Abstract
The putative Cryptococcus neoformans pheromone receptor gene CPRα was isolated and studied for its role in mating and filamentation. CPRα is MATα specific and located adjacent to STE12α at the MATα locus. It encodes a protein which possesses high sequence similarity to the seven-transmembrane class of G-protein-coupled pheromone receptors reported for other basidiomycetous fungi. Strains containing a deletion of the CPRα gene exhibited drastic reductions in mating efficiency but were not completely sterile. Δcprα cells displayed wild-type mating efficiency when reconstituted with the wild-type CPRα gene. Hyphal production on filament agar was not affected in the Δcprα strain, indicating no significant role for CPRα in sensing environmental cues during haploid fruiting. The wild-type MATα CPRα strain produced abundant hyphae in response to synthetic MATa pheromone; however, the hyphal response to pheromone by Δcprα cells was significantly reduced. Exposure of wild-type cells to synthetic MATa pheromone for 2 h induced MFα pheromone expression, whereas unexposed cells showed only basal levels of the MFα transcript. The Δcprα cells, however, exhibited only basal levels of MFα message with or without pheromone exposure, suggesting that CPRα and MFα are components of the same signaling pathway.
Cryptococcus neoformans is the etiologic agent of cryptococcosis, one of the most serious fungal diseases encountered by immunocompromised patients worldwide (26). The fungus is a bipolar heterothallic basidiomycete in which the meiotic cycle is dependent upon interactions between cells of the MATα and MATa types (24). The initial interaction between cells of the two compatible mating strains is believed to involve pheromone-receptor pairs of both mating types (34). In Saccharomyces cerevisiae, MATa cells secrete an a-factor pheromone and express the α-factor receptor Ste2p whereas MATα cells secrete an α-factor pheromone and express the a-factor receptor Ste3p. Both receptors belong to a large class of G-protein-linked seven-transmembrane-domain receptors (reviewed in references 23, 29, and 40) and are expressed mostly at the tips of schmoos, where cell fusions subsequently occur (23). Binding of pheromone to the receptor induces the pheromone response signal transduction pathway via the mitogen-activated protein (MAP) kinase cascade, which leads to activation of genes required for mating (19, 23). For C. neoformans, identification of the STE3 homolog has been reported but detailed information is lacking (30).
The mating system of C. neoformans has received considerable attention due to the preponderance of MATα strains among both environmental and clinical isolates (25, 44) and its increased virulence compared to the MATa type (28). Recently, a physical map of the MATα locus from the C. neoformans strain B-4500 (JEC21), chosen for the C. neoformans genome project, was constructed (21). In contrast to other fungi, homologs of several S. cerevisiae pheromone response MAP kinase cascade genes such as STE20α, STE11α, and STE12α, as well as three copies of the MATα pheromone gene, were found embedded in the MATα locus (21). Partial sequencing of the MATα locus also revealed a gene located at the 5′ end of STE12α that had high degrees of homology with the pheromone receptors of basidiomycetous fungi such as rcb3 of Coprinus cinereus (35), bbr2 of Schizophyllum commune (16), and pra1 of Ustilago maydis (4) and a lesser degree of homology to the yeast STE3 sequence (18). The gene was named CPRα (cryptococcal pheromone receptor, α mating type). Interestingly, a similar genomic organization of the locus bearing the STE3 homolog was recently reported for Pneumocystis carinii, a species phylogenetically unrelated to C. neoformans (38). The P. carinii ortholog of STE3 was present in the midst of a cluster of pheromone response signal transduction cascade genes, including an STE12 homolog. Although a sexual life cycle has been proposed for P. carinii, there has been no clear indication that this organism is heterothallic and it is unknown whether these genes are mating type specific.
The known pheromone receptors of basidiomycetous fungi also contain the seven potential membrane-spanning domains characteristic of receptors that couple to heterotrimeric G proteins. In homobasidiomycete species such as Coprinus cinereus and Schizophyllum commune, however, pheromones and receptors play no role in mate attraction and fusion but promote the formation and maintenance of a dikaryon after cell fusion. In U. maydis, a heterobasidiomycete species, pheromones and receptors are essential for mate attraction and haploid cell fusion, which lead to the formation of infectious dikaryons (6). For this reason, pheromone and receptor genes of U. maydis are considered pathogenicity genes. Although the CPRα gene was predicted to encode a functional receptor protein for the MATa pheromone, it has not been characterized. In this paper, we report the isolation and characterization of the pheromone receptor gene CPRα, expressed by MATα strains of C. neoformans.
MATERIALS AND METHODS
Strains and media.
All strains used in this study were serotype D, MATα or MATa mating type, derived from the congenic set B-4500 (JEC21; MATα) and B-4476 (JEC20; MATa). YEPD medium contained 1% yeast extract, 2% Bacto Peptone, and 2% dextrose. YNB with glucose medium (pH 6.0) was used as a minimal medium (MIN) and contained 6.7 g of yeast nitrogen base without amino acids (Difco) and 20 g of glucose per liter. Synthetic low-ammonium dextrose (SLAD) agar (17) with slight modification (11) was used to test the morphological response of cultures to synthetic C. neoformans MATa pheromone (Mfap) as well as the accumulation of MFα transcript in response to synthetic Mfap. To observe the formation of hyphae in B-4500 and the Δcprα mutant in response to the presence of the opposite mating type, SLAD agar (11) was used. Filament agar (45) was used for haploid fruiting, and V8 juice (27) agar was used for crossing.
Identification of the CPRα gene.
A 20-kb BamHI fragment containing the STE12α gene was cloned from a cosmid library of B-4500 (21) and sequenced by standard methods (37). Databases were searched to identify genes homologous to those found on the fragment. A gene that encoded a putative protein with high sequence identity (2e-41) with several pheromone receptors of basidiomycetous fungi was identified and named CPRα (see Fig. 1). A partial cDNA clone lacking the 5′ end of CPRα was initially isolated from a cDNA library of B-3501 (15). The complete cDNA sequence was determined by using the SMART RACE cDNA amplification kit (Clontech). The two primers used for the 5′ rapid amplification of cDNA ends were 5′-G GCG GTA GAC GAT GCA ATC ATC AC-3′ and 5′-GCT GAA ATC CCA ACA GGT ACT CCG-3′.
FIG. 1.
Protein sequence alignment of pheromone receptors. CcRcb3, Rcb3 of Coprinus cinereus (GenBank accession number AF186385); ScBbr2, Bbr2 of S. commune (GenBank accession number AF148501); UmPra1, Pra1 of U. maydis (GenBank accession number U37795); CnCPRα, Cprα of C. neoformans (GenBank accession number AF259519). Black boxes indicate the regions of identity, and gray boxes indicate the regions of similarity. Putative transmembrane domains 1 to 7 are indicated by dotted underlines.
Deletion and reconstitution of CPRα.
To generate the deletion construct pMK1, the 5′ (nucleotides −873 to +127, where the first ATG is at +1) and 3′ (nucleotides +3873 to +4885) flanking regions of CPRα were each amplified by PCR and cloned into pYCC76 so that they flanked the ADE2 gene in the plasmid (8). Cells of strain LP1 (MATα ade2 ura5) derived from B-4500 were transformed by the biolistic method (41) and plated onto MIN agar supplemented with uracil. A PCR protocol was used to detect the putative deletion mutants resulting from homologous recombination as described previously (11). Deletions of CPRα were confirmed by Southern blot analysis (37). The strain with the deleted cprα locus was subsequently reconstituted back to the wild type by biolistic cotransformation (11) with plasmids pYCC331 (9) and pMK2. Plasmid pMK2 contained the 3.0-kb BstXI/SalI fragment of CPRα, which was cloned into the EcoRV/SalI site of pBluescript II SK(+). Putative adenine auxotrophs (pink colonies) were isolated from the transformation plates and screened by Southern blot analysis to identify the reconstituted clones.
Quantitative assay for mating frequency.
The mating frequencies of various strains were assayed by a method devised in our laboratory (11). All cultures used for this experiment were less than 24 h old. Briefly, about 5 × 106 cells of each of the MATa (JEC30 and JEC32) and MATα (Δcprα and Δcprα::CPRα) strains expressing different auxotrophic markers were suspended in 1 ml of saline (0.9%) and mixed thoroughly by vortexing. The cells were collected on a 0.45-μm-pore-size nitrocellulose filter membrane by suction, placed on V8 juice agar, and incubated for 6 h at room temperature. The cells were washed off the filters, plated onto MIN agar, and incubated for 4 days at 30°C before the number of hyphal colonies was counted. The mating frequency of each strain was determined as the total number of hypha-producing colonies divided by the total number of input cells from both mating type strains. The relative mating frequency was expressed as a percentage of the mating frequency of the CPRα wild-type strain LP1 (MATα ura5 ade2) crossed with JEC30 (MATa lys2) or LP1 crossed with JEC32 (MATa lys2). Data were the average of the results derived from mating each strain with both JEC30 and JEC32. The experiments were repeated three times to establish reproducibility.
Synthesis of MATa pheromone (Mfap).
The presumptive mature peptide portion of Mfap, Glu-Glu-Ala-Tyr-Gly-Ser-Gly-Gln-Gly-Pro-Thr-Tyr-Ser-Cys (32), was synthesized as follows: the 13-mer sequence fragment missing the C-terminal cysteine residue was assembled by standard 9-fluorenylmethoxy carbonyl (Fmoc) peptide synthesis methodology (22) using Fmoc-based solid-phase peptide synthesis on a 2-chlorotrityl chloride resin. The fully protected 13-amino-acid peptide was then cleaved from the solid support and condensed with the suitably protected cysteine (22) as follows: the protected peptide fragment and 2 eq of S-trityl-cysteine methyl ester (22) were dissolved in dimethylformamide (∼150 μl/μmol of peptide), and 5 eq of N-methyl morpholine (NMM) was added to an ice bath with stirring and cooling. To the resulting solution, N-ethyl-N′-(3-dimethylaminopropyl)-carbodiimide hydrochloride (1.5 eq) was added. After 1 h, the solution was allowed to warm to 25°C and stirred for 12 more hours. Most of the solvent was removed in vacuo. The residue was then dissolved in ethyl acetate and washed with a mixture of 2% citric acid, 2% sodium bicarbonate, and saturated aqueous NaCl. Drying of this solution with anhydrous MgSO4, filtration, and solvent removal in vacuo yielded the crude product. This material was then treated with a mixture of 87.5% trifluoroacetic acid, 2.5% ethanedithiol (EDT), 5% phenol, and 5% thioanisole (by volume; ∼50 μl/μmol of peptide) for 3 h at room temperature. After removal of the bulk of the solvent in vacuo, the peptide was precipitated with methyl tert-butyl ether and the solid was filtered off, washed with methyl tert-butyl ether, and dried, yielding the crude peptide Fmoc-Glu-Glu-Ala-Tyr-Gly-Ser-Gly-Gln-Gly-Pro-Thr-Tyr-Ser-Cys(SH)-OMe.
In order to remove Fmoc and to S-farnesylate the cysteine residue, the following procedure was used. While the solution of the Fmoc-protected peptide in dimethylformamide was cooling with ice and stirring, 2 eq of farnesyl bromide (Sigma-Aldrich, St. Louis, Mo.) and 8 eq of diisopropylethylamine (Sigma-Aldrich) were added at a concentration of 1 μmol/100 μl. After 2 h, 4 eq of ethanedithiol was added to the reaction mixture, and the mixture was stirred for 30 min.
Next, neat piperidine (∼36 μl/μmol of peptide) was added to the reaction mixture and the Fmoc group removal was performed for an additional 20 min at room temperature. The reaction mixture was subsequently diluted with 3 volumes of water and acidified to pH 6 with acetic acid. The resulting solution was applied to a semipreparative high-performance liquid chromatograph column (DELTA PAK C18 reverse phase; Waters) (10-μm inside diameter, 300 by 19 mm), and the peptides were eluted with a linear acetonitrile gradient with 15 mM ammonium acetate in both solvents (solvent A, 15 mM ammonium acetate in water; solvent B, 15 mM ammonium acetate-80% acetonitrile-20% water). The fractions with the best high-performance liquid chromatograph analytical profile were pooled, frozen, and lyophilized to afford the final products a purity of >90%. The identity of the expected product was confirmed by matrix-assisted laser desorption ionization-time of flight and electrospray mass spectrometry analyses (molecular mass, 1,667 Da).
Response to MATa pheromone.
Three different methods were used to observe the response of a Δcprα strain to the MATa pheromone. In the first method, the Δcprα and Δcprα::CPRα strains, as well as the wild-type MATα and MATa strains, were streaked in parallel with the MATa wild-type strain in close proximity on SLAD agar, incubated for 48 to 72 h in the dark, and then observed for hyphal formation (11). The second and third methods employed the putative synthetic Mfap. The synthetic pheromone (3.9 mg) was dissolved in 1 ml of acetonitrile solution (acetonitrile and distilled water; 1:1 [vol/vol]). Ten aliquots (100 μl each) of Mfap were lyophilized and stored in a −20°C freezer until needed. To test the response of the wild-type strains and the Δcprα strain to Mfap, the previously described method (14) was used with some modifications. The contents of each lyophilized tube of pheromone were dissolved in 6 μl of dimethyl sulfoxide for 10 min and diluted with 6 μl of distilled water. SLAD agar plates were spotted with 20 μl of the pheromone solution and left overnight to dry. A small loopful of cells from a 48-h YEPD culture of the Δcprα mutant and MATα (B-4500) and MATa (B-4476) wild-type strains were patched on the center of the Mfap spots on the agar. An identical culture plate of SLAD agar without pheromone was used as a control. The plates were incubated in the dark for 24 h at 25°C and examined under a microscope. The same method was used for detection of MFα transcripts in cells exposed to pheromone except that approximately 108 cells of a 24-h YEPD culture were spread on SLAD agar over the area of synthetic Mfap application. After 2 h of exposure to Mfap, RNAs were extracted and subjected to Northern analysis with the MFα1 gene as a probe. The quantity of RNA was assessed by hybridizing the same filter with the ACTIN gene.
RESULTS
Isolation of the CPRα gene.
During mapping of the MATα locus in B-4500, an open reading frame present only in MAΤα strains (21) was observed approximately 1 kb upstream of the STE12α gene and named CPRα. In this study, the 1.3-kb CPRα gene present in the MAΤα locus of strain B-4500 was isolated and characterized. CPRα cDNA encodes a 42-kDa protein that shows high degrees of homology with the pheromone receptor genes of Coprinus cinereus (rcb3), Schizophyllum commune (bbr2), and U. maydis (pra1) (4, 16, 35). As is the case with these pheromone receptors, the Cprαp sequence was suggestive of it being a seven-transmembrane G-protein-coupled receptor. Comparisons between the genomic and cDNA sequences revealed three introns (GenBank accession number AF259519). Alignment of the Cprαp amino acid sequence with pheromone receptors of other basidiomycetous fungi showed high similarities: 46% with the Rcb3 protein of Coprinus cinereus, 43% with the Bbr2 protein of S. commune, and 42% with the Pra1 protein of U. maydis (Fig. 1).
Constructions of Δcprα and reconstituted CPRα strains.
In order to study the function of CPRα, the gene was deleted in the MATα strain LP1 (MATα ura5 ade2) (7) by biolistic transformation (41) with the deletion plasmid construct pMK1. Putative transformants resulting from homologous recombination between the deletion construct and the genomic CPRα locus were first identified by PCR and subsequently confirmed by Southern blot hybridization. DNA was digested with EcoRV, blotted, and hybridized with a PCR probe of the deleted portion of CPRα in pMK1 (data not shown). Hybridization signals corresponding to the CPRα gene were observed for the wild-type MATα strain (B-4500), whereas none were detected in either the MATa strain (B-4476) or the Δcprα strain. The membrane was stripped and hybridized with the 5′ and 3′ flanking regions of CPRα in pMK1. The putative deletion mutant strain also showed signals corresponding to fragments of predicted size (data not shown).
To avoid the possible introduction of gene disruptions resulting from ectopic integrations, the wild-type gene was reintroduced into the homologous site by transforming the Δcprα strain with the reconstitution construct, pMK2, by the cotransformation method described previously (11). The putative transformants from this replacement event were isolated and analyzed by Southern hybridization. The putative reconstituted CPRα strain exhibited the same hybridization pattern as B-4500 (data not shown). This result indicated that the Δcprα locus was reconstituted to the CPRα allele.
Mating of the Δcprα strain.
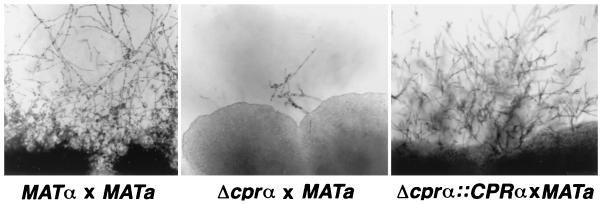
The Δcprα strain (MATα ura5) and the reconstituted strains both produced hyphae and basidiospores when crossed with B-4476 on V8 juice agar supplemented with uracil. The mating frequency of the Δcprα strain, however, was drastically reduced relative to those of the CPRα or Δcprα::CPRα strains (Fig. 2). To quantify the extent of the reduction in mating frequency in Δcprα, the strains were mated with JEC30 and JEC32, both lys2 mutants derived from B-4476. The Δcprα strain showed 0.3% of the mating frequency exhibited by the CPRα wild-type strain, whereas the reconstituted strain exhibited an 89% mating frequency. To confirm that the rare mating observed in the Δcprα × MATa strains resulted only from the interactions between the two opposite mating type cells, the Δcprα strain was also crossed with JEC31 (MATα lys2). No mating was observed in this cross. In order to prove that the basidiospores were the product of meiosis, single basidiospores were isolated by micromanipulation from the cross of the Δcprα and JEC30 strains. Recombination of genetic markers was observed among the single-basidiospore cultures, and a prototrophic Δcprα strain was obtained (data not shown). Growth rates of the prototrophic Δcprα strains at both 30 and 37°C were the same as those for the wild-type strains.
FIG. 2.
Mating behavior of the wild type (B-4500), the Δcprα mutant, and the Δcprα::CPRα mutant crossed with B-4476. Δcprα × B-4476 shows drastic reduction in mating efficiency compared to B-4500 × B-4476 or Δcprα::CPRα.
Response of the Δcprα strain to MATa pheromone.
To observe the response of the Δcprα strain to the MATa pheromone, the Δcprα strain, as well as the Δcprα strain reconstituted with the wild-type CPRα gene and the MATα and MATa strains, was streaked in parallel with the MATa wild-type strain in close proximity on SLAD agar and incubated for 48 to 72 h in the dark (11). Hyphal formation was observed in the Δcprα, Δcprα::CPRα, and wild-type MATα strains within 72 h. As shown in Fig. 3A, the number of hyphae produced toward the streak of a MATa strain by the Δcprα strain was considerably less than the number produced by the Δcprα::CPRα strain. The abundance of hyphae produced by the wild-type MATα strain was similar to that of the Δcprα::CPRα strain, whereas no hyphal production was observed between the streaks of the MATα and MATα strains (data not shown).
FIG. 3.
Response of the Δcprα mutant toward MATa culture or MATa pheromone (Mfap). (A) Formation of hyphae on SLAD agar. Left panel, Δcprα strain (lower side) produced some hyphae toward MATa strain (upper side). Right panel, Δcprα strain reconstituted with the wild-type CPRα gene (lower side) produced abundant hyphae toward MATa strain (upper side). (B) Synthetic Mfap triggered hyphal formation in MATα wild-type cells and with a markedly reduced rate in the Δcprα mutant. No hyphal formation was observed in the same strains cultured on the same plate where Mfap was not applied.
The putative Mfap confirmed by matrix-assisted laser desorption ionization-time of flight and electrospray mass spectrometry analyses (molecular mass, 1,667 Da) (data not shown) was dissolved in dimethyl sulfoxide and used to test the induction of hyphae in both the wild-type strain and the Δcprα strain on SLAD agar (see Materials and Methods). Colonies of both mating types grown outside of the pheromone spot as well as MATa colonies grown on the pheromone spot failed to produce any hyphae. However, Mfap induced short hyphal growth in colonies of the B-4500 and Δcprα strains. Predictably, the amount of hyphae induced in the Δcprα mutant strain was considerably less than that in the strain with the wild-type CPRα gene (Fig. 3B).
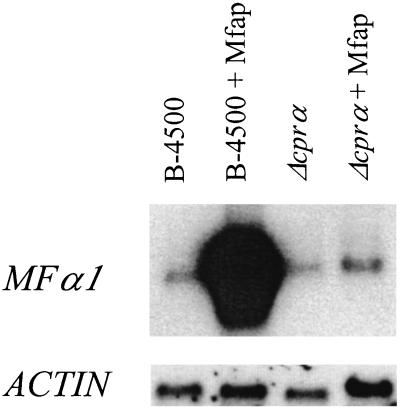
When exposed to Mfap for 2 h on SLAD agar, the cells of B-4500 accumulated abundant MFα message, whereas in the absence of pheromone, they exhibited only basal levels of MFα message (Fig. 4). Transcription of the MFα genes in the Δcprα strain remained at basal levels regardless of exposure to pheromone (Fig. 4).
FIG. 4.
Induction of MFα gene expression by synthetic MATa pheromone. (A) The wild-type (B-4500) and Δcprα strains were grown on SLAD agar plates for 2 h with or without Mfap. Total RNAs were extracted, fractionated on an agarose gel, and transferred to a nitrocellulose membrane. The resulting blot was hybridized with the MFα1 gene probe. (B) The membrane described for panel A was stripped and hybridized with the ACTIN gene.
Effect of the CPRα gene deletion in haploid fruiting.
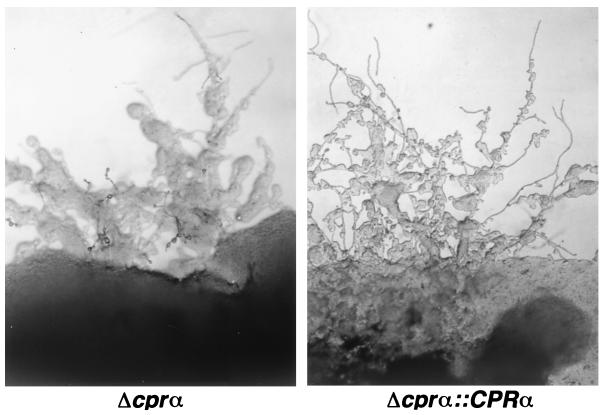
Haploid fruiting, a phenomenon believed to be MATα strain specific (45), was tested in the Δcprα strain in order to evaluate the role of pheromone receptor protein-sensing environmental cues that induce haploid fruiting. This test was performed since haploid fruiting (induced on filament agar) and mating (induced on V8 agar) in C. neoformans share common environmental conditions: nitrogen starvation and dehydration (4% agar). The Δcprα strain produced hyphae almost as abundantly as the Δcprα::CPRα (Fig. 5) or wild-type strain on filament agar (data not shown). These results indicated that the pheromone receptor does not play a significant role in sensing the environmental cues conducive to haploid fruiting in C. neoformans.
FIG. 5.
Haploid fruiting in the Δcprα strain and the Δcprα::CPRα strain on filament agar. No significant difference was found in the formation of haploid fruiting between the two strains.
DISCUSSION
We have identified and characterized a MATα-specific pheromone receptor gene embedded in the MATα locus of C. neoformans (21). Our study suggests that the CPRα gene of C. neoformans plays an important role in mating, presumably by mediating the signal transduction response to pheromone. The drastic reduction in mating efficiency in the Δcprα strain but not in the Δcprα::CPRα strain corroborates this conclusion. Furthermore, while the expression of the MFα genes in the wild-type strain was drastically upregulated upon exposure to synthetic MATa pheromone (Mfap), Δcprα cells exhibited only basal levels of MFα transcript in spite of exposure to the MATa pheromone. These findings support the notion that CPRα and MFα are components of the same signaling pathway. Since the Δcprα strain is able to mate, albeit poorly, and produce viable basidiospores, it indicates that the Δcprα strain still responds to the presence of its mating partner and transmits the signals downstream to genes involved in sexual morphogenesis. This notion is supported by the failure of mating between Δcprα and CPRα cells. It is further supported by the results of the SLAD agar test (11) and the test with synthesized Mfap. Although rare, Δcprα cells streaked side by side and in close proximity to B-4476 (MATa) produced short hyphae toward B-4476 cells. Since such hyphae are produced by MATα cells on SLAD agar only when they are streaked in proximity to MATa strains (11), it was considered to be a pheromone response. Our tests with the synthetic putative Mfap (MATa pheromone) supported this assumption. Though rare, Mfap did induce hyphal formation in a Δcprα colony. It is not unusual that Δcprα cells of C. neoformans are still able to mate since none of the other S. cerevisiae STE gene homologs of C. neoformans such as STE20 (31), STE11 (12), and STE12 (10) caused complete sterility upon deletion. Unlike S. cerevisiae, C. neoformans contains mating type-specific alleles of the STE gene homologs listed above. Of the three genes, the STE11α gene is the only one that caused a drastic reduction in mating efficiency upon deletion (12). Deletion of either alleles of STE20 (J. Heitman, personal communication) or STE12 (10, 11), however, showed no dramatic mating defects. Mating efficiency between Δste20α and Δste20a strains (J. Heitman, personal communication) or Δste12α and Δste12a strains (10), on the other hand, was markedly reduced. These observations suggest that the signaling pathway for mating in C. neoformans is different from that in S. cerevisiae. C. neoformans may contain other sets of genes that supplement the defects caused by deletion of STE20 and STE12 but not STE11. It is also possible that STE20 and STE12 of C. neoformans belong to different pathways that cross talk with the pheromone response pathway, where STE11 presumably functions. The cumulative effect of deletions in both alleles of STE20 and STE12 upon mating also suggests that the mating type-specific alleles supplement each other in signaling. Our experience with the STE12 alleles supports this notion. When a Δste12α strain is reconstituted with the wild-type STE12a allele, the mutant phenotype of the ste12α strain is complemented (10).
A phenomenon similar to our observation on the mating ability of the Δcprα strain has also been reported for another heterobasidiomycetous fungus, Ustilago hordei (2). Deletion of a receptor gene in the species produced occasional dikaryotic hyphae when crossed with wild-type strains of a compatible mating type. It would be interesting to know whether the efficiency of dikaryote formation between a pheromone receptor deletion mutant strain and the wild-type strain of U. hordei is as dramatically reduced as reported for S. cerevisiae or less severely reduced as is the case for C. neoformans.
Fungal cells sense pheromones through G-protein-coupled receptors (3). It is believed that these receptors anchor to the plasma membrane using seven-transmembrane helices and regulate the expression of second messengers through their interactions with heterotrimeric G protein. As is the case in S. cerevisiae, the MAP kinase pathway regulating hyphal formation in C. neoformans shares many signaling elements with the mating pathway (11, 30). In C. neoformans, the β subunit of a heterotrimeric G protein (Gpb1p) has been shown to play a critical role in mating and haploid fruiting, presumably through a pheromone-triggered MAP kinase cascade (42). Although the receptor-coupled α subunit of the GPB1 gene has not been characterized, a model depicted for signal transduction pathways by Lengeler et al. (30) suggests that it is GPA3. STE20α (J. Heitman, personal communication) and STE11α (MAP kinase kinase kinase) also appear to play crucial roles in haploid hyphal formation and mating (12) in C. neoformans. Our results indicated that haploid fruiting is not impeded in the cprα deletion mutant strain, suggesting that the Cprαp does not play an important role in sensing the environmental cues conducive to haploid fruiting. This result concurs with previous observations that pheromone receptors do not sense the environmental stresses that induce filamentation in other yeasts (3). We, however, performed the test since the environmental conditions such as nitrogen starvation and dehydration that promote sexual reproduction also promote haploid fruiting in C. neoformans. This observation indicates that other molecular components are involved in the sensing and transduction of the signals that trigger haploid hyphal growth. It has been suggested that the MAP kinase cascade functions during mating in both MATα and MATa cells (42). The involvement of Ras1 and Gpa1 pathways in mating as well as filamentation has also been reported (1). The cross talk between these two pathways and CPR- and pheromone-initiated signal transduction requires further dissection in order to elucidate precise networks of signaling for mating versus haploid filamentation.
We synthesized the predicted mature pheromone peptide of mating type a (Mfap) based on the sequence of the MFa genes (32). When MATα and MATa cells were exposed to synthetic Mfap, the pheromone triggered hyphal formation only in MATα strains. This result suggests that the synthesized Mfap is an active form of MATa pheromone. Furthermore, the synthetic pheromone induced MFα gene expression in the CPRα wild-type strain, whereas the wild-type strain not exposed to pheromone and the Δcprα strain exposed to pheromone showed only basal levels of MFα transcript. The drastic reduction of hyphal formation in the Δcprα strain in response to MATa pheromone suggests that the decrease in mating efficiency of the Δcprα strain is due to the loss of the Cpr α protein. This assumption was further supported by the recovery of mating efficiency in the Δcprα::CPRα strain. The MATα pheromone is reported to be an undecapeptide, and synthetic Mfαp induced hyphae in only the MATa strain (14). It was noticed that fungal pheromones identified in ascomycetes and basidiomycetous fungi encode CAAX motifs at the carboxyl termini of the proteins (reviewed in reference 6). The carboxyl-terminal CAAX motif has been believed to direct posttranslational farnesylation and carboxymethylation. Without exception, pheromone receptors from these fungi have sequence similarity to Ste3p, which responds to the a-factor-containing CAAX motif. Though Cprαp showed lower sequence similarity to Ste3p than to the pheromone receptor proteins of basidiomycetous fungi, it still showed 36% similarity. It is interesting to note that the mature MATa pheromone contains three more amino acids (32) than the MATα pheromone (14). The Mfap appears to be larger than most of the well-characterized fungal lipopeptide mating factors that are usually 9- to 13-amino-acid peptides (2, 4, 5, 13, 14, 20, 33, 36, 39, 43).
Since the Δcprα strain still responded to synthetic MATa pheromone and mated with MATa wild-type strains, though with low efficiency, one may suspect genetic redundancy of pheromone-sensing proteins in C. neoformans. We searched the Cryptococcal Genome Database created by the Stanford Genome Technology Center (http://www.sequence.Stanford.edu) and found one more pheromone receptor-like gene, which we named CPR2. Unlike CPRα, the CPR2 gene was present in strains of both mating types and was not associated with the MAT locus (Y. C. Chang and K. J. Kwon-Chung, Abstr. 101st Gen. Meet. Am. Soc. Microbiol. 2001, abstr. F-55, 2001). We are currently studying the role of CPR2 in mating by constructing a Δcpr2 strain and a Δcprα Δcpr2 double deletion strain. Phenotypes of these strains would reveal the role of CPR2 in mating and offer an explanation as to whether there is genetic redundancy in C. neoformans pheromone receptors.
Acknowledgments
We thank Joseph Heitman for sharing his experience and notes on synthesis of the cryptococcal pheromone, Ashok Varma for his reading of the manuscript, and Lisa Penoyer for DNA sequencing.
Brian L. Wickes is a Burroughs Wellcome new investigator in molecular pathogenic mycology and is supported by U.S. Public Health Service grant R29AI43522 from the National Institutes of Health.
Seyung Chung and Marvin Karos contributed equally to this work.
REFERENCES
- 1.Alspaugh, J. A., L. M. Cavallo, J. R. Perfect, and J. Heitman. 2000. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 36:352-365. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, C. M., D. A. Willits, P. J. Kosted, E. J. Ford, A. D. Martinez-Espinoza, and J. E. Sherwood. 1999. Molecular analysis of the pheromone and pheromone receptor genes of Ustilago hordei. Gene 240:89-97. [DOI] [PubMed] [Google Scholar]
- 3.Banuett, F. 1998. Signalling in the yeasts: an informational cascade with links to the filamentous fungi. Microbiol. Mol. Biol. Rev. 62:249-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolker, M., M. Urban, and R. Kahmann. 1992. The a mating type locus of Ustilago maydis specifies cell signaling components. Cell 68:441-450. [DOI] [PubMed] [Google Scholar]
- 5.Brake, A. J., C. Brenner, R. Najarian, P. Laybourn, and J. Merryweather. 1985. Structure of genes encoding precursors of the yeast peptide mating pheromone a factor, p. 103-108. In M. J. Gething (ed.), Protein transport and secretion. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 6.Casselton, L. A., and N. S. Olesnicky. 1998. Molecular genetics of mating recognition in basidiomycete fungi. Microbiol. Mol. Biol. Rev. 62:55-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, Y. C., and K. J. Kwon-Chung. 1994. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell. Biol. 14:4912-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, Y. C., and K. J. Kwon-Chung. 1998. Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect. Immun. 66:2230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, Y. C., and K. J. Kwon-Chung. 1999. Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J. Bacteriol. 181:5636-5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, Y. C., L. Penoyer, and K. J. Kwon-Chung. 2001. The second STE12 homologue of Cryptococcus neoformans is MATa-specific and plays an important role in virulence. Proc. Natl. Acad. Sci. USA 98:3258-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, Y. C., B. L. Wickes, G. F. Miller, L. A. Penoyer, and K. J. Kwon-Chung. 2000. Cryptococcus neoformans STE12α regulates virulence but is not essential for mating. J. Exp. Med. 191:871-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke, D. L., G. L. Woodlee, C. M. McClelland, T. S. Seymour, and B. L. Wickes. 2001. The Cryptococcus neoformans STE11α gene is similar to other fungal mitogen-activated protein kinase kinase kinase (MAPKKK) genes but is mating type specific. Mol. Microbiol. 40:200-213. [DOI] [PubMed] [Google Scholar]
- 13.Davey, J. 1992. Mating pheromones of the fission yeast Schizosaccharomyces pombe: purification and structural characterization of M-factor and isolation and analysis of two genes encoding the pheromone. EMBO J. 11:951-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson, R. C., T. D. E. Moore, A. R. Odom, and J. Heitman. 2000. Characterization of the MFα pheromone of the human fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 38:1017-1026. [DOI] [PubMed] [Google Scholar]
- 15.Edman, J. C., and K. J. Kwon-Chung. 1990. Isolation of the URA5 gene from Cryptococcus neoformans var. neoformans and its use as a selective marker for transformation. Mol. Cell. Biol. 10:4538-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowler, T. J. D., S. M. DeSimone, M. F. Mitton, J. Kurjan, and C. A. Raper. 1999. Multiple sex pheromones and receptors of a mushroom- producing fungus elicit mating in yeast. Mol. Biol. Cell 10:2559-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gimeno, C. J., P. O. Ljungdahl, C. A. Styles, and G. R. Fink. 1992. Unipolar cell divisions in the yeast Saccharomyces cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077-1090. [DOI] [PubMed] [Google Scholar]
- 18.Hagen, D. C., G. McCaffrey, and G. F. Sprague, Jr. 1986. Evidence the yeast STE3 gene encodes a receptor for the peptide pheromone a factor: gene sequence and implications for the structure of the presumed receptor. Proc. Natl. Acad. Sci. USA 83:1418-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herskowitz, I. 1995. MAP kinase pathways in yeast: for mating and more. Cell 80:187-197. [DOI] [PubMed] [Google Scholar]
- 20.Kamiya, Y., A. Sakurai, S. Tamura, and N. Takahashi. 1978. Structure of rhodotorucine A, a novel lipopeptide, inducing mating tube formation in Rhodosporidium toruloides. Biochem. Biophys. Res. Commun. 83:1077-1083. [DOI] [PubMed] [Google Scholar]
- 21.Karos, M., Y. C. Chang, C. M. McClelland, D. L. Clark, J. Fu, B. L. Wickes, and K. J. Kwon-Chung. 2000. Mapping of the Cryptococcus neoformans MATα locus: presence of mating type-specific mitogen-activated protein kinase cascade homologs. J. Bacteriol. 182:6222-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koppitz, M., T. Spellig, R. Kahmann, and H. Kessler. 1996. Lipoconjugates: structure-activity studies for pheromone analogues of Ustilago maydis with varied lipophilicity. Int. J. Pept. Protein Res. 48:377-390. [DOI] [PubMed] [Google Scholar]
- 23.Kurjan, J. 1993. The pheromone response pathway in Saccharomyces cerevisiae. Annu. Rev. Genet. 27:147-179. [DOI] [PubMed] [Google Scholar]
- 24.Kwon-Chung, K. J. 1976. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68:821-833. [PubMed] [Google Scholar]
- 25.Kwon-Chung, K. J., and J. E. Bennett. 1978. Distribution of α and a mating types of Cryptococcus neoformans among natural and clinical isolates. Am. J. Epidemiol. 108:337-340. [DOI] [PubMed] [Google Scholar]
- 26.Kwon-Chung, K. J., and J. E. Bennett. 1992. Medical mycology. Lea & Febiger, Philadelphia, Pa.
- 27.Kwon-Chung, K. J., J. E. Bennett, and J. C. Rhodes. 1982. Taxonomic studies on Filobasidiella species and their anamorphs. Antonie Leeuwenhoek 48:25-38. [DOI] [PubMed] [Google Scholar]
- 28.Kwon-Chung, K. J., J. C. Edman, and B. L. Wickes. 1992. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect. Immun. 60:602-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leberer, E., D. Y. Thomas, and M. Whiteway. 1997. Pheromone signalling and polarized morphogenesis in yeast. Curr. Opin. Genet. Develop. 7:59-66. [DOI] [PubMed] [Google Scholar]
- 30.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W.-C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lengeler, K. B., P. Wang, G. M. Cox, J. R. Perfect, and J. Heitman. 2000. Identification of the MATa mating-type locus of Cryptococcus neoformans reveals a serotype A MATa strain thought to have been extinct. Proc. Natl. Acad. Sci. USA 97:14455-14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClelland, C. M., J. Fu, G. L. Woodlee, T. S. Seymour, and B. L. Wickes. 2002. Isolation and characterization of the Cryptococcus neoformans MATa pheromone gene. Genetics 160:935-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyakawa, T., M. Tabata, E. Tsuchiya, and S. Fukui. 1985. Biosynthesis and secretion of tremerogen A-10, a polyisoprenyl peptide mating pheromone of Tremella mesenterica. Eur. J. Biochem. 147:489-493. [DOI] [PubMed] [Google Scholar]
- 34.Moore, T. D., and J. C. Edman. 1993. The α-mating type locus of Cryptococcus neoformans contains a peptide pheromone gene. Mol. Cell. Biol. 13:1962-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Shea, S. F., P. T. Chaure, J. H. Halsall, N. S. Olesnicky, A. Leibbrandt, I. F. Connerton, and L. A. Casselton. 1998. A large pheromone and receptor gene complex determines multiple B mating type specificities in Coprinus cinereus. Genetics 148:1081-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakagami, Y., A. Isogai, S. Suzuki, C. Tamura, C. Kitada, and S. Fujino. 1979. Structure of tremerogen A-10, a peptidal hormone inducing conjugation tube formation in Tremella mesenterica. Agric. Biol. Chem. 43:2643-2645. [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Smulian, A. G., T. Seterhenn, R. Tanaka, and M. T. Cushion. 2001. The ste3 pheromone receptor gene of Pneumocystis carinii is surrounded by a cluster of signal transduction genes. Genetics 157:991-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spelling, T., M. Bolker, F. Lottspeich, R. W. Frank, and R. Kahmann. 1994. Pheromones trigger filamentous growth in Ustilago maydis. EMBO J. 13:1620-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sprague, G. F., and J. W. Thorner. 1992. Pheromone response and signal transduction during the mating process of Saccharomyces cerevisiae. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Toffaletti, D. L., T. H. Rude, S. A. Johnston, D. T. Durack, and J. R. Perfect. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, P., J. R. Perfect, and J. Heitman. 2000. The G-protein beta subunit GPB1 is required for mating and haploid fruiting in Cryptococcus neoformans. Mol. Cell. Biol. 20:352-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wendland, J., L. J. Vaillancourt, J. Hegner, K. B. Lengeler, K. J. Laddison, C. A. Specht, C. A. Raper, and E. Kothe. 1995. The mating-type locus Bα1 of Schizophyllum commune contains a pheromone receptor gene and putative pheromone gene. EMBO J. 14:5271-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wickes, B. L., U. Edman, and J. C. Edman. 1997. The Cryptococcus neoformans STE12α gene: a putative Saccharomyces cerevisiae STE12 homologue that is mating type specific. Mol. Microbiol. 26:951-960. [DOI] [PubMed] [Google Scholar]
- 45.Wickes, B. L., M. E. Mayorga, U. Edman, and J. C. Edman. 1996. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the alpha-mating type. Proc. Natl. Acad. Sci. USA 93:7327-7331. [DOI] [PMC free article] [PubMed] [Google Scholar]