–838. doi: 10.1113/jphysiol.1977.sp011839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M. The mammalian muscle spindle and its central control. Rev Physiol Biochem Pharmacol. 1984;101:1–110. doi: 10.1007/BFb0027694. [DOI] [PubMed] [Google Scholar]
- Hulliger M. The responses of primary spindle afferents to fusimotor stimulation at constant and abruptly changing rates. J Physiol. 1979 Sep;294:461–482. doi: 10.1113/jphysiol.1979.sp012941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C. C., Ottoson D. Responses of primary and secondary endings of isolated mammalian muscle spindles to sinusoidal length changes. J Neurophysiol. 1977 Sep;40(5):1113–1120. doi: 10.1152/jn.1977.40.5.1113. [DOI] [PubMed] [Google Scholar]
- Hunt C. C., Wilkinson R. S. An analysis of receptor potential and tension of isolated cat muscle spindles in response to sinusoidal stretch. J Physiol. 1980 May;302:241–262. doi: 10.1113/jphysiol.1980.sp013240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A. The frequency response of frog muscle spindles under various conditions. J Physiol. 1972 Apr;222(1):135–160. doi: 10.1113/jphysiol.1972.sp009791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in the human thumb. J Physiol. 1976 May;257(1):1–44. doi: 10.1113/jphysiol.1976.sp011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. Evolving views on the internal operation and functional role of the muscle spindle. J Physiol. 1981 Nov;320:1–30. doi: 10.1113/jphysiol.1981.sp013931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Stein R. B. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol. 1969 Feb;200(3):723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppele R. E., Bowman R. J. Quantitative description of linear behavior of mammalian muscle spindles. J Neurophysiol. 1970 Jan;33(1):59–72. doi: 10.1152/jn.1970.33.1.59. [DOI] [PubMed] [Google Scholar]
- Poppele R. E., Kennedy W. R., Quick D. C. A determination of static mechanical properties of intrafusal muscle in isolated cat muscle spindles. Neuroscience. 1979;4(3):401–411. doi: 10.1016/0306-4522(79)90103-9. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Hulliger M., Zangger P., Appenteng K. 'Fusimotor set': new evidence for alpha-independent control of gamma-motoneurones during movement in the awake cat. Brain Res. 1985 Jul 22;339(1):136–140. doi: 10.1016/0006-8993(85)90632-8. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Muscle spindle function during normal movement. Int Rev Physiol. 1981;25:47–90. [PubMed] [Google Scholar]
- Prochazka A., Stephens J. A., Wand P. Muscle spindle discharge in normal and obstructed movements. J Physiol. 1979 Feb;287:57–66. doi: 10.1113/jphysiol.1979.sp012645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. B. Peripheral control of movement. Physiol Rev. 1974 Jan;54(1):215–243. doi: 10.1152/physrev.1974.54.1.215. [DOI] [PubMed] [Google Scholar]


