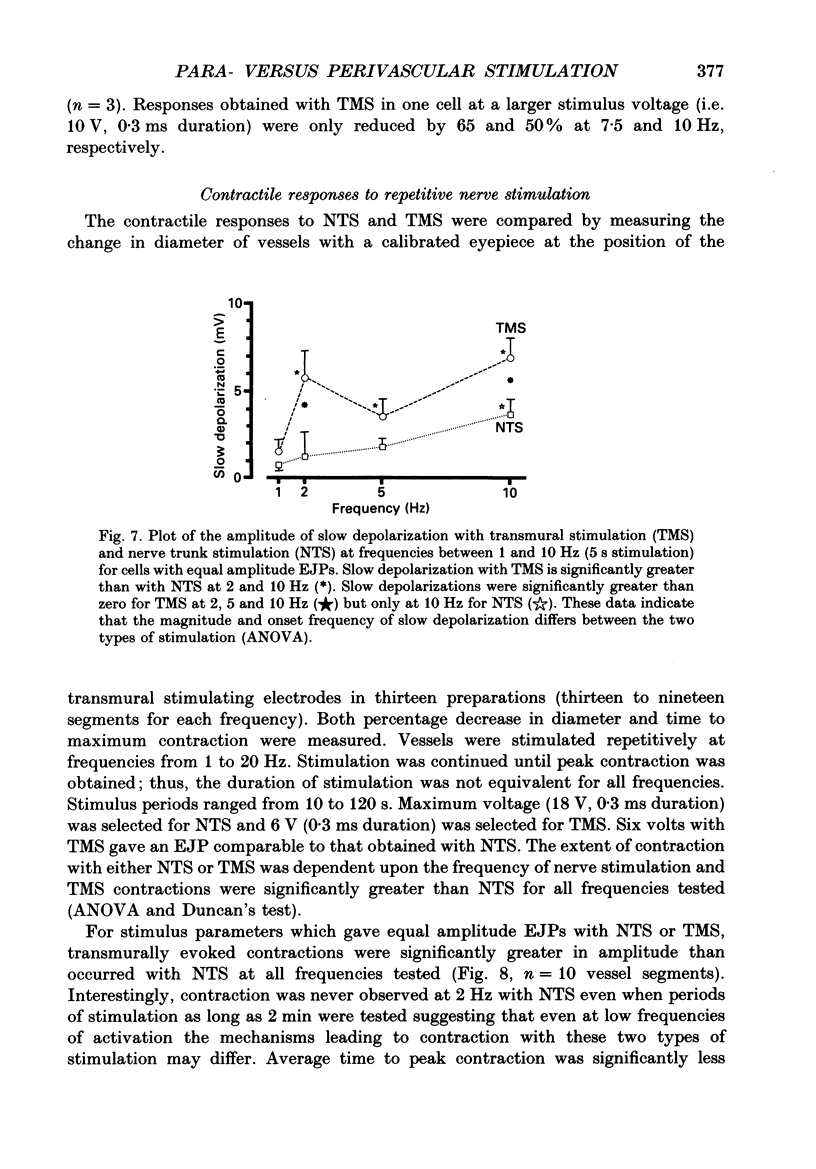
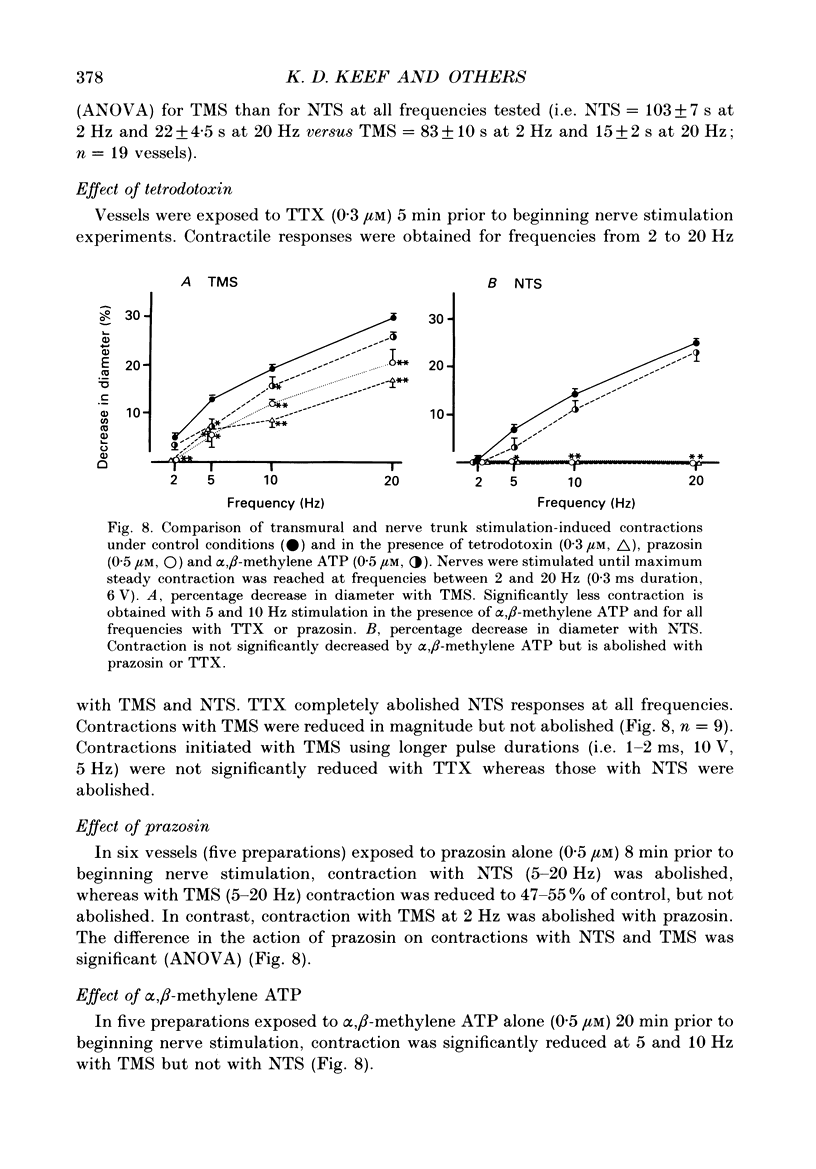
Abstract
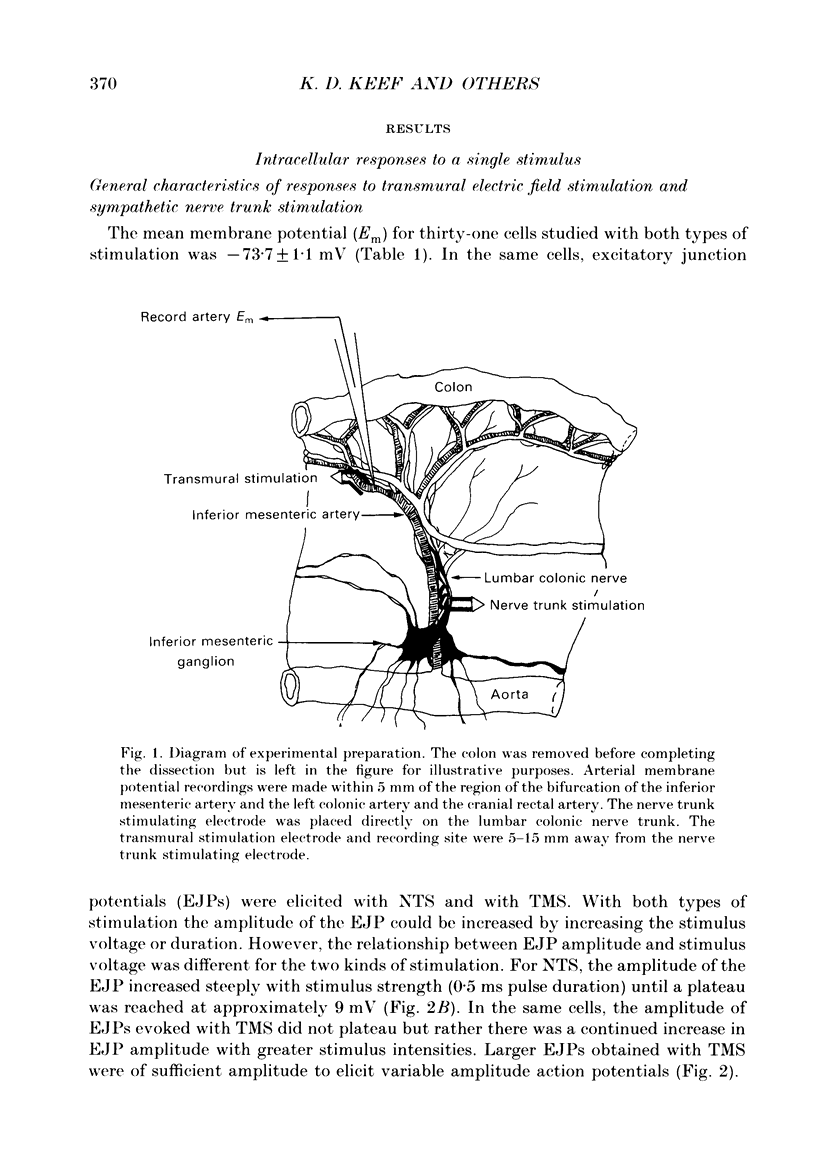
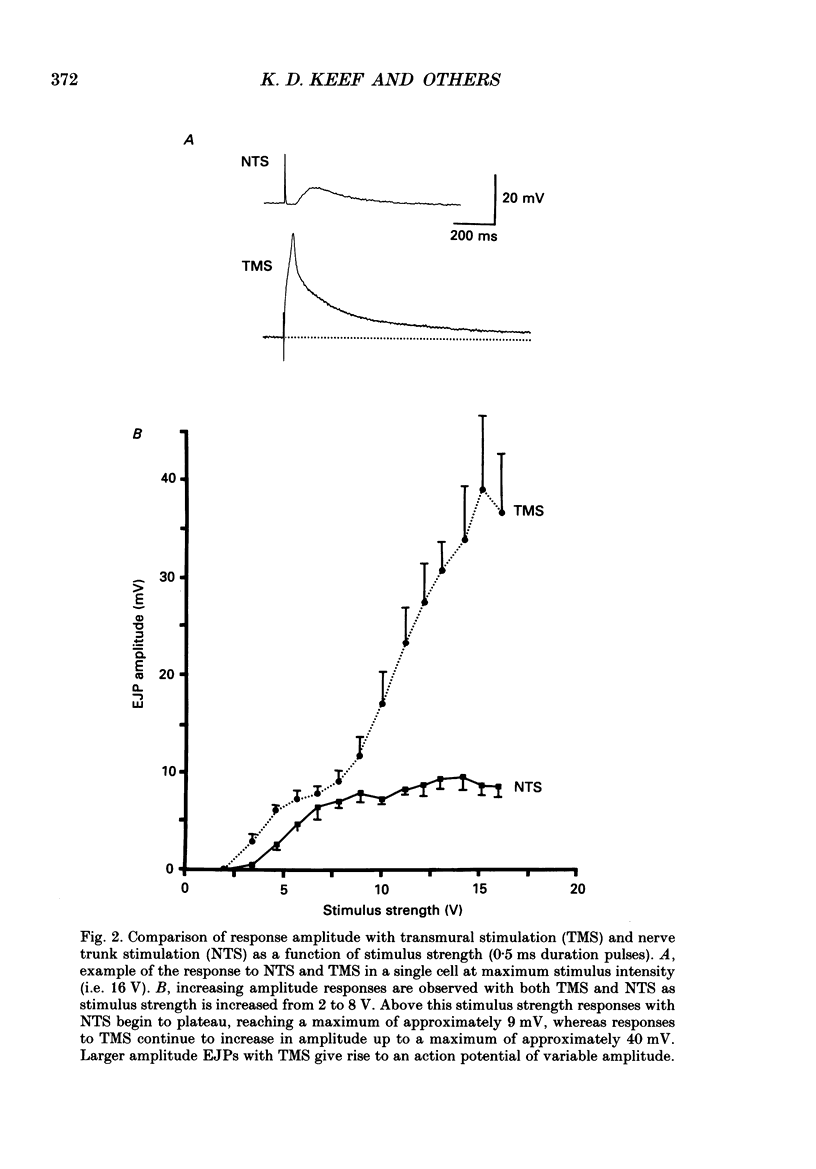
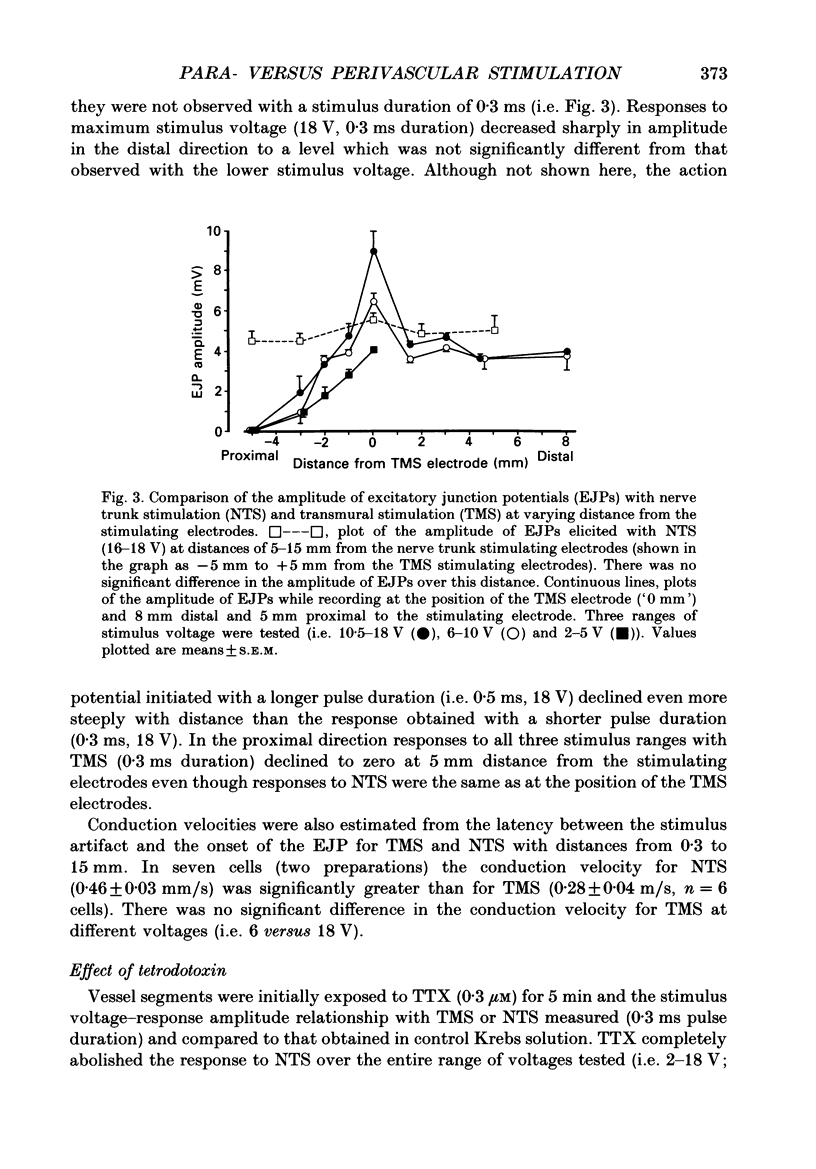
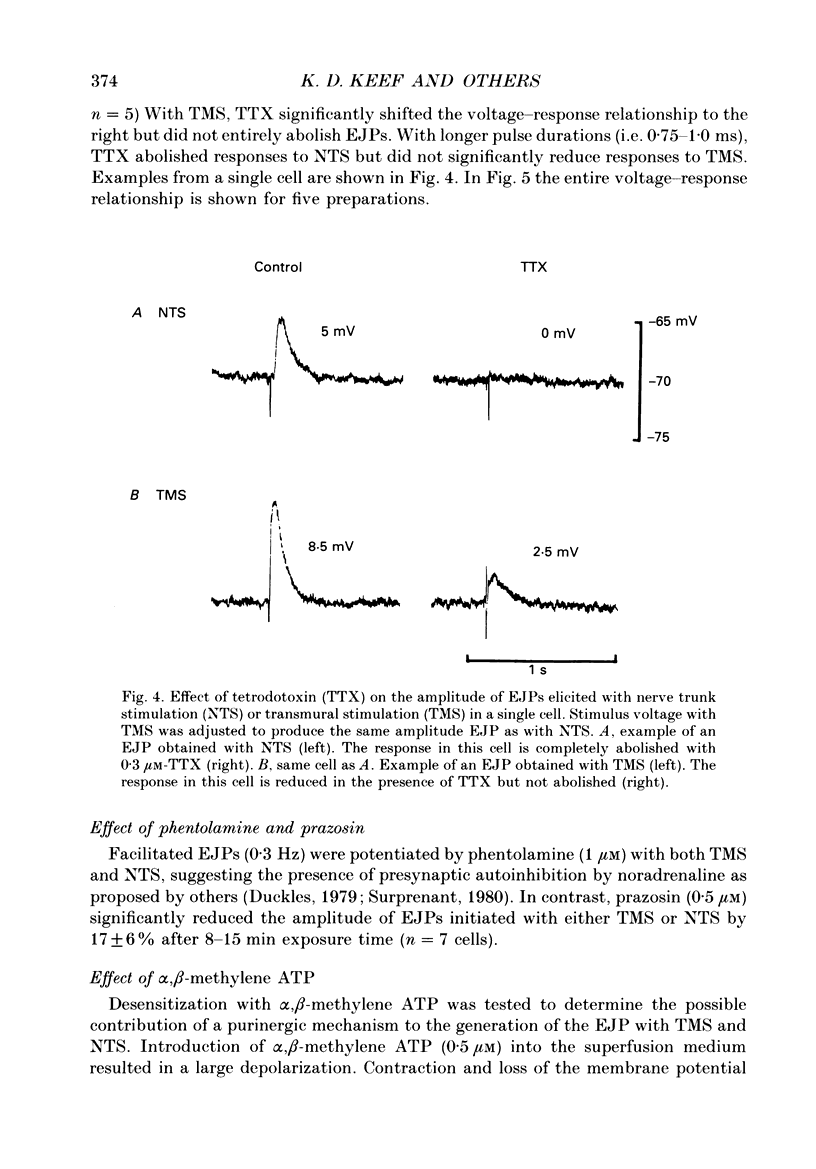
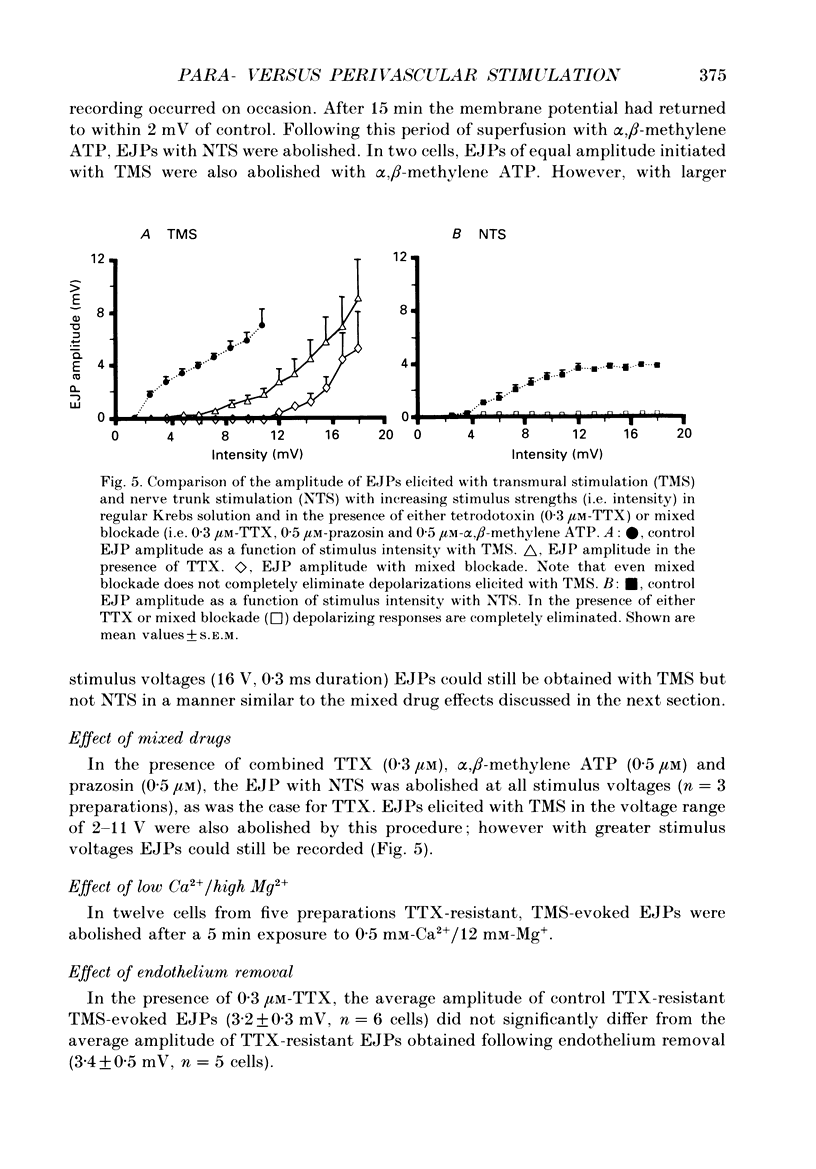
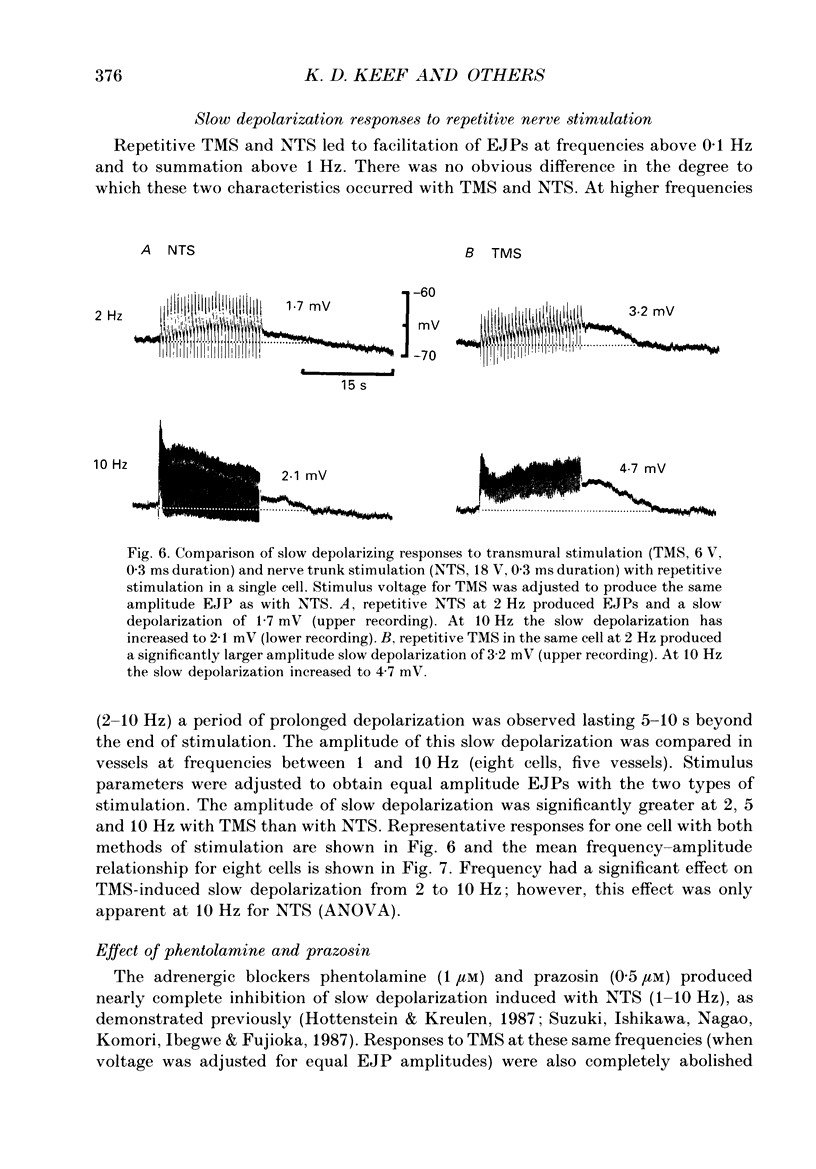
1. Intracellular electrical and contractile responses to sympathetic nerve trunk stimulation (NTS) and transmural electrical field stimulation (TMS) were compared in the guinea-pig mesenteric artery in vitro. 2. Step increases in voltage with NTS gave rise to excitatory junctional potentials (EJPs) which reached a plateau amplitude of 5-10 mV, whereas with TMS larger amplitude EJPs and sometimes action potentials were obtained. 3. EJPs of equal amplitude (1-7 mV) elicited with TMS and NTS had the same rise time, duration and decay half-time. 4. Slow depolarization obtained with repetitive stimulation was significantly greater in amplitude with TMS than with NTS. 5. Equal amplitude EJPs were obtained throughout the preparation with NTS. With TMS, the amplitude of responses declined substantially with distance from the stimulating electrodes. 6. Tetrodotoxin (TTX) completely blocked EJPs, slow depolarization and contraction with NTS; however, with TMS a TTX-resistant component was observed. The TTX-resistant response to TMS was abolished in the presence of a low-Ca2+ superfusion solution but was not affected by endothelium removal. 7. Phentolamine or prazosin abolished slow depolarization but not EJPs with NTS or TMS. Prazosin abolished contraction with NTS and reduced but did not abolish contraction with TMS. 8. alpha, beta-Methylene ATP abolished EJPs with NTS, whereas with TMS only EJPs obtained with low stimulus intensities were abolished. alpha, beta-Methylene ATP did not block contraction with either NTS or TMS. 9. Combined TTX, prazosin and alpha, beta-methylene ATP abolished EJPs initiated with TMS at all but the highest stimulus intensities (12-20 V, 0.3 ms duration). 10. It is concluded that responses obtained with NTS can be reliably attributed to the release of transmitter by the conduction of action potentials in paravascular nerves, whereas activation by TMS is a more complex phenomenon dependent upon stimulus strength and probably involving multiple forms of activation.
Full text
PDF


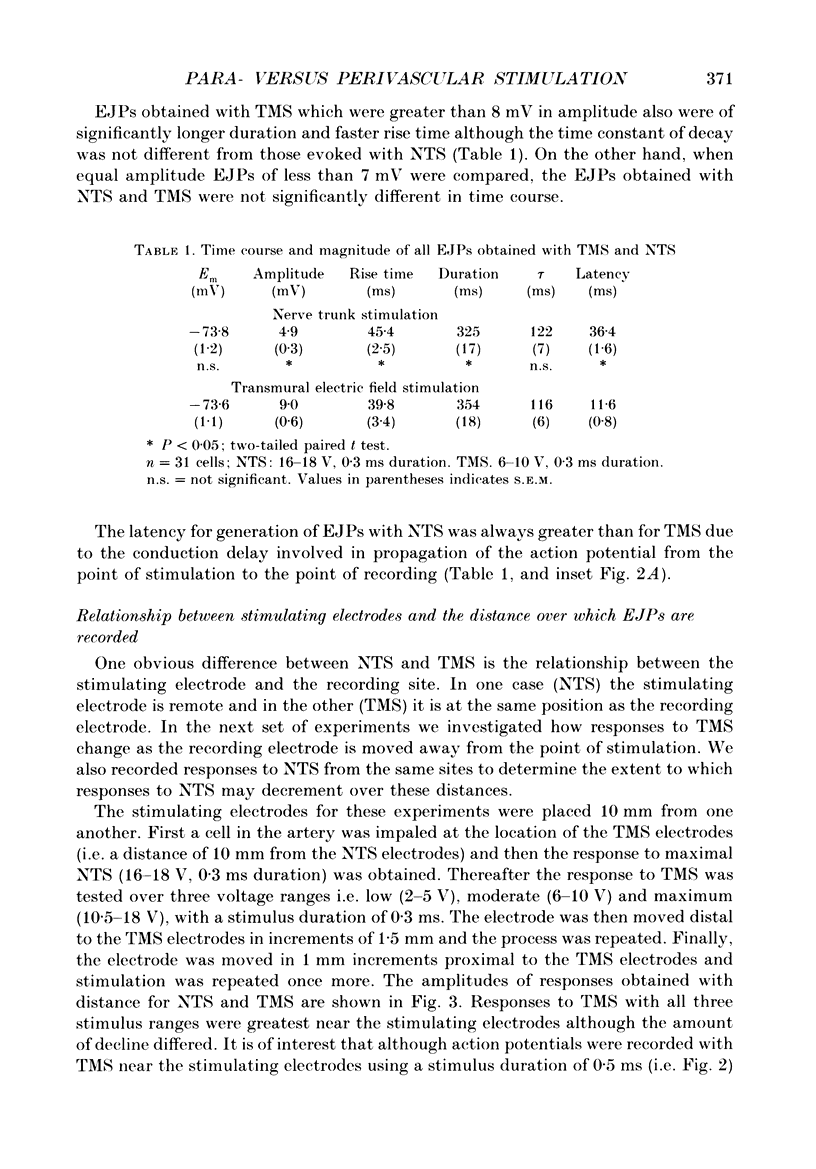





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEVAN J. A. Some characteristics of the isolated sympathetic nerve-pulmonary artery preparation of the rabbit. J Pharmacol Exp Ther. 1962 Aug;137:213–218. [PubMed] [Google Scholar]
- De la Lande I. S., Rand M. J. A simple isolated nerve-blood vessel preparation. Aust J Exp Biol Med Sci. 1965 Oct;43(5):639–656. doi: 10.1038/icb.1965.48. [DOI] [PubMed] [Google Scholar]
- Duckles S. P. Neurogenic dilator and constrictor responses of pial arteries in vitro. Differences between dogs and sheep. Circ Res. 1979 Apr;44(4):482–490. doi: 10.1161/01.res.44.4.482. [DOI] [PubMed] [Google Scholar]
- Fedan J. S., Hogaboom G. K., O'Donnell J. P., Colby J., Westfall D. P. Contribution by purines to the neurogenic response of the vas deferens of the guinea pig. Eur J Pharmacol. 1981 Jan 5;69(1):41–53. doi: 10.1016/0014-2999(81)90600-2. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Holman M. E., McLean A. The innervation of sheep mesenteric veins. J Physiol. 1967 May;190(1):55–69. doi: 10.1113/jphysiol.1967.sp008192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. An electrophysiological analysis of the effects of noradrenaline and alpha-receptor antagonists on neuromuscular transmission in mammalian muscular arteries. Br J Pharmacol. 1980;71(2):651–661. doi: 10.1111/j.1476-5381.1980.tb10986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. Effects of tetraethylammonium chloride on sympathetic neuromuscular transmission in saphenous artery of young rabbits. J Physiol. 1980 Aug;305:451–465. doi: 10.1113/jphysiol.1980.sp013375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottenstein O. D., Kreulen D. L. Comparison of the frequency dependence of venous and arterial responses to sympathetic nerve stimulation in guinea-pigs. J Physiol. 1987 Mar;384:153–167. doi: 10.1113/jphysiol.1987.sp016448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S. Actions of ATP and alpha, beta-methylene ATP on neuromuscular transmission and smooth muscle membrane of the rabbit and guinea-pig mesenteric arteries. Br J Pharmacol. 1985 Dec;86(4):777–787. doi: 10.1111/j.1476-5381.1985.tb11099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kitamura K., Kuriyama H. Roles of extrajunctional receptors in the response of guinea-pig mesenteric and rat tail arteries to adrenergic nerves. J Physiol. 1983 Dec;345:409–422. doi: 10.1113/jphysiol.1983.sp014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keef K. D., Kreulen D. L. Electrical responses of guinea pig coronary artery to transmural stimulation. Circ Res. 1988 Mar;62(3):585–595. doi: 10.1161/01.res.62.3.585. [DOI] [PubMed] [Google Scholar]
- Keef K., Neild T. O. Modification of the response to nerve stimulation in small arteries of guinea-pig caused by distension of the artery. J Physiol. 1982 Oct;331:355–365. doi: 10.1113/jphysiol.1982.sp014376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreulen D. L. Activation of mesenteric arteries and veins by preganglionic and postganglionic nerves. Am J Physiol. 1986 Dec;251(6 Pt 2):H1267–H1275. doi: 10.1152/ajpheart.1986.251.6.H1267. [DOI] [PubMed] [Google Scholar]
- Moulds R. F. Techniques for testing isolated blood vessels. Gen Pharmacol. 1983;14(1):47–53. doi: 10.1016/0306-3623(83)90062-9. [DOI] [PubMed] [Google Scholar]
- Muramatsu I., Kigoshi S., Oshita M. Nonadrenergic nature of prazosin-resistant, sympathetic contraction in the dog mesenteric artery. J Pharmacol Exp Ther. 1984 May;229(2):532–538. [PubMed] [Google Scholar]
- Nagao T., Suzuki H. Non-neural electrical responses of smooth muscle cells of the rabbit basilar artery to electrical field stimulation. Jpn J Physiol. 1987;37(3):497–513. doi: 10.2170/jjphysiol.37.497. [DOI] [PubMed] [Google Scholar]
- Neild T. O. The relation between the structure and innervation of small arteries and arterioles and the smooth muscle membrane potential changes expected at different levels of sympathetic nerve activity. Proc R Soc Lond B Biol Sci. 1983 Dec 22;220(1219):237–249. doi: 10.1098/rspb.1983.0097. [DOI] [PubMed] [Google Scholar]
- Neild T., Kotecha N. Two-component responses to sympathetic nerve stimulation in the rat tail artery. Comp Biochem Physiol C. 1985;81(2):311–317. doi: 10.1016/0742-8413(85)90012-x. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984 Oct 30;106(1):149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Surprenant A. A comparative study of neuromuscular transmission in several mammalian muscular arteries. Pflugers Arch. 1980 Jul;386(1):85–91. doi: 10.1007/BF00584192. [DOI] [PubMed] [Google Scholar]
- Surprenant A., Neild T. O., Holman M. E. Membrane properties of rabbit basilar arteries and their responses to transmural stimulation. Pflugers Arch. 1987 Sep;410(1-2):92–101. doi: 10.1007/BF00581901. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Ishikawa S., Nagao T., Komori K., Ibengwe J. K., Fujioka M. Effects of bunazosin on electrical responses of smooth muscle cells of the guinea-pig mesenteric artery and vein to perivascular nerve stimulation and to noradrenaline. Gen Pharmacol. 1987;18(2):171–177. doi: 10.1016/0306-3623(87)90245-x. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline and adenosine triphosphate as co-transmitters of neurogenic vasoconstriction in rabbit mesenteric artery. J Physiol. 1985 Oct;367:435–455. doi: 10.1113/jphysiol.1985.sp015834. [DOI] [PMC free article] [PubMed] [Google Scholar]