Abstract
The pathogenic yeast Candida albicans can undergo a dramatic change in morphology from round yeast cells to long filamentous cells called hyphae. We have cloned the CaMYO5 gene encoding the only myosin I in C. albicans. A strain with a deletion of both copies of CaMYO5 is viable but cannot form hyphae under all hypha-inducing conditions tested. This mutant exhibits a higher frequency of random budding and a depolarized distribution of cortical actin patches relative to the wild-type strain. We found that polar budding, polarized localization of cortical actin patches, and hypha formation are dependent on a specific phosphorylation site on myosin I, called the “TEDS-rule” site. Mutation of this serine 366 to alanine gives rise to the null mutant phenotype, while a S366D mutation, the product of which mimics a phosphorylated serine, allows hypha formation. However, the S366D mutation still causes a depolarized distribution of cortical actin patches in budding cells, similar to that in the null mutant. The localization of CaMyo5-GFP together with cortical actin patches at the bud and hyphal tips is also dependent on serine 366. Intriguingly, the cortical actin patches in the majority of the hyphae of the mutant expressing Camyo5S366D were depolarized, suggesting that although their distribution is dependent on myosin I localization, polarized cortical actin patches may not be required for hypha formation.
Polarized growth is a regulated cellular expansion which underlies many processes, such as phagocytosis in mammalian cells, morphogenesis of root hair and other specialized cell types in plants, cell locomotion in Acanthamoeba and Dictyostelium, and hypha formation in fungi (19, 24, 37, 49). Saccharomyces cerevisiae cells can elongate into pseudohyphae in response to specific environmental cues, and polarization of the actin cytoskeleton is essential for this differentiation (11). Similarly, hyphal morphogenesis in other fungi, such as Saprolegnia ferax, Neurospora crassa, and Aspergillus nidulans, requires filamentous actin, while microtubules play a secondary role (20, 51, 52). The pathogenic yeast Candida albicans can undergo a dramatic change in morphogenesis when round yeast cells form highly elongated filaments called hyphae, but little is known about the role of the actin cytoskeleton during hypha formation in this organism (25). Treatment of germinating cells with cytochalasin A prevents further hyphal growth, suggesting that filamentous actin is critical to hyphal growth (2). Nocodazole, in contrast, does not prevent apical cell elongation, suggesting that microtubules are not critical for polarized growth in C. albicans (61).
Two forms of actin appear to be important during polarized growth. First, actin cables serve as tracks for the vesicular transport of molecular components of the plasma membrane and cell wall toward the site of growth, i.e., the bud tip (23, 44, 45) and presumably the hyphal tip of C. albicans. These run along the longitudinal axis of yeast and hyphal cells in S. cerevisiae and C. albicans (4, 44). Second, cortical actin patches correlate with sites of targeted secretion and endocytosis, critical during cell wall biogenesis (44). These localize to the tips of emerging buds as well to growing hyphal tips in C. albicans (4). Proteins that modulate the structure of the actin cytoskeleton are key factors in determining cell polarity (44). Myosin I, one of these factors, is a single-headed molecular motor that functions in actin-based processes such as polarized growth, cell motility, phagocytosis, endocytosis and exocytosis, and contractile vacuolar activity in several organisms (12, 13, 32, 37, 40, 46, 50, 58). In S. cerevisiae, myosin I was shown to promote actin polymerization at cortical patches, which correlate with sites of growth (1, 14, 29). This myosin I regulation of actin polymerization was shown to be achieved by its interaction with and activation of the Arp2/3 complex, which nucleates the assembly of actin filaments (14, 29, 33, 34). Similarly, in Schizosaccharomyces pombe, myosin I is required for a polarized actin cytoskeleton and was shown to bind to the Arp2/Arp3 complex and activate its actin nucleation activity (32, 53). Jung et al. (22) also found that Dyctiostelium myosin I interacts with the Arp2-Arp3 complex via the CARMIL protein and may localize actin polymerization to sites of cellular growth. In accordance with its proposed role in polarized growth, myosin I colocalizes with cortical actin patches at the tips of buds in S. cerevisiae and of growing cells in S. pombe (3, 32, 53) and localizes as well to the tips of hyphae in Aspergillus (36).
The actin-dependent ATPase activity of Acanthamoeba myosin I and Dictyostelium myosin I is activated by the phosphorylation of a unique site, called the “TEDS-rule” site, by members of the p21-activated kinase (PAK) kinases (7, 9, 56). The corresponding phosphorylation site of S. cerevisiae myosin I is essential for its function in vivo and for its ability to polymerize actin in vitro (29, 57). It is also a target of the Ste20p and Cla4p kinases in vitro (57). These latter proteins are members of the PAK family of protein kinases and function to regulate cell morphology (6, 27, 47). Homologues of these kinases are involved in hypha formation in C. albicans (8, 26, 28).
In this study, we took a genetic approach to define the role of myosin I in C. albicans. We found that myosin I is required for hypha but not pseudohypha formation and that the PAK phosphorylation site (serine 366) is critical for myosin I function during budding yeast and hyphal growth.
MATERIALS AND METHODS
DNA manipulations.
The oligonucleotides used for cloning are listed in Table 1. The CaMYO5 gene was amplified by PCR from genomic DNA prepared from C. albicans strain SC5314 by using the UO5 and UO6 oligonucleotides. The PCR products were digested with BamHI and HindIII as well as with HindIII and XbaI. The 3.75- and 1.5-kb fragments were subcloned into pBluescript KS (Stratagene)to give pU14 and pU15, respectively. Several independent clones were verified by sequencing. The alignment of these sequences revealed the existence of two alleles for CaMYO5 in SC5314, as expected for a diploid organism. The allele that was used for subsequent cloning was different from the sequence available in the Stanford genomic database for amino acids R354K, T585A, A596T, and I954V and for a short deletion, Δ1046-1051. The complete gene was reconstituted by subcloning a 3.75-kb BamHI-HindIII fragment from pU14 and a 1.5-kb HindIII-XbaI fragment from pU15 into pKS and pVEC (35) digested with BamHI and XbaI to give pU46A and pU67, respectively. These fragments were also subcloned into pRS316 and pRS426 digested with BamHI and XbaI to give pU47 and pU48, respectively.
TABLE 1.
Oligonucleotides
| Oligonucleotide | Sequencea |
|---|---|
| UO5 | GCGGATCCCTGACCTTACACTGTCGTTGG |
| UO6 | GCTCTAGACATTGGCCACTTGTGGAGGTTC |
| UO9 | CGGGGTACCCAATACTAGATCCTCTGG |
| UO10 | CGGGGATCCAGACTGCAGCCTCTTTTCACAATAGCC |
| UO11 | CGGGGATCCGAAGAAGAAGACGATGATG |
| UO19 | GCGGATCCATGGCTATTGTGAAAAGAGG |
| UO20 | GCAGATCTCATTGGCCACTTGTGGAGGTTC |
| UO26 | GCAAGCTTACGCCTGCAGTAGGTAAGAACTTGTTATATTTTG |
| UO27 | CTGCAGGCGTAAGCTTGCCCAATCATCATCGTCTTC |
| UO28 | GGGATGAGACGAGGTGCAACTTATCAT |
| UO29 | GGTGAATGATAAGTTGCACCTCGTCTCATCCC |
| UO30 | GGGATGAGACGAGGTGACACTTATCATTCACC |
| UO31 | GGTGAATGATAAGTGTCACCTCGTCTCATCCC |
| UO36 | CGGGGTACCAAGCTTCCAAAGATTGG |
| UO37 | CGGGGATCCAGAGCGGCCGCCCTCTTTTCACAATAGCC |
| CB20F | CGGGCGGCCGCGGTACCTACCTGGAGGTGAGGAG |
| CB20R | CGGGGATCCAATATTTATGAGAAACTATCACTTC |
| Reverse | GGAAACAGCTATGACCATG |
Restriction sites used for cloning are underlined in UO5 to UO27 and UO36 to CB20R. Codons mutated for site-directed mutagenesis are underlined in UO28 and UO29.
To construct the disruption cassette containing the hisG::URA3::hisG blaster (15), the 5′ noncoding region of CaMYO5 was PCR amplified from genomic DNA by using UO9 and UO10, and the 3′ noncoding region was amplified with UO11 and UO6. These PCR products were digested with KpnI and BamHI and with BamHI and XbaI, respectively, and subcloned together into pKS digested with KpnI and XbaI (pU20). p5921 (15) was digested with PstI and BamHI, and the 4.0-kb fragment containing the hisG::URA3::hisG blaster was cloned into pU20 digested with BamHI and PstI. To construct the disruption cassette containing the HIS1 gene, a 5′-flanking sequence was PCR amplified from genomic DNA by using UO36 and UO37. The 2-kb product was digested with KpnI and BamHI and subcloned into pU18 containing the 3′-flanking sequence of CaMYO5 (pU72). Then, the HIS1 gene was PCR amplified by using oligonucleotides CBF20 and CB20R (kind gifts from Catherine Bachewich), and the PCR product was digested with NotI and BamHI and subcloned into pU72 digested first with BamHI and then partially with NotI to generate pU74.
To construct CaMYO5-GFP, pU46A was digested with HindIII and XbaI, and the 1.5-kb fragment containing the sequences of the CaMYO5 tail domain was subcloned into pKS (pU15). A HindIII-PstI site was created 5′ to the stop codon of CaMYO5 in pU15 by fusion-PCR by using the reverse primer with UO26 and by using UO27 with UO6. The 1.5-kb PCR product was subcloned as a HindIII-XbaI fragment into pKS (pU50). A 700-base HindIII-PstI fragment encoding green fluorescent protein (GFP) from pGFP26 (38) and a 1-kb KpnI-HindIII partial fragment from pU50 were subcloned into pU50 digested with KpnI and PstI (pU88). Several clones were verified by sequencing. Finally, a 2.2-kb XbaI-HindIII partial fragment from pU88 and a 3.5-kb HindIII-BamHI fragment from pU46A were subcloned together into pVEC digested with BamHI and XbaI to generate pU93.
The S366A and S366D mutant alleles of CaMYO5 were obtained by site-directed mutagenesis by using a Quick Change kit from Stratagene. First, the mutations were introduced into pU14 by PCR with Pfu polymerase and by using primer UO28 with primer UO29 (S366A mutation; pU55) and primer UO30 with primer UO31 (S366D mutation; pU56). These clones were sequenced to ensure that the S366A and S366D mutations were introduced while no other mutations had occurred. A 3.75-kb BamHI-HindIII fragment from each of pU55 and pU56 and a 1.5-kb HindIII-XbaI fragment from pU15 were subcloned into pVEC digested with BamHI and XbaI (pU77 and pU78, respectively). Similarly, the 3.75-kb BamHI-HindIII fragments and a 2.2-kb HindIII-XbaI fragment from pU88 were subcloned into pVEC to create pU97 and pU98, which contain the S366A and S366D mutant alleles of CaMYO5 in frame with the GFP sequence, respectively.
To express the S366A and S366D mutant alleles of CaMYO5 under the control of the PCK1 regulatable promoter, 4.75-kb fragments were PCR amplified from pU77 and pU78 by using UO19 and UO20. These fragments were digested with BglII and BamHI and subcloned into pJA24 (PCK1 promoter in p5921; a kind gift from J. Ash) digested with BglII (pU95 and pU96, respectively). Clones were kept in which the fragments were oriented such that the ATG of CaMYO5 was immediately downstream of the PCK1 promoter.
Transformations in C. albicans and S. cerevisiae.
The strains used in this study are listed in Table 2. Transformation of C. albicans and S. cerevisiae strains was done by the lithium acetate method (21). To create a mutant in which both copies of the CaMYO5 gene were deleted, the Ura− strain CAI4 (15) and the Ura− His− strain RM1000 (39) were transformed with pU21 digested with KpnI and SacI. Genomic DNA was prepared from Ura+ transformants, digested with SpeI, and analyzed for the correct integration event by Southern blotting with a digoxigenin system (Boehringer Mannheim). Positive transformants derived from strain CAI4 were plated on medium containing 5-fluoroorotic acid (Diagnostics Chemicals Ltd., Charlottetown, Prince Edward Island, Canada), a drug which inhibits the growth of Ura+ strains. Ura− strains thus obtained were analyzed similarly for the loop-out event that occurs by homologous recombination between the hisG direct repeats flanking the URA3 gene. The CaMYO5/Camyo5::hisG strain was transformed with pU21 digested with KpnI and SacI. Ura+ transformants were analyzed similarly for the correct integration event resulting in the disruption of the second CaMYO5 allele with the hisG::URA3::hisG blaster. A positive Camyo5::hisG/Camyo5::hisG::URA3::hisG transformant, called COU42, was analyzed for the correct loop-out event by plating on 5-fluoroorotic acid as described above. In parallel, positive transformants derived from the disruption of the first allele of CaMYO5 in RM1000 were transformed with 5 μg of a PCR-amplified Camyo5::CaHIS1 cassette by using UO6 and UO36. Transformants that were Ura+ and His+ were screened by Southern blot analysis for the disruption of the second allele with the CaHIS1 marker as described above. Nineteen positive transformants were thus obtained that displayed phenotypes similar to that of COU42.
TABLE 2.
Strains
| Strain | Genotype | Reference or source |
|---|---|---|
| C. albicans | ||
| SC5314 | CaMYO5/CaMYO5 CaURA3/CaURA3 | 15 |
| CAI4 | ura3::1 imm434/ura3::1 imm434 | 15 |
| RM1000 | CAI4 Cahis1::hisG/Cahis1::hisG | 39 |
| COU13 | CAI4 CaMYO5/Camyo5::hiG-URA3-hisG | This study |
| COU42 | CAI4 Camyo5::hisG/Camyo5::hisG-URA3-hisG | This study |
| COU46 | CAI4 Camyo5::hisG/Camyo5::hisG | This study |
| COU73 | CAI4 Camyo5::hisG/Camyo5 (CaMYO5) | This study |
| COU186 | CAI4 Camyo5::hisG/Camyo5 (CaMYO5-GFP) | This study |
| COU190 | CAI4 Camyo5::hisG/Camyo5 (Camyo5S366A) | This study |
| COU201 | CAI4 Camyo5::hisG/Camyo5 (Camyo5S366D) | This study |
| COU232 | CAI4 Camyo5::hisG/Camyo5 (Camyo5S366D-GFP) | This study |
| COU243 | CAI4 Camyo5::hisG/Camyo5 (Camyo5S366A-GFP) | This study |
| CLJ4 | CAI4 Cacla4::hisG/Cacla4::hisG | 28 |
| S. cerevisiae HA31-9c | MATacan1-100 ade2-1 his3-11 leu2-3, 112 ura3-1 trp1-1 myo3::HIS3 myo5::TRP1 | 18 |
The CaMYO5, CaMYO5S366A, CaMYO5S366D, CaMYO5-GFP, CaMYO5S366A-GFP, and CaMYO5S366D-GFP alleles were integrated into the Camyo5/Camyo5 Ura− mutant. First, pU67, pU77, pU78, pU93, pU97, and pU98 were linearized with BglII and transformed by the lithium acetate method into COU46 (COU42 Ura−). The integration of each of these CaMYO5 alleles at the CaMYO5::hisG locus was verified by Southern blot analysis. The overexpressed CaMYO5S366A and CaMYO5S366D alleles were integrated into the Cacla4/Cacla4 Ura− mutant (CLJ4) (28). For this purpose, 10 μg each of pU95 and pU96 was linearized with XhoI and transformed by the lithium acetate method into CLJ4. The integration of these alleles at the PCK1 locus was also verified by Southern blot analysis.
To determine functional complementation of the CaMYO5 gene in the S. cerevisiae myo3 myo5 mutant strain, pRS316/426, pVL62 (MYO5 in pRS316; a kind gift from Cunle Wu), pU47, and pU48 were transformed into HA31-9c (18). Single colonies were streaked on 1% yeast extract-2% peptone-2% d-glucose (YPD) plates and grown at either 30 or 37°C for 3 days.
Phenotypic analyses.
Media used for the hypha induction experiments were as follows: YPD supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco BRL), 2% agar supplemented with 10% FBS, Spider medium (1% nutrient broth, 1% mannitol, 0.2% potassium phosphate [pH 7.2]), SLAHD medium (16), and Lee's medium (30).
Strains were grown in YPD overnight, and 30 μl of a 10−5 dilution was spread on one-sixth of a plate (agar-FBS, Spider, SLAHD, or Lee's medium). Plates were incubated for 4 days at 37°C. Colonies were visualized by using an inverted Nikon TMS microscope with a ×2 objective and a ×10 projection lens. Strains from the overnight cultures were also diluted to 1:20 in YPD supplemented with 10% fetal calf serum and were incubated for different times at 37°C. Cells were visualized by Nomarski optics by using an upright Leitz Aristoplan microscope with a ×40 objective or a ×100 immersion oil objective and a ×10 projection lens. For testing the phenotypes of the derived cla4 deletion strains, these and wild-type strain SC5314 were grown overnight in YPD or YPCa (1% yeast extract-2% peptone-2% Casamino Acids). Cultures were diluted to 1:20 in YPD or YPCa, respectively, supplemented with 10% FBS and were incubated for 3 h at 37°C.
Doubling times for each strain were determined starting from three independent colonies grown overnight in YPD and diluted to an optical density at 600 nm (OD600) of 0.1 at the beginning of the time course. The OD600 values were determined every hour for up to 7 h.
Fluorescence.
Overnight cultures in YPD of the various strains were diluted to 1:20 in either YPD or YPD with 10% FBS and grown for 3 h at 30 or 37°C, respectively. For GFP fluorescence, cells were washed once in phosphate-buffered saline (PBS) and mounted on slides for visualization with the appropriate filter by epifluorescence microscopy by using an upright Leitz Aristoplan microscope with a ×100 immersion oil objective and a ×10 projection lens. For 4′,6′-diamino-2-phenylindole (DAPI; Sigma) and Calcofluor White (Sigma) staining, cells were fixed in 70% ethanol for 20 min and washed once with PBS. Cells were then stained for 5 to 10 min with either a 1:1,000 dilution of DAPI at 1 mg/ml or a 1:1,000 dilution of Calcofluor White at 1 mg/ml. Cells were washed extensively with PBS before visualization with the appropriate filter. For rhodamine-phalloidin (Molecular Probes, Eugene, Oreg.) staining, cells were fixed in medium with 3.7% formaldehyde (final concentration) for 30 min. After the cells were resuspended in 50 mM potassium phosphate buffer (pH 6.6) (PK buffer), they were fixed with 3.7% formaldehyde for 1 h. After one wash in PK buffer, cells were incubated for 30 min in PK buffer containing 0.1% Triton X-100. Cells were washed twice with PBS and incubated with a 1:10 dilution of rhodamine-phalloidin (200 U/ml) in PBS overnight at 4°C. After the supernatant was removed, cells were resuspended in PBS and visualized directly with the appropriate filter. DAPI staining was done at the last step with no further washing.
Video microscopy.
Overnight cultures of COU73, COU186, COU201, COU232, and SC5314 were diluted to1:1,600 in YPD supplemented with 10% FBS. Live video microscopy was done at 37°C by using a Leica DM-IRB inverted microscope equipped with a Ludl motorized stage, a temperature-controlled Δt culture dish system (Bioptechs), and a Sensys charge-coupled device camera. Pictures were taken with a ×40 objective and a ×10 projection lens every 15 min and in five different locations. Lengths of hyphae at different time points for more than 25 hyphae per strain were measured by using Openlab 3.1 software. The hyphal growth rate distribution for each strain was then determined. t tests were performed to determine the difference of means for COU73 versus COU201 and for COU186 versus COU232. The means were found to be significantly different in each set (P < 10−6).
Protein extracts and Western blot analysis.
Overnight cultures of COU186, COU232, COU243, and SC5314 grown in YPD were diluted to 1:100 in fresh YPD and grown to late exponential phase (OD600, 2 to 3). Cells were centrifuged and frozen as a pellet at −80°C. Cells were subsequently lysed by vortexing with glass beads 10 times for 15 s each time at 4°C in lysis buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 10 mM EDTA, 0.1% Triton X-100) containing protease inhibitors (10 mM Pefabloc, 50 μg of pepstatin A/ml, 50 μg of E64/ml, and 50 μg of aprotinin/ml) (Boehringer Mannheim). Lysates were cleared by centrifugation for 2 min at 10,000 × g. Protein extracts (50 μg) were loaded on a 6% acrylamide gel and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were then transferred to a nitrocellulose membrane (Bio-Rad) for Western blot analysis with a 1:1,000 dilution of anti-GFP polyclonal antibody (a kind gift from B. Massie) followed by a 1:2,000 dilution of anti-rabbit horseradish peroxidase-conjugated immunoglobulin G (Santa Cruz Biotechnology). GFP fusion proteins were detected by enhanced chemiluminescence (Roche Diagnostics GmbH).
RESULTS
A single gene encodes myosin I in C. albicans.
A search of the C. albicans sequence database (Stanford University) identified a single gene, CaMYO5, that encodes myosin I in C. albicans. In contrast, two such genes, MYO3 and MYO5, are found in S. cerevisiae (18). The presence of a single myosin I-encoding gene was confirmed by low-stringency Southern blotting of C. albicans genomic DNA probed with a sequence corresponding to the SH3 domain of CaMYO5 (data not shown). CaMYO5 was amplified from genomic DNA prepared from wild-type strain SC5314 by PCR with specific oligonucleotides designed according to the sequence in the database. The PCR products were cloned, and four independent clones were sequenced to confirm their identities. The amino acid sequence was aligned with those of the Myo3 and Myo5 proteins of S. cerevisiae. On average, the amino acid sequence of CaMyo5 is 40% identical with those of Myo3 and Myo5. The highest conservation is found for residues in the N-terminal head domain involved in motor activity and including the TEDS-rule site (data not shown).
To determine if CaMYO5 is a functional homologue of the S. cerevisiae MYO3 and MYO5 genes, the C. albicans gene was introduced into S. cerevisiae strain HA31-9c (18), which is temperature sensitive due to deletion of the MYO3 and MYO5 genes. This strain could grow at the restrictive temperature when carrying MYO5 on a centromeric plasmid (pVL62) but not when carrying the vector alone (pRS316) (data not shown). When this strain carried the CaMYO5 gene on a multicopy plasmid, it could grow at the restrictive temperature, but it barely grew when carrying CaMYO5 on a centromeric plasmid (data not shown). Thus, the overexpression of CaMYO5 can complement the growth defect of myo3 myo5 strains.
Myosin I is not essential for viability in C. albicans.
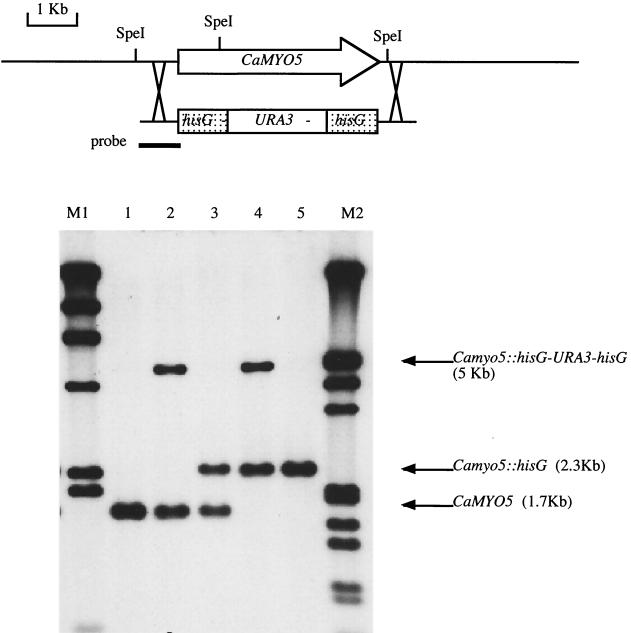
To study the role of myosin I in C. albicans, we created a strain in which both copies of CaMYO5 were deleted (Δ/Δ Camyo5). Figure 1 shows the sequential disruption of both alleles of CaMYO5 in C. albicans (for details, see Materials and Methods). A single Δ/Δ Camyo5 transformant was obtained starting from strain CAI4 (15), and 19 Δ/Δ Camyo5 transformants were obtained starting from strain RM1000 (39). The latter strain is auxotrophic for histidine as well as uracil, enabling the disruption of each allele with a different marker (CaHIS1 and CaURA3). These 20 Δ/Δ Camyo5 mutants displayed similar phenotypes, as described below. The ability to generate viable double-mutant strains indicates that the myosin I gene is not an essential gene in C. albicans.
FIG. 1.
Sequential disruption of both alleles of CaMYO5. Genomic DNA from strains was digested with SpeI and analyzed by Southern blotting with a probe encompassing the 5′ noncoding region of CaMYO5 (thick line). Lanes: 1,CAI4 (wild type; Ura−); 2, CaMYO5/Camyo5::hisG-URA3-hisG; 3, CaMYO5/Camyo5::hisG; 4, Camyo5::hisG-URA3-hisG/Camyo5::hisG; 5, Camyo5::hisG/Camyo5::hisG; M1, bands of 23, 9.4, 6.6, 4.4, 2.3, and 2.0 kb; M2, bands of 21, 5.1, 5.0, 4.3, 3.5, 2.0, 1.9, 1.6, 1.4, 0.9, and 0.8 kb.
Myosin I is required for the polarized distribution of cortical actin patches.
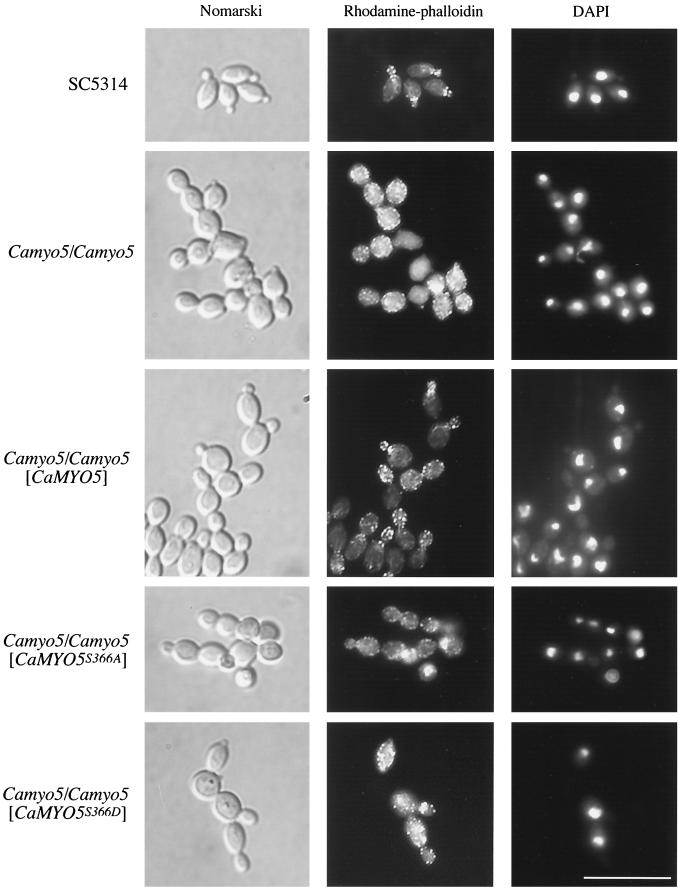
We examined cells of the Δ/Δ Camyo5 mutant and the wild-type strain (SC5314) microscopically for phenotypes that would indicate the role played by myosin I in budding yeast cells. Cells of the Δ/Δ Camyo5 mutant viewed by Nomarski optics looked abnormally round, in some instances enlarged, and were clumped compared to wild-type cells (Fig. 2). To determine whether cortical actin patches were mislocalized in the Δ/Δ Camyo5 mutant, we treated cells grown exponentially with rhodamine-phalloidin to stain filamentous actin. Localization of cortical actin patches in budding cells of S. cerevisiae and C. albicans has been described extensively (4, 44). In summary, cortical actin patches localize to the growing bud tip, redistribute evenly in an isotropic growing bud, and finally localize at the mother bud neck after cytokinesis. In our experiments, cortical actin patches in 69% of wild-type cells were entirely localized to the buds during early bud emergence and growth (Fig. 2 and Table 3). These patches remained in the buds of 18% of cells during and after nuclear division. These patches also were localized at the mother bud neck in 10% of cells. Strikingly, for the Δ/Δ Camyo5 mutant, only 3% of cells retained cortical actin patches exclusively in the buds. In this mutant, cortical actin patches were localized evenly in both the mother cell and the buds for 44% of cells before nuclei had divided and in 31% of cells during nuclear division. Finally, in 7% of cells, the patches were localized at the mother bud neck while also localizing in daughter and mother cells. Overall, cortical actin patches are dramatically mislocalized in the Δ/Δ Camyo5 mutant, suggesting that myosin I plays a role in the organization of the actin cytoskeleton.
FIG. 2.
Cortical actin patch patterns in wild-type and mutant cells. Cells were fixed and stained with rhodamine-phalloidin. Nuclear DNA was stained with DAPI. Scale bar, 10 μm.
TABLE 3.
Cortical actin patch patterns in yeast cellsa
DAPI, nuclear staining; RP, rhodamine phalloidin, F-actin staining. Polarized in bud, cortical actin patches localize exclusively in bud; loose, cortical actin patches localize preferentially in bud but also in mother cell; depolarized, cortical actin patches localize equally in bud and mother cell; nuclear division, nuclei dividing; bud neck, cortical actin patches localize exclusively at neck; bud neck, loose, cortical actin patches localize at bud neck preferentially but also in daughter and mother cells; cytokinesis, nuclei have completed division, and mother and daughter cells are about the same size; G1, no apparent budding. Scale bar, 1 μm.
Myosin I plays a role in chitin deposition in the cell wall and in the budding pattern.
To determine whether myosin I also plays a role in cell wall biogenesis in C. albicans, cells of the mutant and wild-type strains were labeled for chitin with Calcofluor White. In the mutant, a small proportion of cells that were unusually enlarged and round showed aberrant chitin deposition in the cell wall (Fig. 3). These findings were not observed in the wild-type strain. At the same time, we found that nearly 20% of mutant cells exhibited a random budding pattern; the value for wild-type cells was 2% (Table 4). Mutations in actin or other cytoskeletal proteins lead to a high frequency of random budding patterns in diploid S. cerevisiae cells (11, 60). The higher incidence of random budding in the Δ/Δ Camyo5 mutant than in the wild-type strain correlate with these data, suggesting that the actin-dependent mechanisms for bud site selection are similar in C. albicans and S. cerevisiae. In addition, C. albicans myosin I may be important for the regulation of cell wall biogenesis, based on the abnormal chitin deposition observed in the cell wall of the mutant.
FIG. 3.
Budding patterns of wild-type and Camyo5/Camyo5 mutant strains. Cells were fixed and stained for chitin with Calcofluor White. (Panel a) Nomarski view. (Other panels) Calcofluor White staining. (a to c) SC5314. (d to f) reintegrant Δ/Δ (Camyo5) . Arrows indicate cells exhibiting random budding. In the large panel (Camyo5/Camyo5), the enlarged cell in the center shows abnormal chitin deposition in its cell wall. Scale bar, 4 μm.
TABLE 4.
Budding patternsa
Cells with more than two buds and/or bud scars were counted. Scale bar, 1 μm.
Reintegration of wild-type myosin I in the null mutant leads to the recovery of wild-type characteristics.
To determine whether the phenotypes of the mutant strain are due to the deletion of both copies of myosin I, the wild-type CaMYO5 gene was reintegrated at one of the CaMYO5 loci in a Ura− strain (see Materials and Methods). Six transformants or “reintegrant” strains were thus obtained, and all exhibited normal cell shape. One reintegrant (COU73) was selected for more detailed analysis. This reintegrant exhibited normal cell shape (Fig. 2), cortical actin patch distribution (Fig. 2 and Table 3), and budding pattern (Fig. 3 and Table 4). Thus, the deletion of myosin I alone is responsible for the phenotypes of the Δ/Δ Camyo5 strain.
Myosin I is required for the formation of hyphae on solid and in liquid media.
We examined the ability of the Δ/Δ Camyo5 mutant to form hyphae under different hypha-inducing conditions. The Δ/Δ Camyo5 and wild-type (SC5314) strains were plated for single colonies on agar containing 10% FBS, Spider medium, SLAHD medium, or Lee's medium and were grown for 4 to 5 days at 37°C. The wild-type strain could form extensive hyphae on all media (Fig. 4A and data not shown). In contrast, the Δ/Δ Camyo5 strain did not form hyphae on any medium (Fig. 4A and data not shown). Occasional extensions could be observed on parts of a Δ/Δ Camyo5 colony grown on agar containing 10% FBS, but microscopic examination showed that these were formed by pseudohyphal cells (data not shown). To confirm that the deletion of myosin I alone was the cause of this nonhyphal phenotype, we tested the ability of the reintegrant strain to form hyphae under these conditions. This strain could form hyphae to the same extent as wild-type strain SC5314 (Fig. 4A and data not shown). These results indicate that myosin I is required for hypha formation on solid hypha-inducing media.
FIG. 4.
Hypha formation in wild-type and mutant strains. (A) Hypha formation on solid media was determined by plating single colonies of each strain on 10% FBS (a) or Spider medium (b) plates. Plates were incubated for 4 days at 37°C. Scale bar, 1 mm. (B) Hypha formation in liquid YPD supplemented with 10% FBS was determined at 37°C after 2.5 h of incubation. Scale bar, 10 μm.
To determine cellular morphology under hypha-inducing conditions, cells were also incubated in liquid YPD medium containing 10% FBS or in Lee's medium at 37°C. After 2 to 3 h of incubation, cells of the wild-type strain formed germ tubes that extended into hyphae (Fig. 4B and data not shown). In contrast, cells of the Δ/Δ Camyo5 strain occasionally formed germ tubes but were unable to form hyphae after more than 6 h of incubation in YPD medium containing serum or overnight incubation in Lee's medium, by which time the wild-type and reintegrant strains had formed extensive hyphae (data not shown). Pseudohyphal cells could be observed in the Δ/Δ Camyo5 mutant instead (Fig. 4B). Because the Δ/Δ Camyo5 strain could form pseudohyphae but not hyphae, it is possible that myosin I is required for maintaining polarized growth but not for initiating it.
Myosin I localizes to the bud and hyphal tips and partially colocalizes with cortical actin patches.
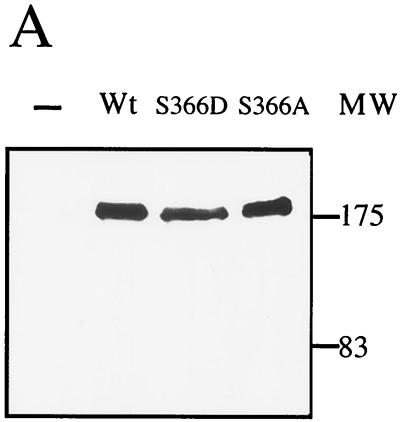
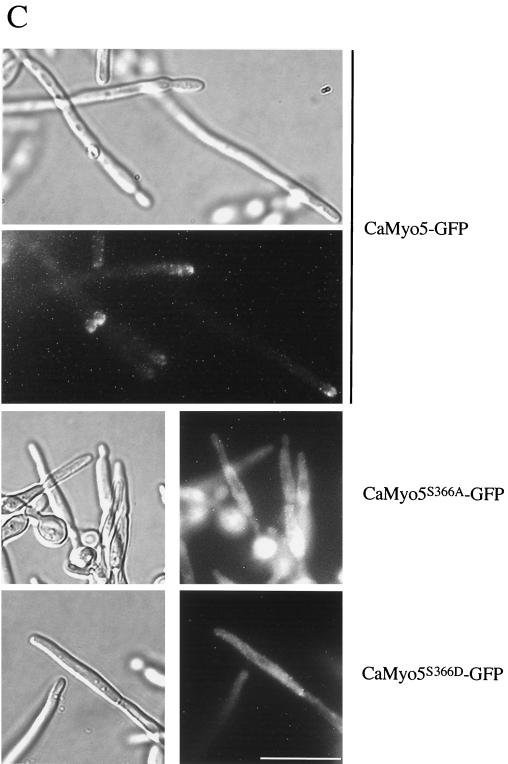
To determine the localization of myosin I in C. albicans, we fused in frame the sequence encoding GFP (38) at the 3′ end of the CaMYO5 open reading frame. We then introduced CaMYO5-GFP into the Ura− Δ/Δ Camyo5 strain, such that CaMyo5-GFP was the sole source of myosin I. Two independent transformants that were examined could form hyphae in the presence of serum. The CaMyo5-GFP protein in these transformants was detected by Western blot analysis as a band of 175 kDa (Fig. 5A). These results suggest that CaMyo5-GFP functions properly in the Δ/Δ Camyo5 strain. Figure 5B shows the localization of CaMyo5-GFP in exponentially growing cells. CaMyo5-GFP clearly localized in patches at the tip of emerging buds. CaMyo5-GFP localization could also be observed at the mother bud neck after nuclear division was completed (Fig. 5B and data not shown). The localization of CaMyo5-GFP in hyphal cells was examined as well (Fig. 5C). CaMyo5-GFP localized in patches at the hyphal tip. The patch-like distribution of CaMyo5-GFP in budding and hyphal cells was similar to that of actin in cortical patches. To determine whether CaMyo5-GFP colocalizes with actin, we stained yeast cells and hyphal cells with rhodamine-phalloidin (Fig. 6). In most of the cells examined (108 of 120), there was either very good colocalization or partial colocalization of CaMyo5-GFP and cortical actin patches (two or more spots coinciding) either at the bud or hyphal tips or at the mother bud neck or septae (Fig. 6 and data not shown).
FIG. 5.
Localization of wild-type and mutant CaMyo5-GFP. (A) Protein extracts (50 μg) prepared from cells expressing CaMYO5-GFP (Wt), CaMYO5S366D-GFP (S366D), or CaMYO5S366A-GFP (S366A) or from SC5314 (−) grown in YPD to late exponential phase were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 6% acrylamide gel. GFP fusion proteins were analyzed by Western blotting. Molecular weight markers (MW; in thousands) are indicated on the right. (B and C) Cells growing exponentially (B) or induced with 10% FBS at 37°C for 3 h (C) were visualized for GFP fluorescence. One of the wild-type hyphal cells moved during exposure, leading to a double exposure. Scale bars, 10 μm.
FIG. 6.
Overlay of rhodamine-phalloidin and GFP signals. Budding and hyphal cells expressing CaMyo5-GFP were fixed and stained with rhodamine-phalloidin (RP) and DAPI. Pictures were taken with the appropriate filters to visualize CaMyo5-GFP (GFP), actin (RP), and nuclei (a). Overlays of the images were created with Adobe Photoshop, version 6.0. Scale bar, 4 μm.
Serine 366 in myosin I is essential for normal growth and hypha formation.
In several organisms, the ATPase activity of myosin I is increased by phosphorylation of a conserved serine residue in the head domain (10, 31, 56). In S. cerevisiae, this phosphorylation is catalyzed by activated Ste20 and Cla4 kinases (57). Ste20 and Cla4 are members of the PAK family of protein kinases which may be activated by Cdc42p and function to regulate cell morphology (26, 27). To determine whether the phosphorylation of myosin I by C. albicans PAK kinases is important for hypha formation, we created mutant alleles of CaMYO5 that code for alanine or aspartate instead of serine in the unique PAK phosphorylation site (serine 366). These alleles were reintroduced into the Δ/Δ Camyo5 strain at the Camyo5::hisG loci (see Materials and Methods). Proper integration and expression of these alleles in several transformants were confirmed by Southern and Northern blot analyses, respectively (data not shown).
To determine whether Camyo5S366D and Camyo5S366A can rescue the phenotypes of the Δ/Δ Camyo5 strain, we measured the growth rates of strains carrying these alleles and characterized their budding patterns. We found that the wild-type strain and the strain expressing Camyo5S366D (COU201) divided every 55 min at 37°C in 2× YPD (Table 5). In contrast, the Δ/Δ Camyo5 strain and the strain expressing Camyo5S366A (COU190) divided every 69 min under the same growth conditions (Table 5). We also found that the S366A mutation increased random budding 10-fold, similar to the complete deletion of myosin I, while the S366D mutation increased random budding 5-fold (Table 4). Although there was no abnormal chitin deposition in cells expressing Camyo5S366D, abnormal chitin staining could be observed in cells expressing Camyo5S366A (data not shown). These results suggest that the phosphorylation of serine 366 is important for optimal growth and for cell wall biogenesis. Furthermore, phosphorylation of serine 366 may also be important, albeit to a lesser extent, for localization of the bud site. We also observed that the mutant expressing Camyo5S366D did not form hyphal cells in YPD (Fig. 2), suggesting that other components of the actin cytoskeleton need to be activated to allow hypha formation under non-hypha-inducing conditions.
TABLE 5.
Doubling times
| Strain | Genotype | Doubling time (min, mean ± SD)a |
|---|---|---|
| SC5314 | CaMYO5/CaMYO5 | 54.3 ± 1.5 |
| COU42 | Camyo5/Camyo5 | 68.2 ± 2.0 |
| COU73 | Camyo5/Camyo5 (CaMYO5) | 54.8 ± 1.3 |
| COU190 | Camyo5/Camyo5 (CaMYO5S366A) | 69.6 ± 2.0 |
| COU201 | Camyo5/Camyo5 (CaMYO5S366D) | 57.7 ± 3.0 |
Calculated from three independent colonies per strain.
The mutant strains were plated for single colonies on agar containing 10% FBS and on Spider medium to determine their ability to form hyphae. All six transformants that expressed Camyo5S366D formed fuzzy colonies similar to those of the wild-type and reintegrant strains (Fig. 4A). Under liquid hypha-inducing conditions, these cells were able to form hyphae as well. However, the numbers and rates of growth of the hyphae formed were lower than those of the wild-type strain (Fig. 4B and Table 6). The majority of seven transformants that expressed Camyo5S366A formed fuzzy colonies with much shorter fringes than the wild-type and reintegrant strains on agar containing 10% FBS, but on the whole periphery of the colony, unlike the Δ/Δ Camyo5 strain (Fig. 4A). On Spider medium, the colonies of the strain expressing Camyo5S366A were consistently larger than those of the wild-type and Δ/Δ Camyo5 strains. Microscopic examination revealed that colonies of this strain consisted of pseudohyphal cells (data not shown). In liquid YPD supplemented with 10% FBS or in Lee's medium, cells of the strain expressing Camyo5S366A were unable to form hyphae but formed pseudohyphae to a greater extent than cells of the Δ/Δ Camyo5 strain (Fig. 4B and data not shown). These results suggest that CaMyo5S366A still retains some activity sufficient for more uniform pseudohyphal growth. Overall, the phosphorylation of serine 366 appears to be important for the proper function of CaMyo5 during hyphal growth.
TABLE 6.
Hyphal growth ratesa
| Strain | Genotype | Growth rate (μm/h) | No. of hyphae |
|---|---|---|---|
| SC5314 | Wild type | 10.5 | 26 |
| COU73 | Camyo5/Camyo5 (CaMYO5) | 11.7 | 29 |
| COU186 | Camyo5/Camyo5 (CaMYO5-GFP) | 11.1 | 28 |
| COU201 | Camyo5/Camyo5 (Camyo5S366D) | 7.7 | 29 |
| COU232 | Camyo5/Camyo5 (CaMYO5S366D-GFP) | 8.4 | 30 |
The differences in the means for the hyphal growth rates for COU73 versus COU201 and for COU186 versus COU232 were significant at a P value of <10−6.
To determine whether the TEDS-rule site serine 366 is important for the localization of myosin I, we tested the localization of CaMyo5S366A-GFP and CaMyo5S366D-GFP in normal cells and elongated pseudohyphal or hyphal cells. These proteins were also detected by Western blot analysis as 175-kDa bands, and their levels were similar to the CaMyo5-GFP protein level (Fig. 5A). We found that the majority of CaMyo5S366A-GFP did not localize in cortical actin patches but localized in the cytoplasm (Fig. 5B and C). The majority of CaMyo5S366D-GFP did not localize to cortical actin patches either (Fig. 5B and C). Occasionally, some patches could be observed at the tips of buds, but these were not observed at hyphal tips. Mainly cytoplasmic staining and minor punctate staining at the periphery could be observed in these hyphae. These results suggest that serine 366 is important for the proper localization of myosin I and, surprisingly, that the proper localization of myosin I at the hyphal tip is not required for hyphal growth.
Polarized distribution of cortical actin patches is dependent on serine 366.
Because myosin I appeared mislocalized in the phosphorylation site mutants, we also determined the localization of cortical actin patches in these strains. Cortical actin patches were dramatically mislocalized to the mother cells (Fig. 2). The cortical actin patch distribution in cells expressing Camyo5S366D was quantified more precisely (Table 3). Cortical actin patches were localized uniquely in the buds in only 7% of cells. They were also distributed in both the buds and the mother cell in 42% of cells prior to nuclear division and in 21% of cells after nuclear division. Finally, in 8% of cells, cortical actin patches were localized at the mother bud neck, while in most of these cells, they also were localized in the daughter and mother cells. Overall, cortical actin patches are mislocalized when serine 366 is mutated to alanine or aspartate, and the patterns of actin distribution are similar to those of the Δ/Δ Camyo5 strain.
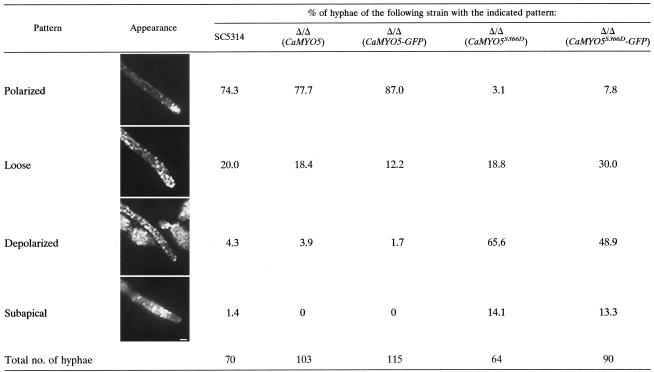
We also compared cortical actin patch distribution in hyphal cells expressing Camyo5S366D, in pseudohyphal cells of both the Δ/Δ Camyo5 strain and the strain expressing Camyo5S366A, and in wild-type hyphal cells. In wild-type hyphal cells of C. albicans, cortical actin patches always localize at the tip throughout hyphal elongation (4). In contrast, cortical actin patch distribution in pseudohyphal cells of S. cerevisiae and C. albicans resembles that found in yeast cells (11) (data not shown). Surprisingly, we found that cortical actin patches localized evenly throughout pseudohyphal cells of the Δ/Δ Camyo5 strain and strains expressing Camyo5S366A and Camyo5S366D (Fig. 7 and data not shown). In these mutants, very few tips of emerging pseudohyphal cells contained more cortical actin patches than the mother cells, in accordance with cortical actin patch patterns in yeast cells (data not shown). Surprisingly also, we found that cortical actin patches were localized throughout 66% of hyphal cells expressing Camyo5S366D (Fig. 7 and Table 7). These patches localized at the tip of only 3% of hyphal cells in this mutant, while they localized at the tip of 75% of wild-type hyphal cells (Table 7). In addition, 14% of mutant hyphal cells showed an abnormal actin patch distribution rarely seen in wild-type cells. In these cells, cortical actin patches were found behind the hyphal tip (subapical). Overall, cortical actin patches were depolarized in the hyphae of the strain expressing Camyo5S366D. These data indicate that cortical actin patch distribution is dependent on serine 366 in myosin I and suggest that a “tip-high” localization of cortical actin patches is not required for hyphal growth. Moreover, there is a strong correlation between myosin I localization and cortical actin patch distribution, because the strain expressing CaMYO5S366D-GFP also exhibited a depolarized cortical actin patch distribution in 49% of hyphal cells; this value was 2% for the strain expressing CaMYO5-GFP (Table 7).
FIG. 7.
Cortical actin patch patterns in wild-type and mutant hyphal and pseudohyphal cells. Hypha formation was induced with 10% FBS at 37°C for 3 h. Cells were then fixed and stained with rhodamine-phalloidin. The Nomarski image and the corresponding rhodamine-phalloidin image are shown for each strain. Scale bar, 10 μm.
TABLE 7.
Cortical actin patch patterns in hyphaea
Polarized, cortical actin patches localize exclusively at the hyphal tip; loose, cortical actin patches localize preferentially at the tip but also throughout the hyphae; depolarized, cortical actin patches localize throughout the hyphae; subapical, cortical actin patches localize beneath the tip. Scale bar, 1 μm.
DISCUSSION
We used C. albicans as a model system to study polarized growth because this yeast is a human pathogen and can switch from the yeast form to the hyphal form under defined conditions. Moreover, through genetic screens and manipulations, several components have been identified that reveal aspects of the mechanisms involved in this transition (8, 55). The structural roles of the actin cytoskeleton and associated regulatory proteins are critical to hypha formation; mutations affecting these components have dramatic effects on polarized growth in different organisms (24, 41, 44). For instance, a homologue of a cortical actin patch component in S. cerevisiae, CaSla2p, is required for hypha formation in C. albicans (5).
Several observations reported here support a role for myosin I during polarized growth in C. albicans. First, cells with a deletion of for myosin I (Δ/Δ Camyo5 strain) can form buds, but they are abnormally round and some are enlarged. Moreover, the Δ/Δ Camyo5 strain fails to form true hyphae under hypha-inducing conditions. Second, CaMyo5-GFP in wild-type cells colocalizes with cortical actin patches at sites of polarized growth at the tips of buds and of hyphae. This localization pattern has been found for Myo5p and Myo3-GFP in S. cerevisiae and for MyoA-GFP in Aspergillus (3, 14, 59). Finally, while buds of wild-type S. cerevisiae and C. albicans strains grow apically initially and have localized actin patches at the tips (4, 44), the Δ/Δ Camyo5 strain exhibits mislocalized cortical actin patches. In this mutant, cortical actin patches are largely dispersed throughout the bud as well as in the mother cell. This distribution could account for excessive isotropic growth resulting in the round and enlarged shape of the mutant cells. Overall, these results suggest that C. albicans myosin I is required for polarized cortical actin patch localization and are consistent with the role of myosin I in other organisms (3, 18, 32, 48).
We made several surprising observations relative to pseudohyphal and hyphal cells of C. albicans, suggesting that some differences in the mechanisms controlling polarized growth may exist between C. albicans and other organisms, including S. cerevisiae. First, we found that myosin I is not essential and does not significantly impair growth in C. albicans. In S. cerevisiae, the two redundant genes encoding myosin I are either essential or important for growth, depending on the strain background (18, 57). Second, we found that myosin I is not required for pseudohypha formation in C. albicans, in contrast to the situation for Aspergillus, where deletion of myoA causes the complete inability to form polarized structures (36). Third, pseudohyphal cells of the Δ/Δ Camyo5 mutant show mislocalized cortical actin patches, in contrast to S. cerevisiae, where pseudohypha formation requires a highly polarized actin cytoskeleton (11). This delocalized pattern of cortical actin patch distribution is also observed in the majority of hyphal cells of the mutant expressing Camyo5S366D. This finding could explain the slow growth of these hyphae. Finally, we found that localization of myosin I to tips is not required for hypha formation, because hyphal cells were observed in the absence of polarized localization of myosin I in the strain expressing Camyo5S366D. Thus, the formation of pseudohyphae and hyphae does not necessarily depend on polarized cortical actin patch and myosin I localization. However, treatment with cytochalasin A of cells of the wild-type, the Δ/Δ Camyo5 strain, and the mutant expressing Camyo5S366D inhibits germ tube formation (data not shown). These results suggest that some form of the actin cytoskeleton is essential for polarized growth.
To explain the formation of elongated cells despite the lack of cortical actin patch polarization, we suggest several possibilities. Perhaps only actin patches localizing at the hyphal tips of the mutant expressing Camyo5S366D are functional. This would be the case if cortical actin patch components required for patch function preferentially localize at the hyphal tips of the mutant. Together with the small proportion of CaMyo5S366D also localizing at the tips, this distribution may be sufficient to direct the growth of the hyphal form. On the other hand, the absence of myosin I and cortical actin patch components whose localization is dependent on myosin I may explain the hyphal defect of the Δ/Δ Camyo5 mutant. In this regard, it would be interesting to determine the localization of key cortical actin patch components required for patch function in the various myosin I mutants. At least one cortical actin patch component, cofilin, is not found associated with actin patches in an S. cerevisiae myosin I mutant (48). Alternatively, other forms of actin that may specify polarized growth in C. albicans, such as actin cables, could be dependent on myosin I. The S. cerevisae myo3 myo5 mutant shows defects in the organization of actin cables (18). It is therefore possible that the absence of hypha formation in the Δ/Δ Camyo5 mutant can be explained by a defect in the organization of actin cables. Although the polarized localization of cortical actin patches is dependent on the presence of actin cables in S. cerevisiae and S. pombe (43, 45), the orientation of the cables may be restored and allow the formation of slowly growing hyphae in the mutant expressing Camyo5S366D in the absence of localized cortical actin patches. The importance of actin cables during polarized growth in C. albicans is presently uncertain, as we could not directly observe cables in the myosin I mutants, possibly because these were masked by cortical actin patches. Finally, it has been suggested that the transport of secretory vesicles occurs by dual microtubule- and actin-based systems (17). It is possible that when the actin-based system is impaired, vesicles are transported by a microtubule-based system.
Regulation of C. albicans myosin I function.
We suggest that serine 366 of C. albicans myosin I is phosphorylated and that phosphorylation is required for the activity of myosin I, either by controlling the actin-dependent ATPase activity of the motor (56) or by controlling the interaction of myosin I with actin. That serine 366 is subject to phosphorylation is consistent with previous studies and is based on mutant phenotypes produced by mutations affecting this residue. Previous studies demonstrated that the phosphorylation of the TEDS-rule site in myosin I of different organisms is important for function. In one particular S. cerevisiae strain background (W303α), the phosphorylation of serine 357 in myosin I is essential (57). In A. nidulans, an S371E mutation introduces a residue that mimics a phosphorylated serine and leads to the accumulation of membranes in growing hyphae as a result of the hyperactivation of endocytosis. In contrast, an S371A mutation does not drastically affect this process (58). However, both of these mutations affect other aspects of cell growth and morphogenesis to the same extents, suggesting that the phosporylation of serine 371 per se does not regulate all functions of myosin I. In Dictyostelium, serine 332 in myoB is essential for function, although it is not clear if phosphorylation plays a critical role (42). In C. albicans, an S366A mutation produces a slow-growth phenotype similar to that of the Δ/Δ Camyo5 strain, while an S366D mutation does not impair growth in the yeast form. These data are analogous to the function of serine 361 in myosin I of fission yeast, where mutation to an alanine does not rescue the growth defect produced by a myosin I deficiency, but mutation to an aspartate does (53). Finally, a C. albicans myosin I mutant allele with the S366A mutation also does not allow the formation of hyphae, while the S366D mutation restores significant hyphal growth in response to hypha-inducing conditions. However, it appears that myosin I with aspartate 366 is less functional than wild-type myosin I. As discussed above, the S366D mutation results in the cytoplasmic localization of myosin I, as observed for myosin I fused to GFP. Moreover, the S366D mutation does not restore the polarized distribution of cortical actin patches in budding or hyphal cells. These results suggest that myosin I localization is required for the polarized distribution of cortical actin patches but is not a prerequisite for polarized growth.
To determine if the CaCla4 PAK kinase regulates the activity of myosin I by phosphorylating serine 366, we assessed whether the hyphal defect of the Δ/Δ Cacla4 mutant can be overcome by overexpressing the phosphorylation mimic allele of myosin I, CaMYO5S366D. We detected no major morphological differences between the Δ/Δ Cacla4 mutants expressing wild-type levels of CaMYO5 or overexpressing CaMYO5S366D under hypha-inducing conditions (data not shown). Based on observations with S. cerevisiae, where the expression of Myo3S357D could not overcome the lethality caused by the deletion of both STE20 and CLA4 (57), our results suggest that other targets of CaCla4 must be activated to bypass the hyphal defect of the Δ/Δ Cacla4 mutant.
Mechanism of C. albicans myosin I function.
Overall, it appears that the molecular mechanism by which myosin I acts in C. albicans is well conserved, as are its putative interacting partners, such as the Arp2/3 complex components (http://alces.med.umn.edu/Candida.html) (54). First, as in S. cerevisiae (3), in C. albicans myosin I appears not to be required for cortical actin patch formation but is required for the polarized localization of cortical actin patches in the bud and hyphal tips. Hence, C. albicans myosin I may regulate the activity of the Arp2/3 complex and target the polymerization of cortical actin patches to sites where it itself localizes. This interaction may be particularly critical for true hypha formation and may be regulated by phosphorylation of the TEDS-rule site by a homologue of the PAK kinases. We are currently investigating the role of serine 366 in the interaction of C. albicans myosin I with other proteins modulating actin polymerization.
Acknowledgments
We thank Daniel Dignard for the sequence alignment, Bernard Massie for antibodies, Josée Ash and Cunle Wu for plasmids, Catherine Bachewich for oligonucleotides, Doreen Harcus for help with processing C. albicans, and Jürg Oberholzer for analysis of data. We also thank Stanford University for making the C. albicans myosin I sequence available from the database. We are especially grateful to Catherine Bachewich, Cunle Wu, and Bill Zerges for critical reading of the manuscript. Finally, we thank members of our laboratories for fruitful discussions.
U.O. was the recipient of Swiss National Foundation and NSERC visiting fellowships.
Footnotes
NRCC publication 44811.
REFERENCES
- 1.Adams, A. E., and J. R. Pringle. 1984. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 98:934-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akashi, T., T. Kanbe, and K. Tanaka. 1994. The role of the cytoskeleton in the polarized growth of the germ tube in Candida albicans. Microbiology 140:271-280. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, B. L., I. Boldogh, M. Evangelista, C. Boone, L. A. Greene, and L. A. Pon. 1998. The Src homology domain 3 (SH3) of a yeast type I myosin, Myo5p, binds to verprolin and is required for targeting to sites of actin polarization. J. Cell Biol. 141:1357-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, J. M., and D. R. Soll. 1986. Differences in actin localization during bud and hypha formation in the yeast Candida albicans. J. Gen. Microbiol. 132:2035-2047. [DOI] [PubMed] [Google Scholar]
- 5.Asleson, C. M., E. S. Bensen, C. A. Gale, A. S. Melms, C. Kurischko, and J. Berman. 2001. Candida albicans INT1-induced filamentation in Saccharomyces cerevisiae depends on Sla2p. Mol. Cell. Biol. 21:1272-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagrodia, S., and R. A. Cerione. 1999. Pak to the future. Trends Cell Biol. 9:350-355. [DOI] [PubMed] [Google Scholar]
- 7.Bement, W. M., and M. S. Mooseker. 1995. TEDS rule: a molecular rationale for differential regulation of myosins by phosphorylation of the heavy chain head. Cell Motil. Cytoskel. 31:87-92. [DOI] [PubMed] [Google Scholar]
- 8.Brown, A. J., and N. A. Gow. 1999. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 7:333-338. [DOI] [PubMed] [Google Scholar]
- 9.Brzeska, H., and E. D. Korn. 1996. Regulation of class I and class II myosins by heavy chain phosphorylation. J. Biol. Chem. 271:16983-16986. [DOI] [PubMed] [Google Scholar]
- 10.Brzeska, H., B. M. Martin, and E. D. Korn. 1996. The catalytic domain of Acanthamoeba myosin I heavy chain kinase. I. Identification and characterization following tryptic cleavage of the native enzyme. J. Biol. Chem. 271:27049-27055. [DOI] [PubMed] [Google Scholar]
- 11.Cali, B. M., T. C. Doyle, D. Botstein, and G. R. Fink. 1998. Multiple functions for actin during filamentous growth of Saccharomyces cerevisiae. Mol. Biol. Cell 9:1873-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doberstein, S. K., I. C. Baines, G. Wiegand, E. D. Korn, and T. D. Pollard. 1993. Inhibition of contractile vacuole function in vivo by antibodies against myosin-I. Nature 365:841-843. [DOI] [PubMed] [Google Scholar]
- 13.Durrbach, A., K. Collins, P. Matsudaira, D. Louvard, and E. Coudrier. 1996. Brush border myosin-I truncated in the motor domain impairs the distribution and the function of endocytic compartments in an hepatoma cell line. Proc. Natl. Acad. Sci. USA 93:7053-7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evangelista, M., B. M. Klebl, A. H. Tong, B. A. Webb, T. Leeuw, E. Leberer, M. Whiteway, D. Y. Thomas, and C. Boone. 2000. A role for myosin-I in actin assembly through interactions with Vrp1p, Bee1p, and the Arp2/3 complex. J. Cell Biol. 148:353-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gimeno, C. J., P. O. Ljungdahl, C. A. Styles, and G. R. Fink. 1992. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077-1090. [DOI] [PubMed] [Google Scholar]
- 17.Goode, B. L., D. G. Drubin, and G. Barnes. 2000. Functional cooperation between the microtubules and actin cytoskeletons. Curr. Opin. Cell Biol. 12:63-71. [DOI] [PubMed] [Google Scholar]
- 18.Goodson, H. V., B. L. Anderson, H. M. Warrick, L. A. Pon, and J. A. Spudich. 1996. Synthetic lethality screen identifies a novel yeast myosin I gene (MYO5): myosin I proteins are required for polarization of the actin cytoskeleton. J. Cell Biol. 133:1277-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall, A., and C. D. Nobes. 2000. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:965-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heath, I. B., G. Gupta, and S. Bai. 2000. Plasma membrane-adjacent actin filaments, but not microtubules, are essential for both polarization and hyphal tip morphogenesis in Saprolegnia ferax and Neurospora crassa. Fungal Genet. Biol. 30:45-62. [DOI] [PubMed] [Google Scholar]
- 21.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung, G., K. Remmert, X. Wu, J. M. Volosky, and J. A. Hammer III. 2001. The Dictyostelium CARMIL protein links capping protein and the Arp2/3 complex to type I myosins through their SH3 domains. J. Cell Biol. 153:1479-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karpova, T. S., S. L. Reck-Peterson, N. B. Elkind, M. S. Mooseker, P. J. Novick, and J. A. Cooper. 2000. Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Mol. Biol. Cell 11:1727-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kost, B., R. J. Mathu, and N. H. Chua. 1999. Cytoskeleton in plant development. Curr. Opin. Plant Biol. 2:462-470. [DOI] [PubMed] [Google Scholar]
- 25.Kurischko, C., and R. K. Swoboda. 2000. Cytoskeletal proteins and morphogenesis in Candida albicans and Yarrowia lipolytica, p. 173-184. In J. F. Ernst and A. Schmidt (ed.), Dimorphism in human pathogenic and apathogenic yeasts, vol. 5. S. Karger, Basel, Switzerland. [DOI] [PubMed] [Google Scholar]
- 26.Leberer, E., D. Harcus, I. D. Broadbent, K. L. Clark, D. Dignard, K. Ziegelbauer, A. Schmidt, N. A. Gow, A. J. Brown, and D. Y. Thomas. 1996. Signal transduction through homologs of the Ste20p and Ste7p protein kinases can trigger hyphal formation in the pathogenic fungus Candida albicans. Proc. Natl. Acad. Sci. USA 93:13217-13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leberer, E., C. Wu, T. Leeuw, A. Fourest-Lieuvin, J. E. Segall, and D. Y. Thomas. 1997. Functional characterization of the Cdc42p binding domain of yeast Ste20p protein kinase. EMBO J. 16:83-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leberer, E., K. Ziegelbauer, A. Schmidt, D. Harcus, D. Dignard, J. Ash, L. Johnson, and D. Y. Thomas. 1997. Virulence and hyphal formation of Candida albicans require the Ste20p-like protein kinase CaCla4p. Curr. Biol. 7:539-546. [DOI] [PubMed] [Google Scholar]
- 29.Lechler, T., A. Shevchenko, and R. Li. 2000. Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization. J. Cell Biol. 148:363-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, K. L., H. R. Buckley, and C. C. Campbell. 1975. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouradia 13:148-153. [DOI] [PubMed] [Google Scholar]
- 31.Lee, S. F., T. T. Egelhoff, A. Mahasneh, and G. P. Côté. 1996. Cloning and characterization of a Dictyostelium myosin I heavy chain kinase activated by Cdc42 and Rac. J. Biol. Chem. 271:27044-27048. [DOI] [PubMed] [Google Scholar]
- 32.Lee, W. L., M. Bezanilla, and T. D. Pollard. 2000. Fission yeast myosin-I, Myo1p, stimulates actin assembly by Arp2/3 complex and shares functions with WASp. J. Cell Biol. 151:789-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machesky, L. M. 2000. The tails of two myosins. J. Cell Biol. 148:219-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machesky, L. M., and R. H. Insall. 1999. Signaling to actin dynamics. J. Cell Biol. 146:267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magee, B. B., and P. T. Magee. 1997. WO-2, a stable aneuploid derivative of Candida albicans strain WO-1, can switch from white to opaque and form hyphae. Microbiology 143:289-295. [DOI] [PubMed] [Google Scholar]
- 36.McGoldrick, C. A., C. Gruver, and G. S. May. 1995. myoA of Aspergillus nidulans encodes an essential myosin I required for secretion and polarized growth. J. Cell Biol. 128:577-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mermall, V., P. L. Post, and M. S. Mooseker. 1998. Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science 279:527-533. [DOI] [PubMed] [Google Scholar]
- 38.Morschhauser, J., S. Michel, and J. Hacker. 1998. Expression of a chromosomally integrated, single-copy GFP gene in Candida albicans, and its use as a reporter of gene regulation. Mol. Gen. Genet. 257:412-420. [DOI] [PubMed] [Google Scholar]
- 39.Negredo, A., L. Monteoliva, C. Gil, J. Pla, and C. Nombela. 1997. Cloning, analysis and one-step disruption of the ARG5,6 gene of Candida albicans. Microbiology 143:297-302. [DOI] [PubMed] [Google Scholar]
- 40.Neuhaus, E. M., and T. Soldati. 2000. A myosin I is involved in membrane recycling from early endosomes. J. Cell Biol. 150:1013-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noegel, A. A., and M. Schleicher. 2000. The actin cytoskeleton of Dictyostelium: a story told by mutants. J. Cell Sci. 113:759-766. [DOI] [PubMed] [Google Scholar]
- 42.Novak, K. D., and M. A. Titus. 1998. The myosin I SH3 domain and TEDS rule phosphorylation site are required for in vivo function. Mol. Biol. Cell 9:75-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelham, R. J. J., and F. Chang. 2001. Role of actin polymerization and actin cables in actin-patch movement in Schizosaccharomyces pombe. Nat. Cell Biol. 3:235-244. [DOI] [PubMed] [Google Scholar]
- 44.Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. II. The role of the cortical actin cytoskeleton. J. Cell Sci. 113:571-585. [DOI] [PubMed] [Google Scholar]
- 45.Pruyne, D. W., D. H. Schott, and A. Bretscher. 1998. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 143:1931-1945. [DOI] [PubMed] [Google Scholar]
- 46.Raposo, G., M. N. Cordonnier, D. Tenza, B. Menichi, A. Durrbach, D. Louvard, and E. Coudrier. 1999. Association of myosin I alpha with endosomes and lysosomes in mammalian cells. Mol. Biol. Cell 10:1477-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sells, M. A., and J. Chernoff. 1997. Emerging from the PAK: the p21-activated protein kinase family. Trends Cell Biol. 7:162-167. [DOI] [PubMed] [Google Scholar]
- 48.Smith, M. G., S. R. Swamy, and L. A. Pon. 2001. The life cycle of actin patches in mating yeast. J. Cell Sci. 114:1505-1513. [DOI] [PubMed] [Google Scholar]
- 49.Soldati, T., H. Geissler, and E. C. Schwarz. 1999. How many is enough? Exploring the myosin repertoire in the model eukaryote Dictyostelium discoideum. Cell Biochem. Biophys. 30:389-411. [DOI] [PubMed] [Google Scholar]
- 50.Temesvari, L. A., J. M. Bush, M. D. Peterson, K. D. Novak, M. A. Titus, and J. A. Cardelli. 1996. Examination of the endosomal and lysosomal pathways in Dictyostelium discoideum myosin I mutants. J. Cell Sci. 109:663-673. [DOI] [PubMed] [Google Scholar]
- 51.Torralba, S., M. Raudaskoski, and A. Pedegrosa. 1998. Effects of methyl benzimidazole-2-yl carbamate on microtubule and actin cytoskeleton in Aspergillus nidulans. Protoplasma 202:54-64. [Google Scholar]
- 52.Torralba, S., M. Raudaskoski, A. Pedegrosa, and F. Laborda. 1998. Effect of cytochalasin A on apical growth, actin cytoskeleton organization and enzyme secretion in Aspergillus nidulans. Microbiology 144:45-53. [DOI] [PubMed] [Google Scholar]
- 53.Toya, M., F. Motegi, K. Nakano, I. Mabuchi, and M. Yamamoto. 2001. Identification and functional analysis of the gene for type I myosin in fission yeast. Genes Cells 6:187-199. [DOI] [PubMed] [Google Scholar]
- 54.Tzung, K. W., R. M. Williams, S. Scherer, N. Federspiel, T. Jones, N. Hansen, V. Bivolarevic, L. Huizar, C. Komp, R. Surzycki, R. Tamse, R. W. Davis, and N. Agabian. 2001. Genomic evidence for a complete sexual cycle in Candida albicans. Proc. Natl. Acad. Sci. USA 98:3249-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whiteway, M. 2000. Transcriptional control of cell type and morphogenesis in Candida albicans. Curr. Opin. Microbiol. 3:582-588. [DOI] [PubMed] [Google Scholar]
- 56.Wu, C., S. F. Lee, E. Furmaniak-Kazmierczak, G. P. Côté, D. Y. Thomas, and E. Leberer. 1996. Activation of myosin-I by members of the Ste20p protein kinase family. J. Biol. Chem. 271:31787-31790. [DOI] [PubMed] [Google Scholar]
- 57.Wu, C., V. Lytvyn, D. Y. Thomas, and E. Leberer. 1997. The phosphorylation site for Ste20p-like protein kinases is essential for the function of myosin-I in yeast. J. Biol. Chem. 272:30623-30626. [DOI] [PubMed] [Google Scholar]
- 58.Yamashita, R. A., and G. S. May. 1998. Constitutive activation of endocytosis by mutation of myoA, the myosin I gene of Aspergillus nidulans. J. Biol. Chem. 273:14644-14648. [DOI] [PubMed] [Google Scholar]
- 59.Yamashita, R. A., N. Osherov, and G. S. May. 2000. Localization of wild type and mutant class I myosin proteins in Aspergillus nidulans using GFP-fusion proteins. Cell Motil. Cytoskel. 45:163-172. [DOI] [PubMed] [Google Scholar]
- 60.Yang, S., K. R. Ayscough, and D. G. Drubin. 1997. A role for the actin cytoskeleton of Saccharomyces cerevisiae in bipolar bud-site selection. J. Cell Biol. 136:111-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yokoyama, K., H. Kaji, K. Nishimura, and M. Miyaji. 1990. The role of microfilaments and microtubules in apical growth and dimorphism of Candida albicans. J. Gen. Microbiol. 136:1067-1075. [DOI] [PubMed] [Google Scholar]