Abstract
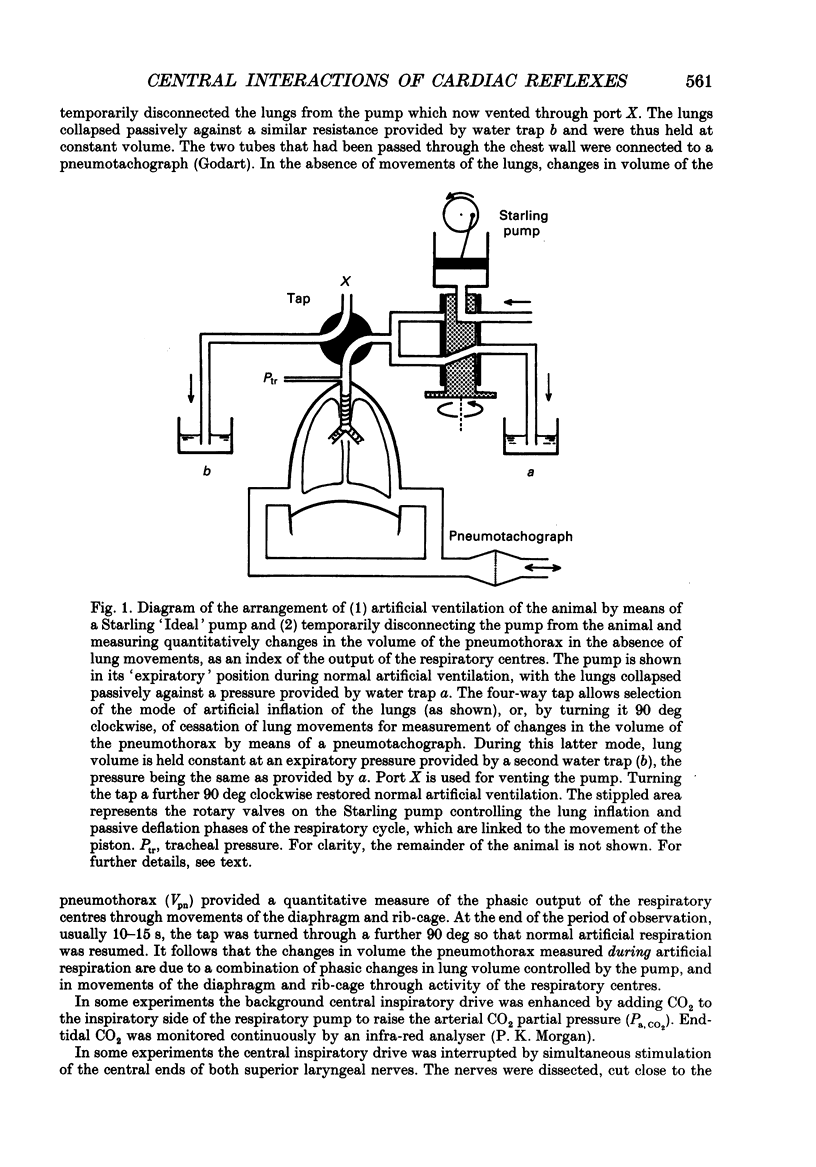
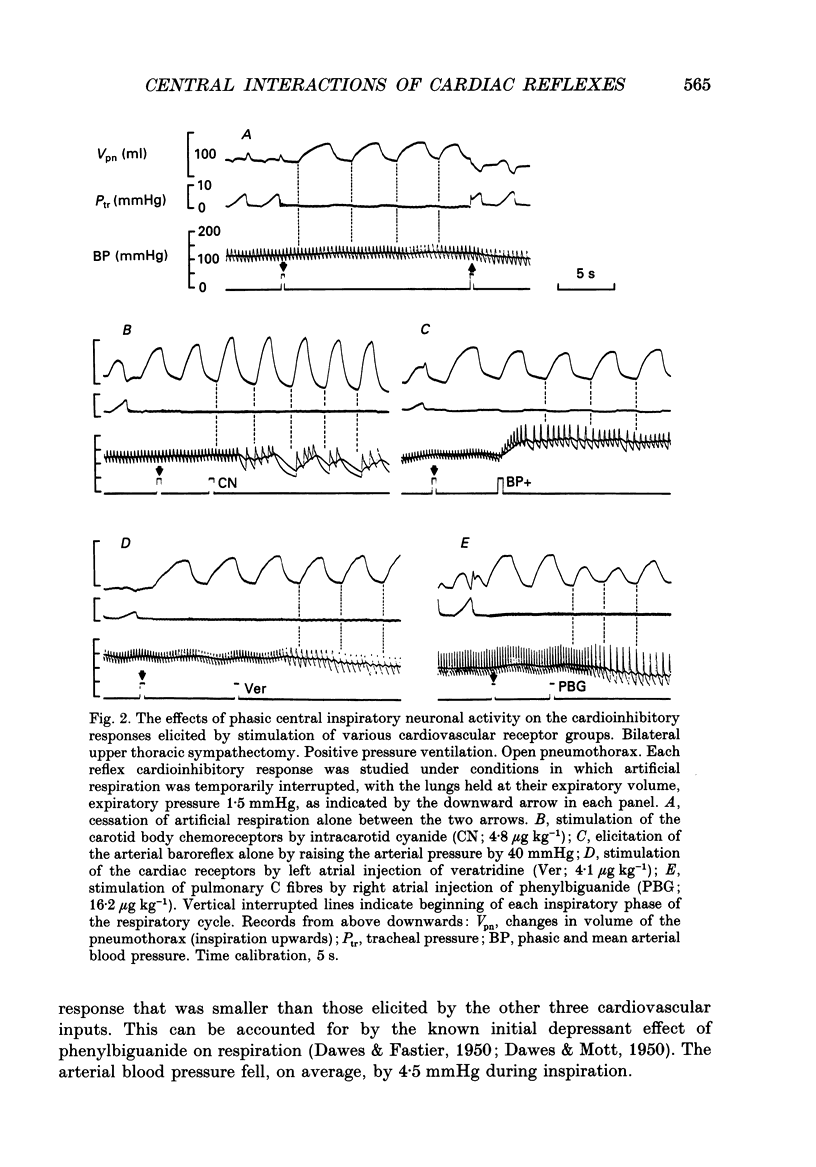
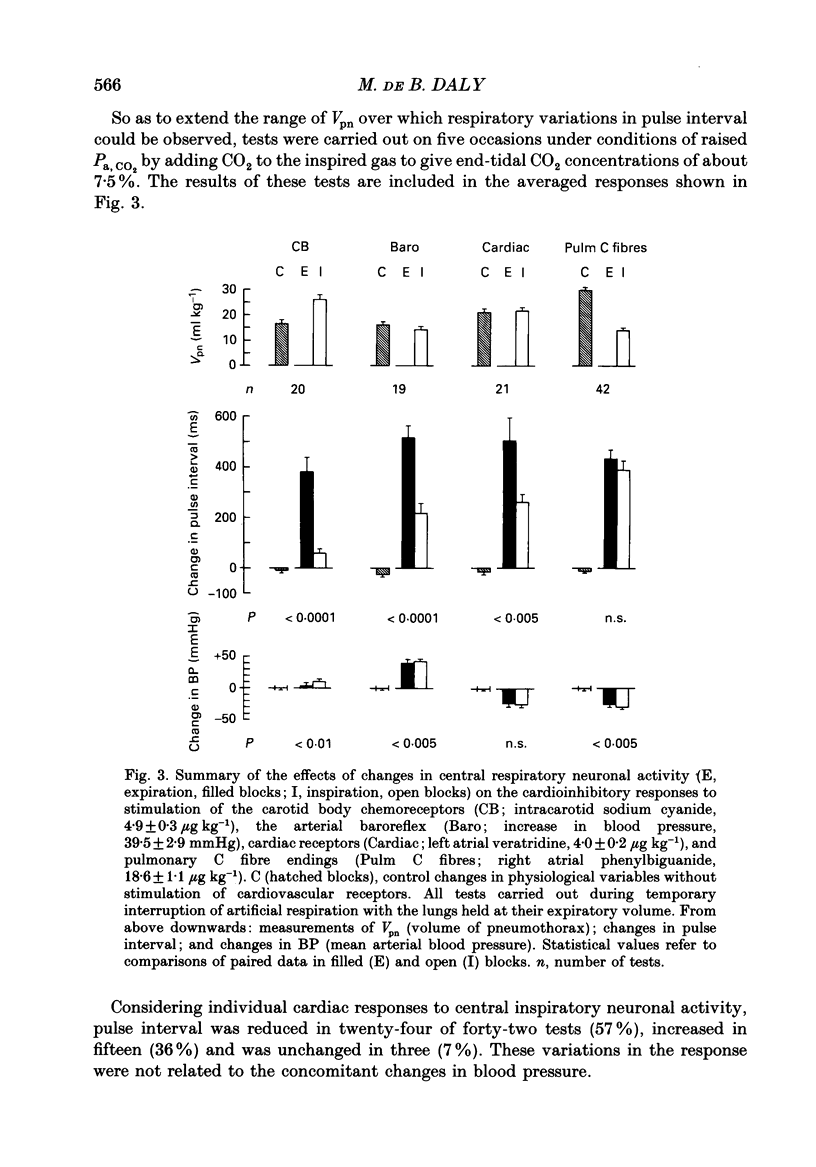
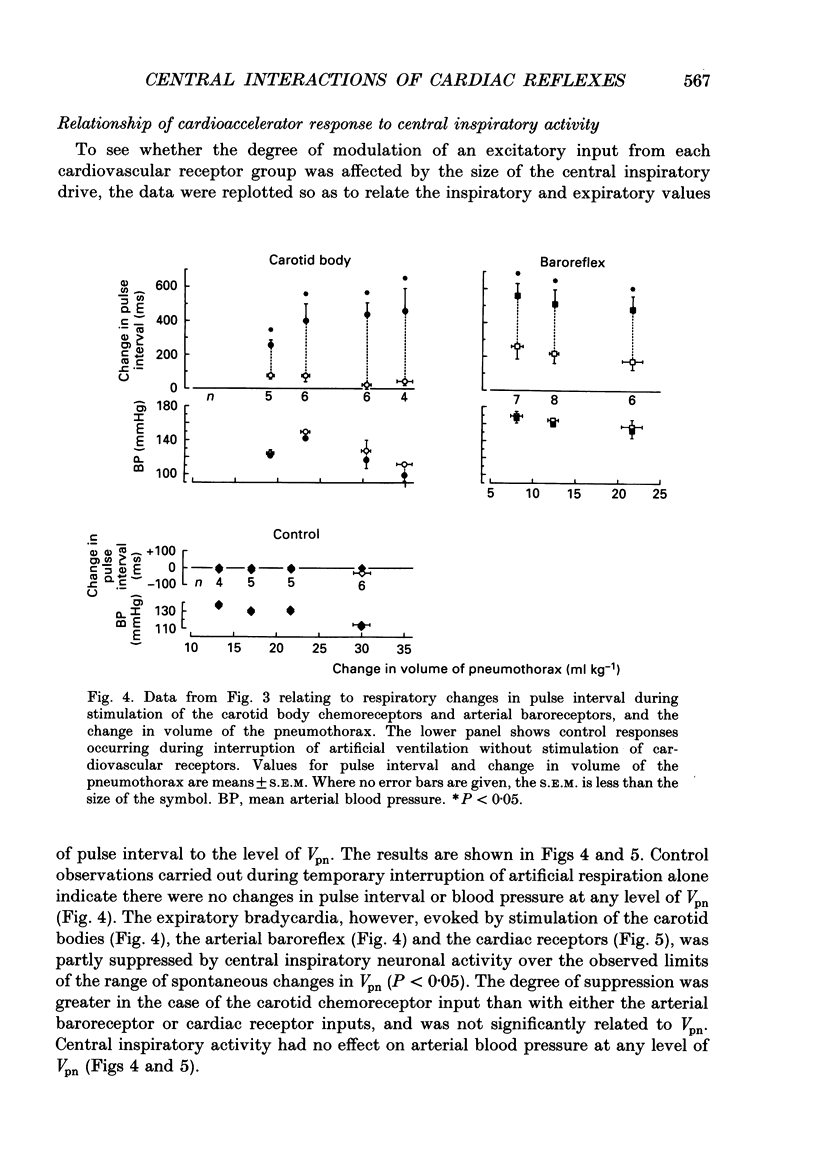
1. Cats were anaesthetized with a mixture of chloralose and urethane, and were artifically ventilated. 2. An open pneumothorax was provided by two large-bore tubes which were sealed in the sixth intercostal space on each side. They were connected to a Fleisch pneumotachograph. Phasic changes in central inspiratory neuronal activity were measured quantitatively as changes in the volume of the pneumothorax during temporary interruption of artificial respiration, the volume of the lungs being held constant at their end-expiratory level. In this way the activity of slowly adapting pulmonary stretch receptors was maintained constant. 3. Reflex cardioinhibitory responses were elicited by stimulation of (a) the carotid body chemoreceptors by intracarotid injections of cyanide; (b) the arterial baroreflex by controlled elevations of the blood pressure; (c) cardiac receptors by left atrial injections of veratridine; and (d) pulmonary C fibres (including J receptors) by right atrial injections of phenylbiguanide. 4. The effects of central inspiratory neuronal activity on pulse interval were assessed by comparing the values observed during the inspiratory and expiratory phases of the respiratory cycle in the control state and during stimulation of each cardiovascular receptor group. 5. The carotid chemoreceptor-induced bradycardia measured during the expiratory phase of respiration was reduced during inspiration to a value of about 15% of control. The central inspiratory drive was less effective in altering the reflex responses from the arterial baroreceptors and cardiac receptors, the corresponding values being 42 and 51% respectively. 6. In contrast, the bradycardia evoked by pulmonary C fibre stimulation was not significantly affected by the central inspiratory drive. 7. The differential nature of the modulation by the central inspiratory drive occurred independently of the integrity of the sympathetic nerve supply to the heart indicating that the cardiac efferents involved were largely fibres in the vagus nerves. 8. The possible explanation of these results in terms of central mechanisms is discussed.
Full text
PDF













Selected References
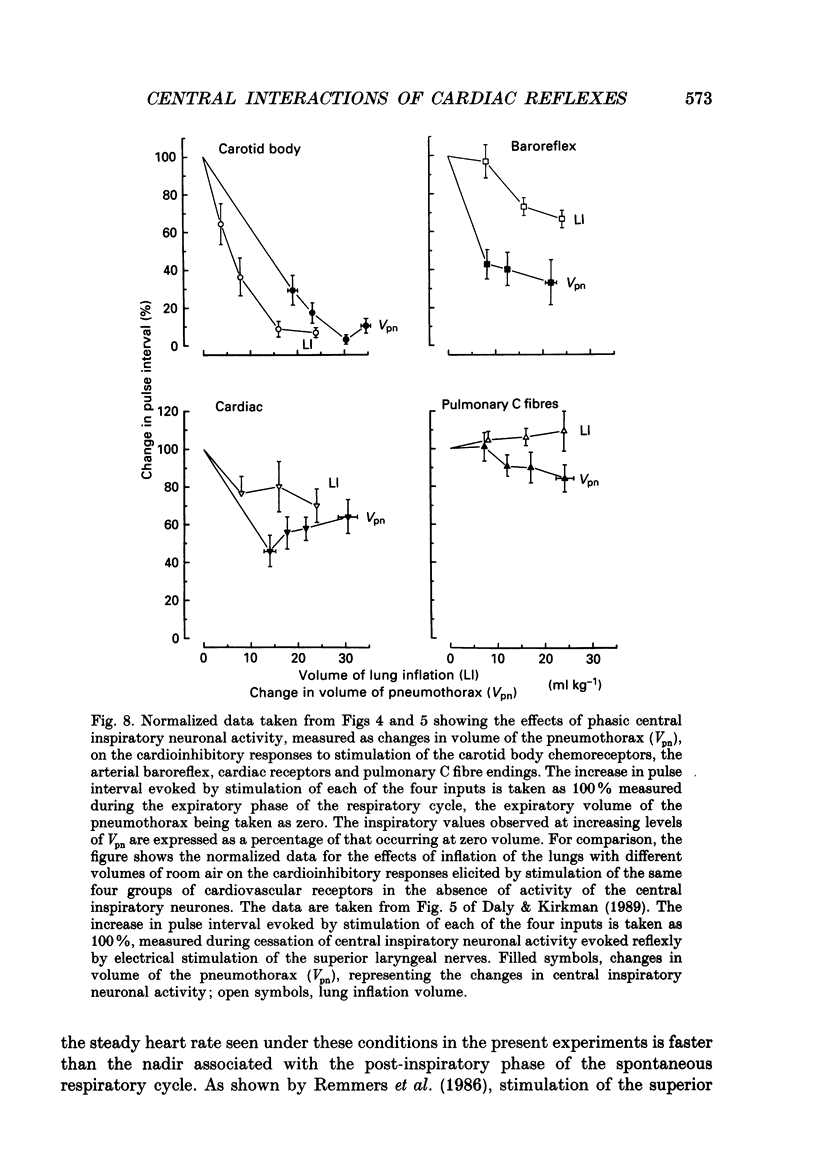
These references are in PubMed. This may not be the complete list of references from this article.
- Angell-James J. E., Daly M. D. The effects of artificial lung inflation on reflexly induced bradycardia associated with apnoea in the dog. J Physiol. 1978 Jan;274:349–366. doi: 10.1113/jphysiol.1978.sp012152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. A., Goodchild C. S., Kidd C., McWilliam P. N. Neurones in the brain stem of the cat excited by vagal afferent fibres from the heart and lungs. J Physiol. 1985 Dec;369:1–15. doi: 10.1113/jphysiol.1985.sp015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. A., Kidd C., Latif A. B., McWilliam P. N. A horseradish peroxidase study of vagal motoneurones with axons in cardiac and pulmonary branches of the cat and dog. Q J Exp Physiol. 1981 Apr;66(2):145–154. doi: 10.1113/expphysiol.1981.sp002541. [DOI] [PubMed] [Google Scholar]
- DAWES G. S., FASTIER F. N. Reflex actions of some isothiourea derivatives on circulation and respiration. Br J Pharmacol Chemother. 1950 Jun;5(2):323–334. doi: 10.1111/j.1476-5381.1950.tb01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES G. S., MOTT J. C. Circulatory and respiratory reflexes caused by aromatic guanidines. Br J Pharmacol Chemother. 1950 Mar;5(1):65–76. doi: 10.1111/j.1476-5381.1950.tb00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DALY M. B., SCOTT M. J. The effects of stimulation of the carotid body chemoreceptors on heart rate in the dog. J Physiol. 1958 Nov 10;144(1):148–166. doi: 10.1113/jphysiol.1958.sp006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M. B., Kirkman E. Differential modulation by pulmonary stretch afferents of some reflex cardioinhibitory responses in the cat. J Physiol. 1989 Oct;417:323–341. doi: 10.1113/jphysiol.1989.sp017804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M. D., Kirkman E. Cardiovascular responses to stimulation of pulmonary C fibres in the cat: their modulation by changes in respiration. J Physiol. 1988 Aug;402:43–63. doi: 10.1113/jphysiol.1988.sp017193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M. D., Ward J., Wood L. M. Modification by lung inflation of the vascular responses from the carotid body chemoreceptors and other receptors in dogs. J Physiol. 1986 Sep;378:13–30. doi: 10.1113/jphysiol.1986.sp016205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson N. S., Goldner S., McCloskey D. I. Respiratory modulation of barareceptor and chemoreceptor reflexes affecting heart rate and cardiac vagal efferent nerve activity. J Physiol. 1976 Jul;259(2):523–530. doi: 10.1113/jphysiol.1976.sp011480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue S., Fox R. E., Kidd C., Koley B. N. The distribution in the cat brain stem of neurones activated by vagal nonmyelinated fibres from the heart and lungs. Q J Exp Physiol. 1981 Oct;66(4):391–404. doi: 10.1113/expphysiol.1981.sp002582. [DOI] [PubMed] [Google Scholar]
- Gandevia S. C., McCloskey D. I., Potter E. K. Inhibition of baroreceptor and chemoreceptor reflexes on heart rate by afferents from the lungs. J Physiol. 1978 Mar;276:369–381. doi: 10.1113/jphysiol.1978.sp012240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbey M. P., Jordan D., Richter D. W., Spyer K. M. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. J Physiol. 1984 Nov;356:65–78. doi: 10.1113/jphysiol.1984.sp015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. E., Daly M. de B. Cardiovascular responses in apnoeic asphyxia: role of arterial chemoreceptors and the modification of their effects by a pulmonary vagal inflation reflex. J Physiol. 1969 Mar;201(1):87–104. doi: 10.1113/jphysiol.1969.sp008744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D., Spyer K. M. Brainstem integration of cardiovascular and pulmonary afferent activity. Prog Brain Res. 1986;67:295–314. doi: 10.1016/s0079-6123(08)62769-7. [DOI] [PubMed] [Google Scholar]
- Jordan D., Spyer K. M. Studies on the excitability of sinus nerve afferent terminals. J Physiol. 1979 Dec;297(0):123–134. doi: 10.1113/jphysiol.1979.sp013031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOEPCHEN H. P., LUX H. D., WAGNER P. H. [Studies on time requirement and central development of the pressor receptor heart reflex]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1961;273:413–430. [PubMed] [Google Scholar]
- KOEPCHEN H. P., WAGNER P. H., LUX H. D. [On the relationships between central excitation, reflex tone and respiratory rhythm in nervous regulation of heart frequency]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1961;273:443–465. [PubMed] [Google Scholar]
- Kalia M., Mesulam M. M. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980 Sep 15;193(2):467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- McAllen R. M., Spyer K. M. The baroreceptor input to cardiac vagal motoneurones. J Physiol. 1978 Sep;282:365–374. doi: 10.1113/jphysiol.1978.sp012469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen R. M., Spyer K. M. The location of cardiac vagal preganglionic motoneurones in the medulla of the cat. J Physiol. 1976 Jun;258(1):187–204. doi: 10.1113/jphysiol.1976.sp011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen R. M., Spyer K. M. Two types of vagal preganglionic motoneurones projecting to the heart and lungs. J Physiol. 1978 Sep;282:353–364. doi: 10.1113/jphysiol.1978.sp012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin S. W., Spyer K. M., Withington-Wray D. J. Baroreceptor inputs to the nucleus tractus solitarius in the cat: postsynaptic actions and the influence of respiration. J Physiol. 1988 May;399:349–367. doi: 10.1113/jphysiol.1988.sp017085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter E. K. Inspiratory inhibition of vagal responses to baroreceptor and chemoreceptor stimuli in the dog. J Physiol. 1981 Jul;316:177–190. doi: 10.1113/jphysiol.1981.sp013781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers J. E., Richter D. W., Ballantyne D., Bainton C. R., Klein J. P. Reflex prolongation of stage I of expiration. Pflugers Arch. 1986 Aug;407(2):190–198. doi: 10.1007/BF00580675. [DOI] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Spyer K. M. Central nervous integration of cardiovascular control. J Exp Biol. 1982 Oct;100:109–128. doi: 10.1242/jeb.100.1.109. [DOI] [PubMed] [Google Scholar]
- St John W. M., Zhou D. Discharge of vagal pulmonary receptors differentially alters neural activities during various stages of expiration in the cat. J Physiol. 1990 May;424:1–12. doi: 10.1113/jphysiol.1990.sp018051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADE O. L. Movements of the thoracic cage and diaphragm in respiration. J Physiol. 1954 May 28;124(2):193–212. doi: 10.1113/jphysiol.1954.sp005099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley D. C., McWilliam P. N., Ford T. W., Clarke R. W. The effect of selective electrical stimulation of non-myelinated vagal fibres on heart rate in the rabbit. J Auton Nerv Syst. 1987 Dec;21(2-3):215–221. doi: 10.1016/0165-1838(87)90024-5. [DOI] [PubMed] [Google Scholar]
- de Burgh Daly M., Kirkman E., Wood L. M. Cardiovascular responses to stimulation of cardiac receptors in the cat and their modification by changes in respiration. J Physiol. 1988 Dec;407:349–362. doi: 10.1113/jphysiol.1988.sp017419. [DOI] [PMC free article] [PubMed] [Google Scholar]


