Abstract
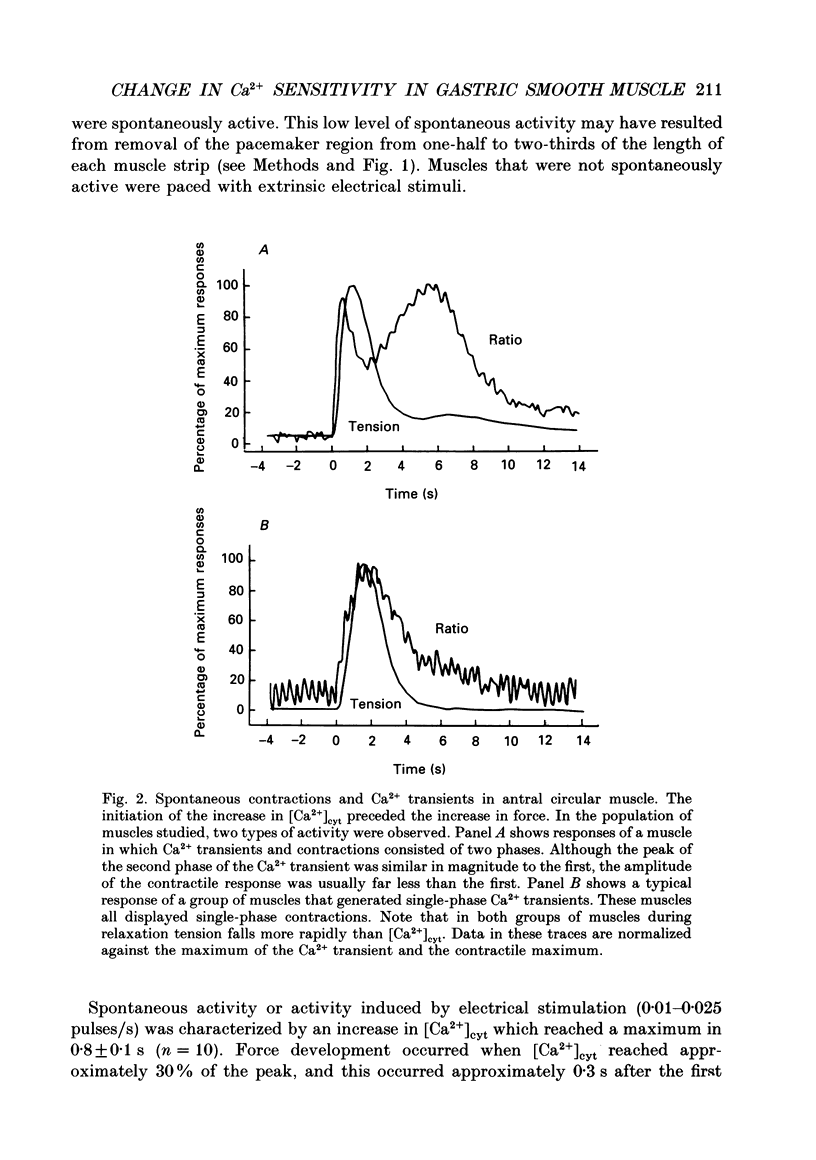
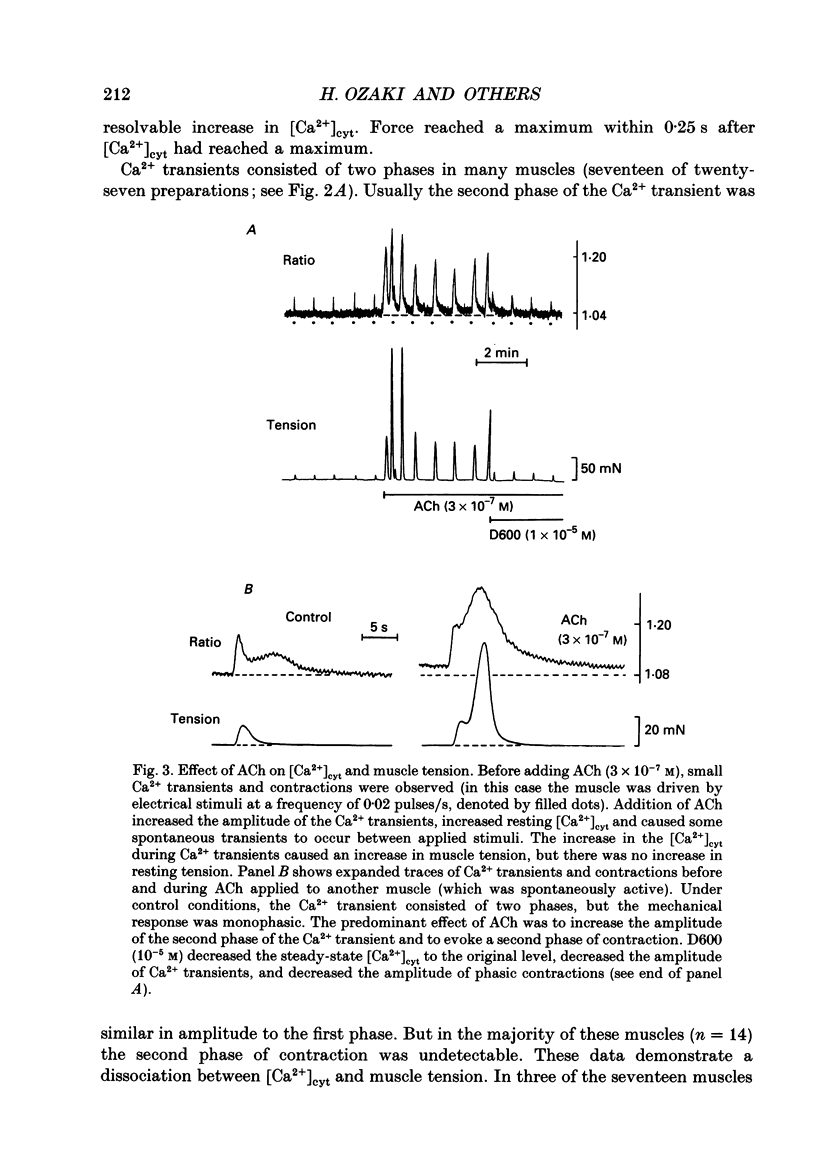
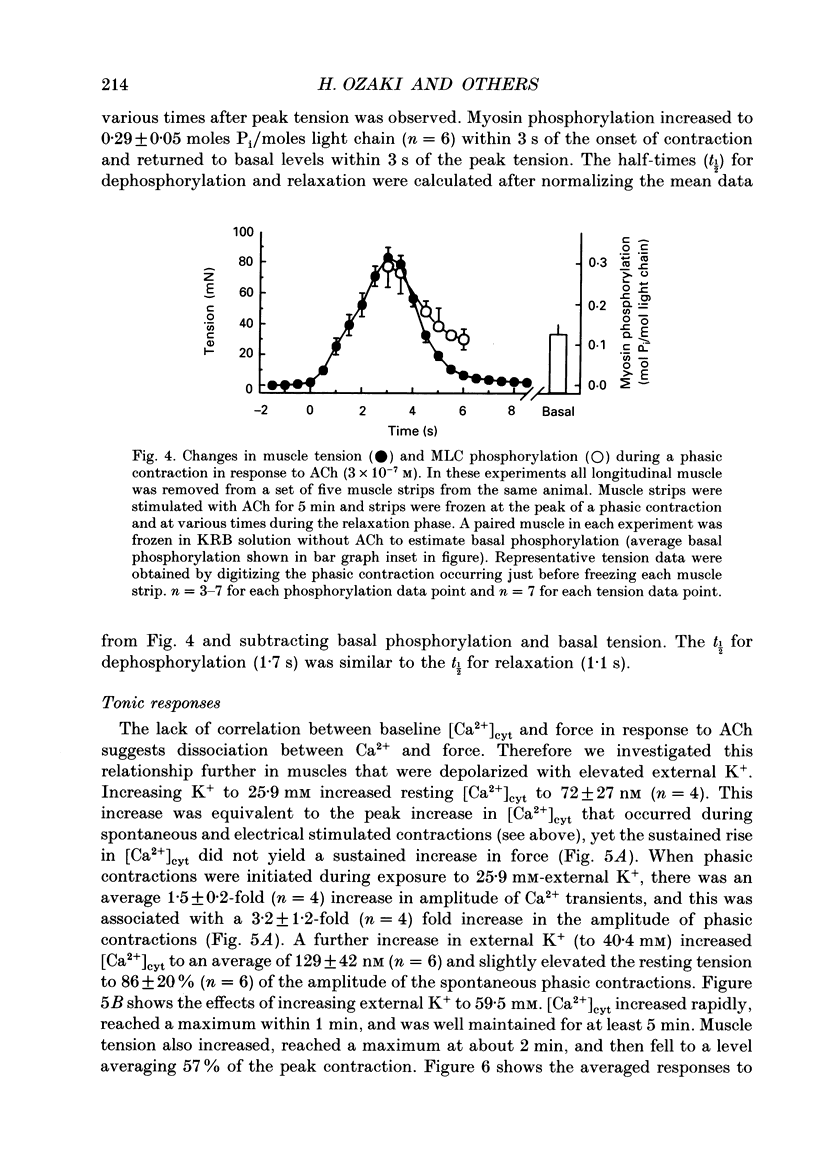
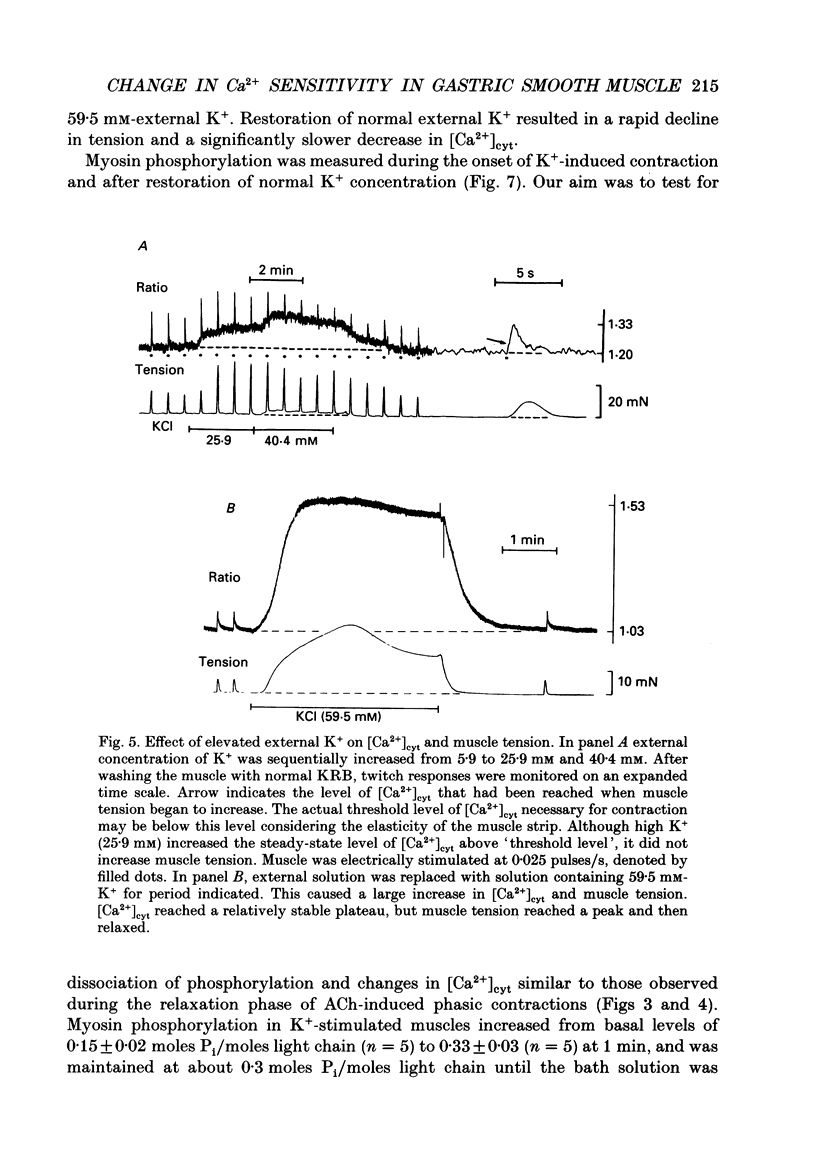
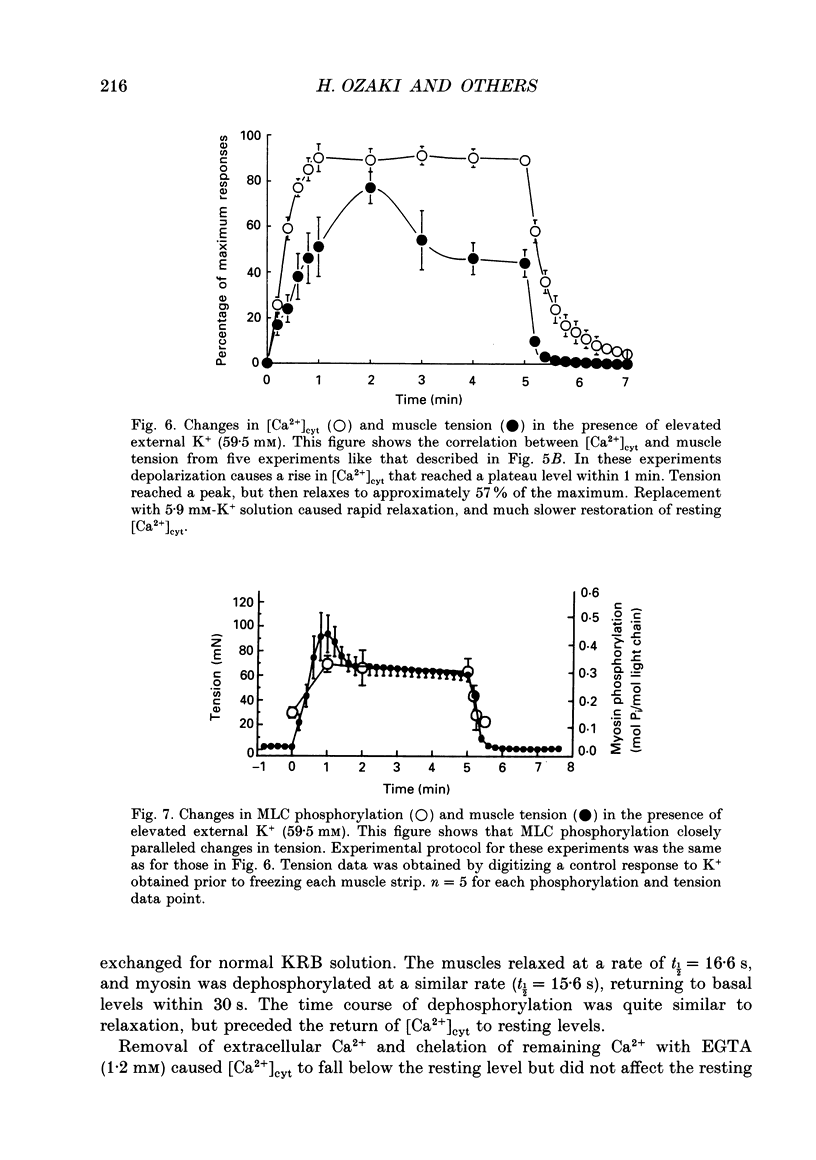
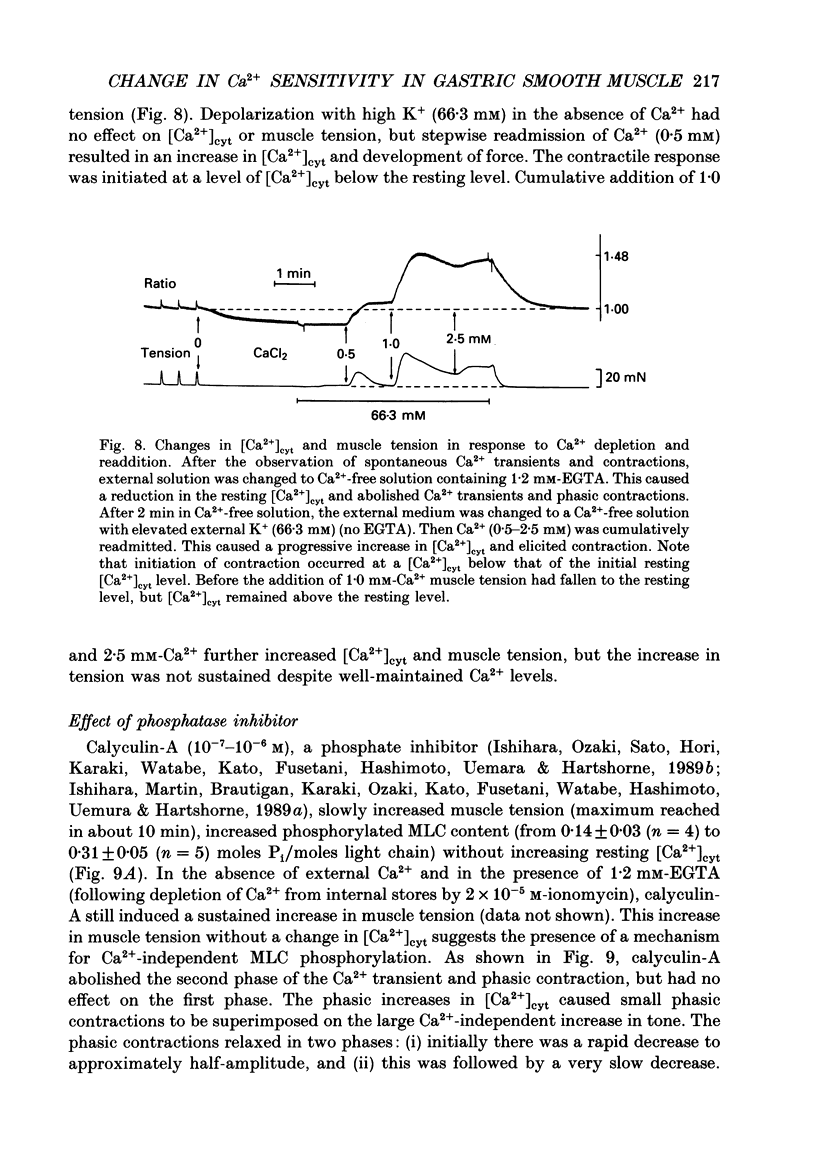
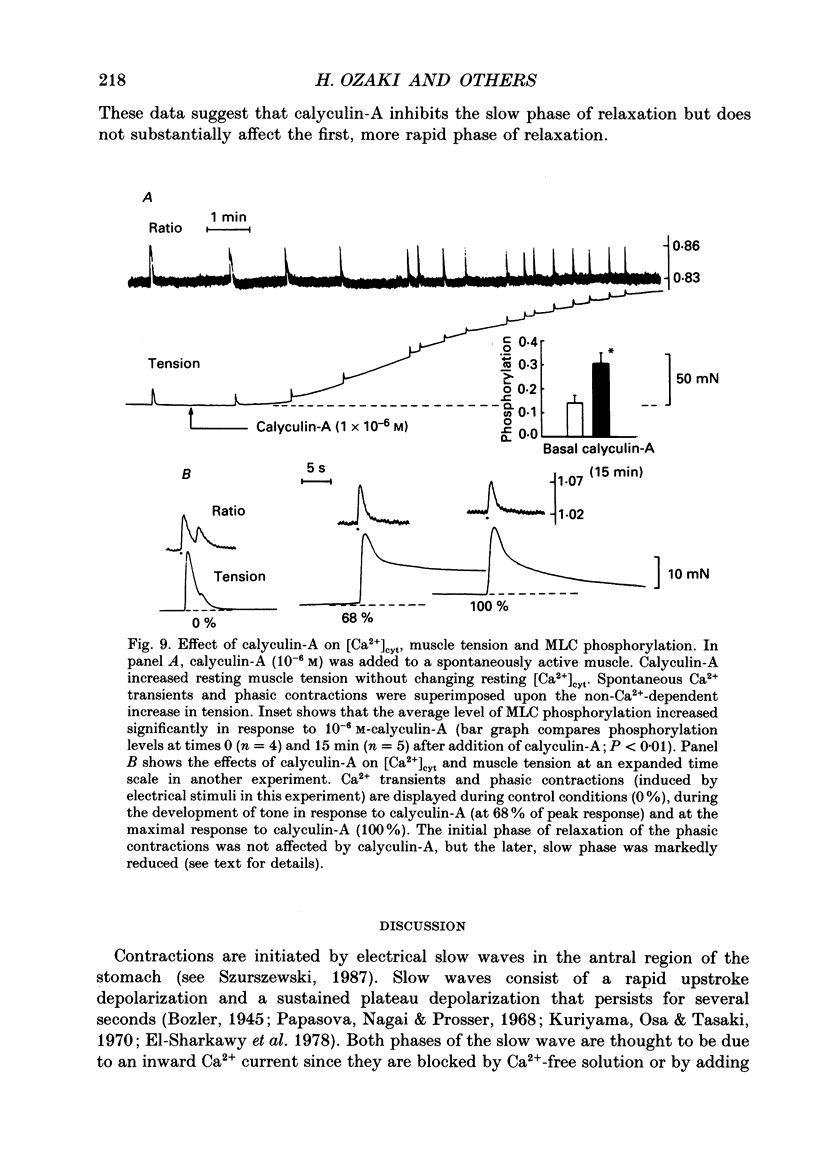
1. Relationships between cytosolic Ca2+ concentration ([Ca2+]cyt), myosin light chain (MLC) phosphorylation and muscle tension were examined in circular smooth muscle of canine gastric antrum. 2. Electrical slow waves induced a transient increase in [Ca2+]cyt and muscle tension. [Ca2+]cyt increased before the initiation of contraction and reached a maximum before the peak of the phasic contractions. Following the first Ca2+ transient, a second rise in [Ca2+]cyt was often observed. The second Ca2+ transient was of similar magnitude to the first, but only in some cases was this increase in [Ca2+]cyt associated with a second phase of contraction. Relaxation occurred more rapidly than the restoration of resting levels of [Ca2+]cyt. 3. Acetylcholine (ACh; 3 x 10(-7) M) increased the amplitude of Ca2+ transients, caused MLC phosphorylation and increased the force of contraction. The decay of contraction and MLC dephosphorylation preceded that of [Ca2+]cyt. 4. Increasing external K+ (to 25-40 mM) caused a sustained increase in [Ca2+]cyt, but little change in resting tension. This suggests that the Ca2+ sensitivity decreased as [Ca2+]cyt increased. Increasing K+ to 59.5 mM further increased the level of [Ca2+]cyt, induced MLC phosphorylation and caused a transient contraction. When normal levels of K+ were restored, the rates of MLC dephosphorylation and relaxation exceeded the rate of decay in [Ca2+]cyt. 5. Removal of external Ca2+ in depolarized muscles decreased [Ca2+]cyt below the resting level without affecting resting tension. Readmission of Ca2+ to depolarized muscles caused force to develop at [Ca2+]cyt levels below the original resting level, suggesting that Ca2+ sensitivity was increased when the resting level of [Ca2+]cyt was decreased. 6. The phosphatase inhibitor, calyculin-A (10(-6) M), induced tonic contraction and MLC phosphorylation without an increase in [Ca2+]cyt. During these contractures, electrical activity caused transient increases in [Ca2+]cyt and phasic contractions which were superimposed upon the Ca(2+)-independent contracture. In the presence of calyculin-A, relaxation occurred in two phases. The initial, rapid phase of relaxation was not significantly affected by calyculin-A, but the slow phase was significantly decreased. 7. These results suggest that the relationship between [Ca2+]cyt, MLC phosphorylation and contraction changes as a function of [Ca2+]cyt in canine antral muscles. This may be due to a Ca(2+)-and time-dependent phosphatase that regulates the level of myosin phosphorylation.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. J., Publicover N. G., Sanders K. M. Origin and spread of slow waves in canine gastric antral circular muscle. Am J Physiol. 1985 Dec;249(6 Pt 1):G800–G806. doi: 10.1152/ajpgi.1985.249.6.G800. [DOI] [PubMed] [Google Scholar]
- Bauer A. J., Reed J. B., Sanders K. M. Slow wave heterogeneity within the circular muscle of the canine gastric antrum. J Physiol. 1985 Sep;366:221–232. doi: 10.1113/jphysiol.1985.sp015793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. J., Sanders K. M. Gradient in excitation-contraction coupling in canine gastric antral circular muscle. J Physiol. 1985 Dec;369:283–294. doi: 10.1113/jphysiol.1985.sp015901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeo T. T., Morgan K. G. Calcium-force relationships as detected with aequorin in two different vascular smooth muscles of the ferret. J Physiol. 1985 Dec;369:269–282. doi: 10.1113/jphysiol.1985.sp015900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerthoffer W. T., Murphey K. A., Gunst S. J. Aequorin luminescence, myosin phosphorylation, and active stress in tracheal smooth muscle. Am J Physiol. 1989 Dec;257(6 Pt 1):C1062–C1068. doi: 10.1152/ajpcell.1989.257.6.C1062. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hathaway D. R., Haeberle J. R. A radioimmunoblotting method for measuring myosin light chain phosphorylation levels in smooth muscle. Am J Physiol. 1985 Sep;249(3 Pt 1):C345–C351. doi: 10.1152/ajpcell.1985.249.3.C345. [DOI] [PubMed] [Google Scholar]
- Hathaway D. R., Haeberle J. R. Selective purification of the 20,000-Da light chains of smooth muscle myosin. Anal Biochem. 1983 Nov;135(1):37–43. doi: 10.1016/0003-2697(83)90726-1. [DOI] [PubMed] [Google Scholar]
- Himpens B., Casteels R. Different effects of depolarization and muscarinic stimulation on the Ca2+/force relationship during the contraction-relaxation cycle in the guinea pig ileum. Pflugers Arch. 1990 Apr;416(1-2):28–35. doi: 10.1007/BF00370218. [DOI] [PubMed] [Google Scholar]
- Himpens B., Matthijs G., Somlyo A. P. Desensitization to cytoplasmic Ca2+ and Ca2+ sensitivities of guinea-pig ileum and rabbit pulmonary artery smooth muscle. J Physiol. 1989 Jun;413:489–503. doi: 10.1113/jphysiol.1989.sp017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. The protein phosphatases involved in cellular regulation. 1. Classification and substrate specificities. Eur J Biochem. 1983 May 2;132(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07357.x. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Ozaki H., Sato K., Hori M., Karaki H., Watabe S., Kato Y., Fusetani N., Hashimoto K., Uemura D. Calcium-independent activation of contractile apparatus in smooth muscle by calyculin-A. J Pharmacol Exp Ther. 1989 Jul;250(1):388–396. [PubMed] [Google Scholar]
- Ito Y., Kuriyama H., Parker I. Calcium transients evoked by electrical stimulation of smooth muscle from guinea-pig ileum recorded by the use of Fura-2. J Physiol. 1988 Dec;407:117–134. doi: 10.1113/jphysiol.1988.sp017406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Karaki H. Ca2+ localization and sensitivity in vascular smooth muscle. Trends Pharmacol Sci. 1989 Aug;10(8):320–325. doi: 10.1016/0165-6147(89)90066-7. [DOI] [PubMed] [Google Scholar]
- Kitazawa T., Somlyo A. P. Desensitization and muscarinic re-sensitization of force and myosin light chain phosphorylation to cytoplasmic Ca2+ in smooth muscle. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1291–1297. doi: 10.1016/0006-291x(90)91589-k. [DOI] [PubMed] [Google Scholar]
- Konishi M., Olson A., Hollingworth S., Baylor S. M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J. 1988 Dec;54(6):1089–1104. doi: 10.1016/S0006-3495(88)83045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Tasaki H. Electrophysiological studies of the antrum muscle fibers of the guinea pig stomach. J Gen Physiol. 1970 Jan;55(1):48–62. doi: 10.1085/jgp.55.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui M., Karaki H. Dual effects of carbachol on cytosolic Ca2+ and contraction in intestinal smooth muscle. Am J Physiol. 1990 May;258(5 Pt 1):C787–C793. doi: 10.1152/ajpcell.1990.258.5.C787. [DOI] [PubMed] [Google Scholar]
- Obara K., Takai A., Ruegg J. C., de Lanerolle P. Okadaic acid, a phosphatase inhibitor, produces a Ca2+ and calmodulin-independent contraction of smooth muscle. Pflugers Arch. 1989 Jun;414(2):134–138. doi: 10.1007/BF00580954. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Ishihara H., Kohama K., Nonomura Y., Shibata S., Karaki H. Calcium-independent phosphorylation of smooth muscle myosin light chain by okadaic acid isolated from black sponge (Halichondria okadai). J Pharmacol Exp Ther. 1987 Dec;243(3):1167–1173. [PubMed] [Google Scholar]
- Ozaki H., Kohama K., Nonomura Y., Shibata S., Karaki H. Direct activation by okadaic acid of the contractile elements in the smooth muscle of guinea-pig taenia coli. Naunyn Schmiedebergs Arch Pharmacol. 1987 Mar;335(3):356–358. doi: 10.1007/BF00172811. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Kwon S. C., Tajimi M., Karaki H. Changes in cytosolic CA2+ and contraction induced by various stimulants and relaxants in canine tracheal smooth muscle. Pflugers Arch. 1990 Jun;416(4):351–359. doi: 10.1007/BF00370740. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Ohyama T., Sato K., Karaki H. Ca2(+)-dependent and independent mechanisms of sustained contraction in vascular smooth muscle of rat aorta. Jpn J Pharmacol. 1990 Mar;52(3):509–512. doi: 10.1254/jjp.52.509. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Sato K., Satoh T., Karaki H. Simultaneous recordings of calcium signals and mechanical activity using fluorescent dye fura 2 in isolated strips of vascular smooth muscle. Jpn J Pharmacol. 1987 Nov;45(3):429–433. doi: 10.1254/jjp.45.429. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Satoh T., Karaki H., Ishida Y. Regulation of metabolism and contraction by cytoplasmic calcium in the intestinal smooth muscle. J Biol Chem. 1988 Oct 5;263(28):14074–14079. [PubMed] [Google Scholar]
- Papasova M. P., Nagai T., Prosser C. L. Two-component slow waves in smooth muscle of cat stomach. Am J Physiol. 1968 Apr;214(4):695–702. doi: 10.1152/ajplegacy.1968.214.4.695. [DOI] [PubMed] [Google Scholar]
- Persechini A., Kamm K. E., Stull J. T. Different phosphorylated forms of myosin in contracting tracheal smooth muscle. J Biol Chem. 1986 May 15;261(14):6293–6299. [PubMed] [Google Scholar]
- Rembold C. M. Desensitization of swine arterial smooth muscle to transplasmalemmal Ca2+ influx. J Physiol. 1989 Sep;416:273–290. doi: 10.1113/jphysiol.1989.sp017760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata K., Ozaki H., Kwon S. C., Karaki H. Effects of endothelin on the mechanical activity and cytosolic calcium level of various types of smooth muscle. Br J Pharmacol. 1989 Oct;98(2):483–492. doi: 10.1111/j.1476-5381.1989.tb12621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator fura 2. J Pharmacol Exp Ther. 1988 Jul;246(1):294–300. [PubMed] [Google Scholar]
- Scanlon M., Williams D. A., Fay F. S. A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Assessment and accurate Ca2+ measurement. J Biol Chem. 1987 May 5;262(13):6308–6312. [PubMed] [Google Scholar]
- Shibata S., Ishida Y., Kitano H., Ohizumi Y., Habon J., Tsukitani Y., Kikuchi H. Contractile effects of okadaic acid, a novel ionophore-like substance from black sponge, on isolated smooth muscles under the condition of Ca deficiency. J Pharmacol Exp Ther. 1982 Oct;223(1):135–143. [PubMed] [Google Scholar]
- Somlyo A. P., Himpens B. Cell calcium and its regulation in smooth muscle. FASEB J. 1989 Sep;3(11):2266–2276. doi: 10.1096/fasebj.3.11.2506092. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Flash photolysis studies of excitation-contraction coupling, regulation, and contraction in smooth muscle. Annu Rev Physiol. 1990;52:857–874. doi: 10.1146/annurev.ph.52.030190.004233. [DOI] [PubMed] [Google Scholar]
- Stewart A. A., Ingebritsen T. S., Cohen P. The protein phosphatases involved in cellular regulation. 5. Purification and properties of a Ca2+/calmodulin-dependent protein phosphatase (2B) from rabbit skeletal muscle. Eur J Biochem. 1983 May 2;132(2):289–295. doi: 10.1111/j.1432-1033.1983.tb07361.x. [DOI] [PubMed] [Google Scholar]
- Yagi S., Becker P. L., Fay F. S. Relationship between force and Ca2+ concentration in smooth muscle as revealed by measurements on single cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4109–4113. doi: 10.1073/pnas.85.11.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Sharkawy T. Y., Morgan K. G., Szurszewski J. H. Intracellular electrical activity of canine and human gastric smooth muscle. J Physiol. 1978 Jun;279:291–307. doi: 10.1113/jphysiol.1978.sp012345. [DOI] [PMC free article] [PubMed] [Google Scholar]


