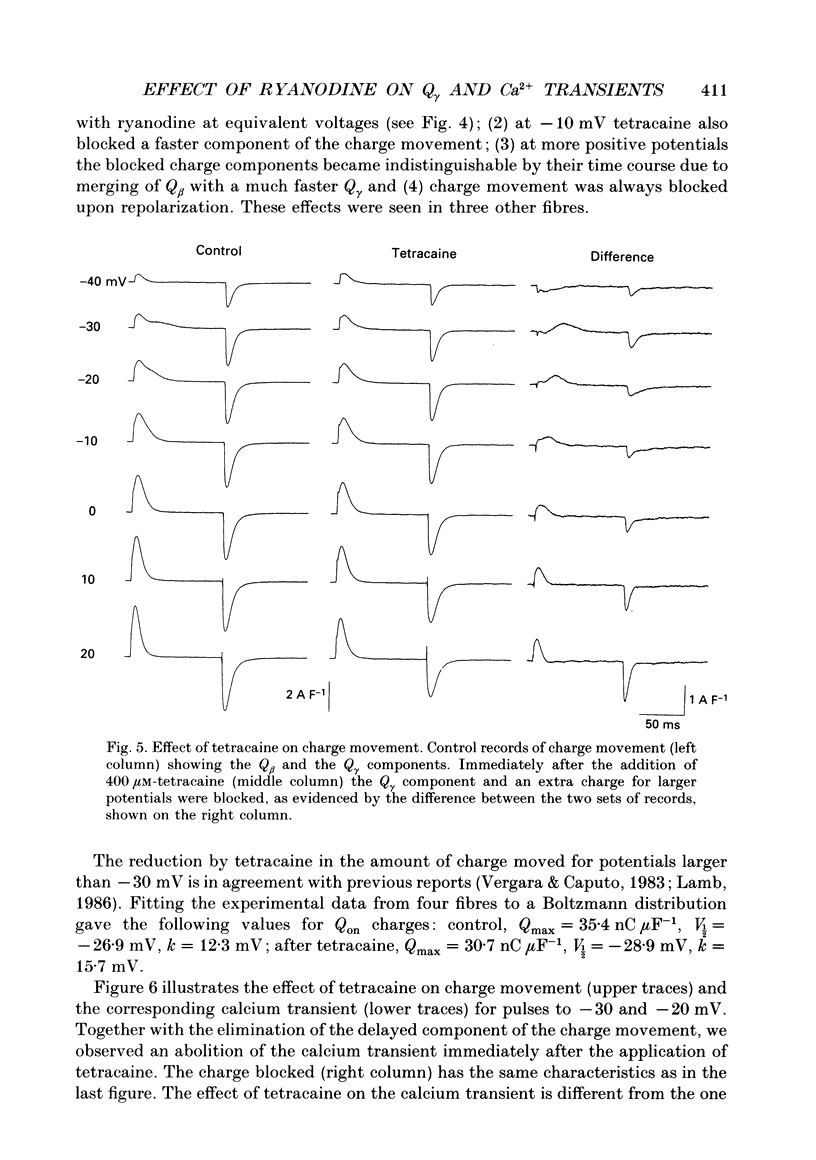
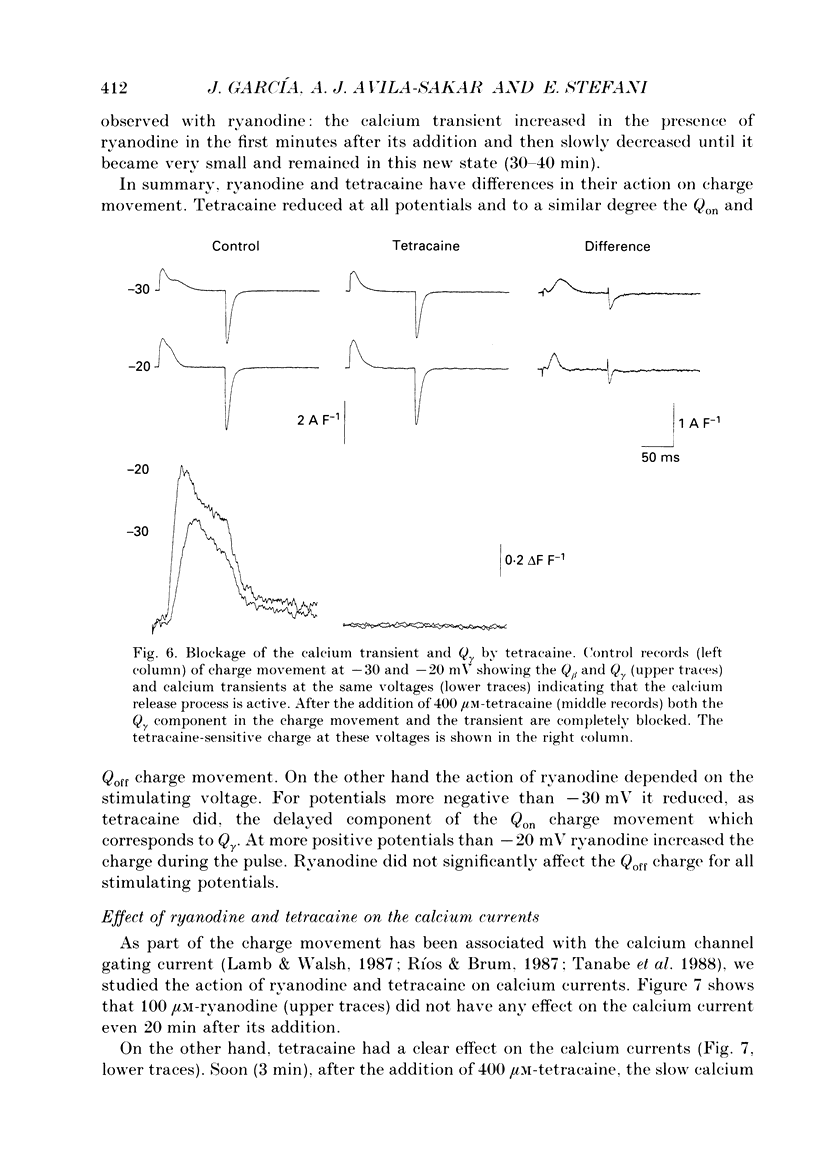
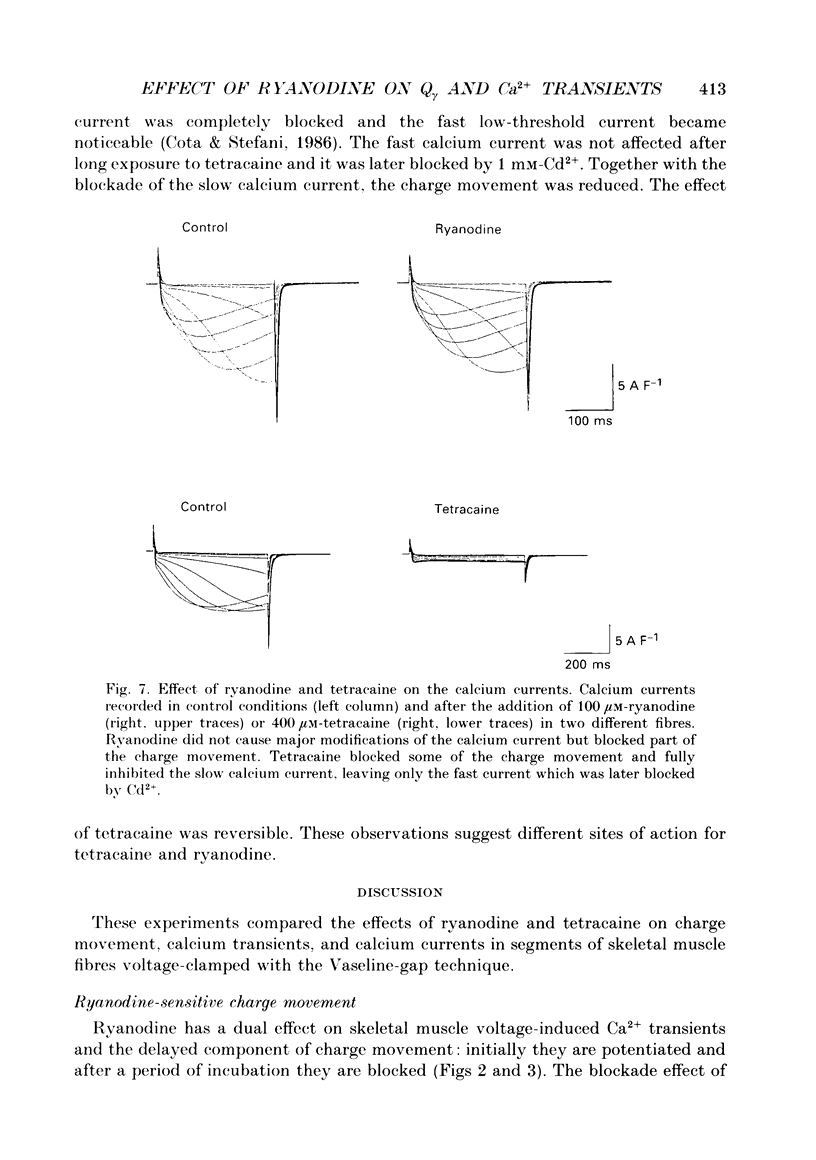
Abstract
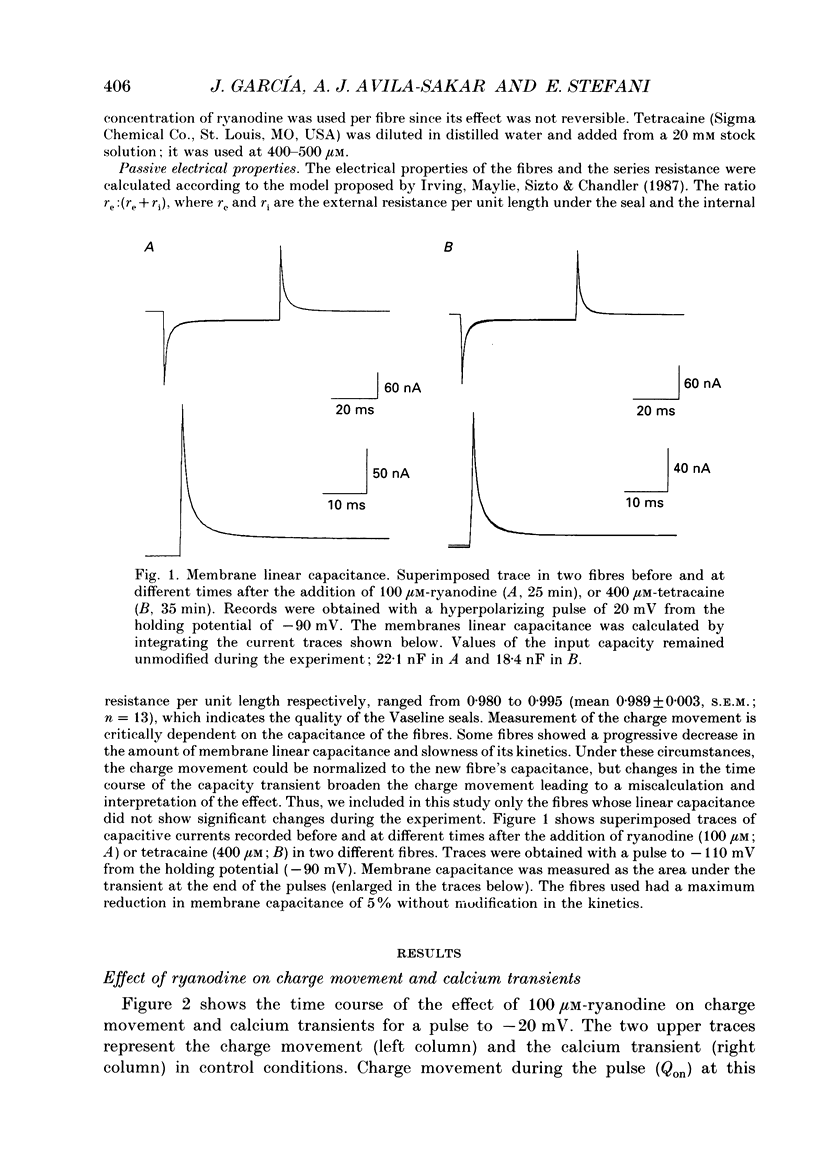
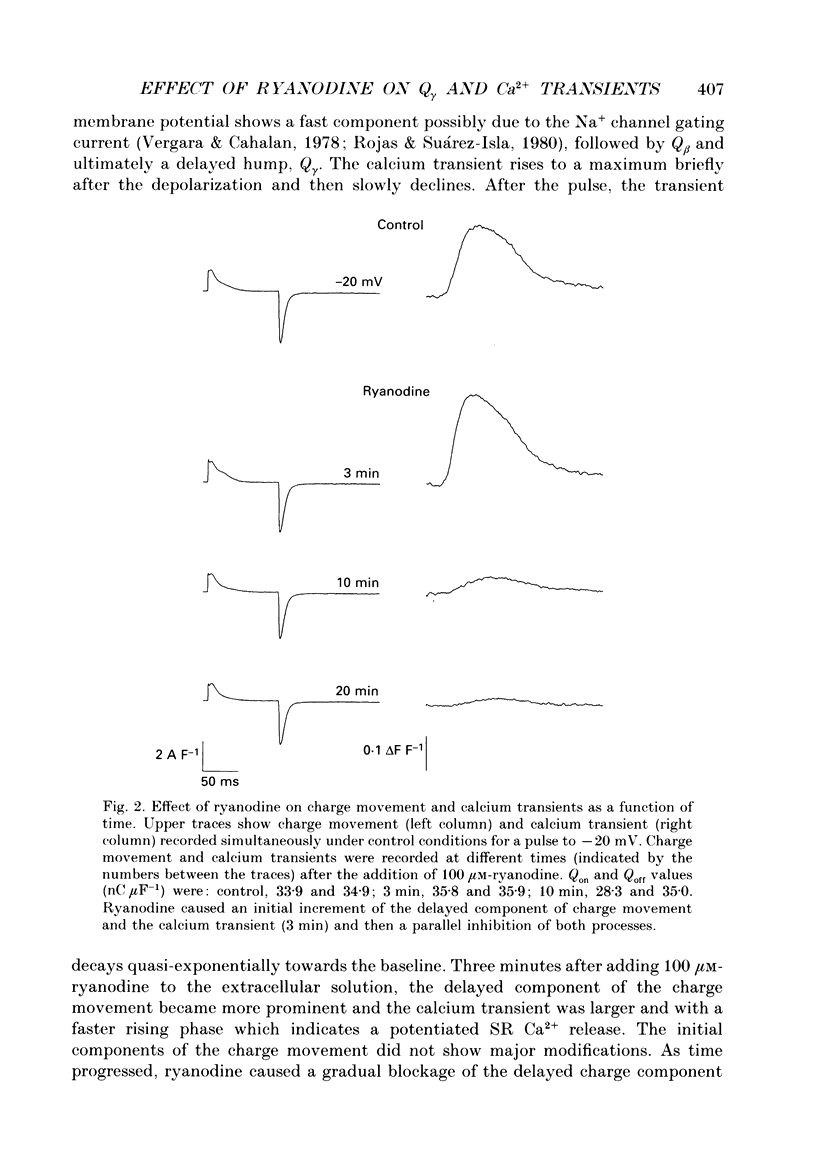
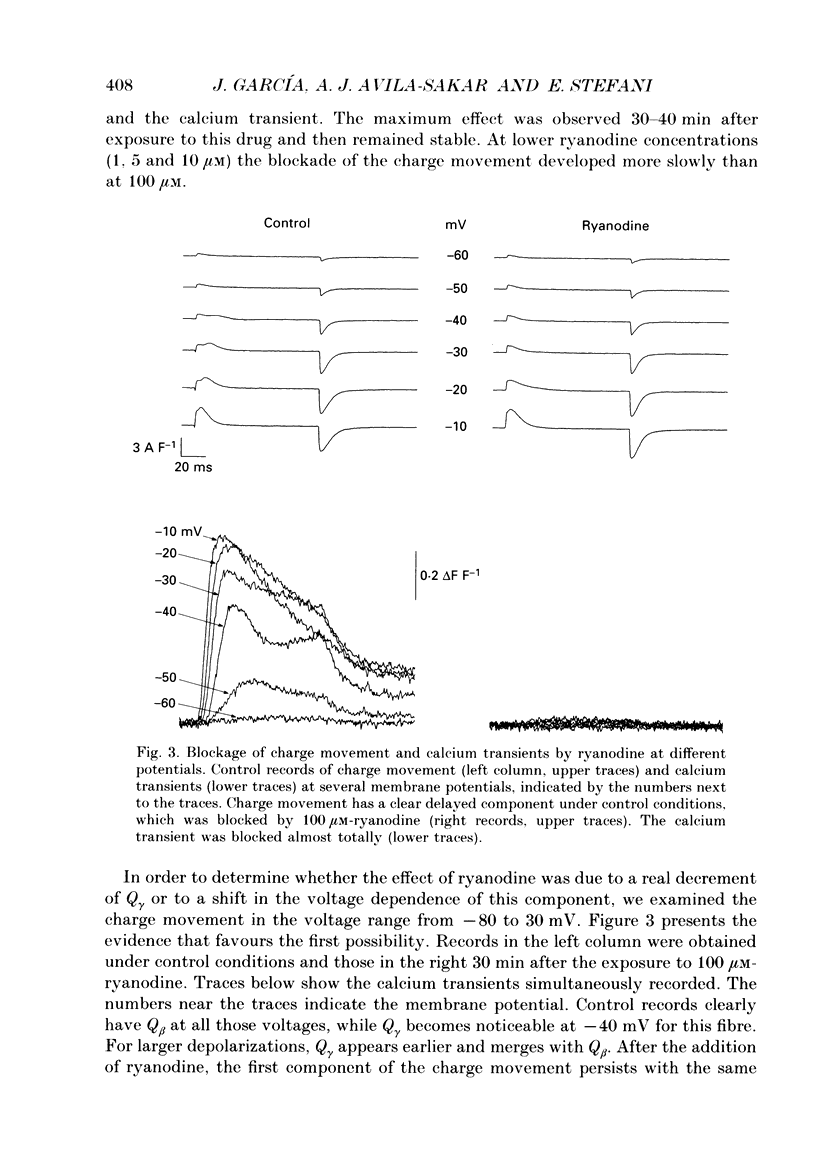
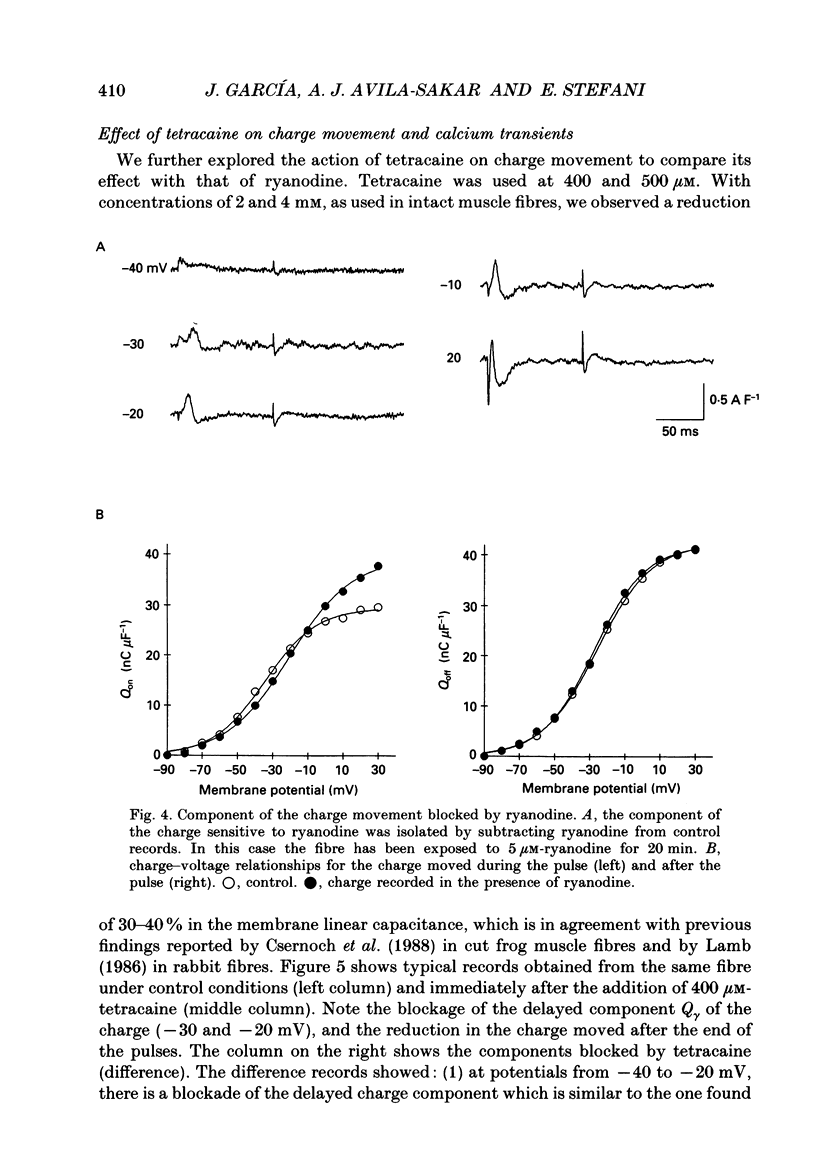
1. Charge movement and myoplasmic calcium transients were simultaneously recorded from frog skeletal muscle fibres by using the double-seal Vaseline-gap technique. Calcium transients were monitored with the fluorescent indicator Rhod-2. 2. Ryanodine modified the kinetics and the total amount of charge moved during depolarizing pulses (Q(on)), while it did not significantly modify the charge after repolarization (Q(off)). The extracellular application of 100 microM-ryanodine elicited a temporary initial increase of the delayed component of charge movement (Q gamma) and the calcium transient. Both phenomena were later blocked with the same temporal course and to the same extent. 3. The blockade of Q gamma and the calcium transient was also observed with ryanodine concentrations of 1-10 microM. For membrane potentials positive to -10mV, the Qon measured was larger in the presence of ryanodine; Qoff was not modified. 4. Tetracaine (400-500 microM) blocked a similar delayed component of Qon, identified as Q gamma, as well as the calcium transient monitored simultaneously. This effect was observed in the first minutes after the addition of tetracaine to the extracellular solution. 5. Tetracaine blocked a faster initial component of Qon for voltages positive to -10 mV, corresponding to the voltage range of activation of the calcium current. At these same membrane potentials, Qoff was also reduced to a similar extent to Qon. 6. Ryanodine and tetracaine showed different effects on calcium current. Whereas the slow calcium current was not modified upon the addition of ryanodine, it was completely blocked in the presence of tetracaine. The blockade of the slow calcium current made evident the fast calcium current. The effects of tetracaine on the charge movement, the calcium transient and the slow calcium current were reversible. 7. These results suggest that ryanodine and tetracaine may act at different sites. Ryanodine exerts its effect on the sarcoplasmic reticulum ryanodine receptor, blocking calcium release and Q gamma, while tetracaine at these concentrations may affect the release channel and the dihydropyridine receptor, causing a blockade of the charge movement, calcium transient and calcium current.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Peres A. Charge movement and membrane capacity in frog muscle. J Physiol. 1979 Apr;289:83–97. doi: 10.1113/jphysiol.1979.sp012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota G., Stefani E. A fast-activated inward calcium current in twitch muscle fibres of the frog (Rana montezume). J Physiol. 1986 Jan;370:151–163. doi: 10.1113/jphysiol.1986.sp015927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L., Huang C. L., Szucs G., Kovacs L. Differential effects of tetracaine on charge movements and Ca2+ signals in frog skeletal muscle. J Gen Physiol. 1988 Nov;92(5):601–612. doi: 10.1085/jgp.92.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. A., Weiant E. A., Slocombe A. G., Roeder K. D. The Action of Ryanodine on the Contractile Process in Striated Muscle. Science. 1948 Sep 24;108(2804):330–332. doi: 10.1126/science.108.2804.330. [DOI] [PubMed] [Google Scholar]
- Francini F., Stefani E. Decay of the slow calcium current in twitch muscle fibers of the frog is influenced by intracellular EGTA. J Gen Physiol. 1989 Nov;94(5):953–969. doi: 10.1085/jgp.94.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Nunzi G. Junctional feet and particles in the triads of a fast-twitch muscle fibre. J Muscle Res Cell Motil. 1983 Apr;4(2):233–252. doi: 10.1007/BF00712033. [DOI] [PubMed] [Google Scholar]
- Fryer M. W., Lamb G. D., Neering I. R. The action of ryanodine on rat fast and slow intact skeletal muscles. J Physiol. 1989 Jul;414:399–413. doi: 10.1113/jphysiol.1989.sp017695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth S., Marshall M. W., Robson E. The effects of tetracaine on charge movement in fast twitch rat skeletal muscle fibres. J Physiol. 1990 Feb;421:633–644. doi: 10.1113/jphysiol.1990.sp017966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowicz P., Schneider M. F. Membrane charge moved at contraction thresholds in skeletal muscle fibres. J Physiol. 1981 May;314:595–633. doi: 10.1113/jphysiol.1981.sp013726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Dielectric components of charge movements in skeletal muscle. J Physiol. 1981;313:187–205. doi: 10.1113/jphysiol.1981.sp013658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L., Peachey L. D. Anatomical distribution of voltage-dependent membrane capacitance in frog skeletal muscle fibers. J Gen Physiol. 1989 Mar;93(3):565–584. doi: 10.1085/jgp.93.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L. Pharmacological separation of charge movement components in frog skeletal muscle. J Physiol. 1982 Mar;324:375–387. doi: 10.1113/jphysiol.1982.sp014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S. Pharmacological studies of charge movement in frog skeletal muscle. J Physiol. 1983 Apr;337:509–529. doi: 10.1113/jphysiol.1983.sp014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M., Maylie J., Sizto N. L., Chandler W. K. Intrinsic optical and passive electrical properties of cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):1–40. doi: 10.1085/jgp.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D. Components of charge movement in rabbit skeletal muscle: the effect of tetracaine and nifedipine. J Physiol. 1986 Jul;376:85–100. doi: 10.1113/jphysiol.1986.sp016143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Walsh T. Calcium currents, charge movement and dihydropyridine binding in fast- and slow-twitch muscles of rat and rabbit. J Physiol. 1987 Dec;393:595–617. doi: 10.1113/jphysiol.1987.sp016843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Chandler W. K. Calcium signals recorded from cut frog twitch fibers containing antipyrylazo III. J Gen Physiol. 1987 Jan;89(1):83–143. doi: 10.1085/jgp.89.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Melzer W., Schneider M. F., Simon B. J., Szucs G. Intramembrane charge movement and calcium release in frog skeletal muscle. J Physiol. 1986 Apr;373:481–511. doi: 10.1113/jphysiol.1986.sp016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Nakajima S., Parker I., Takahashi T. Effects of membrane polarization on sarcoplasmic calcium release in skeletal muscle. Proc R Soc Lond B Biol Sci. 1981 Sep 17;213(1190):1–13. doi: 10.1098/rspb.1981.0049. [DOI] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Pessah I. N., Francini A. O., Scales D. J., Waterhouse A. L., Casida J. E. Calcium-ryanodine receptor complex. Solubilization and partial characterization from skeletal muscle junctional sarcoplasmic reticulum vesicles. J Biol Chem. 1986 Jul 5;261(19):8643–8648. [PubMed] [Google Scholar]
- Pessah I. N., Waterhouse A. L., Casida J. E. The calcium-ryanodine receptor complex of skeletal and cardiac muscle. Biochem Biophys Res Commun. 1985 Apr 16;128(1):449–456. doi: 10.1016/0006-291x(85)91699-7. [DOI] [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Valdivia H. H., Coronado R. Inhibition of dihydropyridine-sensitive calcium channels by the plant alkaloid ryanodine. FEBS Lett. 1989 Feb 27;244(2):333–337. doi: 10.1016/0014-5793(89)80557-5. [DOI] [PubMed] [Google Scholar]
- Vergara J., Caputo C. Effects of tetracaine on charge movements and calcium signals in frog skeletal muscle fibers. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1477–1481. doi: 10.1073/pnas.80.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]


