Abstract
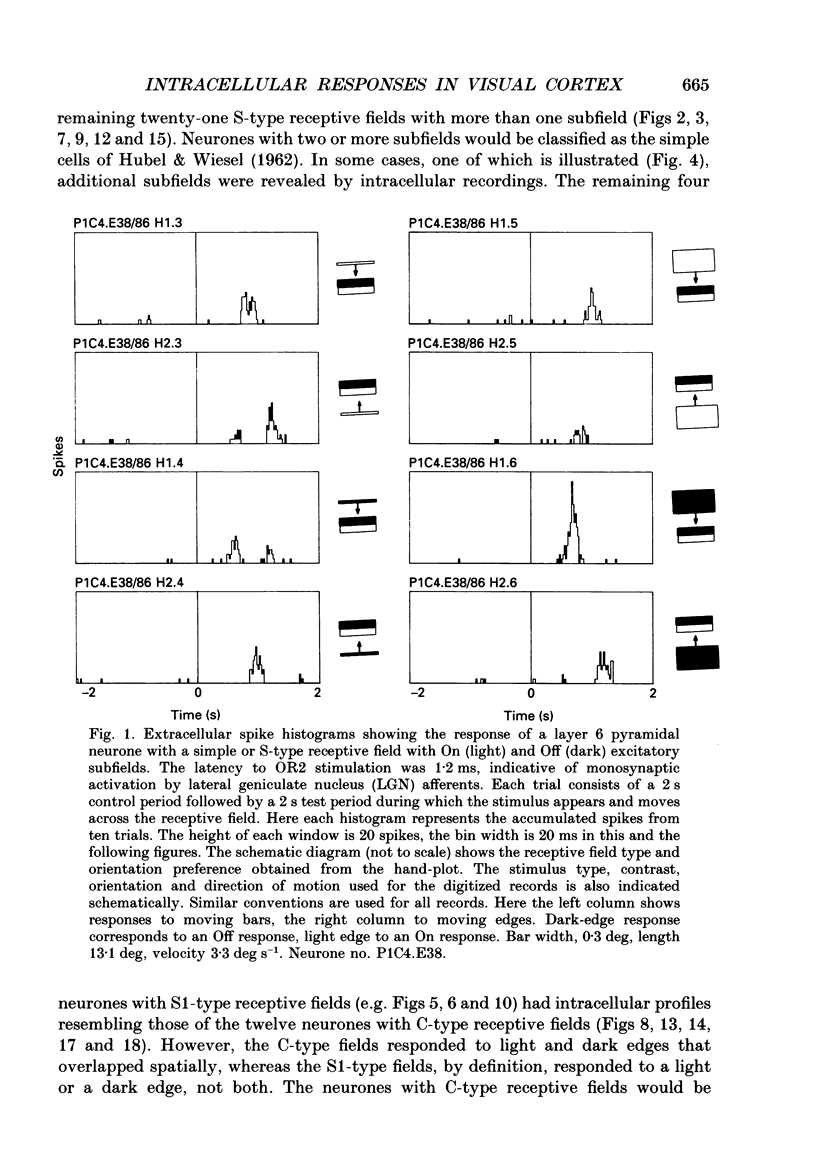
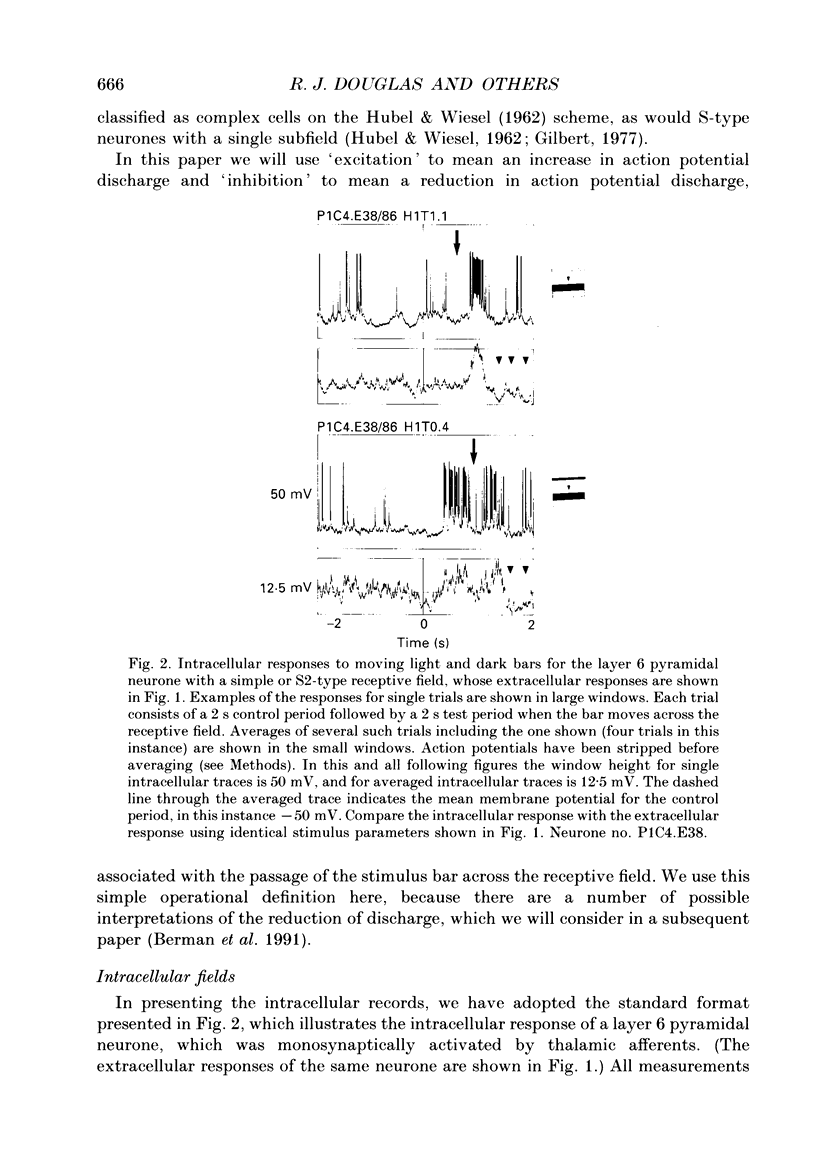
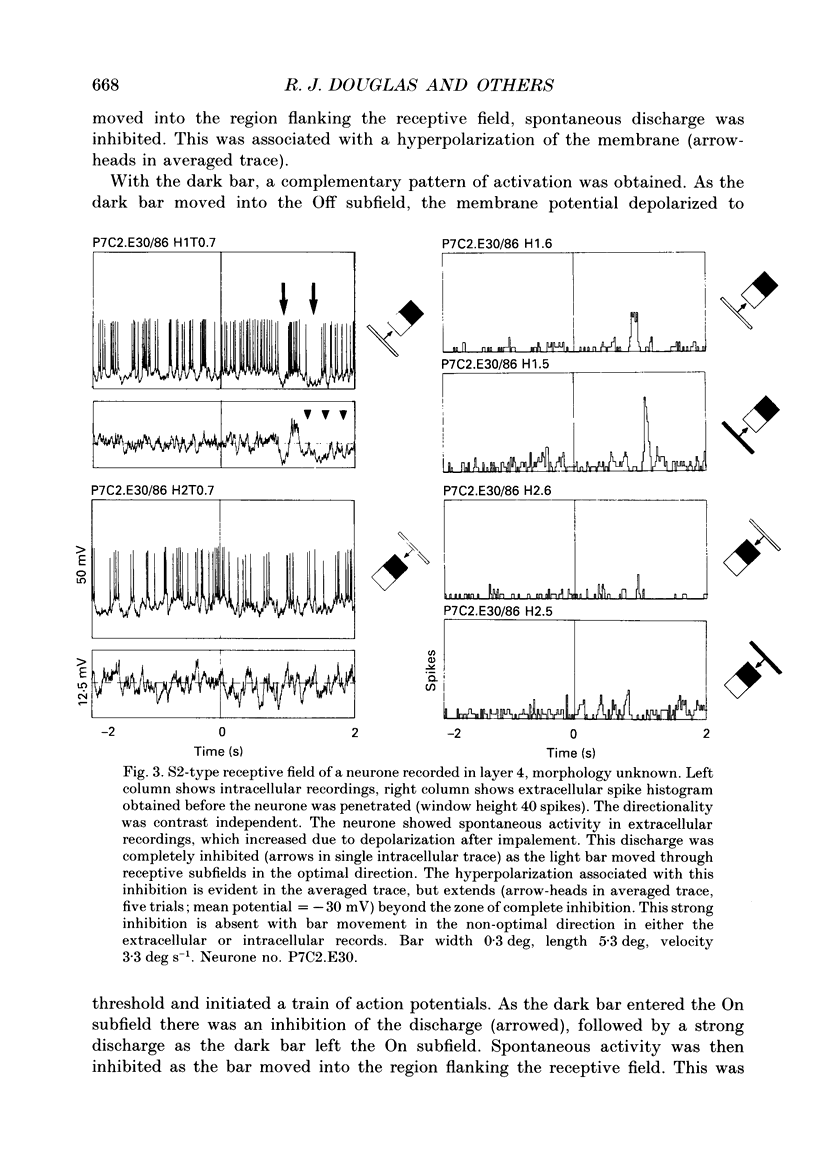
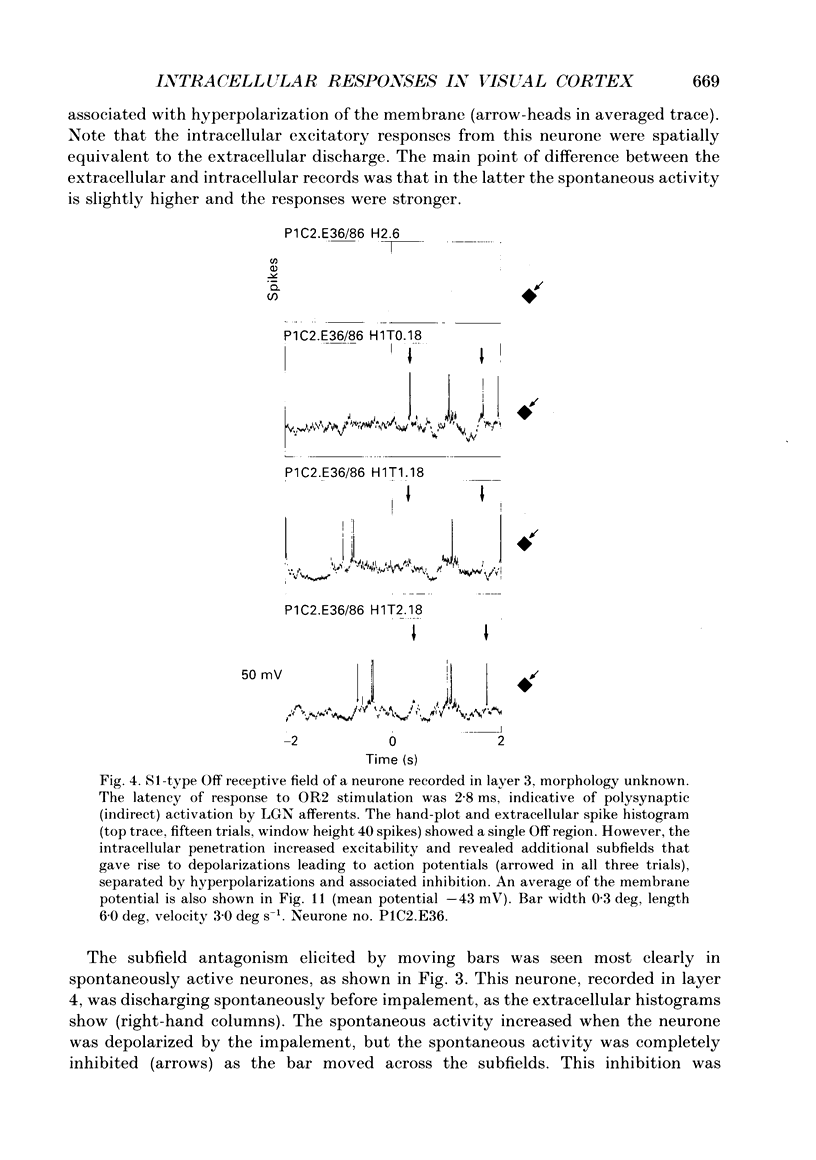
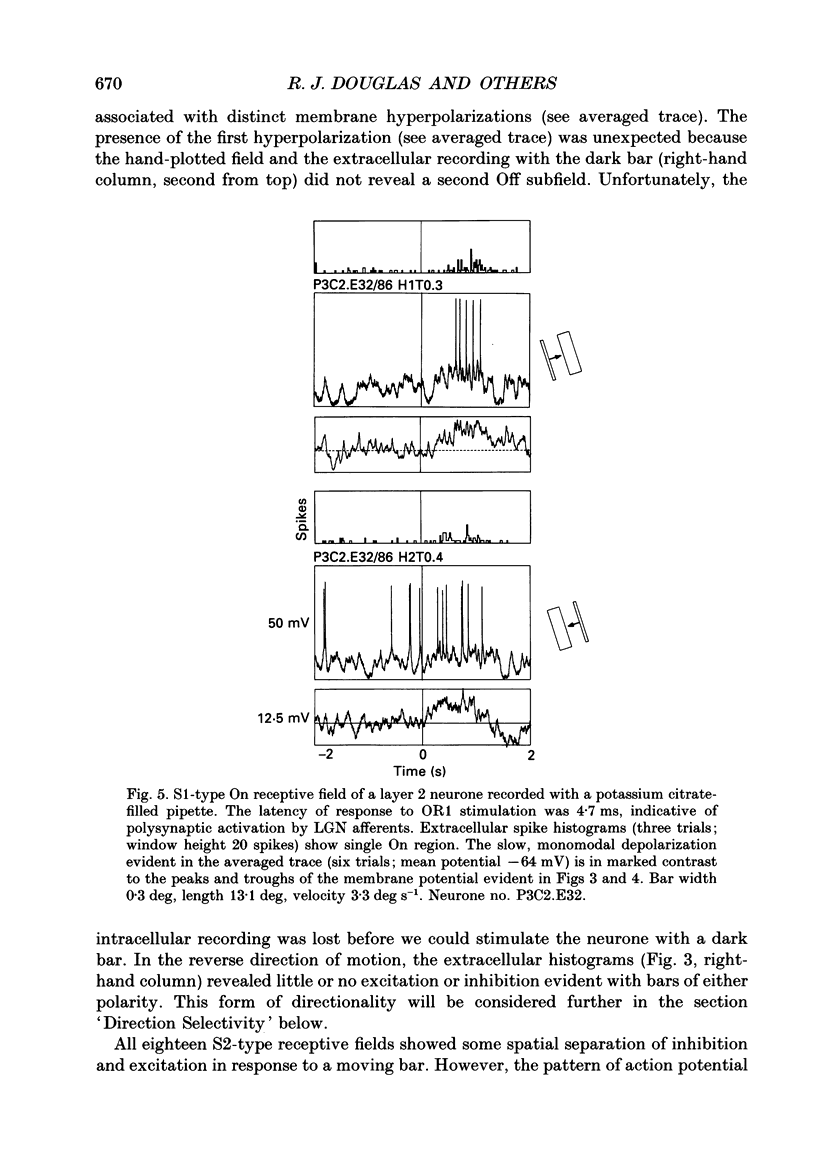
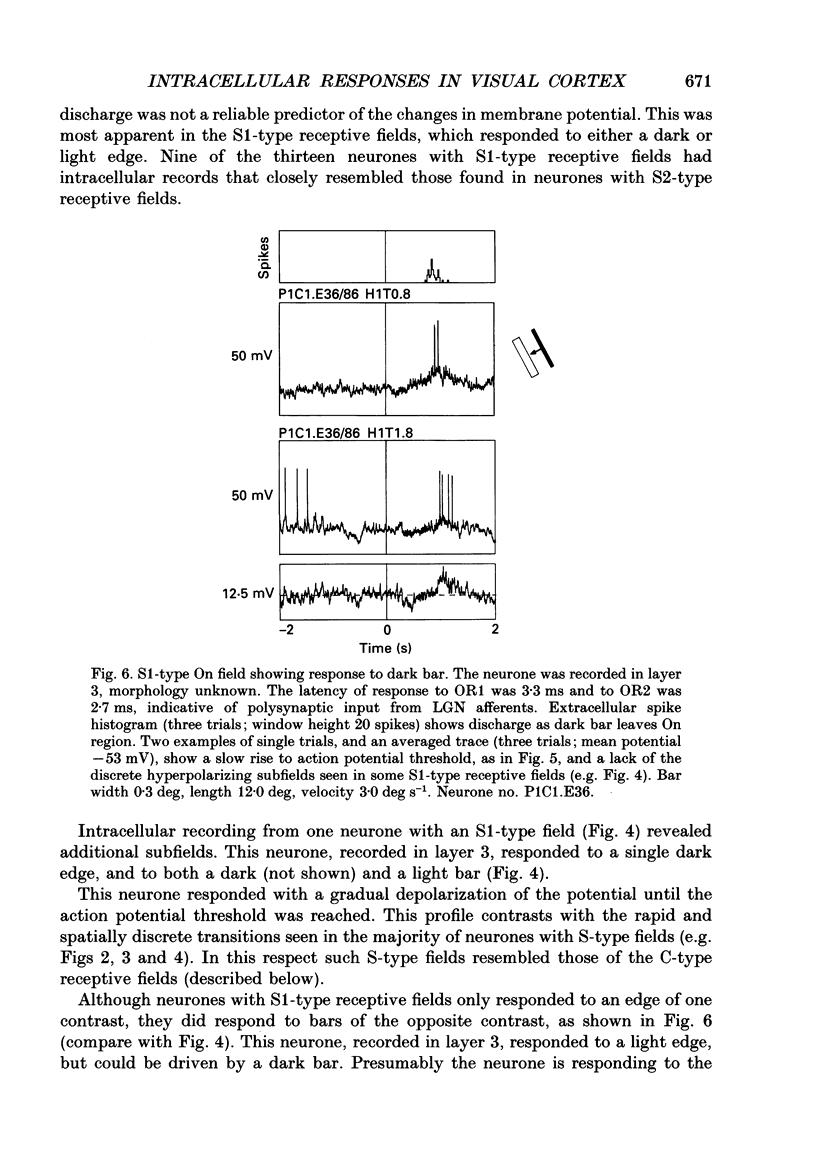
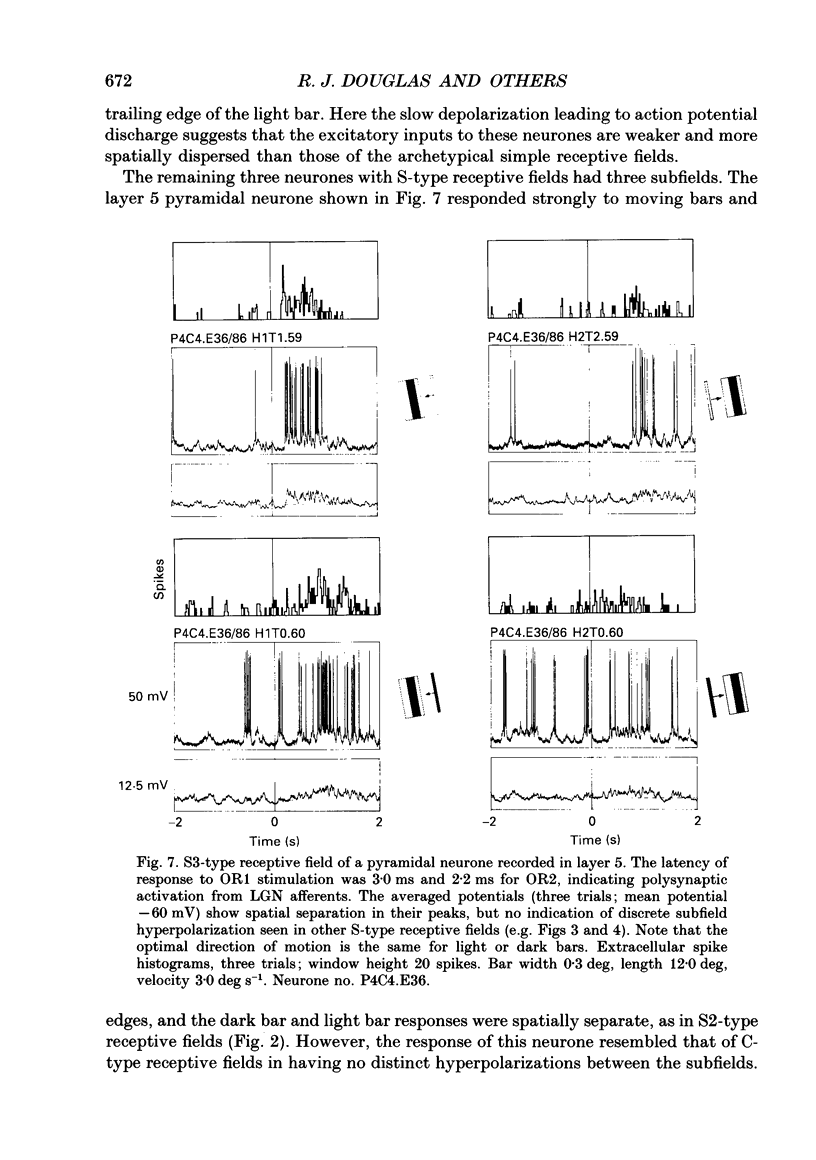
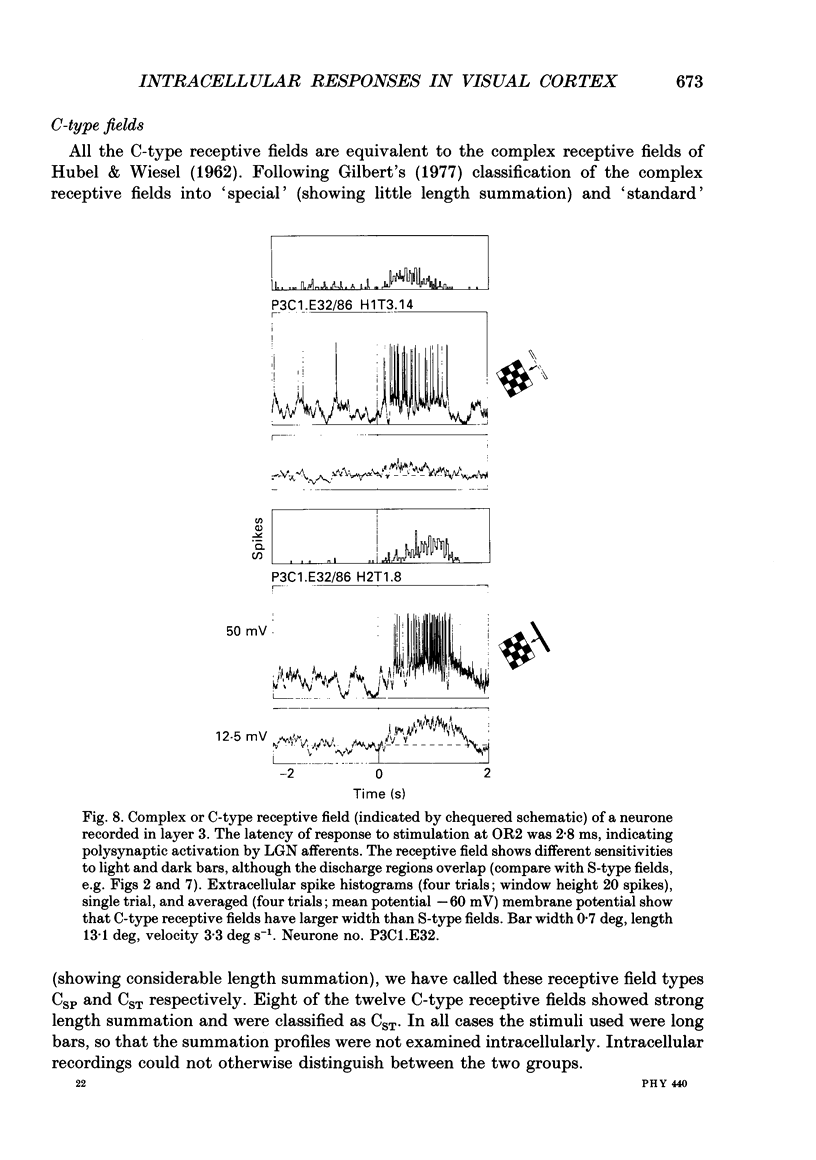
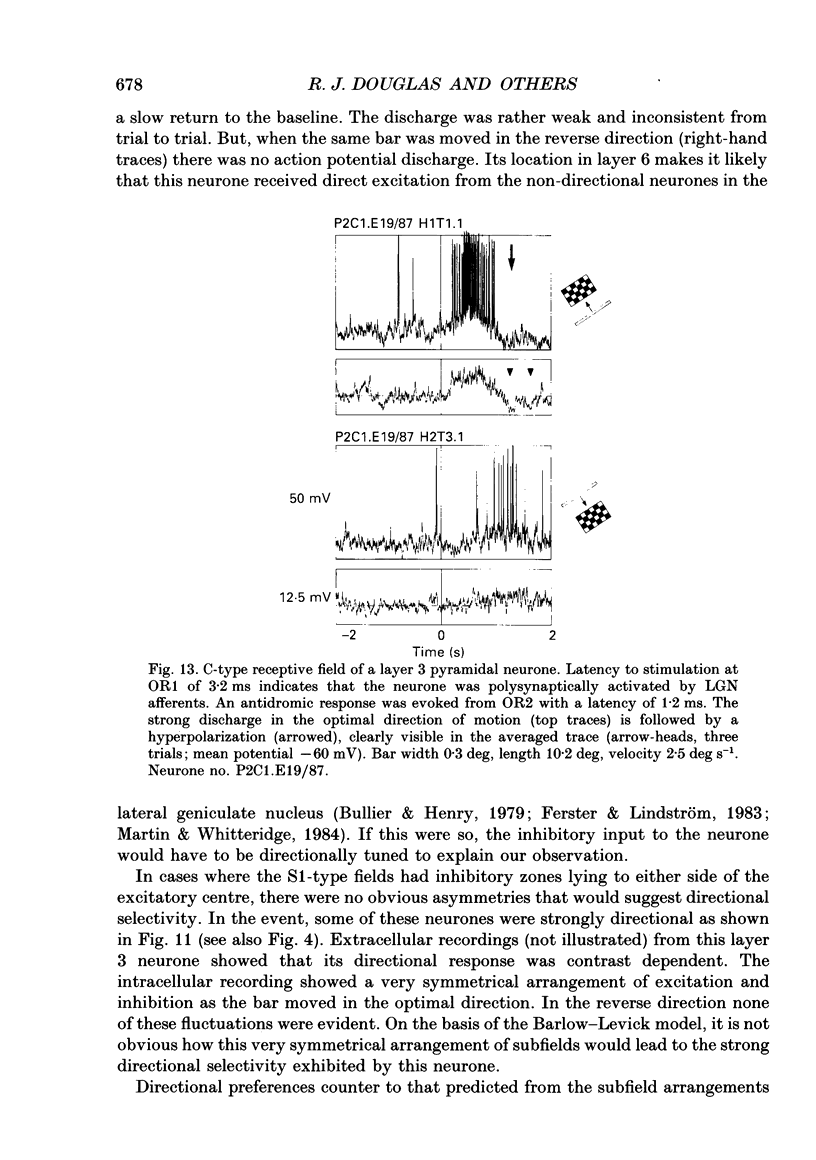
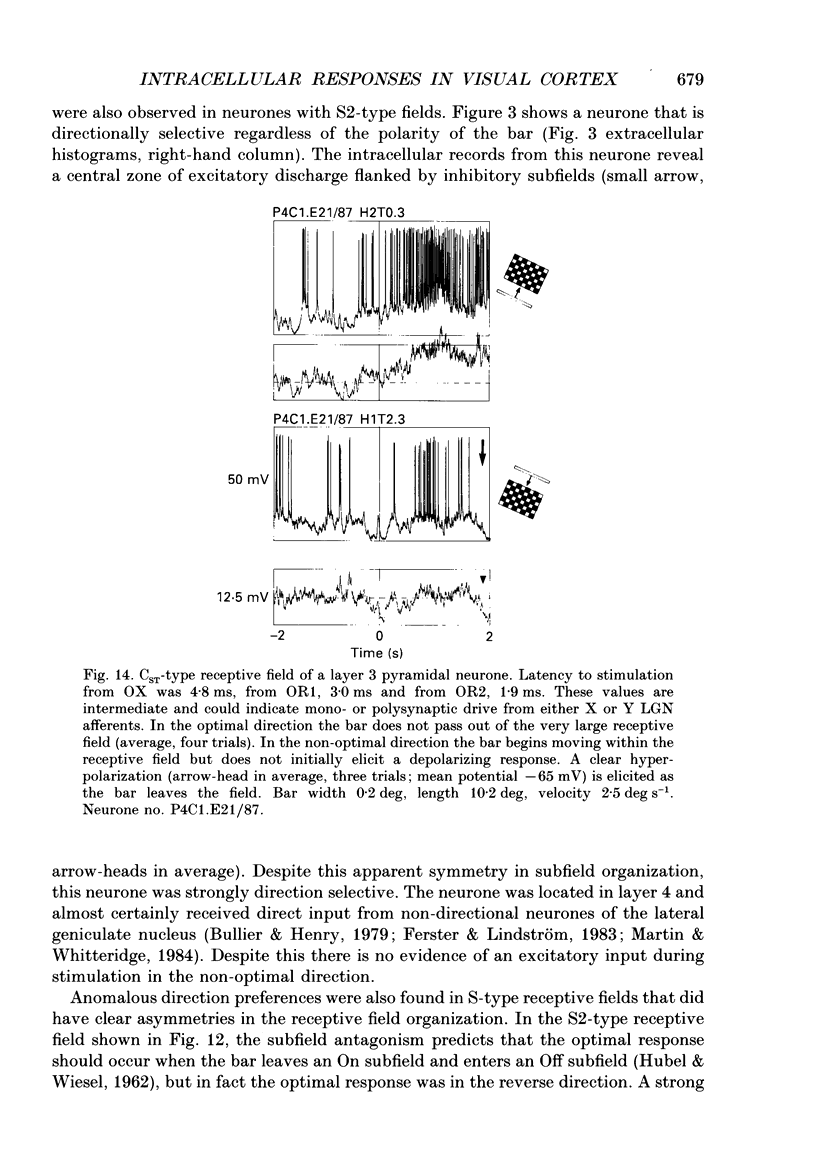
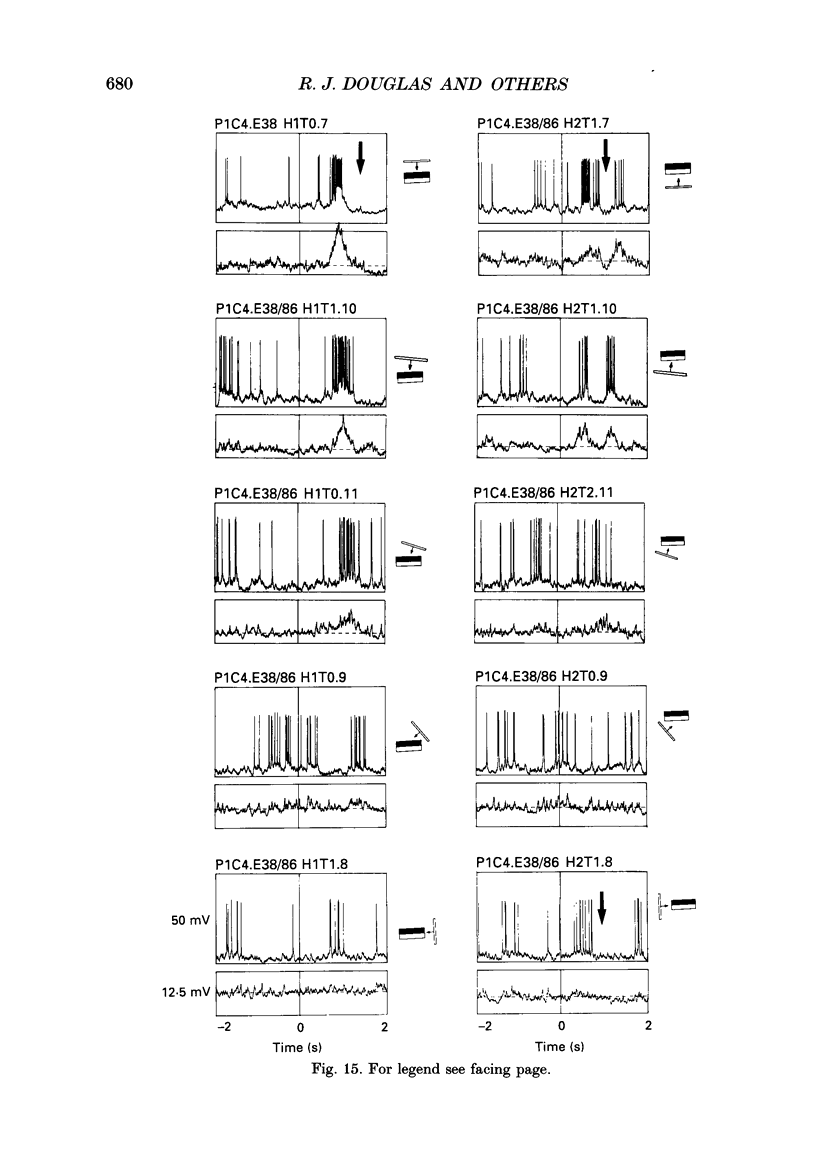
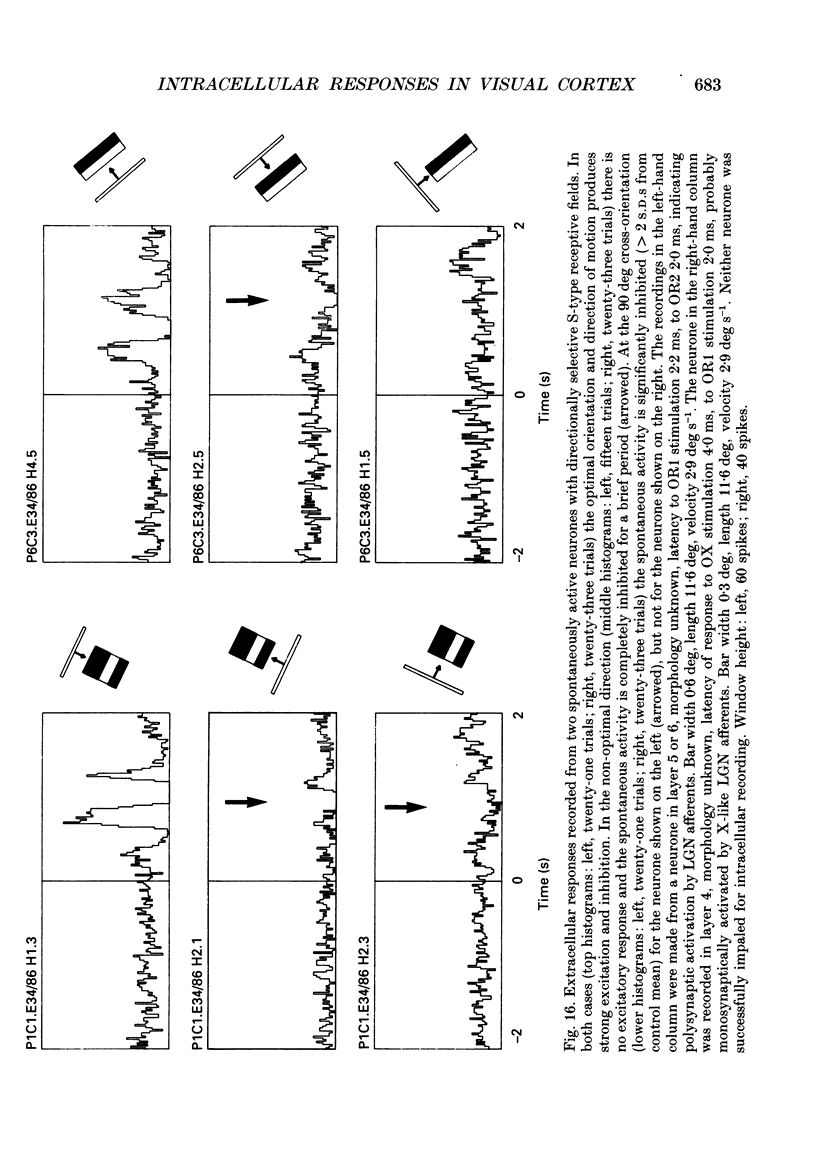
1. Extracellular and intracellular recordings were made from neurones in the visual cortex of the cat in order to compare the subthreshold membrane potentials, reflecting the input to the neurone, with the output from the neurone seen as action potentials. 2. Moving bars and edges, generated under computer control, were used to stimulate the neurones. The membrane potential was digitized and averaged for a number of trials after stripping the action potentials. Comparison of extracellular and intracellular discharge patterns indicated that the intracellular impalement did not alter the neurones' properties. Input resistance of the neurone altered little during stable intracellular recordings (30 min-2 h 50 min). 3. Intracellular recordings showed two distinct patterns of membrane potential changes during optimal visual stimulation. The patterns corresponded closely to the division of S-type (simple) and C-type (complex) receptive fields. Simple cells had a complex pattern of membrane potential fluctuations, involving depolarizations alternating with hyperpolarizations. Complex cells had a simple single sustained plateau of depolarization that was often followed but not preceded by a hyperpolarization. In both simple and complex cells the depolarizations led to action potential discharges. The hyperpolarizations were associated with inhibition of action potential discharge. 4. Stimulating simple cells with non-optimal directions of motion produced little or no hyperpolarization of the membrane in most cases, despite a lack of action potential output. Directional complex cells always produced a single plateau of depolarization leading to action potential discharge in both the optimal and non-optimal directions of motion. The directionality could not be predicted on the basis of the position of the hyperpolarizing inhibitory potentials found in the optimal direction. 5. Stimulation of simple cells with non-optimal orientations occasionally produced slight hyperpolarizations and inhibition of action potential discharge. Complex cells, which had broader orientation tuning than simple cells, could show marked hyperpolarization for non-optimal orientations, but this was not generally the case. 6. The data do not support models of directionality and orientation that rely solely on strong inhibitory mechanisms to produce stimulus selectivity.
Full text
PDF








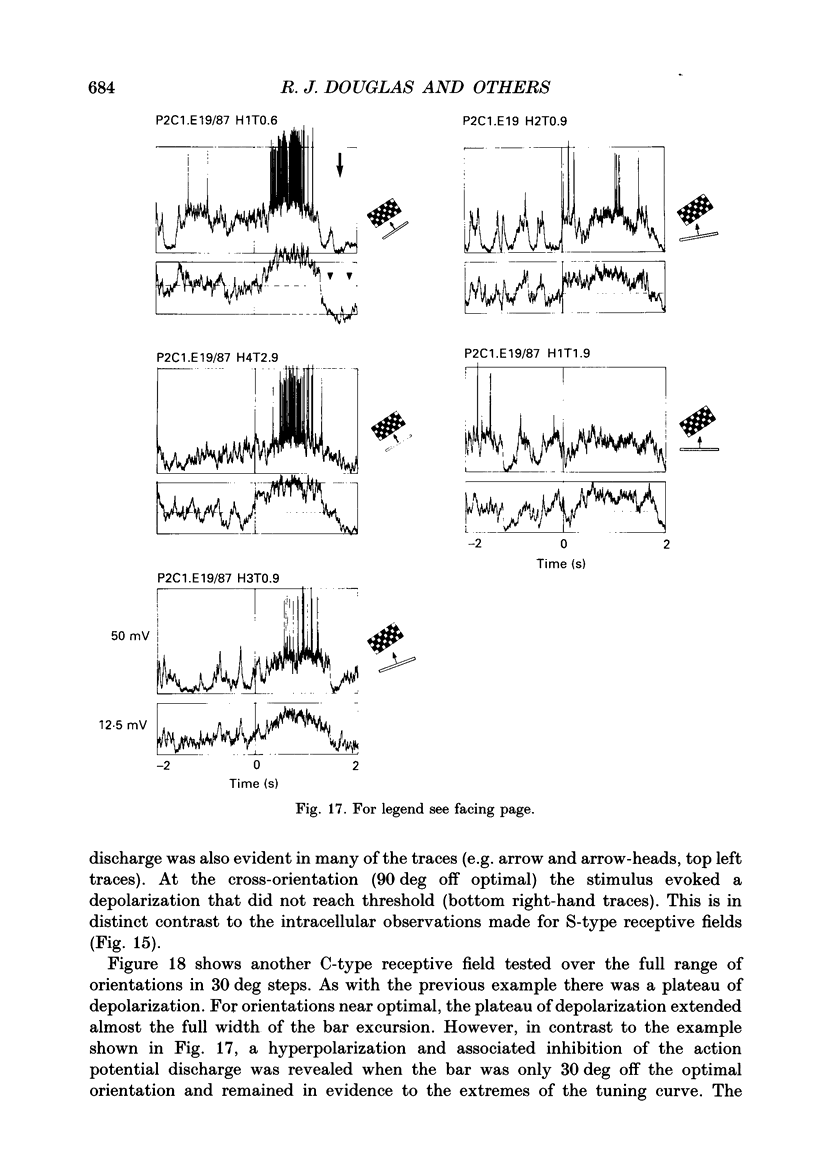
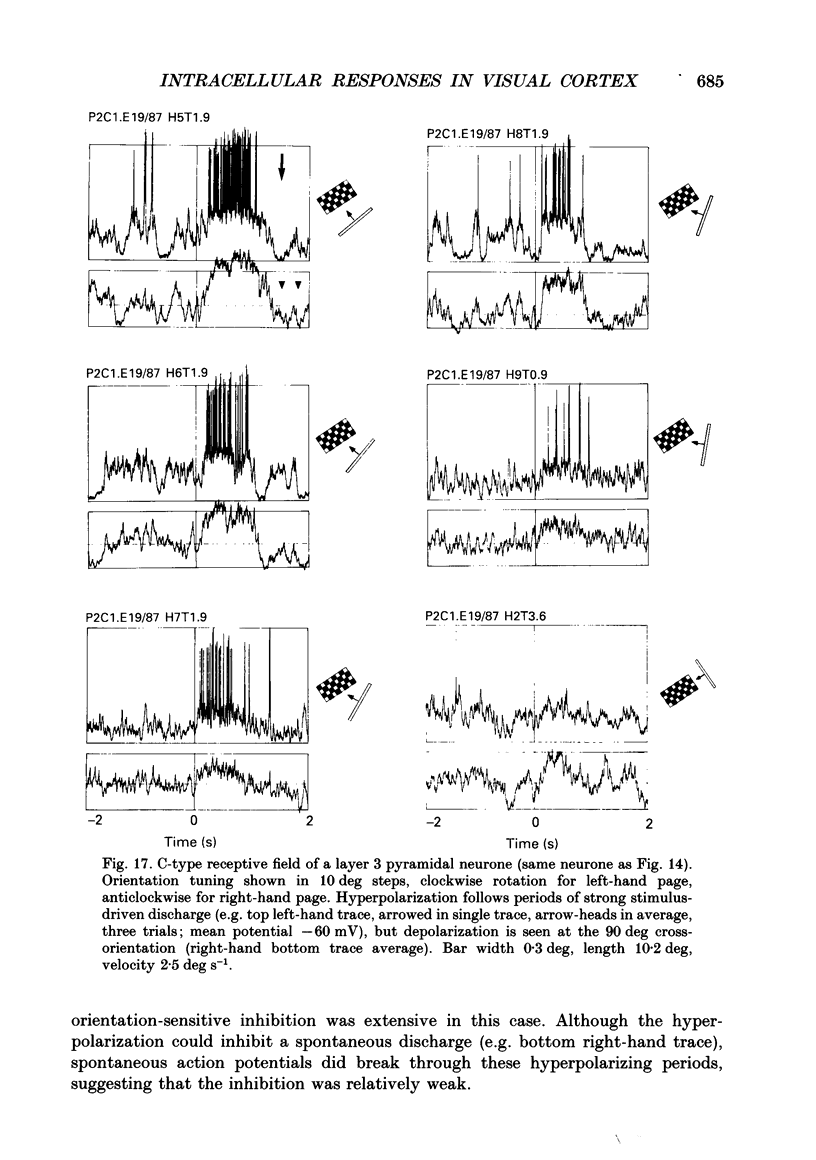
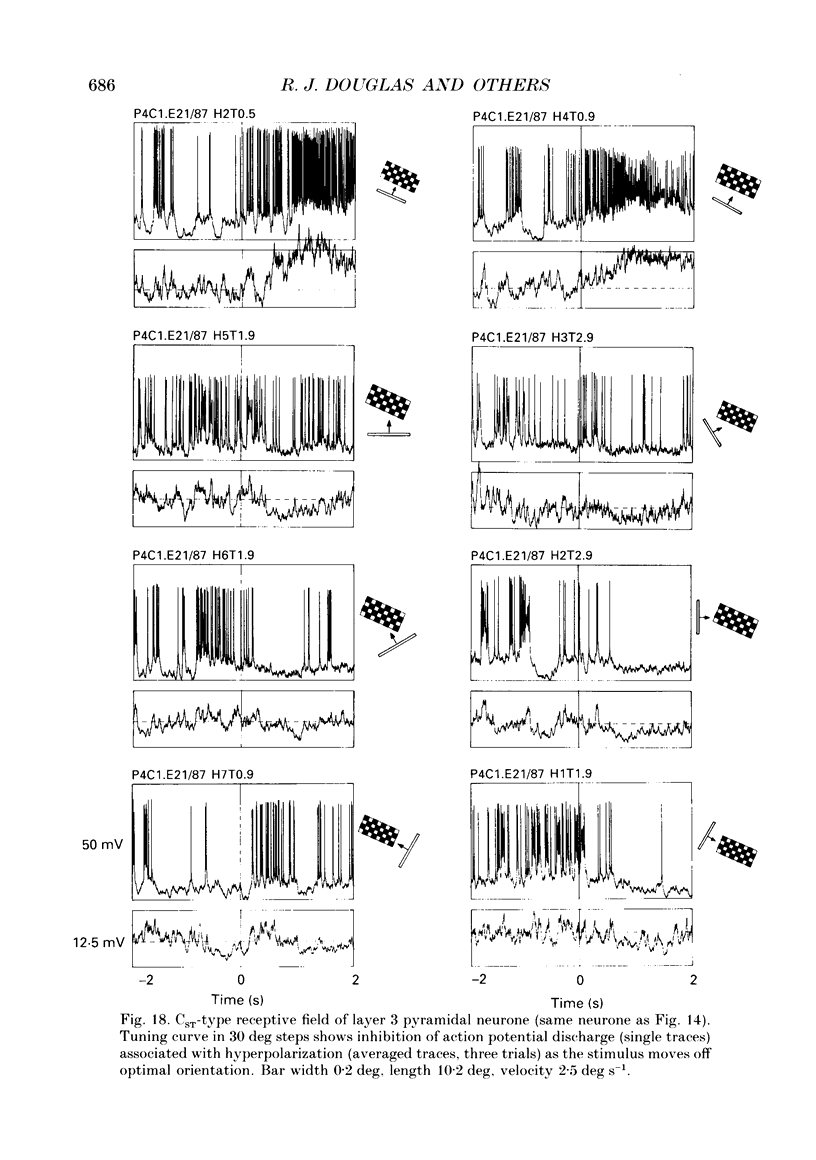










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K. The detection of movement direction and effects of contrast reversal in the cat's striate cortex. Vision Res. 1980;20(4):289–293. doi: 10.1016/0042-6989(80)90015-2. [DOI] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965 Jun;178(3):477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B. The Ferrier Lecture, 1980. Critical limiting factors in the design of the eye and visual cortex. Proc R Soc Lond B Biol Sci. 1981 May 7;212(1186):1–34. doi: 10.1098/rspb.1981.0022. [DOI] [PubMed] [Google Scholar]
- Benevento L. A., Creutzfeldt O. D., Kuhnt U. Significance of intracortical inhibition in the visual cortex. Nat New Biol. 1972 Jul 26;238(82):124–126. doi: 10.1038/newbio238124a0. [DOI] [PubMed] [Google Scholar]
- Berman N. J., Bush P. C., Douglas R. J. Adaptation and bursting in neocortical neurones may be controlled by a single fast potassium conductance. Q J Exp Physiol. 1989 Mar;74(2):223–226. doi: 10.1113/expphysiol.1989.sp003260. [DOI] [PubMed] [Google Scholar]
- Berman N. J., Douglas R. J., Martin K. A., Whitteridge D. Mechanisms of inhibition in cat visual cortex. J Physiol. 1991;440:697–722. doi: 10.1113/jphysiol.1991.sp018731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Interaction effects of visual contours on the discharge frequency of simple striate neurones. J Physiol. 1971 Dec;219(3):659–687. doi: 10.1113/jphysiol.1971.sp009682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Receptive fields of simple cells in the cat striate cortex. J Physiol. 1973 May;231(1):31–60. doi: 10.1113/jphysiol.1973.sp010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Kato H., Orban G. A. Direction-selective cells in complex family in cat striate cortex. J Neurophysiol. 1980 May;43(5):1266–1283. doi: 10.1152/jn.1980.43.5.1266. [DOI] [PubMed] [Google Scholar]
- Bolz J., Gilbert C. D. Generation of end-inhibition in the visual cortex via interlaminar connections. 1986 Mar 27-Apr 2Nature. 320(6060):362–365. doi: 10.1038/320362a0. [DOI] [PubMed] [Google Scholar]
- Bonds A. B. Role of inhibition in the specification of orientation selectivity of cells in the cat striate cortex. Vis Neurosci. 1989;2(1):41–55. doi: 10.1017/s0952523800004314. [DOI] [PubMed] [Google Scholar]
- Bullier J., Henry G. H. Ordinal position of neurons in cat striate cortex. J Neurophysiol. 1979 Sep;42(5):1251–1263. doi: 10.1152/jn.1979.42.5.1251. [DOI] [PubMed] [Google Scholar]
- Burr D., Morrone C., Maffei L. Intra-cortical inhibition prevents simple cells from responding to textured visual patterns. Exp Brain Res. 1981;43(3-4):455–458. doi: 10.1007/BF00238391. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J., Prince D. A. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982 Dec;48(6):1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Kuhnt U., Benevento L. A. An intracellular analysis of visual cortical neurones to moving stimuli: response in a co-operative neuronal network. Exp Brain Res. 1974;21(3):251–274. doi: 10.1007/BF00235746. [DOI] [PubMed] [Google Scholar]
- De Valois R. L., Yund E. W., Hepler N. The orientation and direction selectivity of cells in macaque visual cortex. Vision Res. 1982;22(5):531–544. doi: 10.1016/0042-6989(82)90112-2. [DOI] [PubMed] [Google Scholar]
- Dehay C., Douglas R. J., Martin K. A., Nelson C. Excitation by geniculocortical synapses is not 'vetoed' at the level of dendritic spines in cat visual cortex. J Physiol. 1991;440:723–734. doi: 10.1113/jphysiol.1991.sp018732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R. J., Martin K. A. A functional microcircuit for cat visual cortex. J Physiol. 1991;440:735–769. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S., Krnjević K. Cortical inhibition and gamma-aminobutyric acid. Exp Brain Res. 1969;9(2):137–154. doi: 10.1007/BF00238327. [DOI] [PubMed] [Google Scholar]
- Duysens J., Orban G. A. Is stimulus movement of particular importance in the functioning of cat visual cortex? Brain Res. 1981 Sep 7;220(1):184–187. doi: 10.1016/0006-8993(81)90223-7. [DOI] [PubMed] [Google Scholar]
- Emerson R. C., Coleman L. Does image movement have a special nature for neurons in the cat's striate cortex? Invest Ophthalmol Vis Sci. 1981 Jun;20(6):766–783. [PubMed] [Google Scholar]
- Emerson R. C., Gerstein G. L. Simple striate neurons in the cat. I. Comparison of responses to moving and stationary stimuli. J Neurophysiol. 1977 Jan;40(1):119–135. doi: 10.1152/jn.1977.40.1.119. [DOI] [PubMed] [Google Scholar]
- Emerson R. C., Gerstein G. L. Simple striate neurons in the cat. II. Mechanisms underlying directional asymmetry and directional selectivity. J Neurophysiol. 1977 Jan;40(1):136–155. doi: 10.1152/jn.1977.40.1.136. [DOI] [PubMed] [Google Scholar]
- Eysel U. T., Muche T., Wörgötter F. Lateral interactions at direction-selective striate neurones in the cat demonstrated by local cortical inactivation. J Physiol. 1988 May;399:657–675. doi: 10.1113/jphysiol.1988.sp017102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D. A comparison of binocular depth mechanisms in areas 17 and 18 of the cat visual cortex. J Physiol. 1981 Feb;311:623–655. doi: 10.1113/jphysiol.1981.sp013608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D., Lindström S. An intracellular analysis of geniculo-cortical connectivity in area 17 of the cat. J Physiol. 1983 Sep;342:181–215. doi: 10.1113/jphysiol.1983.sp014846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D. Orientation selectivity of synaptic potentials in neurons of cat primary visual cortex. J Neurosci. 1986 May;6(5):1284–1301. doi: 10.1523/JNEUROSCI.06-05-01284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D. Origin of orientation-selective EPSPs in simple cells of cat visual cortex. J Neurosci. 1987 Jun;7(6):1780–1791. doi: 10.1523/JNEUROSCI.07-06-01780.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D. Spatially opponent excitation and inhibition in simple cells of the cat visual cortex. J Neurosci. 1988 Apr;8(4):1172–1180. doi: 10.1523/JNEUROSCI.08-04-01172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz L., Felder R. Mechanism of directional selectivity in simple neurons of the cat's visual cortex analyzed with stationary flash sequences. J Neurophysiol. 1984 Feb;51(2):294–324. doi: 10.1152/jn.1984.51.2.294. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D. Microcircuitry of the visual cortex. Annu Rev Neurosci. 1983;6:217–247. doi: 10.1146/annurev.ne.06.030183.001245. [DOI] [PubMed] [Google Scholar]
- Goodwin A. W., Henry G. H., Bishop P. O. Direction selectivity of simple striate cells: properties and mechanism. J Neurophysiol. 1975 Nov;38(6):1500–1523. doi: 10.1152/jn.1975.38.6.1500. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959 Oct;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
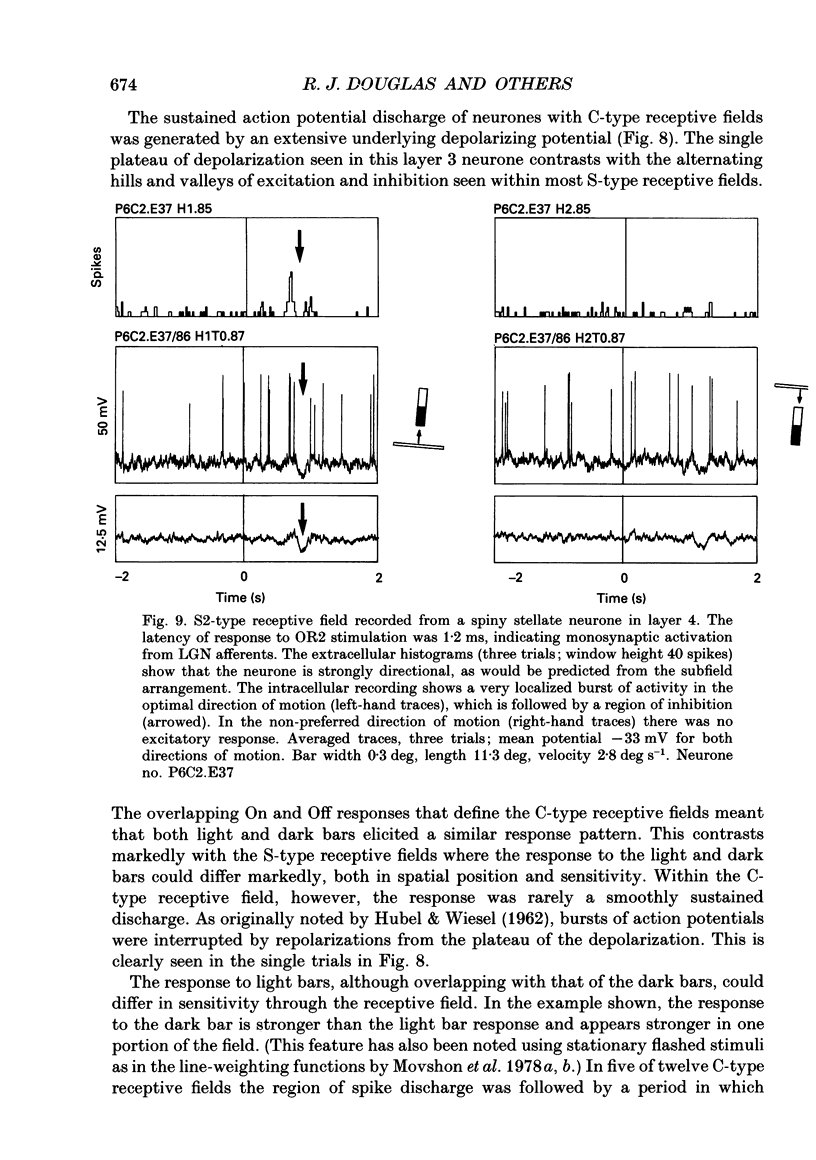
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P., MacKay D. M. Differential responsiveness of simple and complex cells in cat striate cortex to visual texture. Exp Brain Res. 1977 Nov 24;30(2-3):275–296. doi: 10.1007/BF00237256. [DOI] [PubMed] [Google Scholar]
- Heggelund P. Receptive field organization of complex cells in cat striate cortex. Exp Brain Res. 1981;42(1):90–107. [PubMed] [Google Scholar]
- Heggelund P. Receptive field organization of simple cells in cat striate cortex. Exp Brain Res. 1981;42(1):89–98. doi: 10.1007/BF00235733. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Harvey A. R., Lund J. S. The afferent connections and laminar distribution of cells in the cat striate cortex. J Comp Neurol. 1979 Oct 15;187(4):725–744. doi: 10.1002/cne.901870406. [DOI] [PubMed] [Google Scholar]
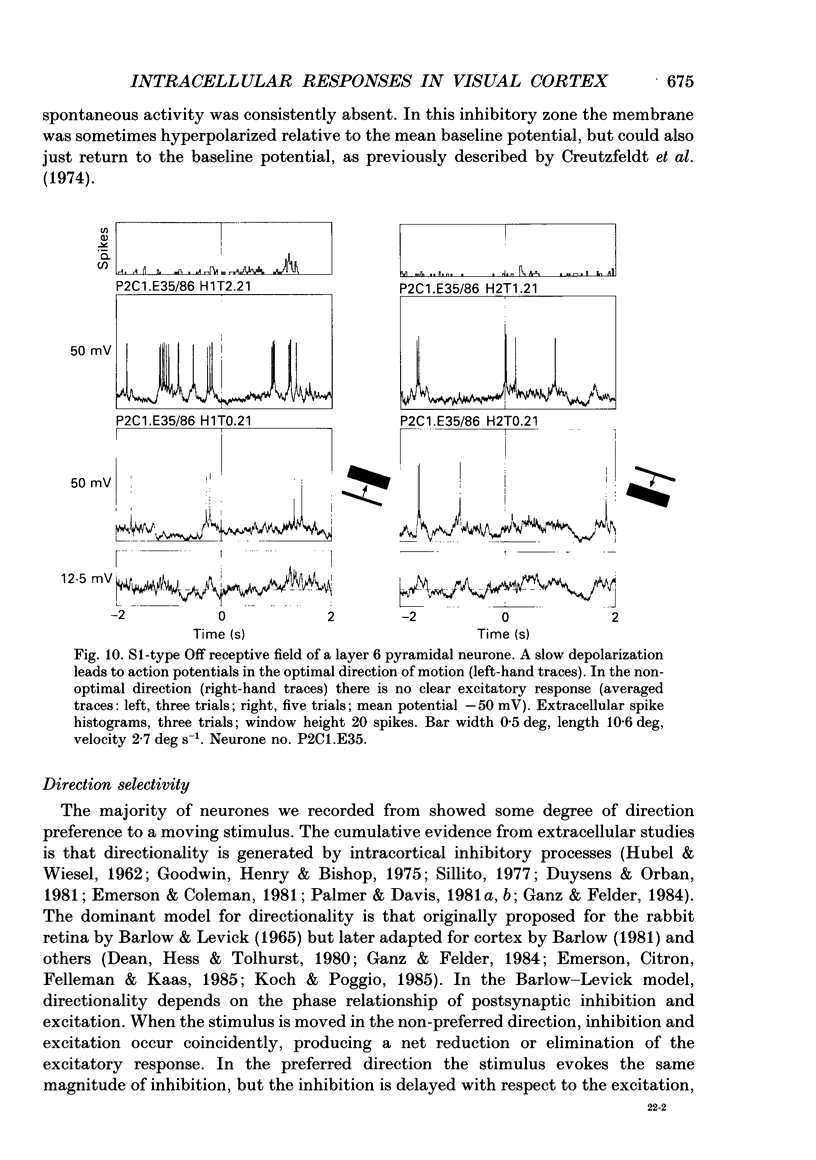
- Henry G. H. Receptive field classes of cells in the striate cortex of the cat. Brain Res. 1977 Sep 9;133(1):1–28. doi: 10.1016/0006-8993(77)90045-2. [DOI] [PubMed] [Google Scholar]
- Hess R., Murata K. Effects of glutamate and GABA on specific response properties of neurones in the visual cortex. Exp Brain Res. 1974;21(3):285–297. doi: 10.1007/BF00235748. [DOI] [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
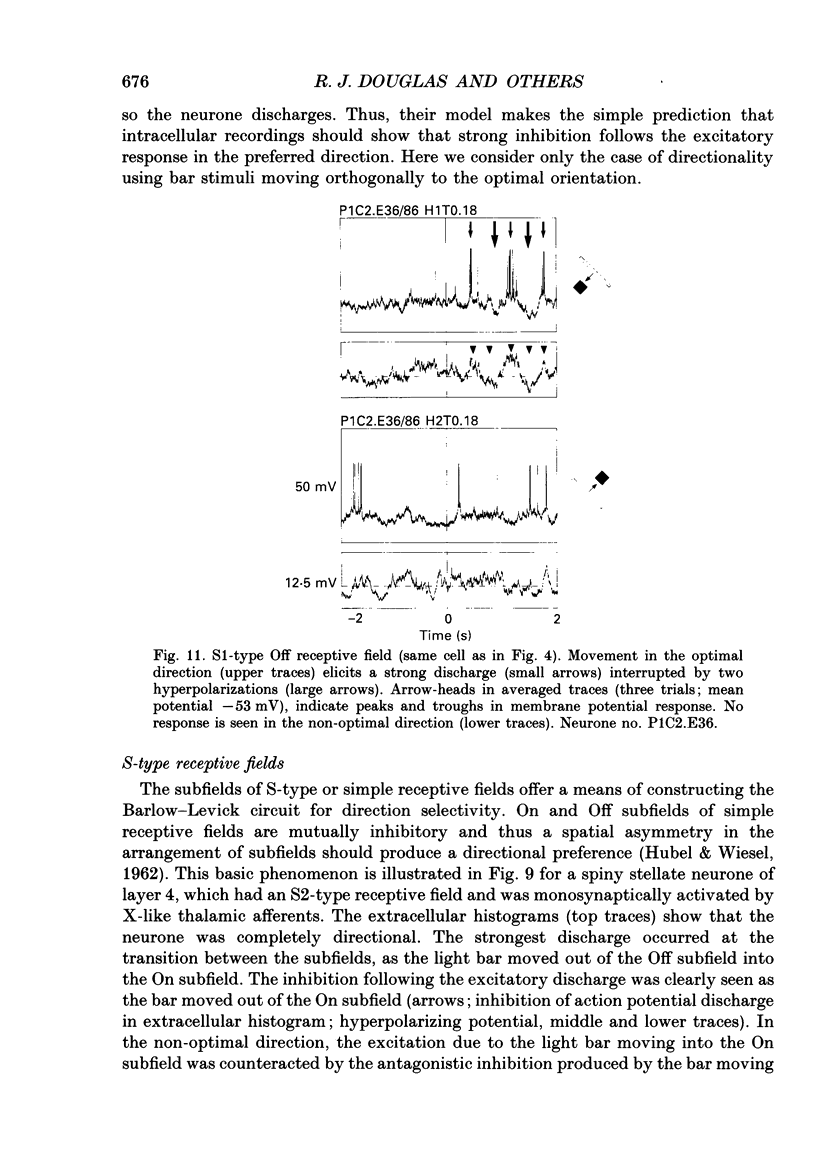
- Innocenti G. M., Fiore L. Post-synaptic inhibitory components of the responses to moving stimuli in area 17. Brain Res. 1974 Nov 8;80(1):122–126. doi: 10.1016/0006-8993(74)90728-8. [DOI] [PubMed] [Google Scholar]
- Koch C., Douglas R., Wehmeier U. Visibility of synaptically induced conductance changes: theory and simulations of anatomically characterized cortical pyramidal cells. J Neurosci. 1990 Jun;10(6):1728–1744. doi: 10.1523/JNEUROSCI.10-06-01728.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J. S. Anatomical organization of macaque monkey striate visual cortex. Annu Rev Neurosci. 1988;11:253–288. doi: 10.1146/annurev.ne.11.030188.001345. [DOI] [PubMed] [Google Scholar]
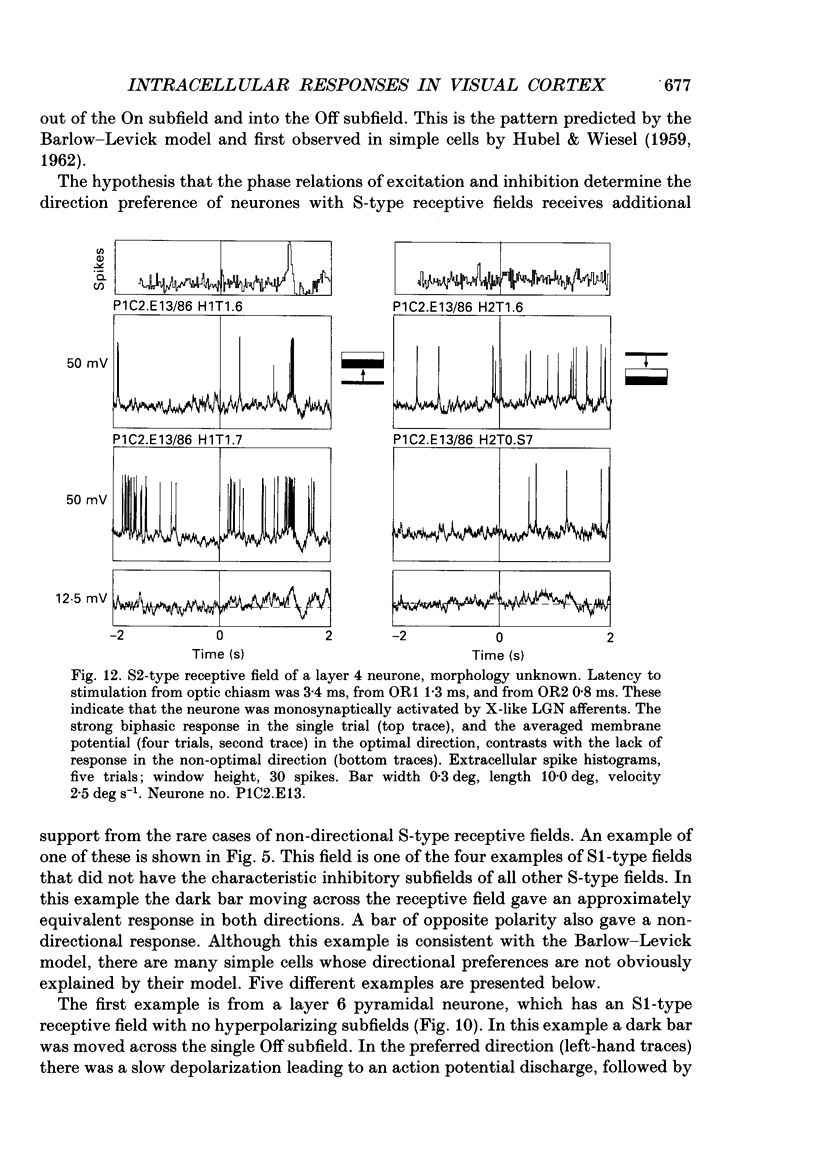
- Martin K. A. The Wellcome Prize lecture. From single cells to simple circuits in the cerebral cortex. Q J Exp Physiol. 1988 Sep;73(5):637–702. doi: 10.1113/expphysiol.1988.sp003190. [DOI] [PubMed] [Google Scholar]
- Martin K. A., Whitteridge D. Form, function and intracortical projections of spiny neurones in the striate visual cortex of the cat. J Physiol. 1984 Aug;353:463–504. doi: 10.1113/jphysiol.1984.sp015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Receptive field organization of complex cells in the cat's striate cortex. J Physiol. 1978 Oct;283:79–99. doi: 10.1113/jphysiol.1978.sp012489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial summation in the receptive fields of simple cells in the cat's striate cortex. J Physiol. 1978 Oct;283:53–77. doi: 10.1113/jphysiol.1978.sp012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. I., Frost B. J. Orientation-selective inhibition from beyond the classic visual receptive field. Brain Res. 1978 Jan 13;139(2):359–365. doi: 10.1016/0006-8993(78)90937-x. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Davis T. L. Comparison of responses to moving and stationary stimuli in cat striate cortex. J Neurophysiol. 1981 Aug;46(2):277–295. doi: 10.1152/jn.1981.46.2.277. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Davis T. L. Receptive-field structure in cat striate cortex. J Neurophysiol. 1981 Aug;46(2):260–276. doi: 10.1152/jn.1981.46.2.260. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Linden R. Evidence for dendritic competition in the developing retina. Nature. 1982 Jun 24;297(5868):683–685. doi: 10.1038/297683a0. [DOI] [PubMed] [Google Scholar]
- Ramoa A. S., Shadlen M., Skottun B. C., Freeman R. D. A comparison of inhibition in orientation and spatial frequency selectivity of cat visual cortex. Nature. 1986 May 15;321(6067):237–239. doi: 10.1038/321237a0. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Spain W. J., Foehring R. C., Chubb M. C., Crill W. E. Slow conductances in neurons from cat sensorimotor cortex in vitro and their role in slow excitability changes. J Neurophysiol. 1988 Feb;59(2):450–467. doi: 10.1152/jn.1988.59.2.450. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. Inhibitory mechanisms influencing complex cell orientation selectivity and their modification at high resting discharge levels. J Physiol. 1979 Apr;289:33–53. doi: 10.1113/jphysiol.1979.sp012723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M. Inhibitory processes underlying the directional specificity of simple, complex and hypercomplex cells in the cat's visual cortex. J Physiol. 1977 Oct;271(3):699–720. doi: 10.1113/jphysiol.1977.sp012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M. The effectiveness of bicuculline as an antagonist of GABA and visually evoked inhibition in the cat's striate cortex. J Physiol. 1975 Sep;250(2):287–304. doi: 10.1113/jphysiol.1975.sp011055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama K., Kimura M., Tanaka K. Cross-Correlation Analysis of Interneuronal Connectivity in cat visual cortex. J Neurophysiol. 1981 Aug;46(2):191–201. doi: 10.1152/jn.1981.46.2.191. [DOI] [PubMed] [Google Scholar]
- Ts'o D. Y., Gilbert C. D., Wiesel T. N. Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. J Neurosci. 1986 Apr;6(4):1160–1170. doi: 10.1523/JNEUROSCI.06-04-01160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumoto T., Eckart W., Creutzfeldt O. D. Modification of orientation sensitivity of cat visual cortex neurons by removal of GABA-mediated inhibition. Exp Brain Res. 1979 Jan 15;34(2):351–363. doi: 10.1007/BF00235678. [DOI] [PubMed] [Google Scholar]


