Abstract
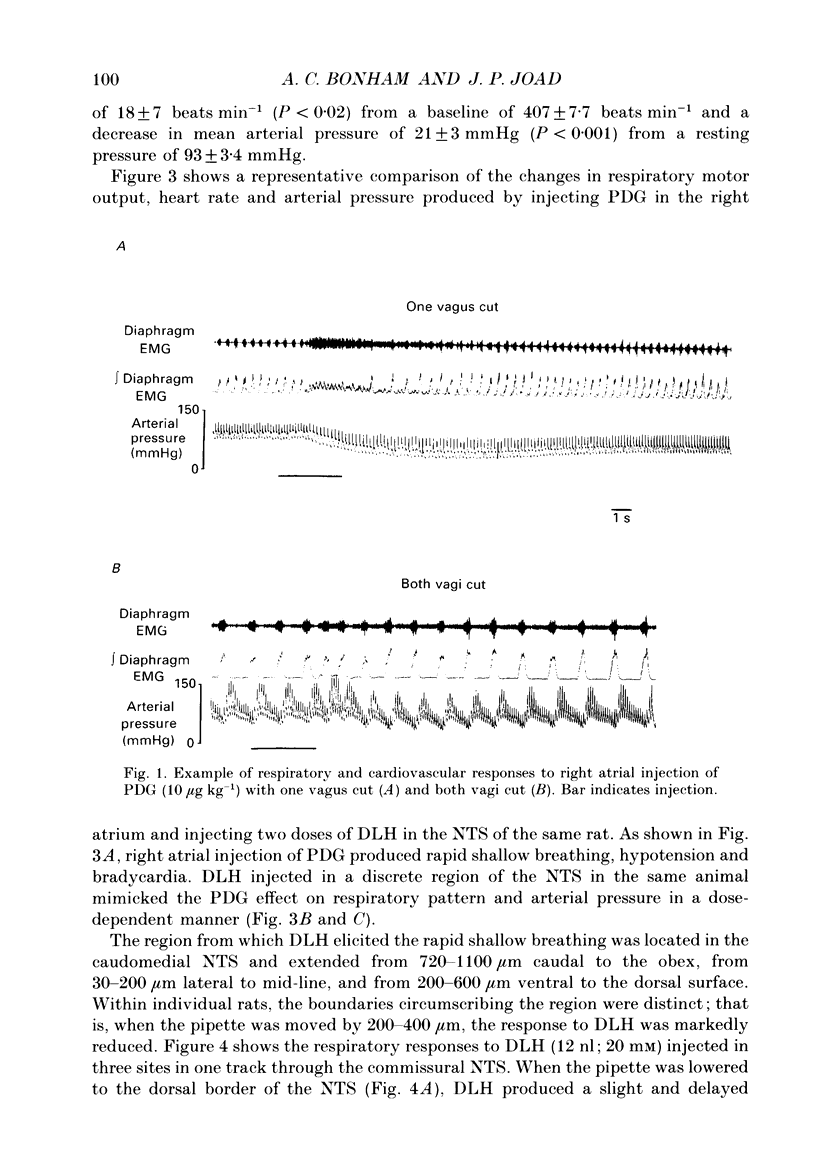
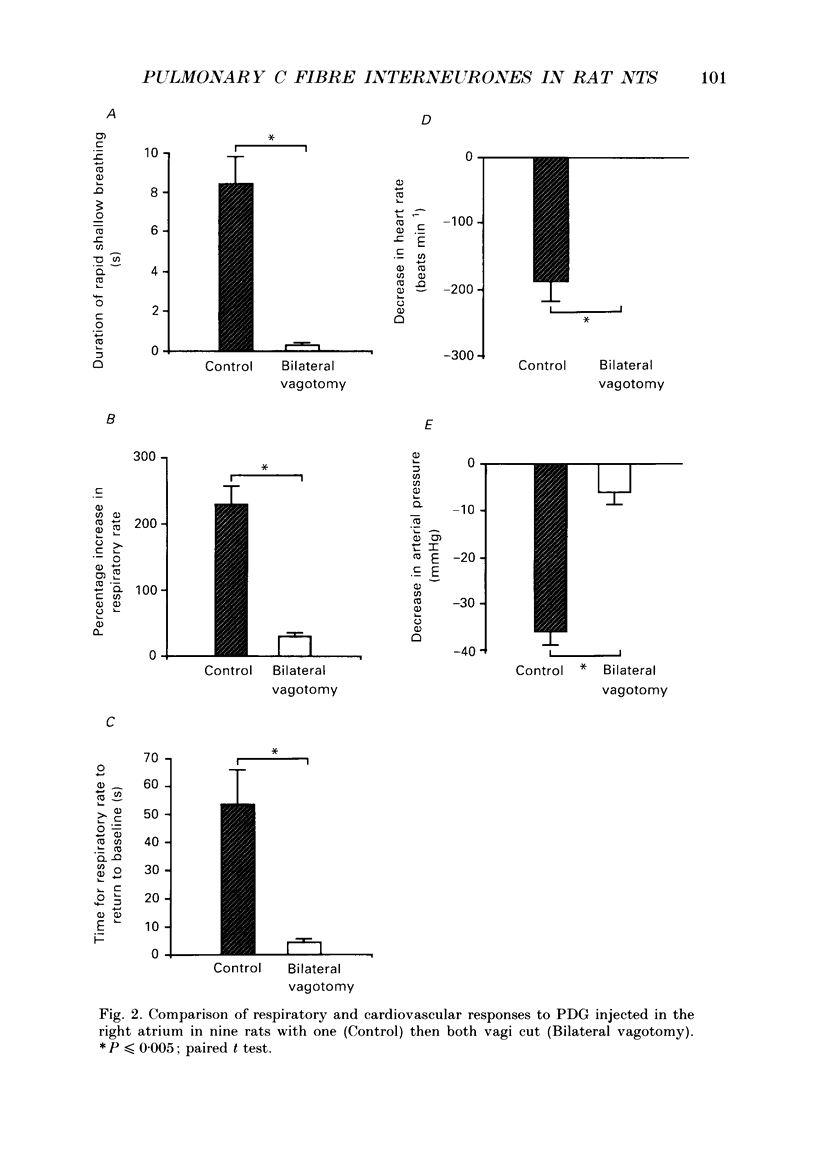
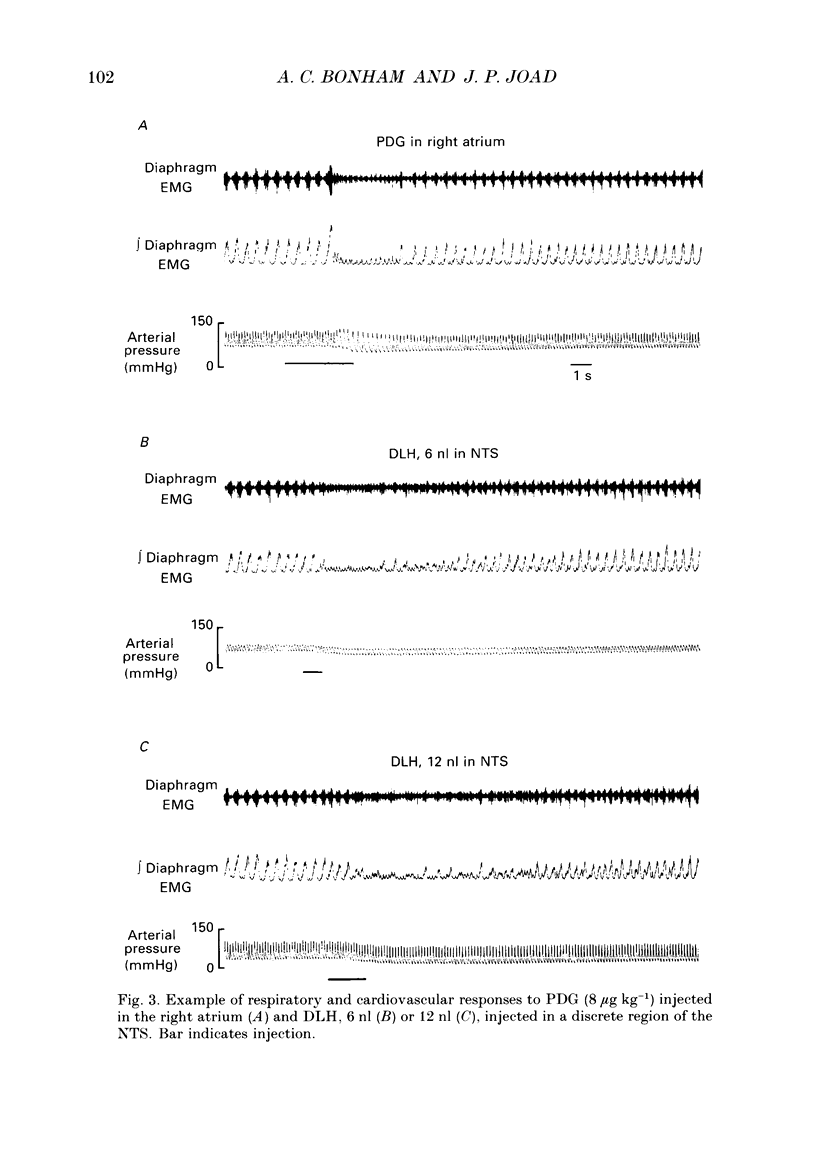
1. The pulmonary C fibre reflex, triggered by activating pulmonary C fibre endings in the lung, consists of rapid shallow breathing (which may be preceded by apnoea), bradycardia, and hypotension. The purpose of this work was to identify proximal synapses in this reflex. From pilot data, we hypothesized that neurones in a discrete region of the commissural nucleus in the nucleus tractus solitarii (NTS) are required for full expression of the pulmonary C fibre reflex. Studies were carried out in urethane-anaesthetized, unilaterally vagotomized, spontaneously breathing rats, in which diaphragm electromyogram, arterial pressure, and blood gases were measured. Phenyldiguanide (PDG) was injected in the right atrium to elicit the pulmonary C fibre reflex. Unilateral NTS injections were made through multibarrelled pipettes containing DL-homocysteic acid (DLH) to mimic the reflex, cobalt chloride to reversibly impair the reflex, and/or dye to mark the injection sites. 2. PDG (5-16 micrograms kg-1) injected in the right atrium of twenty-six rats produced the classic pulmonary C fibre reflex: a vagally mediated, rapid onset of rapid shallow breathing, bradycardia and hypotension. 3. Injection of DLH (3-12 nl of 20 mM for a total of 60-240 pmol) in the dorsomedial aspect of the commissural nucleus of the NTS in thirty rats mimicked the pulmonary C fibre reflex, producing rapid shallow breathing, hypotension, and a slight bradycardia. 4. Interruption of neuronal transmission by injecting cobalt chloride (15-30 nl of 100 mM) in the site where DLH produced rapid shallow breathing, reversibly impaired the rapid shallow breathing and bradycardia produced by right atrial injections of PDG in fifteen rats. The commissural region where DLH produced rapid shallow breathing and cobalt impaired the pulmonary C fibre reflex extended from 720-1100 microns caudal to the obex, 30-200 microns lateral to mid-line, and 200-600 microns ventral to the dorsal surface of the brain stem within the NTS. 5. Taken together, the results suggest that neurones within a discrete region in the dorsomedial commissural nucleus in caudal NTS are required for full expression of the pulmonary C fibre reflex.
Full text
PDF




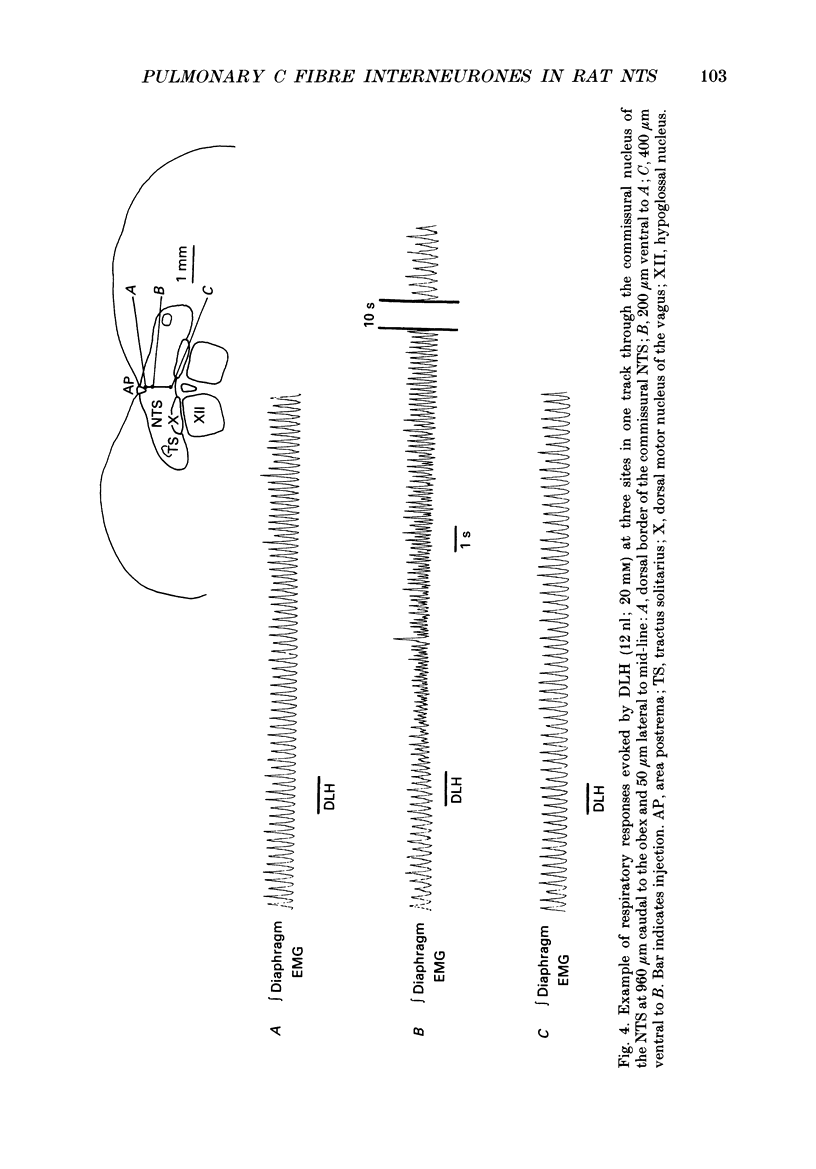
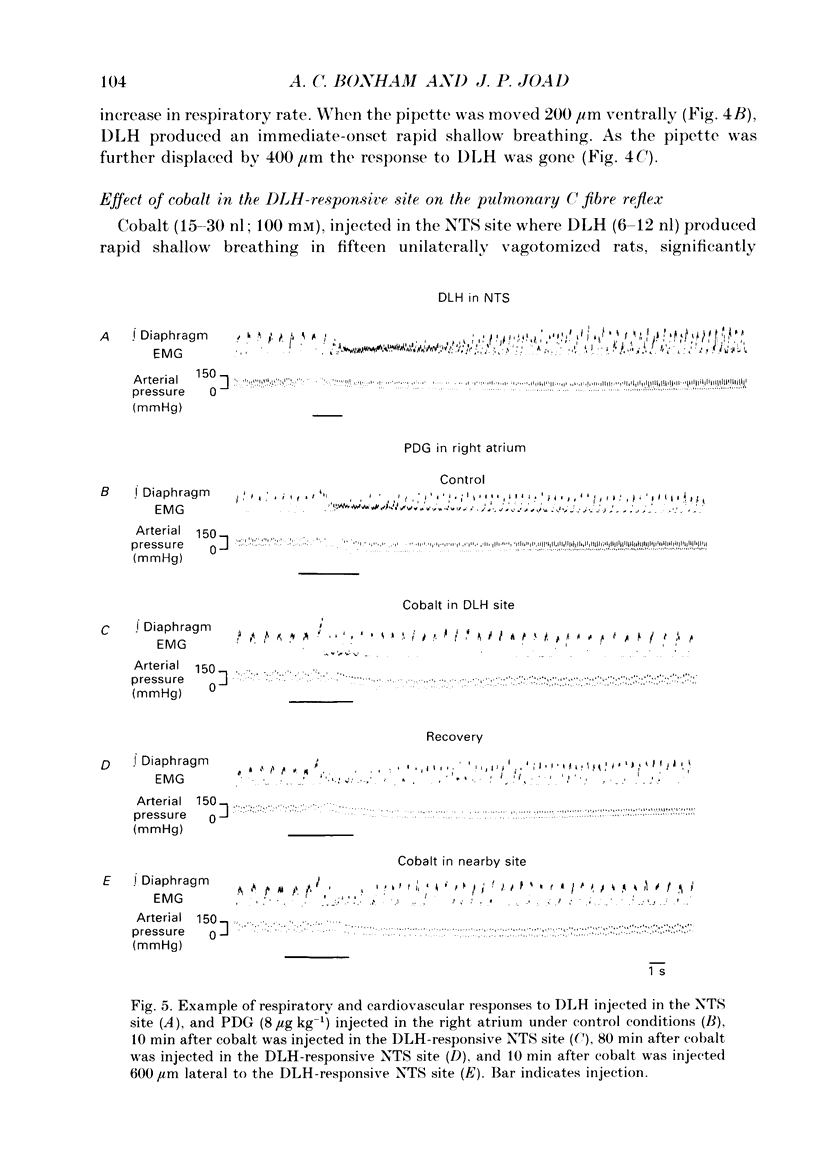

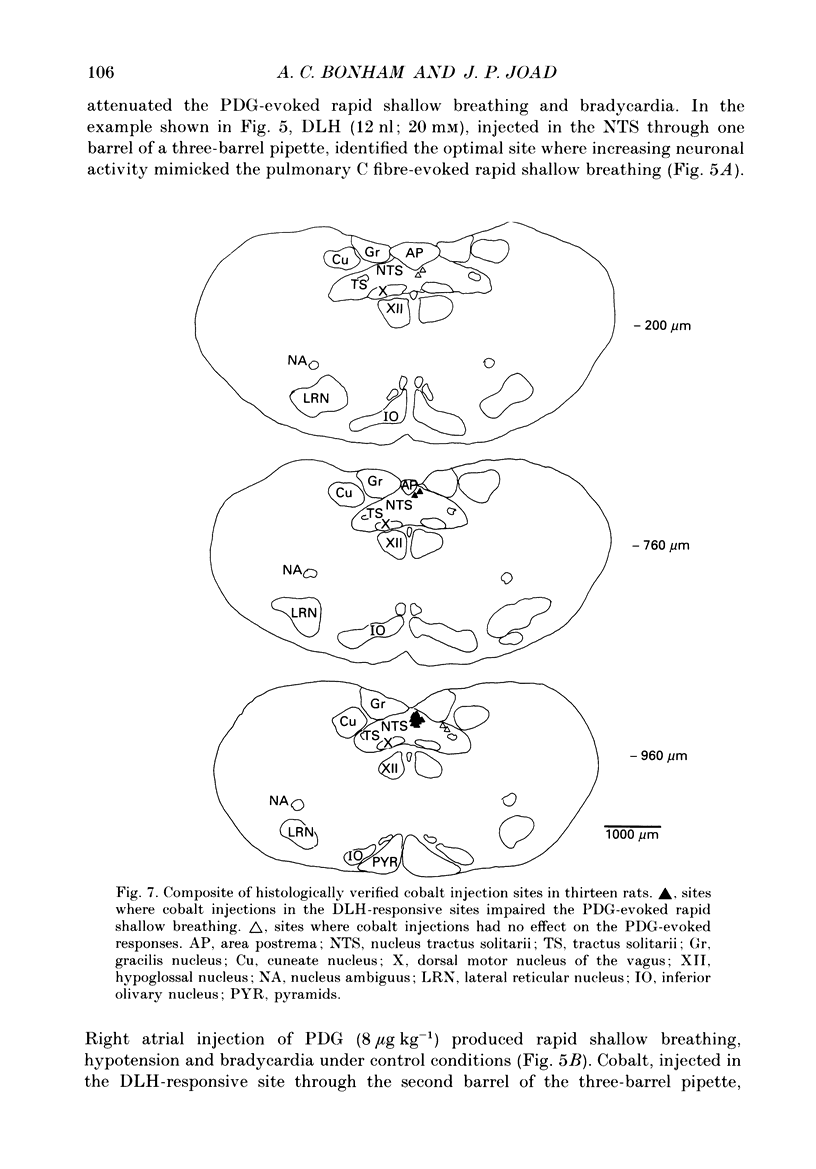






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand A., Paintal A. S. Reflex effects following selective stimulation of J receptors in the cat. J Physiol. 1980 Feb;299:553–572. doi: 10.1113/jphysiol.1980.sp013142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badier M., Jammes Y., Romero-Colomer P., Lemerre C. Tonic activity in inspiratory muscles and phrenic motoneurons by stimulation of vagal afferents. J Appl Physiol (1985) 1989 Apr;66(4):1613–1619. doi: 10.1152/jappl.1989.66.4.1613. [DOI] [PubMed] [Google Scholar]
- Bartlett D., Jr Origin and regulation of spontaneous deep breaths. Respir Physiol. 1971 Jun;12(2):230–238. doi: 10.1016/0034-5687(71)90055-7. [DOI] [PubMed] [Google Scholar]
- COMROE J. H., Jr, MORTIMER L. THE RESPIRATORY AND CARDIOVASCULAR RESPONSES OF TEMPORALLY SEPARATED AORTIC AND CAROTID BODIES TO CYANIDE, NICOTINE, PHENYLDIGUANIDE AND SEROTONIN. J Pharmacol Exp Ther. 1964 Oct;146:33–41. [PubMed] [Google Scholar]
- Coleridge H. M., Coleridge J. C., Luck J. C. Pulmonary afferent fibres of small diameter stimulated by capsaicin and by hyperinflation of the lungs. J Physiol. 1965 Jul;179(2):248–262. doi: 10.1113/jphysiol.1965.sp007660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glogowska M., Richardson P. S., Widdicombe J. G., Winning A. J. The role of the vagus nerves, peripheral chemoreceptors and other afferent pathways in the genesis of augmented breaths in cats and rabbits. Respir Physiol. 1972 Oct;16(2):179–196. doi: 10.1016/0034-5687(72)90050-3. [DOI] [PubMed] [Google Scholar]
- Glogowska M., Widdicombe J. G. The role of vagal reflexes in experimental lung oedema, bronchoconstriction and inhalation of halothane. Respir Physiol. 1973 Jun;18(1):116–128. doi: 10.1016/0034-5687(73)90027-3. [DOI] [PubMed] [Google Scholar]
- Guz A., Trenchard D. W. The role of non-myelinated vagal afferent fibres from the lungs in the genesis of tachypnoea in the rabbit. J Physiol. 1971 Mar;213(2):345–371. doi: 10.1113/jphysiol.1971.sp009386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatridge J., Haji A., Perez-Padilla J. R., Remmers J. E. Rapid shallow breathing caused by pulmonary vascular congestion in cats. J Appl Physiol (1985) 1989 Dec;67(6):2257–2264. doi: 10.1152/jappl.1989.67.6.2257. [DOI] [PubMed] [Google Scholar]
- Housley G. D., Sinclair J. D. Localization by kainic acid lesions of neurones transmitting the carotid chemoreceptor stimulus for respiration in rat. J Physiol. 1988 Dec;406:99–114. doi: 10.1113/jphysiol.1988.sp017371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRIEGER E. M., MARSEILLAN R. F. AORTIC DEPRESSOR FIBERS IN THE RAT: AN ELECTROPHYSIOLOGICAL STUDY. Am J Physiol. 1963 Oct;205:771–774. doi: 10.1152/ajplegacy.1963.205.4.771. [DOI] [PubMed] [Google Scholar]
- Kalia M., Mesulam M. M. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980 Sep 15;193(2):467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Karczewski W., Widdicombe J. G. The role of the vagus nerves in the respiratory and circulatory responses to intravenous histamine and phenyl diguanide in rabbits. J Physiol. 1969 Apr;201(2):271–291. doi: 10.1113/jphysiol.1969.sp008755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman M. P., Coleridge H. M., Coleridge J. C., Baker D. G. Bradykinin stimulates afferent vagal C-fibers in intrapulmonary airways of dogs. J Appl Physiol Respir Environ Exerc Physiol. 1980 Mar;48(3):511–517. doi: 10.1152/jappl.1980.48.3.511. [DOI] [PubMed] [Google Scholar]
- Kretz R. Local cobalt injection: a method to discriminate presynaptic axonal from postsynaptic neuronal activity. J Neurosci Methods. 1984 Jun;11(2):129–135. doi: 10.1016/0165-0270(84)90030-x. [DOI] [PubMed] [Google Scholar]
- Kubin L., Kimura H., Davies R. O. The medullary projections of afferent bronchopulmonary C fibres in the cat as shown by antidromic mapping. J Physiol. 1991 Apr;435:207–228. doi: 10.1113/jphysiol.1991.sp018506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y. L., Tsuya Y., Hildebrandt J. Ventilatory responses to acute CO2 exposure in the rat. J Appl Physiol Respir Environ Exerc Physiol. 1978 Oct;45(4):611–618. doi: 10.1152/jappl.1978.45.4.611. [DOI] [PubMed] [Google Scholar]
- Lee C., Malpeli J. G. Somata-selective lesions induced by cobaltous chloride: a parametric study. Brain Res. 1986 Feb 5;364(2):396–399. doi: 10.1016/0006-8993(86)90855-3. [DOI] [PubMed] [Google Scholar]
- Lee L. Y., Dumont C., Djokic T. D., Menzel T. E., Nadel J. A. Mechanism of rapid, shallow breathing after ozone exposure in conscious dogs. J Appl Physiol Respir Environ Exerc Physiol. 1979 Jun;46(6):1108–1114. doi: 10.1152/jappl.1979.46.6.1108. [DOI] [PubMed] [Google Scholar]
- Lee L. Y., Kou Y. R., Frazier D. T., Beck E. R., Pisarri T. E., Coleridge H. M., Coleridge J. C. Stimulation of vagal pulmonary C-fibers by a single breath of cigarette smoke in dogs. J Appl Physiol (1985) 1989 May;66(5):2032–2038. doi: 10.1152/jappl.1989.66.5.2032. [DOI] [PubMed] [Google Scholar]
- Lipski J., Bellingham M. C., West M. J., Pilowsky P. Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J Neurosci Methods. 1988 Dec;26(2):169–179. doi: 10.1016/0165-0270(88)90166-5. [DOI] [PubMed] [Google Scholar]
- Miserocchi G., Trippenbach T., Mazzarelli M., Jaspar N., Hazucha M. The mechanism of rapid shallow breathing due to histamine and phenyldiguanide in cats and rabbits. Respir Physiol. 1978 Feb;32(2):141–153. doi: 10.1016/0034-5687(78)90105-6. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985 May 6;333(2):325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Paintal A. S. Mechanism of stimulation of type J pulmonary receptors. J Physiol. 1969 Aug;203(3):511–532. doi: 10.1113/jphysiol.1969.sp008877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapru H. N., Willette R. N., Krieger A. J. Stimulation of pulmonary J receptors by an enkephalin-analog. J Pharmacol Exp Ther. 1981 Apr;217(1):228–234. [PubMed] [Google Scholar]


