Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disorders presenting with the pathological hallmarks of amyloid plaques and tau tangles. Over the past few years, great efforts have been made to explore reliable biomarkers of AD. High-throughput omics are a technology driven by multiple levels of unbiased data to detect the complex etiology of AD, and it provides us with new opportunities to better understand the pathophysiology of AD and thereby identify potential biomarkers. Through revealing the interaction networks between different molecular levels, the ultimate goal of multi-omics is to improve the diagnosis and treatment of AD. In this review, based on the current AD pathology and the current status of AD diagnostic biomarkers, we summarize how genomics, transcriptomics, proteomics and metabolomics are all conducing to the discovery of reliable AD biomarkers that could be developed and used in clinical AD management.
Keywords: Alzheimer's disease, Genomics, Epigenomics, Transcriptomics, Proteomics, Metabolomics
Introduction
It is estimated that 50 million people worldwide currently have dementia, 50–70% of whom are AD, and the incidence of AD is expected to triple by 2050. [1]. Although studies on amyloid beta (Aβ) plaques and tau neurofibrillary tangles (NFTs) have provided an understanding of the molecular mechanisms, the pathogenesis of AD is still not fully understood [2]. In addition to the classic pathological features, many emerging new causes/risk factors of AD are discovered. These risks include, but not limited to neurovascular abnormalities, neuroinflammation and immune response disorders, impaired energy metabolism, mitochondrial dysfunction, oxidative stress, compromised autophagy/mitophagy, etc. [3–6]. A comprehensive understanding of the molecular mechanisms under the pathophysiology of AD will help to find out reliable biomarkers to diagnose and track the progression of the disease and provide support for the prevention, treatment and prognosis of the disease [7].
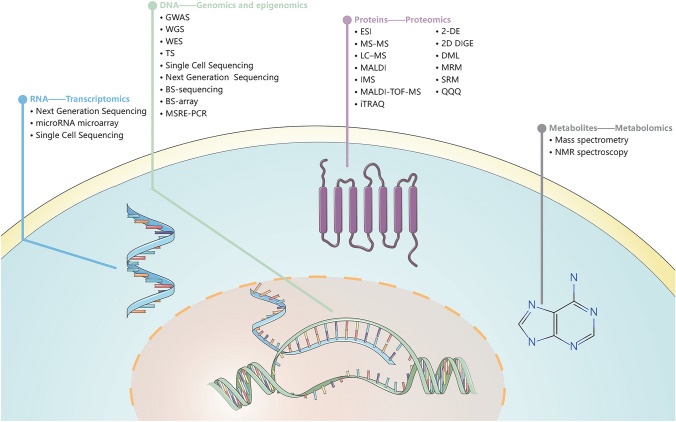
Omics sciences is a holistic, systems-level means to fully expose AD pathophysiology, including genomics, transcriptomics, proteomics and metabolomics [8] The technological innovations of various omics have become the most promising tool to investigate AD (Fig. 1). Genomics main purpose is to collectively characterize and quantify all genes and mutations in an organism, identifying new loci that affect the risk of AD is crucial for us to understand the underlying causes of AD [9]. Transcriptomics is a powerful way to study the potential gene regulation mechanisms of complex trait associations to identify groups of co-expressed genes representing cellular processes and transcriptional programs associated with AD phenotypes [10]. Proteomics studies protein expression levels, posttranslational modifications (PTM), protein–protein interactions, etc., so as to identify the proteins and biological processes that play roles in AD [11]. Metabolomics studies the collection of all metabolites in a cell at a certain moment, reflecting the environment in which the cell is located. It reveals ongoing pathological processes of AD [12]. Thus, omics research can describe the entire biological continuum of AD and identify networks during AD progression.
Fig. 1.
Multi-omics approaches in AD. We list the methods currently available in each omics. GWAS Genome-wide association studies, WGS Whole-genome sequencing, WES Whole-exome sequencing, TS Targeted Sequencing, BS-sequencing Oxi- and Bisulfite sequencing, BS-array Bisulfite-modified DNA-based arrays, MSRE-PCR Methylation-sensitive restriction enzyme-PCR, ESI Soft ionization techniques, MS–MS Tandem-Mass Spectrometry, LC–MS Liquid chromatography-mass spectrometry, MALDI Matrix-assisted laser desorption/ionization, IMS MALDI-imaging mass spectrometry, MALDI-TOF–MS Matrix-Assisted Laser Desorption Ionization Time-of-Flight, iTRAQ Isobaric Tag for Relative and Absolute Quantification, 2-DE Two-dimensional gel electrophoresis, 2D DIGE Differential Gel Electrophoresis, DML Dimethyl labels, MRM Multiple-reaction monitoring, SRM Selected reaction monitoring, QQQ Triple quadrupole mass spectrometer
In this review, we outline the multi-omics interrelated with AD in order to identify its potential biomarkers, and these new biomarkers may potentially facilitate the headway of early diagnosis and therapeutic interventions for the disease.
Available biomarkers for AD diagnosis
The current biomarkers used to diagnose AD in clinical practice are cerebrospinal fluid (CSF) protein markers and brain neuroimaging markers. The core CSF biomarker of AD is Aβ42, which is used to show cortical amyloid deposition. Besides, total tau (t-tau) represents the intensity of neurodegeneration, and phosphorylated tau (p-tau) is related to pathological changes of neurofibrillars. Potential imaging biomarkers that have been approved can be used to observe structural and functional changes, as well as synapses and cell degeneration through positron emission tomography (PET) or functional magnetic resonance imaging (fMRI) [13]. F-fluorodeoxyglucose (FDG) PET can measure the glucose uptake of neurons and glial cells and is sensitive to synaptic function. Amyloid PET has a very high preciseness for cortical amyloidosis. Studies on plasma Aβ, tau and neurofilament light chain (NfL) also have some good results, but none of them has been clinically verified yet [14]. In addition, tau-PET has only been used for a few years. Although it is being actively included in many clinical studies, longitudinal data on accumulated changes in tau are still limited [15].
The combination of proven multiple markers has good predictive value for distinguishing between normal aging in patients with mild cognitive impairment (MCI) and AD [16]. Currently, the diagnosis of AD is determined by experienced clinicians using a series of cognitive tests, coupled with various structural and functional imaging and CSF biomarkers, although these markers for early diagnosis of AD has high sensitivity and specificity, but more biomarkers need to be determined in order to make a diagnosis at an earlier stage of the disease, so that it can monitor disease modification therapies and disease-related processes, and provide novel perceptions in disease mechanisms.
The features of genomics in AD
Genome-wide association study (GWAS) usually examines a large number of single nucleotide polymorphisms (SNPs) in the entire genome to identify disease-related genetic variation, so that thousands of genetic variations (including non-coding regions) can be evaluated simultaneously without the need to make prior assumptions about biological pathways. Next-generation genome sequencing (NGS) technologies, such as whole-exome sequencing (WES) and whole-genome sequencing (WGS), can identify rare SNPs associated with AD, and these genes usually cannot be detected by GWAS [17]. Rare variants are more likely to increase the risk of AD than common variants.
Late-onset Alzheimer's disease
AD that develops after the age of 65 is often referred to as late onset AD (LOAD). LOAD is considered to be a polygenic disease and is sporadic. The APOEɛ4 allele is considered to be the strongest genetic risk factor for LOAD, and its genetic variation greatly increases the risk of AD [18–20].To date, more than 50 common low-risk genetic loci in LOAD have been revealed [21–27] (Fig. 2, Table 1), and their individual contribution to the total heritability of AD is relatively small. Jansen et al. and Kunkle et al. identified new risk loci for LOAD using a "proxy phenotype" approach, which the use of genotypes of unaffected individuals and their affected relatives to identify disease risk loci has the advantage of increasing sample size, although it is less sensitive and less specific for the diagnosis of AD [22, 28].
Fig. 2.
AD-related genes and their SNPs. The outer side of the circle is the genetic factors associated with AD in alphabetical order, and the inner side is the SNP sites of each gene associated with AD. Risk factors are marked in red, protective factors are marked in green, and blue stands for both. The circlize package of the R software (http://www.r-project.org/) was used to generate the diagram
Table 1.
Summary of genomic loci and genes associated with AD
| Locus | Chr | Genes | Potential pathogenesis | Cell type |
|---|---|---|---|---|
| ABCA7 | 19 | ABCA7 | Aβ degradation/clearance, lipid metabolism and phagocytosis, immune response and inflammation | Ubiquitous |
| ABI3 | 17 | ABI3 | Cell growth | Microglia |
| AC074212.3 | 19 | AC074212.3 | – | – |
| AC099552.4 | 7 | AC099552.4 | – | – |
| ACE | 17 | ACE, CYB561, TANC2 | Aβ degradation/clearance | Neurons |
| ADAMTS4 | 1 | ADAMTS4 | APP processing to Aβ, tau pathology | Microglia |
| ADAMTS1 | 21 | ADAMTS1 | Synapse regulation | Microglia |
| ADAM10 | 15 | ADAM10 | APP processing to Aβ | Ubiquitous |
| AKAP9 | 7 | AKAP9 | Kinase signaling | Ubiquitous |
| ALPK2 | 18 | ALPK2 | – | Microglia |
| APH1B | 15 | APH1B | APP processing to Aβ | Ubiquitous |
| APOE | 19 | APOE | Aβ degradation/clearance, lipid metabolism and phagocytosis | Ubiquitous |
| B1N1 | 2 | B1N1 | Endocytosis, tau pathology | Ubiquitous |
| BHMG1 | 19 | BHMG1, FBX046 | – | – |
| BZRAP1-AS1 | 17 | BZRAP1-AS1, RNF43, SUPT4H1, TSPOAP1 | – | – |
| CASS4 | 20 | CASS4, AURKA, CSTF1, FAM209A, FAM209B, GCNT7, RTFDC1 | Cell adhesion/actin cytoskeleton, tau pathology | Microglia |
| CD2AP | 6 | CD2AP, ADGRF2, ADGRF4, GPR111, GPR115, OPN5, TNFRSF21 | APP processing to Aβ, Aβ degradation/clearance, cell adhesion/actin cytoskeleton, endocytosis, immune response, tau pathology | Ubiquitous |
| CD33 | 19 | CD33, SIGLEC7, SIGLEC9, SIGLECL1 | Aβ degradation/clearance, immune response and phagocytosis | Microglia |
| CELF1/SPI1 | 11 | CELF1, ACP2, AGBL2, C1QTNF4, DDB2, FAM180B, FNBP4, KBTBD4, MADD, MTCH2, MYBPC3, NDUFS3, NR1H3, NUP160, PACSIN3, PSMC3, PTPMT1, RAPSN, SLC39A13, SPI1, LOC101928943 |
Cell adhesion/actin cytoskeleton, myeloid lineage determination, tau pathology |
Myeloid cells |
| CLNK | 4 | CLNK | – | – |
| CLU | 8 | CLU, EPHX2, SCARA3 | Aβ degradation/clearance, lipid metabolism, immune response and inflammation | Astrocytes |
| CNTNAP2 | 7 | CNTNAP2 | Cell adhesion/actin cytoskeleton | Neurons |
| CR1 | 1 | CR1 | Aβ degradation/clearance, immune response and phagocytosis | Microglia |
| DSG2 | 18 | DSG2, DSG2-AS1, DSG3 | – | – |
| ECHDC3 | 10 | ECHDC3, USP6NL, PROSER2 | – | Microglia |
| EPHA1 | 7 | EPHA1, CASP2, CLCN1, EPHA-AS1, FAM131B, LOC100507507, TAS2R41, TAS2R60, TAS2R62P, ZYX | Synapse regulation, cell adhesion/actin cytoskeleton, immune response and inflammation | – |
| FERMT2 | 14 | FERMT2, ERO1A, PSMC6, STYX, GNPNAT1 | Cell adhesion/actin cytoskeleton, tau pathology | Microglia |
| FRMD4A | 10 | FRMD4A | Tau pathology | Ubiquitous |
| HESX1 | 3 | HESX1, IL17RD, APPL1 | Synapse regulation | Microglia |
| HLA-DRB1/HLA-DRB5 | 6 | HLA-DRB1, HLA-DRB5, C6orf10, BTNL2, HLA-DRA, HLA- DRB6, HLA- DQA1, HLA-DQB1, HLA- DQA2, HLA-DQB2, HLA- DOB | Immune response and inflammation | Microglia |
| HS3ST1 | 4 | HS3ST1 | – | Ubiquitous |
| INPP5D | 2 | INPP5D, NEU2, NGEF | Immune response and inflammation | Microglia |
| IQCK | 16 | IQCK, KNOP1, C16orf62 | Endocytosis and sorting | Microglia |
| KAT8 | 16 | KAT8 | – | Ubiquitous |
| MAPT | 17 | MAPT | Synapse regulation, Aβ toxicity | Ubiquitous |
| MEF2C | 5 | MEF2C, TMEM161B, MIR9-2, LINC00461, MEF2C-AS1 | Immune response and inflammation, tau pathology | – |
| MS4A | 11 | MS4A1, MS4A2, MS4A3, MS4A4, MS4A4E, MS4A5, MS4A6A, MS4A6E, MS4A7, MS4A14, OOSP2 | Chemosensory receptors, immune response and inflammation | Microglia |
| NME8 | 7 | NME8, EPDR1, GPR141, SFRP4 | Cell adhesion/actin cytoskeleton | – |
| PFDN1/HBEGF | 5 | PFDN1, HBEGF | Immune response, cell adhesion/actin cytoskeleton | Ubiquitous |
| PICALM | 11 | PICALM, CCDC83, EED | APP processing to Aβ, Aβ degradation/clearance, endocytosis, tau pathology | Ubiquitous |
| PLCG2 | 16 | PLCG2 |
Phospholipase signaling, tau pathology |
Microglia |
| PLD3 | 19 | PLD3 | – | Ubiquitous |
| PTK2B | 8 | PTK2B, CHRNA2, STMN4, TRIM35 | Synapse regulation, tau pathology | Neurons |
| RBFOX1 | 16 | RBFOX1 | Aβ degradation | – |
| SCIMP | 17 | SCIMP, C1QBP, DHX33, NUP88, RABEP1, RPAIN, USP6, ZFP3, ZNF232, ZNF594 | Immune response | Microglia |
| SLC24A4/RIN3 | 14 | SLC24A4, RIN3, LGMN | Endocytosis and sorting | Endothelial cell |
| SORL1 | 11 | SORL1 | APP processing to Aβ, endocytosis and sorting, lipid metabolism | Ubiquitous |
| SPPL2A | 15 | SPPL2A, USP8, USP50, TRPM7 | Immune response | Ubiquitous |
| TM2D3 | 15 | TM2D3 | Aβ degradation/clearance | Ubiquitous |
| TREM2 | 6 | TREM2 | Aβ degradation/clearance, immune response and inflammation, tau pathology | Microglia |
| TREML2 | 6 | TREML2 | Immune response | Microglia |
| TRIP4 | 15 | TRIP4, CSNK1G1, KIAA0101, ZNF609 | – | – |
| TSPOAP1 | 17 | TSPOAP1 | Lipid metabolism | – |
| UNC5C | 4 | UNC5C | Response to neurotoxic stimuli, cell death | Neurons |
| WWOX/MAF | 16 | WWOX, MAF | Immune response, tau pathology | Ubiquitous |
| ZCWPW1/NYAP1 | 7 | ZCWPW1, ACTL6B, AGFG2, AP4M1, AZGP1, C7orf43, C7orf61, CNPY4, COPS6, CYP3A43, FBXO24, GAL3ST4, GATS, GASTOR3, GIGYF1, GJC3, GNB2, GPC2, LAMTPR4, LOC100128334, LRCH4, MBLAC1, MCM7, MEPCE, MOSPD3, NYAP, OR2AE1, PCOLCE, PCOLCE-AS1, PILRA, PILRB, PPP1R35, PVRIG, SAP25, SPDYE3, STAG3, TAF6, TFR2, TR1M4, TSC22D4, ZKSCAN1, ZNF3, ZSCAN21 | Immune response and inflammation, tau pathology | Microglia |
| ZNF655 | 7 | ZNF655 | – | Ubiquitous |
We summarized the genomic loci in chromosome order, genes contained in gene locus, potential pathogenesis to which these genes have been functionally linked and cell type-specific expression of these genes [24]. Copyright© 2019, Springer Nature)
“–”The information has not been obtained from the primary publication or has not been found yet
Rare variants are more likely to increase the risk of LOAD than common variants [29] and can affect different pathological processes [30–33]. The first genome-wide copy number variations (CNVs) study on LOAD identified 3,012 AD-specific CNVs, suggesting that accumulation of CNVs may lead to AD-related pathological changes [34].
The KnockoffScreen-AL approach, recently found to be validated in the AD cohort, is particularly effective in identifying rare variants [35]. NGS approach will identify additional low frequency and rare variants associated with AD, which is important for identifying key pathways in disease pathogenesis and discovering potential targets for drug therapy.
Currently, most GWAS are conducted in European populations, in the largest gene-based cross-ethnic meta-analysis in LOAD to date, the final analysis results are different from the published results [36, 37]. Although the main pathways of AD etiology in other ethnic groups are similar to those in European populations, many disease-associated loci are different [38–41]. The reason for these inconsistencies may be the result of cross-ethnic transfer of risk genotypes described by different populations. However, it has also been argued that the possibility of combining individuals from different populations to obtain a larger sample cannot be considered completely certain [42].
Early-onset Alzheimer's disease
Patients with early onset of AD, usually before age 65, are referred to as early-onset AD (EOAD). EOAD is an almost entirely genetic disorder with heritability ranging from 92 to 100%. Through genetic studies on single genes, high-risk mutations were found in three genes encoding APP, Presenilin 1 and Presenilin 2 (PSEN1 and PSEN2) [43], which mainly regulate Aβ production and degradation [9].
However, most pathogenic variants have not been evaluated by in vitro functional assays. In the case of insufficient genetic evidence, the definite pathogenicity of a variant may therefore still be uncertain. A PSEN1 mutation carrier who did not develop MCI until age 70 was found to have two rare Christchurch (APOEch) mutations in APOE3 after WES, considering that the APOEch homozygous gene was her most likely genetic modifier, which has a strong protective effect [44]. Therefore, can it be considered that the safe and effective editing of APOE may have a profound impact on the treatment and prevention of AD?
Studies on the role of rare variants in EOAD are scarce. In a WGS, FERMT2 was found to regulate AD risk by regulating APP metabolism and Aβ peptide production [45]. A novel missense variant in ACAA1 impairs lysosomal function and promotes Aβ pathology and cognitive decline [46]. It can be seen that the genetic determinants of EOAD involve rare mutations in Aβ network genes. The main advantage of these NGS technologies is that they can identify putative disease genes by directly analyzing the data of related family members belonging to small families.
Genomics related to pathological processes
Aβ is produced by sequentially dividing APPs by β- and γ-secretase enzymes. PSEN1 and PSEN2 form the active core of the γ-secretase complex, which affects the activity of endopeptidases or carboxypeptidases, allowing the production of Aβ40 and Aβ42 to be transferred to longer, more neurotoxic species [9]. Whole-brain amyloid deposition using Pittsburgh compound-B (PiB)-PET imaging confirmed the interrelationship between the APOE locus and brain amyloidosis in vivo [47].
Mutations of tau have been majorly associated with/contribute to other tau proteinopathies; however, there is no evidence that the moment that mutation of tau happens in AD [48]. Thus, we will be focusing on the mutations of other genes that could activate/inhibit tau pathologies in AD. Lots of known autosomal-dominant mutations in MAPT highlight the importance of tau protein in AD, and the gene encodes for tau [49]. Identifying other pathogenic genes that perturb tau accumulation, such as those involved in tau clearance and protein quality control, has the latent to disclose previously unknown mechanisms of AD [49].
A subset of genes has been identified to be associated with classical AD pathology or other pathways (Table 1), and subsequent genetic and functional studies are needed to confirm the role of candidate genes in the relevant pathological processes. Many studies currently focus on one variant or some variants, neglecting the complex polygenic disease context. Further understanding of polygenic risk factors still requires additional efforts and validating the discovery of these genes and elaborating them into targetable mechanisms remains an important issue.
Alzheimer's disease epigenomics
Epigenomics does not alter nucleotide sequences, and DNA methylation and histone modifications are highly variable and play an important role in AD [50].
The large-scale epigenome-wide association study (EWAS) found that although the different brain areas cells are different in composition and have different sensitivities to AD pathology, AD has similar associations with DNA methylation across different brain regions [51]. Through the combination of GWAS and EWAS, not only the contribution of DNA methylation in the AD risk loci to the disease was studied, but also the SNPs related to changes in DNA methylation were identified, which is helpful to determine the functional results of proven risk genes, provides a complement to causal variation at risk loci for AD [52, 53]. A recent epigenomic study found that an epigenomic factor associated with a reduced proportion of activated microglia appears to attenuate the risk of APOEε4 for AD [54].
There are relatively few studies on histone modification. Based on previous studies that determined the correlation between SNPs and gene expression, DNA methylation and histone modification levels, it was found that SNPs have an effect on RNA expression. The effect was exclusively mediated by epigenetic features in 9% of these loci, indicating that genetic variation affects these three factors together [55, 56], as well as a close interaction between epigenetic changes and RNA.
Challenges and opportunities
There is an urgent need to collect and process a large amount of data to discover the regulatory mechanism of AD risk-associated variants, and the consequent number and complexity of new treatment strategies. Linking genetic variation to the intra-AD phenotype that characterizes disease progression and is associated with pathology can help prioritize AD risk genes [9] to simplify the follow-up research on treatment. However, it remains unclear how genetic factors are related to the specific progress of the entire AD neuron network pathology. The relationship between the different risk genes and the molecular mechanisms involved in different pathologies still needs further understanding, so our main bottleneck at present is still insufficient understanding of the mechanism of genetic variation.
Sample size in NGS is generally small, and its efficacy is very limited. Therefore, we still need a large number of data sets to find strong evidence for a single gene or variant identified in the entire WGS and WES. Moreover, analysis of postmortem brain tissue has limited exposure to brain tissue in the early stages of the disease. Therefore, mouse models carrying causal mutations in AD have many advantages, such as precise environmental control, early exposure to brain tissue and a clear high-risk genotype and being an important tool [57].
The features of transcriptomics in AD
The traditional methods of transcriptomics research are pooling cell microarrays and single-cell RNA sequencing (scRNA-seq). Transcriptome-wide association analysis (TWAS) is a powerful way to study the potential gene regulation mechanisms of complex trait associations to identify co-expressed genomes representing cellular processes and transcriptional programs associated with disease phenotypes [10].
AD-related genome-wide RNA profiles include protein-coding RNA (cRNA) and non-protein-coding RNA (ncRNA) transcripts. The proportion of conserved transcripts with intact splice sites is smaller than that of general non-coding genes [58], suggesting that non-coding genes associated with AD have faster functional adaptations and are likely to constitute the key to gene regulation.
The alterations of mRNA in AD
Transcriptional analysis of AD-related regions in the human brain reflects the relevant processes of AD pathology [59]. Co-expression analysis of the brain transcriptome effectively links RNA to molecular pathways, organelles and cell types that influence AD, and forms a network, revealing the transcriptional profile of cell types in AD and cell types that are most likely to express candidate AD genes [60]. Using integrative network methods to analyze different brain regions in different cohorts can determine different combinations of multiple dysregulated pathways in specific molecular subtypes of AD [61].
Studies of gene expression profiles in AD brain tissue have also identified transcriptional changes in AD-specific cellular processes. Studies of the transcriptomes of six major brain cell types have identified myelination, inflammation and regulation of neuronal survival as major perturbations [49].
An approach linking genotype and gene expression helps to provide a clearer picture of the pathology of the whole brain [62]. Recently, splicing disruptions have been found to be a common feature of AD. The proteins encoded by splice introns play a role in autophagy-lysosome-related pathways [63] and have also been identified to be associated with neuroinflammatory plaques, Aβ-load and NFTs [55], highlighting a potential role in AD pathology.
The alterations of long non-coding RNA in AD
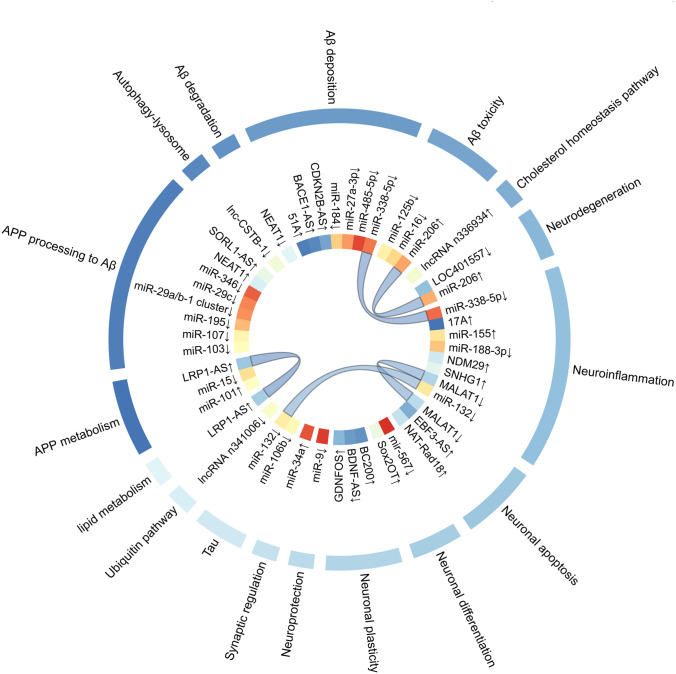
Transcripts longer than 200 nucleotides are referred to as long non-coding RNAs (lncRNAs) and can influence the pathogenesis of AD through various regulatory mechanisms, including transcriptional, posttranscriptional and translational regulation (Fig. 3, Table 2).
Fig. 3.
Transcriptomic biomarkers associated with AD. The diagram shows (from outside to inside): (1) related pathogenesis; (2) potential transcriptomic biomarkers; and (3) connection of different pathogenesis related to the same protein. The circlize package of the R software (http://www.r-project.org/) was used to generate the diagram
Table 2.
RNA transcriptomic biomarkers from different origins
| Source | RNA transcript | Refs. |
|---|---|---|
| PBMC | lnc-AL445989.1–2, TTC39C-AS1, LINC01420, lnc-CSTB-1, LOC401557 | [64] |
| Blood | miR-29c, miR-125b, miR-155, miR-Let-7b, miR-206 | [65, 66] |
| CSF | MALAT1, miR-27a-3p | [67, 68] |
| Brain tissue | BACE1-AS,51 A, 17 A, NDM29, BC200, GDNFOS, BDNF-AS, Sox2OT, EBF3-AS, NAT-Rad18, CDKN2B-AS, lncRNA n336934, lncRNA n341006, LRP1-AS, NEAT1, SORL1-AS, NEAT1, SNHG1, miR-15, miR-16, miR-29a/b-1 cluster, miR-34a, miR-101, miR-106b, miR-184, miR-188-3p, miR-195, miR-338-5p, miR-346, miR-485-5p | [69–71] |
| Blood/CSF | mir-567 | [72] |
| Blood/Brain tissue | miR-9, miR-103, miR-107, miR-206 | [73, 74] |
| Blood/CSF/Brain tissue | miR-132 | [75] |
AD-related lncRNAs in the AD brain may affect coding RNAs at the same site, and some lncRNAs have been identified to be co-expressed with known AD-related genes [76], and studies have also been conducted on its regulatory mechanisms in response to AD, with its corresponding transcripts mainly involved in Aβ metabolism, neuroinflammation, synaptic plasticity, neurotrophic depletion, mitochondrial dysfunction, AD stress response, and other processes [77, 78]. Among the few functionally identified lncRNAs, the lncRNA BACE1-AS is highly expressed in both brain and blood and contributes to the pathogenesis of AD by increasing the levels of Aβ and APP [74]. Recently, the spectrum of lncRNAs in peripheral blood mononuclear cells in AD has also been studied for the first time [64], allowing one to go beyond postmortem brain tissue studies and to open up findings in different blood cells.
The alterations of MicroRNA in AD
MicroRNAs (miRNAs) are non-coding RNAs containing 20–24 nucleotides, which mediates the stability of mRNA and regulates translation inhibition, leading to down-regulation of gene expression [79]. Dysregulation of the transcriptome expressed by miRNAs can be delivered via exosomes and act synergistically with proteins to cause changes in mRNA, providing an early signal to suggest impending neuropathy in the brain.
The expression of miRNAs in blood, CSF and brain has been studied (Fig. 3, Table 2). Inconsistent results have been observed for the same miRNA in different studies analyzing the same brain region, and inconsistent findings regarding the changes in miRNAs in brain, CSF and blood. MiR-567 was currently found to be differentially expressed in all three biological samples from MCI-AD patients [72], but no independently validated miRNA has been found to vary uniformly among the three.
Future directions of transcriptomics
Studies have shown that transcription disorders can be spotted much earlier than the appearance of pathological signs and can be determined as the first sign of brain phenotypic changes [80]. Studies from the whole brain may mask local transcriptome overlap due to the different degree and rate of neurodegeneration in different brain regions. Accurate and consistent pathways in the brain, blood and cerebrospinal fluid from different samples and different disease stages have also not been established. Applying spatial transcriptomics and in situ sequencing techniques to study the cellular stages of AD could complement each other and provide a more comprehensive understanding to unravel the molecular network of AD [81].
In addition, ncRNAs are more readily available and relatively stable. The confirmation of abnormally expressed miRNAs and lncRNAs in AD patients will provide effective support for the early detection, diagnosis and treatment of AD in the future. Future studies on circulating RNAs (circRNAs), PIWI-interacting RNAs (piRNAs) and natural antisense transcripts (NAT) will also help to identify molecular events in the development of AD.
The features of proteomics in AD
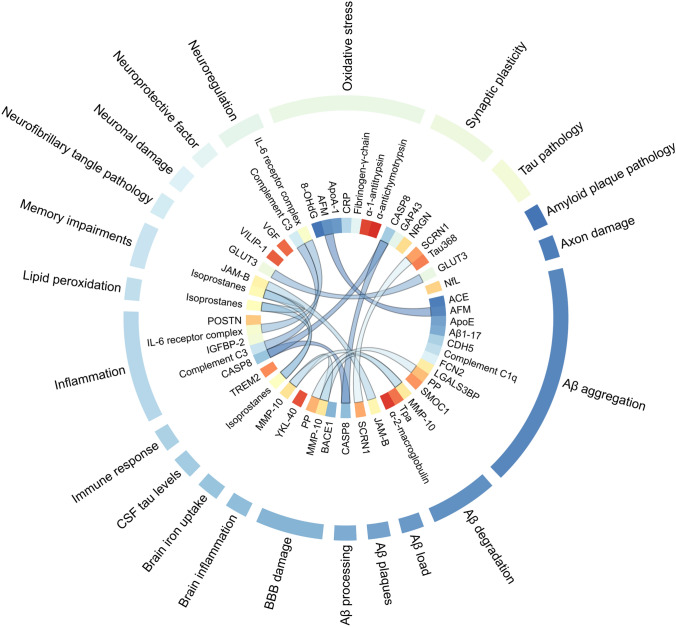
Proteomics is the analysis of proteins in different tissues and fluids of the body. It can identify thousands of proteins in complex biological samples and can be used to study the various pathways that may be affected in the body of early stage of AD patients (Fig. 4, Table 3).
Fig. 4.
Potential proteomic biomarkers associated with AD. The diagram shows (from outside to inside): (1) related pathogenesis; (2) potential proteomic biomarkers; and (3) connection of different pathogenesis related to the same protein. The circlize package of the R software (http://www.r-project.org/) was used to generate the diagram
Table 3.
Protein biomarkers from different origins
| Source | Protein | Refs. |
|---|---|---|
| Blood | α-1-antitrypsin, Complement C3, ACE, AFM, BNP, CDH5, FCN2, fibrinogen-γ-chain, Homocysteine, LGALS3BP, NfL, POSTN, PP, α-2-macroglobulin | [82–85] |
| CSF | MMP-10, tau368, TREM2, VILIP-1, YKL-40 | [78, 86–88] |
| Brain tissue | GLUT3, SCRN1 | [89, 90] |
| Blood/CSF | α-antichymotrypsin, Isoprostanes (8,12-isoiPF2α-VI), BACE1, CASP8, JAM-B, NRGN, tPA, 8-hydroxy-deoxyguanine, Complement C1q, CRP, Interleukin-6-receptor complex, Aβ1-17 | [83, 86, 87, 91–95] |
| Blood/Brain tissue | ApoE, SMOC1, VGF, ApoA-1 | [85, 96, 97] |
| CSF/Brain tissue | GAP43 | [92] |
| Blood/CSF/Brain tissue | IGFBP-2 | [98] |
ACE blood angiotensin I-converting enzyme, AFM Afamin, APOA1apolipoprotein A-1, ApoE apolipoprotein E, BACE1Beta-Secretase 1, BNP type B natriuretic peptide, CASP8Caspase 8, Apoptosis-related cysteine peptidase, CDH5Cadherin 5, CRPC‐reactive protein, FCN2Ficolin 2, GAP43 Growth associated protein-43, GLUT3 Glucose transporter 3, IGFBP-2 Insulin-like growth factor binding protein-2, JAM-B Junctional adhesion molecule B, LGALS3BP Galectin 3 binding protein, MMP-10 Matrix metalloproteinase-10, NfL neurofilament light chain, NRGN neurogranin, POSTN Periostin, PP pancreatic polypeptide, SCRN1 Secernin 1, SMOC1 Secreted modular calcium-binding protein 1, tPA tissue plasminogen activator, TREM2 Triggering receptor expressed on myeloid cells 2, VGF Neurosecretory protein VGF, VILIP-1 Visinin-Like protein 1, YKL-40 Chitinase-3-Like Protein 1
Blood-based proteomics
The destruction of the blood–brain barrier (BBB) allows small molecules associated with AD to penetrate into the surrounding area, which makes it possible to discover AD biomarkers in blood.
The identification of a set of blood biomarkers capable of identifying AD at an early stage is pursued, and both plasma and serum samples are currently considered to have sufficient quantitative proteomic properties [99]. Plasma proteins that predict the progression of AD or MCI to AD have been identified based on different phenotypes, such as the degree of hippocampal atrophy, baseline cortical thickness and cognitive abilities; non-amyloid/non-tau proteins markers that can predict brain amyloid deposition as well as other AD phenotypes, or that are associated with cognitive decline and dementia, have also been identified based on different identification and analysis methods [100–102].
Nevertheless, most experiments failed to reproduce the results with similar accuracy during the validation phase. Different stages of AD may be related to the temporal evolution of the pathophysiology of AD in the brain and cerebrospinal fluid, so that different events occur at different time points of disease progression. Recent efforts have also been made in this regard, identifying some proteins that can distinguish the different stages of AD [103].
CSF-based proteomics
It is CSF that is in direct contact with the extracellular space of the brain and has brain tissue-specific proteins whose concentrations can reflect physiological or pathological changes in the AD brain.
Studies have shown that CSF proteomic changes are more representative of tau pathology than amyloid pathology [88, 104]. CSF tau-368 is considered likely to detect pathological changes before the onset of symptoms in cognitively normal individuals [88], and that protein levels involved in neuronal plasticity and blood–brain and blood-CSF barrier dysfunction are associated with CSF t-tau [105]. CSF proteomics can also respond to changes in AD patients such as neuronal proliferation, innate immune response and BBB dysfunction, synaptic, vascular, myelin and metabolic pathway dysfunction [87, 106]; however, no single marker is reflective of the complete pathology and the selection of a set of CSF biomarkers to characterize may be crucial for diagnosis and prognosis. In particular, changes in CSF NPTX2 have been suggested as possible biomarker candidates for AD prognosis [107].
Brain tissue-based proteomics
Proteomics studies of the human brain can only be based on autopsy and are therefore primarily aimed at discovering the biomolecular mechanisms involved in AD, rather than at diagnosis.
Brain degeneration is progressive and dynamic, with very little possibility of continuous observation, and little is known about how pathological changes at different stages of the disease affect the overall expression of brain proteins. However, it has also been found that different disease stages have different AD-related specific proteins [96], and a comprehensive overview of the interaction groups of t-tau and p-tau in the brain at different stages has also been identified [108–110]. Functionally distinct human brain regions show different and region-specific alterations in protein expression, and analysis of the spatial proteome may help to identify specific proteins that represent early states of the disease [111].
A consensus AD brain protein co-expression molecular network was established to look at modules associated with the disease, define different protein pathways, and analyze representative proteins for each pathway, finding that the most closely related to AD is astrocyte/microglia metabolism, and that proteins involved in vesicular endocytosis and secretion pathways begin to change in the early stages of AD [96, 112–116], and in this process gradually led to the derivation of redox proteomics, phosphorylation proteomics, synaptic proteomics, mitochondrial proteomics and microglia proteomics, which together complement the entire proteome.
Limitations and future directions
Proteins are a powerful intermediary for evaluating novel biomarkers for diagnosis and treatment of AD. The proteomics research process is complex and the possible problems and possible solutions for each step have been described in detail [1]. A comprehensive analysis of the ultra-depth proteomes of cerebral cortex, cerebrospinal fluid and serum revealed that the majority of proteins co-present in the three tissues were mitochondrial proteins, but the number of differentially expressed proteins studied and the way they are related in most practical studies lack consistency. The combination of quantitative proteomics, differential expression analysis and co-expression network analysis provides a useful approach to understand the pathogenesis of AD at a systemic level [112]. The combination of nanotechnology and existing analytical tools can enrich the identification of biomarkers of AD [117].
There are currently some studies on urine, saliva, buccal mucosal cells and other samples that also have great potential in the future to provide utterly non-invasive alternative to present CSF and blood sampling procedures. More importantly, the lower molecular parts of blood or CSF proteomics including peptides and protein fragments should also receive more attention and may have more disease-specific information than the full-length proteins themselves.
The features of metabolomics in AD
Metabolomics is the mensuration of small molecules called metabolites in biological samples, such as blood. Understanding how the metabolic changes in various pathways in AD will help us develop effective disease remission therapies (Table 4).
Table 4.
Metabolites associated with AD
| Source | Metabolites | Potential pathogenesis | Refs. |
|---|---|---|---|
| Serum | Lactic acid, α-ketoglutarate, isocitric acid, glucose, oleic acid, adenosine and cholesterol urea, valine, aspartic acid, pyroglutamate, glutamine, phenylalanine, asparagine, ornithine, pipecolic acid, histidine, tyrosine, palmitic and uric acid, tryptophan, stearic acid and cysteine | Energy deficiencies, oxidative stress, dysfunction in mitochondrial activities, hyperammonemia | [118] |
| Serum | Sphingolipids and glycerophospholipids | Tau phosphorylation, Aβ metabolism, calcium homeostasis, acetylcholine biosynthesis, apoptosis | [119] |
| Serum | C12, C14:1, C16:1, C18, PC aeC36:2, PC ae C40:3, PC ae C42:4, PC ae C44:4, SM(OH)C14:1, SM C16:0, SM C20:2, α-aminoadipic acid, valine | Tau phosphorylation, Aβ metabolism, oxidative stress, lipid metabolism | [120] |
| Plasma | Phosphatidylcholines, (PC diacyl (aa) C36:6, PC aa C38:0 PC aa C38:6, PC aa C40:1, PC aaC40:2, PC aa C40:6, PC acyl-alkyl (ae) C40:6), Lysophosphatidylcholine (lysoPC a C18:2), and acylcarnitines (ACs) (Propionyl AC (C3) and C16:1-OH) | Synaptic dysfunction, dysfunction in mitochondrial activities, lipid metabolism | [16] |
| Plasma | Long chain cholesteryl esters (ChEs) (ChE 32:0, ChE 34:0, ChE 34:6, ChE 32:4, ChE 33:6,) | Lipid metabolism, Aβ metabolism, | [121] |
| Plasma | Cholic acid, chenodeoxycholic acid, allocholic acid, indolelactic acid, and tryptophan | Tau phosphorylation, Aβ metabolism, dysfunction in mitochondrial activities, lipid metabolism | [122] |
| Plasma | Branched-chain amino acids (isoleucine, leucine, and valine), creatinine and very low density lipoprotein (VLDL)-specific lipoprotein lipid subclasses | Energy deficiencies, lipid metabolism | [123] |
| Plasma | Anthranilic acid, glutamic acid, taurine, hypoxanthine | Oxidative stress, BBB damage, synapse damage, apoptosis | [124] |
| Plasma | Thymine, arachidonic acid, 2‐aminoadipic acid, N,N‐dimethylglycine, and 5,8‐tetradecadienoic acid | Energy deficiencies, lipid metabolism, DNA damage | [125] |
| Plasma | 16-a-hydroxypregnenolone, stearic acid and PC 16:0/22:5(4Z,7Z,10Z,13Z,16Z) | Inflammation, Aβ metabolism, Tau phosphorylation, oxidative stress | [126] |
| CSF | Uracil, xanthine, uridine, methyl‐salsolinol, nonanoylglycine, dopamine–quinone, caproic acid, vanylglycol, histidine, pipecolic acid, hydroxyphenyl‐pyruvate, creatinine, taurine, sphingosine‐1‐phosphate, tryptophan, and 5′‐methylthioadenosine | Dysfunction in mitochondrial activities, BBB damage, energy deficiencies, oxidative stress | [127] |
| CSF | Total cysteine, kininogen-1 and cholesteryl ester 27:1 16:0 | Energy deficiencies, Aβ metabolism | [128] |
| Brain | Unsaturated fatty acids (UFAs) (linoleic acid, linolenic acid, docosahexaenoic acid, eicosapentaenoic acid, oleic acid and arachidonic acid) | Tau phosphorylation, Aβ metabolism, energy deficiencies, inflammation | [129] |
| Brain | Diacylglycerol (14:0/14:0), triacylglycerol (58:10/FA20:5, 48:4/FA18:3), phosphatidylethanolamine (p-18:0/18:1), phosphatidylserine (PS) (18:1/18:2, 14:0/22:6) | Aβ metabolism, inflammation, apoptosis | [130] |
| Brain | Lauric acid, stearic acid, myristic acid, palmitic acid, palmitoleic acid, and four unidentified mass spectral features | Energy deficiencies | [131] |
| Plasma, CSF | Lysine, cholesterol, and sphingolipids | Energy deficiencies, DNA damage | [132] |
| Urine | Glycine, free cortisol/creatinine, prostaglandinsE2, β-alanyl-l-histidine | Oxidative stress, Aβ metabolism, inflammation | [133–135] |
| Urine, Serum | Serotonin, tryptophan, xanthurenic acid | Inflammation, neurotransmitter regulation | [136] |
| Fecal | Tryptophan metabolites, short-chain fatty acids (SCFAs), and lithocholic acid | Inflammation, neurotransmitter regulation | [137] |
We summarized the metabolites related to the development of AD found in different metabolomic studies from different sources, and each study analyzed the pathogenesis in which these metabolites may be involved
Metabolomics research is mainly predicated on nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). NMR has high repeatability but low sensitivity, whereas MS provides high throughput and sensitivity with good metabolite coverage [138].
Blood-derived metabolites of AD
Lipid metabolism has been found to be the focus of detection of novel potential AD biomarkers, mainly long-chain triglycerides (LCTs) and cholesteryl esters (ChoEs) [139]. Changes in etheric lipid metabolism pathways are a novel finding that may affect downstream mechanisms of AD [140]. Alteration in the levels of some amino acids were thought to be associated with decreased language ability, visuospatial ability, cognitive performance and changes in brain atrophy in AD patients [123, 141]. Gender-specific systemic metabolic changes have been reported in preclinical and clinical AD serum [142–144], and some metabolic effects are specific to female APOE ε4 carriers [145].
Metabolomics and MR analysis can help to identify causal mediators of AD, which is a more feasible approach to explore metabolic risk factors for AD [146, 147], and integrating metabolomic analysis can be of great help in identifying different subgroups of AD patients for future targeted and precise treatment [148, 149]. In addition, different populations have different specificities of blood metabolomes [150, 151], suggesting that identification of relevant metabolic biomarkers for diagnostic purposes requires diversity of sample populations.
CSF-derived metabolites of AD
The most variable in metabolomics in CSF is amino acid. In CSF, the concentration of several aromatic, branched and urea cycle amino acids were significantly correlated with CSF Aβ1-42, tau and p-tau-181 [152]. Metabolic-pathways of CSF metabolites, including caffeine, as well as nicotinic acid and nicotinamide, have also recently been found to be associated with AD-related core pathology [153]. The first study to fully integrate and analyze untargeted CSF metabolomics with structural MRI brain imaging data found that lower levels of gray matter in the hippocampus, thalamus, and parietal cortex were associated with dysregulated urea cycling and amino acid metabolic pathways in the CSF, which is a novel study that could continue to be developed [154], but the association of most blood metabolites with CSF metabolites is not obvious.
Brain tissue-derived metabolites of AD
In brain metabolomics, most of the differential metabolites are sphingolipids and glycerophospholipids. Analysis of various brain regions susceptible to AD lesions identified many different metabolites that are associated with amino acids, lipids, nucleotide-related bases, vitamins, neurotransmitters, energy metabolism, and oxidative stress [89, 143, 155, 156]. Recently, study found differences in gray and white matter metabolomics in the AD brain and showed differences across APOE carriers and in different stages of the disease, suggesting the need for possible personalized treatment in the future [157].
Future development of metabolomics
Current metabolomics studies all have relatively small sample sizes, which will limit our ability to study correlation and replication. It is important to determine the common and different metabolic pathways in different tissue types, which requires us to conduct follow-up studies in larger cohorts and larger data.
In recent years, urine and saliva metabolomics have gradually become very promising and convenient biological samples [158, 159]. Diversity of study specimens can contribute to the versatility of global AD biomarker studies. The comprehensive analysis of the isomers of chiral metabolites is also becoming more and more important. Comprehensive analysis of chiral metabolite isoforms is also becoming increasingly important. In addition, AD has been shown to be associated with gut flora and small molecule metabolites may play a key role in mediating microbial effects on neurotransmission and disease development. The erythrocyte lipid phenotype may be a marker for the risk of AD development [160], which we believe is the direction of strong future development.
Multi-omics science on neuroinflammation
Neuroinflammation is an immune-related response driven by activation of neuroglia. Microglia are the major immune cells in the central nervous system and are thought to be a key factor in the progression of neuroinflammation [161].
As we have previously described, AD GWAS have identified many risk genes that are highly or selectively expressed in microglia, such as ABI3, BIN1, CD3, CLU, CR1, MS4A6A, PLCG2, SPI1 and TREM2, confirming the importance of microglia-mediated neuroinflammation in neurodegeneration.
We have also stated that many AD risk variants exist in noncoding regions of the human genome and introducing genome-wide analyses of chromatin-accessible regions and histone modifications, as well as single-cell analyses, could contribute to the understanding of the microglia regulatoryome and how it is affected by AD risk variants [162], and many efforts have been made to accurately characterize microglia primarily through transcriptomic approaches. The disease-associated microglia (DAM) profile observed in mouse models of AD reflects activation states that can regulate AD risk or progression. Pro-inflammatory DAM appeared earlier in AD mouse models and is characterized by pro-inflammatory genes, surface markers CD44, potassium channel Kv1.3 and regulatory factors (NF-κB, STAT1, RELA), whereas anti-inflammatory DAM expresses phagocytic genes, surface markers with different regulatory factors CXCR4 (LXRα/β, AFTL) [163]. Deficiency of COP1 in microglia promotes changes associated with chronic microglia activation and the development of neuroinflammation [164]. Since TREM2 is the most studied star gene, more microglia-related studies have focused on this gene, and it was further found that increasing TREM2 gene doses altered microglia transcriptional programs as well as morphological and functional responses in the brains of AD mouse models [165]; GAL3 acts as a TREM2 ligand driving AD microglia activation and is thought to be an AD an upstream regulator of microglia immune responses [166]; TREM2 regulates microglia pathology in AD downstream of CD33 [167]; TREM2 deficiency has differential effects on the brain transcriptome of mice expressing different types of human APOE and significantly reduces microglia barrier action around plaques [168]. In an epigenomic study, a key role of DNA methylation in regulating the inflammatory state of microglia was recognized and TET2 was found to drive pro-inflammatory activation of microglia [169]. Deletion of HDAC1 and HDAC2 in microglia reduces amyloid load by enhancing microglial amyloid phagocytosis [170].
Although there are more studies based on transcriptomic and single-cell data, comparison of AD microglia profiles with different mouse microglia profiles showed little similarity between characterized human Alzheimer's microglia (HAM) profiles and the DAM profiles observed in mouse microglia activation profiles [162, 171]. Most of the current histological studies have been performed in mouse models for observational validation, followed by comparison with published human microglia expression profiles, and further by combining the findings with existing GWAS and human postmortem brain proteomes to determine the relevance of DAM modules in human AD, which is a limitation of the current studies, but already in maximizing the opportunity to identify cellular heterogeneity within cell populations. The use of induced pluripotent stem cell (IPSC) technology from humans has been considered as an approach to overcome this problem [161].
For microglia proteomics studies, relatively few studies have been conducted because of the generally low protein yields of their isolated cells, contamination of non-microglia proteins using existing enrichment strategies, and the limitations of the technical requirements of mass spectrometry analysis [172], but some exciting results have been obtained, identifying COTL1, MSN as microglia-specific markers with increased expression in human AD [172, 173]. In a CSF proteomics, SIRT2 and HGF were suggested to be chemotactic for many different immune cells involved in the inflammatory response and activate them [174]. In addition, information on microglia alterations at different stages of Aβ deposition in AD has been investigated [175] to provide help in monitoring the efficacy of microglia repair strategies. Although we were able to obtain data on microglia proteomic changes, it was found that transcript levels do not necessarily reflect the levels of proteins that ultimately control cellular function [176]. This also makes it more difficult to further identify the role of microglial cell proteins in AD for studies related to more readily available cerebrospinal fluid and blood specimens compared to brain tissue. Polyunsaturated fatty acid metabolites were identified by metabolomic analysis corresponding to microglia activation and inflammation [177, 178]. An association between blood metabolomics and neuroinflammation did not identify AD-specific biomarker candidates in a study [179], which further emphasizes the complexity of AD etiology and blood biomarker diagnosis.
Interventions against neuroinflammation exacerbated by over-activated microglia represent a promising therapeutic strategy for AD [180]. Current research efforts need to identify specific pathways that can be actually and effectively targeted, and therefore, further efforts are needed to understand and manipulate microglia in AD, to further understand the various mechanisms by which microglia counteract AD pathology, and the exact timing when microglia-related interventions need to be applied.
Multi-omics integration of AD
Comprehensive analysis of data obtained from different omic approaches is essential to gain insight into disease pathological processes. Systems biology (SB) is to take as much information as possible at every level and integrate them to explain the interactions between molecules [181]. A multi-omics approach identified AD autoantibodies with disease diagnostic capabilities and demonstrated that autoantibodies against isovaleryl coenzyme A dehydrogenase (IVD), ADD2 and CYFIP1 were able to distinguish AD patients from healthy controls [182]. Madrid, L et al. found that blood and cortical biomarkers showed opposite profiles in APOE2 and APOE4 carriers [183]. A study by Clark, C et al. found that total cysteine, kininogen-1 and cholesteryl ester 27:1 16:0 were associated with AD pathology [128]. Two important genes (ABCA1 and CPT1A) and two proteins (lipocalin and neutrophil gelatinase-associated lipid transport protein (NGAL)) involved in acylcarnitine and amine regulation were identified by Horgusluoglu, E et al. through an integrated multi-omics approach [184]. These cross-omics pathways suggest that the development of AD may be the result of genetic variation with potential cascading effects on the downstream transcriptome and proteome levels as well as on the metabolome. Multi-omics studies provide a better understanding of the disease, from the original cause of the disease to functional consequences or related interactions.
Many recent advances in statistics have made it possible to integrate information from multiple data modalities to thoroughly explore disease-related endophenotypic networks and biological interactions. In this context, multifaceted and integrated research networks applied to AD have been developed. One idea that unifies these different approaches is to use it to address the relationships between multiple biological pathways and to correlate them. The Genome-wide Positioning Systems Platform for Alzheimer's Drug Discovery (AlzGPS) is used to accelerate the discovery of biologically relevant targets and clinically relevant drug candidates for AD by combining systems pharmacology and integrated web-based analysis of multi-omics data [185]. Another similar platform that uses gene regulatory networks as targets for integrating multiple levels of data to identify key disease pathways and driver genes is superior to the former in that it also includes in vitro and in vivo functional validation within the platform [186]. An open-source comparative analysis of computational pipelines called scGRNom (single-cell Gene Regulatory Network prediction from multi-omics) reveals interactions between genomic function, pathways, cell types, and disease, enabling the ability to predict the clinical phenotype of AD from its cellular type disease genes [187]. Considering that AD is a heterogeneous disease, understanding how molecular interactions evolve over time can help determine the optimal time point for biomarker measurements or the time window of therapeutic action of a specific drug, it has been shown that by a comprehensive analysis of multi-omics data with animal genome-scale metabolic models (GEM), that is, by correlating gene expression changes with subsystem and metabolite levels, AD mice show tissue- and time-dependent changes [188], which will also help to translate into diagnostic or therapeutic approaches. In addition, identifying its association with AD-related changes in brain function and structure through integration of multi-omics studies is also an approach to help guide the interpretation of molecular data by providing a global view of the disease [189, 190].
Potential therapeutic approaches based on multi-omics
The omics research has enriched our knowledge of AD at multiple levels, leading us to develop better and more targeted therapies (Table 5).
Table 5.
Potential therapy targets for AD
| Targets | Drugs | Stage | Trial ID | Refs. |
|---|---|---|---|---|
| Genomics and epigenomics | ||||
| ABI3 | – | In vitro | – | [191] |
| ABCA7 | – | – | – | [192] |
| ADAM10 | CRISPR/Cas9 | In vitro | – | [193] |
| ATP6V1A | NCH-51 | In vitro | – | [186] |
| BACE1 | Atabecestat (JNJ-54861911) | IIb | NCT02569398 | [194] |
| Lanabecestat (AZD3293) | I | NCT02005211 | [195] | |
| Verubecestat (MK-8931) | II | NCT01953601 | [196] | |
| Chi3l1/YKL-40 | – | In vitro | – | [196] |
| IL10 | HEK293T cells | In vivo | – | [197] |
| TREM2 | PHSA@PF/pTREM2 | In vitro | – | [180] |
| AL002 | I | NCT03635047 | [198] | |
| TYROBP / DAP12 | – | In vitro | – | [199] |
| UNC5C | HEK293T cells | In vivo | – | [200] |
| H3K27ac, H3K9ac | – | In vitro | – | [56] |
| H3K4me3 | WDR5-0103 | In vitro | – | [201] |
| H3K9me2 | BIX01294 | In vitro | – | [202] |
| Transcriptomics | ||||
| miR-346 | – | In vivo | – | [203] |
| lncRNA XIST | – | In vitro | – | [204] |
| Proteomics | ||||
| AMPKα1 | – | In vitro | – | [205] |
| BDNF | – | In vitro | – | [206] |
| CSF1R |
PLX3397 JNJ-40346527 |
In vitro In vitro |
– – |
[207] [208] |
| Eotaxin-1 | – | – | – | [209] |
| Fyn | Saracatinib | IIa | NCT02167256 | [210] |
| RIPK1 | DNL747 | Ib | NCT03757325 | [211] |
| NEP | PPAR-siSOX9 | In vitro | – | [212] |
| NGF | AAV2-NGF2 | II | NCT00876863 | [213] |
| p38 MAPKβ | VX-745 | In vitro | – | [214] |
| Metabolomics | ||||
| BCAAs | – | – | – | [141] |
| Homocysteine | Vitamin B | III | NCT00056225 | [215] |
| EGCG | – | III | NCT00951834 | [216] |
| LOX | Baicalin | In vitro | – | [217] |
| Oxidative stress products | Vitamin C | I | NCT00117403 | [216] |
| Sterol derivative | NE3107 | III | NCT04669028 | [218] |
“–”The information has not been obtained from the primary publication or has not been found yet
Targeting a specific component of these pathways may bring new directions for drug discovery and treatment. TREM2 is at the forefront of these therapeutic candidates, and a novel TREM2-based treatment for microglia function is currently being clinically tested [198]. Another potential treatment strategy may be based on a special diet or pharmaceutical preparations to maintain the normal level of these metabolites in the early stages of AD [219]. But at the same time, because it is a characteristic of metabolites to affect multiple targets and signaling pathways, which makes it more and more difficult to prove their pharmacological activity when studying pharmaceutical preparations. The results of two large Phase III clinical trials conducted with Aducanumab showed no clinical benefit for AD [220]. In AD, drug trials designed to eliminate toxic amyloid in the brain have so far failed to achieve their main clinical goals. Therefore, targeting the protein co-expression module most relevant to the pathophysiology of AD is a promising method for drug development. Chaperone-mediated autophagy in neurons can maintain protein homeostasis and have a positive impact on Aβ deposition and tau pathology, which is a promising therapeutic approach [221]. What is more, the first anti-tau antibody, semorinemab, did not improve outcomes in a phase II trial in patients with prodromal or mild AD [222]. There are other anti-tau agents that have not yet obtained efficacy data, and we look forward to an inspiring result.
The 2018 ATN criteria were recommended as a standard framework for AD research, which simply combines the three CSF AD biomarkers—Aβ (A), tau (T) and neurodegeneration (N)—to move AD research toward personalized medicine [223]. We believe that the combination of the ATN framework with omics can help high-quality pre-screening of patients for further selection procedures in clinical trials. The potential role of plasma ATN biomarker profiles in clinical trials predicting AD pathology and clinical progression is currently receiving positive results and is considered to act as a therapeutic response marker [224, 225]. The current maximal plasma proteomics study of the ATN framework, which compared plasma protein profiles in various ATN variants and replicated four proteins (FCN2, PAI-1, CRP, and sVCAM1) in relation to the ATN framework and clinical diagnosis of AD, further suggests that these proteins are involved in AD pathological pathways and may be promising targets for future therapy [226]. An MRI-based proteomics study of the ATN framework showed that the underlying biological factors associated with neurodegeneration are different at different levels of disease progression [227]. We know that candidate biomarkers representing "N" encompass different physiological processes, that neurodegeneration is a dynamic process, and that longitudinal measures of neurodegeneration can better capture the brain changes associated with AD. Whereas the multi-omics characterization of neurodegeneration has tremendous value, the proposed ATN framework itself hopes to achieve future flexibility by adding other biomarkers at the time of discovery and validation, the combination with multi-omics can provide information on clinical changes and progression, ensure reliability of biomarker assays, and identify biomarkers with sufficient dynamic range during short-term treatment. In contrast, A and T are considered intermediate targets whose modification may lead to neuroprotection and disease modification through related mechanisms [228], and biomarkers of these related pathological mechanisms identified through multi-omics may play an important role in finding targets for drug therapy for individuals with or at risk of developing AD. Scholars believe that this may change as data accumulate on the impact of treatment on ATN biomarkers and the impact of treatment on clinical outcomes, and that data from emerging biomarker collections may lead to a better understanding of ATN biomarker trajectories and biomarker-clinical outcome measures [229], then multi-omics studies are a very good way to obtain more emerging biomarkers. We believe that this approach will help design clinical trials that stratify subjects by ATN profile, thus enabling multi-omics data to identify biological stages that improve disease.
Although omics is an exciting research to develop treatments for AD, there are still many limiting factors that need to be overcome, the most important one is how to connect the various omics together. At present, the integration of multi-omics and SB in AD is still at an early stage, and the development of SB and gene networks in various molecular systems is relatively mature [230]. Drug development urgently needs a holistic and systematic approach to deal with molecular data, omics data and clinical information, which can effectively provide accurate biomarker-guided targeting methods [8].
The idea of designing AD multi-target drugs (MTD) has become a research hotspot in recent years. Its central goal is to synthesize drugs that combine two or more pharmacophores to simultaneously modulate multiple factors involved in the development of AD, which is more effective in influencing the disease network [231]. There are many studies on neurotransmitter pathways and classical pathological pathways of AD, however, MTD for AD have not achieved the expected effect [232], and most scientists believe that in addition to developing new drugs, redeveloping old drugs, or traditional herbal medicines may a promising treatment [233]. However, it is not clear whether MTD produces a synergistic therapeutic effect. If we can detect the relevant markers that the drug plays a role in the interaction network between the key factors regulating the occurrence and development of AD, it will be of great help for us to evaluate the efficacy or drug design. It is believed that the knowledge transformation from the combination of omics and SB to MTD is expected to bring a breakthrough in the treatment of AD patients in the future.
Conclusions
In general, biomarkers in vivo are critical not only for early diagnosis of diseases but also for prognostic assessment, classification of disease progression and treatment of disease remission. Omics technologies help to provide a more comprehensive view of the molecular pathways involved in disease progression. Through data mining, lists of disease-associated genes are generated and potential biomarkers are discovered through functional enrichment analysis, protein–protein interactions, and analysis of structure and function. Advances in high-throughput technologies have made AD studies more possible and are no longer limited to a single omic analysis, but the data sets generated can be very large and may require increased computational power. Therefore, it is challenging to obtain a large amounts of multi-omics data in a single study. Appropriate bioinformatics tools play an important role in solving this problem. New technologies and platforms have been generated in the course of studies in various omics, a lot of models for predicting or accurately distinguishing AD and control are generated, different work processes and techniques are used to carry out relevant measurements in different studies, so it is necessary to standardize the study design and define a specification to guide the studies in each omics research, and design protocols that are economically parsimonious and capable of finding the best diagnostic performance.
We believed that in the near future, omics-based studies will help to understand how these molecular interactions evolve over time and could help to determine the optimal time point for biomarker measurement or the time window for therapeutic action of a specific drug. The integrated method has great potential in future diagnostic evaluations. Combine omics technology with clinic, developing novel methods that allow clinical trials to be carried out in a pre-clinical stage, and possibly in smaller-scale cohorts, to determine biomarkers that can be easily run in the clinic.
Abbreviations
- AD
Alzheimer's disease
- Aβ
Amyloid beta
- NFTs
Neurofibrillary tangles
- PTM
Posttranslational modification
- CSF
Cerebrospinal fluid
- t-Tau
Total tau
- p-tau
Phosphorylated tau
- PET
Positron emission tomography
- fMRI
Functional magnetic resonance imaging
- FDG
F-fluorodeoxyglucose
- NfL
Neurofilament light chain
- MCI
Mild cognitive impairment
- GWAS
Genome-wide association studies
- SNPs
Single nucleotide polymorphisms
- LOAD
Late-onset AD
- CNVs
Copy number variations
- NGS
Next-generation genome sequencing
- WES
Whole-exome sequencing
- WGS
Whole-genome sequencing
- PSEN1
Presenile hormone 1
- PSEN2
Presenile hormone 2
- EOAD
Early-onset AD
- PiB
Pittsburgh compound-B
- EWAS
Epigenome-wide association study
- scRNA-seq
Single-cell RNA sequencing
- cRNA
Protein-coding RNA
- ncRNA
Non-protein-coding RNA
- TWAS
Transcriptome-wide association studies
- lncRNAs
Long non-coding RNAs
- miRNAs
MicroRNAs
- circRNAs
Circulating RNAs
- piRNAs
PIWI-interacting RNAs
- NAT
Natural antisense transcripts
- BBB
Blood–brain barrier
- NMR
Nuclear magnetic resonance
- MS
Mass spectrometry
- LCTs
Long-chain triglycerides
- ChoEs
Cholesteryl esters
- DAM
Disease-associated microglia
- HAM
Human Alzheimer's microglia
- IPSC
Induced pluripotent stem cell
- SB
Systems biology
- GEM
Genome-scale metabolic models
- MTD
Multi-target drugs
Author contributions
JTY proposed the idea and constructed the structure of the review, as well as revising the manuscript. QA performed the literature search and manuscript writing. ZTW, KMW, XYH and QD participated the literature research and made all illustration. All authors read and approved the final.
Funding
This study was supported by grants from the National Natural Science Foundation of China (82071201,81971032), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01), Shanghai Talent Development Funding for The Project (2019074), Research Start-up Fund of Huashan Hospital (2022QD002), Excellence 2025 Talent Cultivation Program at Fudan University (3030277001), and ZHANGJIANG LAB, Tianqiao and Chrissy Chen Institute, and the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, Fudan University.
Data availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All participants were properly consented.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Molinuevo JL, Ayton S, Batrla R et al (2018) Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol 136(6):821–853. 10.1007/s00401-018-1932-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheltens P, De Strooper B, Kivipelto M et al (2021) Alzheimer’s disease. Lancet (London, England) 397(10284):1577–1590. 10.1016/S0140-6736(20)32205-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Millan MJ (2017) Linking deregulation of non-coding RNA to the core pathophysiology of Alzheimer’s disease: an integrative review. Prog Neurobiol. 10.1016/j.pneurobio.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 4.Bishop NA, Lu T, Yankner BA (2010) Neural mechanisms of ageing and cognitive decline. Nature 464(7288):529–535. 10.1038/nature08983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang EF, Hou Y, Palikaras K et al (2019) Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci 22(3):401–412. 10.1038/s41593-018-0332-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lautrup S, Sinclair DA, Mattson MP et al (2019) NAD in brain aging and neurodegenerative disorders. Cell Metab 30(4):630–655. 10.1016/j.cmet.2019.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peña-Bautista C, Baquero M, Vento M et al (2019) Omics-based biomarkers for the early Alzheimer disease diagnosis and reliable therapeutic targets development. Curr Neuropharmacol 17(7):630–647. 10.2174/1570159X16666180926123722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampel H, Nisticò R, Seyfried NT et al (2021) Omics sciences for systems biology in Alzheimer’s disease: state-of-the-art of the evidence. Ageing Res Rev. 10.1016/j.arr.2021.101346 [DOI] [PubMed] [Google Scholar]
- 9.Pimenova AA, Raj T, Goate AM (2018) Untangling genetic risk for Alzheimer’s disease. Biol Psychiat 83(4):300–310. 10.1016/j.biopsych.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mostafavi S, Gaiteri C, Sullivan SE et al (2018) A molecular network of the aging human brain provides insights into the pathology and cognitive decline of Alzheimer’s disease. Nat Neurosci 21(6):811–819. 10.1038/s41593-018-0154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wesseling H, Mair W, Kumar M et al (2020) Tau PTM profiles identify patient heterogeneity and stages of Alzheimer’s disease. Cell. 10.1016/j.cell.2020.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahajan UV, Varma VR, Griswold ME et al (2020) Dysregulation of multiple metabolic networks related to brain transmethylation and polyamine pathways in Alzheimer disease: a targeted metabolomic and transcriptomic study. PLoS Med 17(1):e1003012. 10.1371/journal.pmed.1003012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Márquez F, Yassa MA (2019) Neuroimaging biomarkers for Alzheimer’s disease. Mol Neurodegener 14(1):21. 10.1186/s13024-019-0325-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zetterberg H, Burnham SC (2019) Blood-based molecular biomarkers for Alzheimer’s disease. Mol Brain 12(1):26. 10.1186/s13041-019-0448-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schonhaut DR, McMillan CT, Spina S et al (2017) F-flortaucipir tau positron emission tomography distinguishes established progressive supranuclear palsy from controls and Parkinson disease: a multicenter study. Ann Neurol 82(4):622–634. 10.1002/ana.25060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mapstone M, Cheema AK, Fiandaca MS et al (2014) Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med 20(4):415–418. 10.1038/nm.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M, Beckmann ND, Roussos P et al (2018) The Mount Sinai cohort of large-scale genomic, transcriptomic and proteomic data in Alzheimer’s disease. Sci Data. 10.1038/sdata.2018.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serrano-Pozo A, Das S, Hyman BT (2021) APOE and Alzheimer’s disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol 20(1):68–80. 10.1016/S1474-4422(20)30412-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Rojas I, Moreno-Grau S, Tesi N et al (2021) Common variants in Alzheimer’s disease and risk stratification by polygenic risk scores. Nat Commun 12(1):3417. 10.1038/s41467-021-22491-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuperlovic-Culf M, Badhwar A (2020) Recent advances from metabolomics and lipidomics application in Alzheimer’s disease inspiring drug discovery. Expert Opin Drug Discov 15(3):319–331. 10.1080/17460441.2020.1674808 [DOI] [PubMed] [Google Scholar]
- 21.Kunkle BW, Grenier-Boley B, Sims R et al (2019) Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet 51(3):414–430. 10.1038/s41588-019-0358-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansen IE, Savage JE, Watanabe K et al (2020) Author Correction: Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet 52(3):354. 10.1038/s41588-019-0573-x [DOI] [PubMed] [Google Scholar]
- 23.Bertram L, Tanzi RE (2019) Alzheimer disease risk genes: 29 and counting. Nat Rev Neurol 15(4):191–192. 10.1038/s41582-019-0158-4 [DOI] [PubMed] [Google Scholar]
- 24.Dourlen P, Kilinc D, Malmanche N et al (2019) The new genetic landscape of Alzheimer’s disease: from amyloid cascade to genetically driven synaptic failure hypothesis? Acta Neuropathol 138(2):221–236. 10.1007/s00401-019-02004-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y, Jun GR, Zhang X et al (2019) Analysis of whole-exome sequencing data for Alzheimer disease stratified by APOE genotype. JAMA Neurol. 10.1001/jamaneurol.2019.1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartzentruber J, Cooper S, Liu JZ et al (2021) Genome-wide meta-analysis, fine-mapping and integrative prioritization implicate new Alzheimer’s disease risk genes. Nat Genet 53(3):392–402. 10.1038/s41588-020-00776-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wightman DP, Jansen IE, Savage JE et al (2021) A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat Genet 53(9):1276–1282. 10.1038/s41588-021-00921-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunkle BW, Grenier-Boley B, Sims R et al (2019) Author Correction: genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat Genet 51(9):1423–1424. 10.1038/s41588-019-0495-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaiteri C, Mostafavi S, Honey CJ et al (2016) Genetic variants in Alzheimer disease - molecular and brain network approaches. Nat Rev Neurol 12(7):413–427. 10.1038/nrneurol.2016.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleineidam L, Chouraki V, Próchnicki T et al (2020) PLCG2 protective variant p.P522R modulates tau pathology and disease progression in patients with mild cognitive impairment. Acta Neuropathol 139(6):1025–1044. 10.1007/s00401-020-02138-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuddy LK, Prokopenko D, Cunningham EP et al (2020) Aβ-accelerated neurodegeneration caused by Alzheimer’s-associated variant R1279Q is rescued by angiotensin system inhibition in mice. Sci Transl Med. 10.1126/scitranslmed.aaz2541 [DOI] [PubMed] [Google Scholar]
- 32.Prokopenko D, Lee S, Hecker J et al (2022) Region-based analysis of rare genomic variants in whole-genome sequencing datasets reveal two novel Alzheimer’s disease-associated genes: DTNB and DLG2. Mol Psychiatry. 10.1038/s41380-022-01475-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann A, Kucukali F, Bos I et al (2022) Rare variants in IFFO1, DTNB, NLRC3 and SLC22A10 associate with Alzheimer’s disease CSF profile of neuronal injury and inflammation. Mol Psychiatry. 10.1038/s41380-022-01437-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ming C, Wang M, Wang Q et al (2021) Whole genome sequencing-based copy number variations reveal novel pathways and targets in Alzheimer’s disease. Alzheimers Dement. 10.1002/alz.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Z, Le Guen Y, Liu L et al (2021) Genome-wide analysis of common and rare variants via multiple knockoffs at biobank scale, with an application to Alzheimer disease genetics. Am J Hum Genet 108(12):2336–2353. 10.1016/j.ajhg.2021.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tosto G, Vardarajan B, Sariya S et al (2019) Association of variants in PINX1 and TREM2 with late-onset Alzheimer disease. JAMA Neurol. 10.1001/jamaneurol.2019.1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bis JC, Jian X, Kunkle BW et al (2020) Whole exome sequencing study identifies novel rare and common Alzheimer’s-Associated variants involved in immune response and transcriptional regulation. Mol Psychiatry 25(8):1859–1875. 10.1038/s41380-018-0112-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunkle BW, Schmidt M, Klein H-U et al (2021) Novel Alzheimer Disease risk loci and pathways in African American individuals using the African genome resources panel: a meta-analysis. JAMA Neurol 78(1):102–113. 10.1001/jamaneurol.2020.3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia L, Li F, Wei C et al (2021) Prediction of Alzheimer’s disease using multi-variants from a Chinese genome-wide association study. Brain 144(3):924–937. 10.1093/brain/awaa364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shigemizu D, Asanomi Y, Akiyama S et al (2022) Whole-genome sequencing reveals novel ethnicity-specific rare variants associated with Alzheimer’s disease. Mol Psychiatry. 10.1038/s41380-022-01483-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Y, Felsky D, Reyes-Dumeyer D et al (2021) Integration of GWAS and brain transcriptomic analyses in a multiethnic sample of 35,245 older adults identifies DCDC2 gene as predictor of episodic memory maintenance. Alzheimers Dement. 10.1002/alz.12524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruni AC, Bernardi L, Gabelli C (2020) From beta amyloid to altered proteostasis in Alzheimer’s disease. Ageing Res Rev. 10.1016/j.arr.2020.101126 [DOI] [PubMed] [Google Scholar]
- 43.De Roeck A, Van Broeckhoven C, Sleegers K (2019) The role of ABCA7 in Alzheimer’s disease: evidence from genomics, transcriptomics and methylomics. Acta Neuropathol 138(2):201–220. 10.1007/s00401-019-01994-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arboleda-Velasquez JF, Lopera F, O’Hare M et al (2019) Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nat Med 25(11):1680–1683. 10.1038/s41591-019-0611-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eysert F, Coulon A, Boscher E et al (2020) Alzheimer’s genetic risk factor FERMT2 (Kindlin-2) controls axonal growth and synaptic plasticity in an APP-dependent manner. Mol Psychiatry. 10.1038/s41380-020-00926-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo R, Fan Y, Yang J et al (2021) A novel missense variant in ACAA1 contributes to early-onset Alzheimer’s disease, impairs lysosomal function, and facilitates amyloid-beta pathology and cognitive decline. Signal Transduct Target Ther 6(1):325. 10.1038/s41392-021-00748-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan Q, Nho K, Del-Aguila JL et al (2021) Genome-wide association study of brain amyloid deposition as measured by Pittsburgh Compound-B (PiB)-PET imaging. Mol Psychiatry 26(1):309–321. 10.1038/s41380-018-0246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goedert M (2020) Tau proteinopathies and the prion concept. Prog Mol Biol Transl Sci. 10.1016/bs.pmbts.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 49.Mathys H, Davila-Velderrain J, Peng Z et al (2019) Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570(7761):332–337. 10.1038/s41586-019-1195-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semick SA, Bharadwaj RA, Collado-Torres L et al (2019) Integrated DNA methylation and gene expression profiling across multiple brain regions implicate novel genes in Alzheimer’s disease. Acta Neuropathol 137(4):557–569. 10.1007/s00401-019-01966-5 [DOI] [PubMed] [Google Scholar]
- 51.Smith RG, Pishva E, Shireby G et al (2021) A meta-analysis of epigenome-wide association studies in Alzheimer’s disease highlights novel differentially methylated loci across cortex. Nat Commun 12(1):3517. 10.1038/s41467-021-23243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corces MR, Shcherbina A, Kundu S et al (2020) Single-cell epigenomic analyses implicate candidate causal variants at inherited risk loci for Alzheimer’s and Parkinson’s diseases. Nat Genet 52(11):1158–1168. 10.1038/s41588-020-00721-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novikova G, Kapoor M, Tcw J et al (2021) Integration of Alzheimer’s disease genetics and myeloid genomics identifies disease risk regulatory elements and genes. Nat Commun 12(1):1610. 10.1038/s41467-021-21823-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Y, Yu L, Olah M et al (2022) Epigenomic features related to microglia are associated with attenuated effect of APOE epsilon4 on Alzheimer’s disease risk in humans. Alzheimers Dement 18(4):688–699. 10.1002/alz.12425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raj T, Li YI, Wong G et al (2018) Integrative transcriptome analyses of the aging brain implicate altered splicing in Alzheimer’s disease susceptibility. Nat Genet 50(11):1584–1592. 10.1038/s41588-018-0238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nativio R, Lan Y, Donahue G et al (2020) An integrated multi-omics approach identifies epigenetic alterations associated with Alzheimer’s disease. Nat Genet 52(10):1024–1035. 10.1038/s41588-020-0696-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neuner SM, Heuer SE, Huentelman MJ et al (2019) Harnessing genetic complexity to enhance translatability of Alzheimer’s disease mouse models: a path toward precision medicine. Neuron. 10.1016/j.neuron.2018.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nitsche A, Arnold C, Ueberham U et al (2020) Alzheimer-related genes show accelerated evolution. Mol Psychiatry. 10.1038/s41380-020-0680-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crist AM, Hinkle KM, Wang X et al (2021) Transcriptomic analysis to identify genes associated with selective hippocampal vulnerability in Alzheimer’s disease. Nat Commun 12(1):2311. 10.1038/s41467-021-22399-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelley KW, Nakao-Inoue H, Molofsky AV et al (2018) Variation among intact tissue samples reveals the core transcriptional features of human CNS cell classes. Nat Neurosci 21(9):1171–1184. 10.1038/s41593-018-0216-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neff RA, Wang M, Vatansever S et al (2021) Molecular subtyping of Alzheimer’s disease using RNA sequencing data reveals novel mechanisms and targets. Sci Adv. 10.1126/sciadv.abb5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gockley J, Montgomery KS, Poehlman WL et al (2021) Multi-tissue neocortical transcriptome-wide association study implicates 8 genes across 6 genomic loci in Alzheimer’s disease. Genome Med 13(1):76. 10.1186/s13073-021-00890-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koch L (2018) Altered splicing in Alzheimer transcriptomes. Nat Rev Genet 19(12):738–739. 10.1038/s41576-018-0064-4 [DOI] [PubMed] [Google Scholar]
- 64.Kurt S, Tomatir AG, Tokgun PE et al (2020) Altered expression of long non-coding RNAs in peripheral blood mononuclear cells of patients with Alzheimer’s disease. Mol Neurobiol 57(12):5352–5361. 10.1007/s12035-020-02106-x [DOI] [PubMed] [Google Scholar]
- 65.Meira-Strejevitch CS, Pereira IS, Hippolito DDC et al (2020) Ocular toxoplasmosis associated with up-regulation of miR-155-5p/miR-29c-3p and down-regulation of miR-21-5p/miR-125b-5p. Cytokine. 10.1016/j.cyto.2020.154990 [DOI] [PubMed] [Google Scholar]
- 66.Kenny A, McArdle H, Calero M et al (2019) Elevated Plasma microRNA-206 levels predict cognitive decline and progression to dementia from mild cognitive impairment. Biomolecules. 10.3390/biom9110734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhuang J, Cai P, Chen Z et al (2020) Long noncoding RNA MALAT1 and its target microRNA-125b are potential biomarkers for Alzheimer’s disease management via interactions with FOXQ1, PTGS2 and CDK5. Am J Transl Res 12(9):5940–5954 [PMC free article] [PubMed] [Google Scholar]
- 68.Sala Frigerio C, Lau P, Salta E et al (2013) Reduced expression of hsa-miR-27a-3p in CSF of patients with Alzheimer disease. Neurology 81(24):2103–2106. 10.1212/01.wnl.0000437306.37850.22 [DOI] [PubMed] [Google Scholar]
- 69.Cortini F, Roma F, Villa C (2019) Emerging roles of long non-coding RNAs in the pathogenesis of Alzheimer’s disease. Ageing Res Rev. 10.1016/j.arr.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 70.Zhou X, Xu J (2015) Identification of Alzheimer’s disease-associated long noncoding RNAs. Neurobiol Aging 36(11):2925–2931. 10.1016/j.neurobiolaging.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 71.Wu Y-Y, Kuo H-C (2020) Functional roles and networks of non-coding RNAs in the pathogenesis of neurodegenerative diseases. J Biomed Sci 27(1):49. 10.1186/s12929-020-00636-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Felice B, Montanino C, Oliva M et al (2020) MicroRNA expression signature in mild cognitive impairment due to Alzheimer’s disease. Mol Neurobiol 57(11):4408–4416. 10.1007/s12035-020-02029-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J, Chen C, Zhang Y (2020) An investigation of microRNA-103 and microRNA-107 as potential blood-based biomarkers for disease risk and progression of Alzheimer’s disease. J Clin Lab Anal 34(1):e23006. 10.1002/jcla.23006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Y, Zhang Y, Zhang L et al (2019) The potential markers of circulating microRNAs and long non-coding RNAs in Alzheimer’s disease. Aging Dis 10(6):1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bekris LM, Lutz F, Montine TJ et al (2013) MicroRNA in Alzheimer’s disease: an exploratory study in brain, cerebrospinal fluid and plasma. Biomarkers 18(5):455–466. 10.3109/1354750X.2013.814073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi Y, Liu H, Yang C et al (2020) Transcriptomic analyses for identification and prioritization of genes associated with Alzheimer’s disease in humans. Front Bioeng Biotechnol. 10.3389/fbioe.2020.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Annese A, Manzari C, Lionetti C et al (2018) Whole transcriptome profiling of late-onset Alzheimer’s disease patients provides insights into the molecular changes involved in the disease. Sci Rep 8(1):4282. 10.1038/s41598-018-22701-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huynh RA, Mohan C (2017) Alzheimer’s disease: biomarkers in the genome, blood, and cerebrospinal fluid. Front Neurol. 10.3389/fneur.2017.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Readhead B, Haure-Mirande J-V, Mastroeni D et al (2020) miR155 regulation of behavior, neuropathology, and cortical transcriptomics in Alzheimer’s disease. Acta Neuropathol 140(3):295–315. 10.1007/s00401-020-02185-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng L, Vella LJ, Barnham KJ et al (2020) Small RNA fingerprinting of Alzheimer’s disease frontal cortex extracellular vesicles and their comparison with peripheral extracellular vesicles. J Extracellular Vesicles 9(1):1766822. 10.1080/20013078.2020.1766822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen W-T, Lu A, Craessaerts K et al (2020) Spatial transcriptomics and in situ sequencing to study Alzheimer’s disease. Cell. 10.1016/j.cell.2020.06.038 [DOI] [PubMed] [Google Scholar]
- 82.Westwood S, Baird AL, Hye A et al (2018) plasma protein biomarkers for the prediction of CSF amyloid and tau and [F]-Flutemetamol PET scan result. Front Aging Neurosci. 10.3389/fnagi.2018.00409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park JC, Han SH, Lee H et al (2019) Prognostic plasma protein panel for Abeta deposition in the brain in Alzheimer’s disease. Prog Neurobiol. 10.1016/j.pneurobio.2019.101690 [DOI] [PubMed] [Google Scholar]
- 84.Elahi FM, Casaletto KB, La Joie R et al (2020) Plasma biomarkers of astrocytic and neuronal dysfunction in early- and late-onset Alzheimer’s disease. Alzheimers Dement 16(4):681–695. 10.1016/j.jalz.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rehiman SH, Lim SM, Neoh CF et al (2020) Proteomics as a reliable approach for discovery of blood-based Alzheimer’s disease biomarkers: a systematic review and meta-analysis. Ageing Res Rev. 10.1016/j.arr.2020.101066 [DOI] [PubMed] [Google Scholar]
- 86.Whelan CD, Mattsson N, Nagle MW et al (2019) Multiplex proteomics identifies novel CSF and plasma biomarkers of early Alzheimer’s disease. Acta Neuropathol Commun 7(1):169. 10.1186/s40478-019-0795-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tijms BM, Gobom J, Reus L et al (2020) Pathophysiological subtypes of Alzheimer’s disease based on cerebrospinal fluid proteomics. Brain 143(12):3776–3792. 10.1093/brain/awaa325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blennow K, Chen C, Cicognola C et al (2020) Cerebrospinal fluid tau fragment correlates with tau PET: a candidate biomarker for tangle pathology. Brain 143(2):650–660. 10.1093/brain/awz346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.An Y, Varma VR, Varma S et al (2018) Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimer’s Dementia 14(3):318–329. 10.1016/j.jalz.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pires G, McElligott S, Drusinsky S et al (2019) Secernin-1 is a novel phosphorylated tau binding protein that accumulates in Alzheimer’s disease and not in other tauopathies. Acta Neuropathol Commun 7(1):195. 10.1186/s40478-019-0848-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perez-Grijalba V, Pesini P, Allue JA et al (2015) Abeta1-17 is a major amyloid-beta fragment isoform in cerebrospinal fluid and blood with possible diagnostic value in Alzheimer’s disease. J Alzheimers Dis 43(1):47–56. 10.3233/JAD-140156 [DOI] [PubMed] [Google Scholar]
- 92.Remnestal J, Just D, Mitsios N et al (2016) CSF profiling of the human brain enriched proteome reveals associations of neuromodulin and neurogranin to Alzheimer’s disease. Proteomics Clin Appl 10(12):1242–1253. 10.1002/prca.201500150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Talwar P, Gupta R, Kushwaha S et al (2019) Viral induced oxidative and inflammatory response in Alzheimer’s disease pathogenesis with identification of potential drug candidates: a systematic review using systems biology approach. Curr Neuropharmacol 17(4):352–365. 10.2174/1570159X16666180419124508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jensen CS, Bahl JM, Ostergaard LB et al (2019) Exercise as a potential modulator of inflammation in patients with Alzheimer’s disease measured in cerebrospinal fluid and plasma. Exp Gerontol. 10.1016/j.exger.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 95.Gyorffy BA, Toth V, Torok G et al (2020) Synaptic mitochondrial dysfunction and septin accumulation are linked to complement-mediated synapse loss in an Alzheimer’s disease animal model. Cell Mol Life Sci 77(24):5243–5258. 10.1007/s00018-020-03468-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bai B, Wang X, Li Y et al (2020) Deep multilayer brain proteomics identifies molecular networks in Alzheimer’s disease progression. Neuron. 10.1016/j.neuron.2019.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jin Y, Chifodya K, Han G et al (2021) High-density lipoprotein in Alzheimer’s disease: From potential biomarkers to therapeutics. J Control Release. 10.1016/j.jconrel.2021.08.018 [DOI] [PubMed] [Google Scholar]
- 98.Bonham LW, Geier EG, Steele NZR et al (2018) Insulin-like growth factor binding protein 2 is associated with biomarkers of Alzheimer’s disease pathology and shows differential expression in transgenic mice. Front Neurosci. 10.3389/fnins.2018.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lan J, Núñez Galindo A, Doecke J et al (2018) Systematic evaluation of the use of human plasma and serum for mass-spectrometry-based shotgun proteomics. J Proteome Res 17(4):1426–1435. 10.1021/acs.jproteome.7b00788 [DOI] [PubMed] [Google Scholar]
- 100.Shi L, Westwood S, Baird AL et al (2019) Discovery and validation of plasma proteomic biomarkers relating to brain amyloid burden by SOMAscan assay. Alzheimer’s Dementia 15(11):1478–1488. 10.1016/j.jalz.2019.06.4951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lindbohm JV, Mars N, Walker KA et al (2022) Plasma proteins, cognitive decline, and 20-year risk of dementia in the Whitehall II and atherosclerosis risk in communities studies. Alzheimers Dement 18(4):612–624. 10.1002/alz.12419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ashton NJ, Nevado-Holgado AJ, Barber IS et al (2019) A plasma protein classifier for predicting amyloid burden for preclinical Alzheimer’s disease. Sci Adv 5(2):eaau7220. 10.1126/sciadv.aau7220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiang Y, Zhou X, Ip FC et al (2021) Large-scale plasma proteomic profiling identifies a high-performance biomarker panel for Alzheimer’s disease screening and staging. Alzheimer’s Dementia. 10.1002/alz.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dayon L, Núñez Galindo A, Wojcik J et al (2018) Alzheimer disease pathology and the cerebrospinal fluid proteome. Alzheimer’s Res Ther 10(1):66. 10.1186/s13195-018-0397-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Visser PJ, Reus LM, Gobom J et al (2022) Cerebrospinal fluid tau levels are associated with abnormal neuronal plasticity markers in Alzheimer’s disease. Mol Neurodegener 17(1):27. 10.1186/s13024-022-00521-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Higginbotham L, Ping L, Dammer EB et al (2020) Integrated proteomics reveals brain-based cerebrospinal fluid biomarkers in asymptomatic and symptomatic Alzheimer’s disease. Sci Adv. 10.1126/sciadv.aaz9360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Libiger O, Shaw LM, Watson MH et al (2021) Longitudinal CSF proteomics identifies NPTX2 as a prognostic biomarker of Alzheimer’s disease. Alzheimer’s Dementia. 10.1002/alz.12353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mendonça CF, Kuras M, Nogueira FCS et al (2019) Proteomic signatures of brain regions affected by tau pathology in early and late stages of Alzheimer’s disease. Neurobiol Dis. 10.1016/j.nbd.2019.104509 [DOI] [PubMed] [Google Scholar]
- 109.Metaxas A, Thygesen C, Kempf SJ et al (2019) Ageing and amyloidosis underlie the molecular and pathological alterations of tau in a mouse model of familial Alzheimer’s disease. Sci Rep 9(1):15758. 10.1038/s41598-019-52357-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Drummond E, Pires G, MacMurray C et al (2020) Phosphorylated tau interactome in the human Alzheimer’s disease brain. Brain 143(9):2803–2817. 10.1093/brain/awaa223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu J, Patassini S, Rustogi N et al (2019) Regional protein expression in human Alzheimer’s brain correlates with disease severity. Commun Biol. 10.1038/s42003-018-0254-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johnson ECB, Dammer EB, Duong DM et al (2020) Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat Med 26(5):769–780. 10.1038/s41591-020-0815-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hampel H, Goetzl EJ, Kapogiannis D et al (2019) Biomarker-drug and liquid biopsy co-development for disease staging and targeted therapy: cornerstones for Alzheimer’s precision medicine and pharmacology. Front Pharmacol. 10.3389/fphar.2019.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Verkhratsky A, Zorec R (2020) Large-scale proteomics highlights glial role in neurodegeneration. Cell Metab 32(1):11–12. 10.1016/j.cmet.2020.06.001 [DOI] [PubMed] [Google Scholar]
- 115.Li X, Tsolis KC, Koper MJ et al (2021) Sequence of proteome profiles in preclinical and symptomatic Alzheimer’s disease. Alzheimer’s Dementia. 10.1002/alz.12345 [DOI] [PubMed] [Google Scholar]
- 116.Johnson ECB, Carter EK, Dammer EB et al (2022) Large-scale deep multi-layer analysis of Alzheimer’s disease brain reveals strong proteomic disease-related changes not observed at the RNA level. Nat Neurosci 25(2):213–225. 10.1038/s41593-021-00999-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hadjidemetriou M, Rivers-Auty J, Papafilippou L et al (2021) Nanoparticle-enabled enrichment of longitudinal blood proteomic fingerprints in Alzheimer’s disease. ACS Nano 15(4):7357–7369. 10.1021/acsnano.1c00658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.González-Domínguez R, García-Barrera T, Gómez-Ariza JL (2015) Metabolite profiling for the identification of altered metabolic pathways in Alzheimer’s disease. J Pharm Biomed Anal. 10.1016/j.jpba.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 119.Varma VR, Oommen AM, Varma S et al (2018) Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: a targeted metabolomics study. PLoS Med 15(1):e1002482. 10.1371/journal.pmed.1002482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Toledo JB, Arnold M, Kastenmüller G et al (2017) Metabolic network failures in Alzheimer’s disease: a biochemical road map. Alzheimer’s Dementia 13(9):965–984. 10.1016/j.jalz.2017.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Proitsi P, Kim M, Whiley L et al (2015) Plasma lipidomics analysis finds long chain cholesteryl esters to be associated with Alzheimer’s disease. Transl Psychiatry. 10.1038/tp.2014.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shao Y, Ouyang Y, Li T et al (2020) Alteration of Metabolic Profile and Potential Biomarkers in the Plasma of Alzheimer’s Disease. Aging Dis 11(6):1459–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tynkkynen J, Chouraki V, van der Lee SJ et al (2018) Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer’s disease: a prospective study in eight cohorts. Alzheimer’s Dementia 14(6):723–733. 10.1016/j.jalz.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chouraki V, Preis SR, Yang Q et al (2017) Association of amine biomarkers with incident dementia and Alzheimer’s disease in the Framingham Study. Alzheimers Dement 13(12):1327–1336. 10.1016/j.jalz.2017.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang G, Zhou Y, Huang FJ et al (2014) Plasma metabolite profiles of Alzheimer’s disease and mild cognitive impairment. J Proteome Res 13(5):2649–2658. 10.1021/pr5000895 [DOI] [PubMed] [Google Scholar]
- 126.Chatterjee P, Cheong YJ, Bhatnagar A et al (2021) Plasma metabolites associated with biomarker evidence of neurodegeneration in cognitively normal older adults. J Neurochem 159(2):389–402. 10.1111/jnc.15128 [DOI] [PubMed] [Google Scholar]
- 127.Ibáñez C, Simó C, Barupal DK et al (2013) A new metabolomic workflow for early detection of Alzheimer’s disease. J Chromatogr A. 10.1016/j.chroma.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 128.Clark C, Dayon L, Masoodi M et al (2021) An integrative multi-omics approach reveals new central nervous system pathway alterations in Alzheimer’s disease. Alzheimers Res Ther 13(1):71. 10.1186/s13195-021-00814-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Snowden SG, Ebshiana AA, Hye A et al (2017) Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: a nontargeted metabolomic study. PLoS Med 14(3):e1002266. 10.1371/journal.pmed.1002266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Akyol S, Ugur Z, Yilmaz A et al (2021) Lipid profiling of Alzheimer’s disease brain highlights enrichment in glycerol(phospho)lipid, and sphingolipid metabolism. Cells. 10.3390/cells10102591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jasbi P, Shi X, Chu P et al (2021) Metabolic profiling of neocortical tissue discriminates Alzheimer’s disease from mild cognitive impairment, high pathology controls, and normal controls. J Proteome Res 20(9):4303–4317. 10.1021/acs.jproteome.1c00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Trushina E, Dutta T, Persson XM et al (2013) Identification of altered metabolic pathways in plasma and CSF in mild cognitive impairment and Alzheimer’s disease using metabolomics. PLoS ONE 8(5):e63644. 10.1371/journal.pone.0063644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fonteh AN, Harrington RJ, Tsai A et al (2007) Free amino acid and dipeptide changes in the body fluids from Alzheimer’s disease subjects. Amino Acids 32(2):213–224. 10.1007/s00726-006-0409-8 [DOI] [PubMed] [Google Scholar]
- 134.Ennis GE, An Y, Resnick SM et al (2017) Long-term cortisol measures predict Alzheimer disease risk. Neurology 88(4):371–378. 10.1212/WNL.0000000000003537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Garcia-Blanco A, Pena-Bautista C, Oger C et al (2018) Reliable determination of new lipid peroxidation compounds as potential early Alzheimer Disease biomarkers. Talanta. 10.1016/j.talanta.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 136.Whiley L, Chappell KE, D’Hondt E et al (2021) Metabolic phenotyping reveals a reduction in the bioavailability of serotonin and kynurenine pathway metabolites in both the urine and serum of individuals living with Alzheimer’s disease. Alzheimers Res Ther 13(1):20. 10.1186/s13195-020-00741-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu L, Han Y, Zheng Z et al (2021) Altered gut microbial metabolites in amnestic mild cognitive impairment and Alzheimer’s disease: signals in host-microbe interplay. Nutrients. 10.3390/nu13010228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Flanagan E, Lamport D, Brennan L et al (2020) Nutrition and the ageing brain: moving towards clinical applications. Ageing Res Rev. 10.1016/j.arr.2020.101079 [DOI] [PubMed] [Google Scholar]
- 139.Proitsi P, Kim M, Whiley L et al (2017) Association of blood lipids with Alzheimer’s disease: a comprehensive lipidomics analysis. Alzheimer’s Dementia 13(2):140–151. 10.1016/j.jalz.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 140.Huynh K, Lim WLF, Giles C et al (2020) Concordant peripheral lipidome signatures in two large clinical studies of Alzheimer’s disease. Nat Commun 11(1):5698. 10.1038/s41467-020-19473-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Polis B, Samson AO (2020) Role of the metabolism of branched-chain amino acids in the development of Alzheimer’s disease and other metabolic disorders. Neural Regen Res 15(8):1460–1470. 10.4103/1673-5374.274328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Varma VR, Wang Y, An Y et al (2021) Bile acid synthesis, modulation, and dementia: a metabolomic, transcriptomic, and pharmacoepidemiologic study. PLoS Med 18(5):e1003615. 10.1371/journal.pmed.1003615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Demarest TG, Varma VR, Estrada D et al (2020) Biological sex and DNA repair deficiency drive Alzheimer’s disease via systemic metabolic remodeling and brain mitochondrial dysfunction. Acta Neuropathol 140(1):25–47. 10.1007/s00401-020-02152-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu J, Green R, Kim M et al (2021) Sex-specific metabolic pathways were associated with Alzheimer’s Disease (AD) endophenotypes in the European Medical Information framework for AD multimodal biomarker discovery cohort. Biomedicines. 10.3390/biomedicines9111610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Arnold M, Nho K, Kueider-Paisley A et al (2020) Sex and APOE ε4 genotype modify the Alzheimer’s disease serum metabolome. Nat Commun 11(1):1148. 10.1038/s41467-020-14959-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sun L, Guo D, Jia Y et al (2022) Association between human blood metabolome and the risk of Alzheimer’s disease. Ann Neurol. 10.1002/ana.26464 [DOI] [PubMed] [Google Scholar]
- 147.Huang SY, Yang YX, Zhang YR et al (2022) Investigating causal relations between circulating metabolites and Alzheimer’s Disease: a Mendelian randomization study. J Alzheimers Dis 87(1):463–477. 10.3233/JAD-220050 [DOI] [PubMed] [Google Scholar]
- 148.Bhawal R, Fu Q, Anderson ET et al (2021) Serum metabolomic and lipidomic profiling reveals novel biomarkers of efficacy for benfotiamine in Alzheimer’s disease. Int J Mol Sci. 10.3390/ijms222413188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chang R, Trushina E, Zhu K et al (2022) Predictive metabolic networks reveal sex- and APOE genotype-specific metabolic signatures and drivers for precision medicine in Alzheimer’s disease. Alzheimers Dement. 10.1002/alz.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.He S, Granot-Hershkovitz E, Zhang Y et al (2022) Blood metabolites predicting mild cognitive impairment in the study of Latinos-investigation of neurocognitive aging (HCHS/SOL). Alzheimers Dement (Amst) 14(1):e12259. 10.1002/dad2.12259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Khan MJ, Chung NA, Hansen S et al (2022) Targeted lipidomics to measure phospholipids and sphingomyelins in plasma: a pilot study to understand the impact of race/ethnicity in Alzheimer’s disease. Anal Chem 94(10):4165–4174. 10.1021/acs.analchem.1c03821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van der Velpen V, Teav T, Gallart-Ayala H et al (2019) Systemic and central nervous system metabolic alterations in Alzheimer’s disease. Alzheimer’s Res Ther 11(1):93. 10.1186/s13195-019-0551-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dong R, Denier-Fields DN, Lu Q et al (2022) Principal components from untargeted cerebrospinal fluid metabolomics associated with Alzheimer’s disease biomarkers. Neurobiol Aging. 10.1016/j.neurobiolaging.2022.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Eldridge RC, Uppal K, Shokouhi M et al (2021) Multiomics analysis of structural magnetic resonance imaging of the brain and cerebrospinal fluid metabolomics in cognitively normal and impaired adults. Front Aging Neurosci. 10.3389/fnagi.2021.796067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yi L, Liu W, Wang Z et al (2017) Characterizing Alzheimer’s disease through metabolomics and investigating anti-Alzheimer’s disease effects of natural products. Ann N Y Acad Sci 1398(1):130–141. 10.1111/nyas.13385 [DOI] [PubMed] [Google Scholar]
- 156.van der Kant R, Langness VF, Herrera CM et al (2019) Cholesterol metabolism is a druggable axis that independently regulates tau and amyloid-β in iPSC-derived Alzheimer’s disease neurons. Cell Stem Cell. 10.1016/j.stem.2018.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hammond TC, Xing X, Yanckello LM et al (2021) Human gray and white matter metabolomics to differentiate APOE and stage dependent changes in Alzheimer’s disease. J Cell Immunol 3(6):397–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yilmaz A, Ugur Z, Bisgin H et al (2020) Targeted metabolic profiling of urine highlights a potential biomarker panel for the diagnosis of Alzheimer’s disease and mild cognitive impairment: a pilot study. Metabolites. 10.3390/metabo10090357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huan T, Tran T, Zheng J et al (2018) Metabolomics analyses of saliva detect novel biomarkers of Alzheimer’s disease. J Alzheimer’s Dis 65(4):1401–1416. 10.3233/JAD-180711 [DOI] [PubMed] [Google Scholar]
- 160.Mill J, Patel V, Okonkwo O et al (2022) Erythrocyte sphingolipid species as biomarkers of Alzheimer’s disease. J Pharm Anal 12(1):178–185. 10.1016/j.jpha.2021.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moore Z, Taylor JM, Crack PJ (2019) The involvement of microglia in Alzheimer’s disease: a new dog in the fight. Br J Pharmacol 176(18):3533–3543. 10.1111/bph.14546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen Y, Colonna M (2021) Microglia in Alzheimer’s disease at single-cell level. Are there common patterns in humans and mice? J Exp Med. 10.1084/jem.20202717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rangaraju S, Dammer EB, Raza SA et al (2018) Identification and therapeutic modulation of a pro-inflammatory subset of disease-associated-microglia in Alzheimer’s disease. Mol Neurodegener 13(1):24. 10.1186/s13024-018-0254-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ndoja A, Reja R, Lee SH et al (2020) Ubiquitin ligase COP1 suppresses neuroinflammation by degrading c/EBPbeta in microglia. Cell 182(5):1156–1169. 10.1016/j.cell.2020.07.011 [DOI] [PubMed] [Google Scholar]
- 165.Lee CYD, Daggett A, Gu X et al (2018) Elevated TREM2 gene dosage reprograms microglia responsivity and ameliorates pathological phenotypes in Alzheimer’s disease models. Neuron 97(5):1032–1048. 10.1016/j.neuron.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Boza-Serrano A, Ruiz R, Sanchez-Varo R et al (2019) Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer’s disease. Acta Neuropathol 138(2):251–273. 10.1007/s00401-019-02013-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Griciuc A, Patel S, Federico AN et al (2019) TREM2 acts downstream of CD33 in modulating microglial pathology in Alzheimer’s disease. Neuron 103(5):820–835. 10.1016/j.neuron.2019.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fitz NF, Wolfe CM, Playso BE et al (2020) Trem2 deficiency differentially affects phenotype and transcriptome of human APOE3 and APOE4 mice. Mol Neurodegener 15(1):41. 10.1186/s13024-020-00394-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Carrillo-Jimenez A, Deniz O, Niklison-Chirou MV et al (2019) TET2 regulates the neuroinflammatory response in microglia. Cell Rep 29(3):697–713. 10.1016/j.celrep.2019.09.013 [DOI] [PubMed] [Google Scholar]
- 170.Datta M, Staszewski O, Raschi E et al (2018) Histone deacetylases 1 and 2 regulate microglia function during development, homeostasis, and neurodegeneration in a context-dependent manner. Immunity 48(3):514–529. 10.1016/j.immuni.2018.02.016 [DOI] [PubMed] [Google Scholar]
- 171.Srinivasan K, Friedman BA, Etxeberria A et al (2020) Alzheimer’s patient microglia exhibit enhanced aging and unique transcriptional activation. Cell Rep 31(13):107843. 10.1016/j.celrep.2020.107843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rayaprolu S, Gao T, Xiao H et al (2020) Flow-cytometric microglial sorting coupled with quantitative proteomics identifies moesin as a highly-abundant microglial protein with relevance to Alzheimer’s disease. Mol Neurodegener 15(1):28. 10.1186/s13024-020-00377-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rangaraju S, Dammer EB, Raza SA et al (2018) Quantitative proteomics of acutely-isolated mouse microglia identifies novel immune Alzheimer’s disease-related proteins. Mol Neurodegener 13(1):34. 10.1186/s13024-018-0266-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gaetani L, Bellomo G, Parnetti L et al (2021) Neuroinflammation and Alzheimer’s disease: a machine learning approach to CSF proteomics. Cells. 10.3390/cells10081930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sebastian Monasor L, Muller SA, Colombo AV et al (2020) Fibrillar Abeta triggers microglial proteome alterations and dysfunction in Alzheimer mouse models. Elife. 10.7554/eLife.54083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bottcher C, Schlickeiser S, Sneeboer MAM et al (2019) Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat Neurosci 22(1):78–90. 10.1038/s41593-018-0290-2 [DOI] [PubMed] [Google Scholar]
- 177.Chen C, Liao J, Xia Y et al (2022) Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut. 10.1136/gutjnl-2021-326269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bazan NG, Molina MF, Gordon WC (2011) Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu Rev Nutr. 10.1146/annurev.nutr.012809.104635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dugger BN, Taha AY (2020) Measuring peripheral markers of neuroinflammation in Alzheimer’s disease - challenges and opportunities. Brain Behav Immun. 10.1016/j.bbi.2020.06.004 [DOI] [PubMed] [Google Scholar]
- 180.Wang P, Yang P, Qian K et al (2022) Precise gene delivery systems with detachable albumin shell remodeling dysfunctional microglia by TREM2 for treatment of Alzheimer’s disease. Biomaterials. 10.1016/j.biomaterials.2021.121360 [DOI] [PubMed] [Google Scholar]
- 181.Zupanic A, Bernstein HC, Heiland I (2020) Systems biology: current status and challenges. Cell Mol Life Sci 77(3):379–380. 10.1007/s00018-019-03410-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.San Segundo-Acosta P, Montero-Calle A, Jernbom-Falk A et al (2021) Multiomics profiling of Alzheimer’s disease serum for the identification of autoantibody biomarkers. J Proteome Res 20(11):5115–5130. 10.1021/acs.jproteome.1c00630 [DOI] [PubMed] [Google Scholar]
- 183.Madrid L, Moreno-Grau S, Ahmad S et al (2021) Multiomics integrative analysis identifies APOE allele-specific blood biomarkers associated to Alzheimer’s disease etiopathogenesis. Aging (Albany NY) 13(7):9277–9329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Horgusluoglu E, Neff R, Song WM et al (2022) Integrative metabolomics-genomics approach reveals key metabolic pathways and regulators of Alzheimer’s disease. Alzheimers Dement 18(6):1260–1278. 10.1002/alz.12468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhou Y, Fang J, Bekris LM et al (2021) AlzGPS: a genome-wide positioning systems platform to catalyze multi-omics for Alzheimer’s drug discovery. Alzheimers Res Ther 13(1):24. 10.1186/s13195-020-00760-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang M, Li A, Sekiya M et al (2021) Transformative network modeling of multi-omics data reveals detailed circuits, key regulators, and potential therapeutics for Alzheimer’s disease. Neuron 109(2):257–272. 10.1016/j.neuron.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jin T, Rehani P, Ying M et al (2021) scGRNom: a computational pipeline of integrative multi-omics analyses for predicting cell-type disease genes and regulatory networks. Genome Med 13(1):95. 10.1186/s13073-021-00908-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang H, Robinson JL, Kocabas P et al (2021) Genome-scale metabolic network reconstruction of model animals as a platform for translational research. Proc Natl Acad Sci USA. 10.1073/pnas.2102344118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xie L, He B, Varathan P et al (2021) Integrative-omics for discovery of network-level disease biomarkers: a case study in Alzheimer’s disease. Brief Bioinform. 10.1093/bib/bbab121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vialle RA, de Paiva LK, Bennett DA et al (2022) Integrating whole-genome sequencing with multi-omic data reveals the impact of structural variants on gene regulation in the human brain. Nat Neurosci 25(4):504–514. 10.1038/s41593-022-01031-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Karahan H, Smith DC, Kim B et al (2021) Deletion of Abi3 gene locus exacerbates neuropathological features of Alzheimer’s disease in a mouse model of Abeta amyloidosis. Sci Adv 7(45):3954. 10.1126/sciadv.abe3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lyssenko NN, Pratico D (2021) ABCA7 and the altered lipidostasis hypothesis of Alzheimer’s disease. Alzheimers Dement 17(2):164–174. 10.1002/alz.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Park H, Hwang Y, Kim J (2021) Transcriptional activation with Cas9 activator nanocomplexes rescues Alzheimer’s disease pathology. Biomaterials. 10.1016/j.biomaterials.2021.121229 [DOI] [PubMed] [Google Scholar]
- 194.Henley D, Raghavan N, Sperling R et al (2019) Preliminary results of a trial of atabecestat in preclinical Alzheimer’s disease. N Engl J Med 380(15):1483–1485. 10.1056/NEJMc1813435 [DOI] [PubMed] [Google Scholar]
- 195.Sakamoto K, Matsuki S, Matsuguma K et al (2017) BACE1 Inhibitor Lanabecestat (AZD3293) in a phase 1 study of healthy Japanese subjects: pharmacokinetics and effects on plasma and cerebrospinal fluid abeta peptides. J Clin Pharmacol 57(11):1460–1471. 10.1002/jcph.950 [DOI] [PubMed] [Google Scholar]
- 196.Egan MF, Kost J, Voss T et al (2019) Randomized trial of verubecestat for prodromal Alzheimer’s disease. N Engl J Med 380(15):1408–1420. 10.1056/NEJMoa1812840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chakrabarty P, Li A, Ceballos-Diaz C et al (2015) IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron 85(3):519–533. 10.1016/j.neuron.2014.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang S, Mustafa M, Yuede CM et al (2020) Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J Exp Med. 10.1084/jem.20200785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Haure-Mirande JV, Wang M, Audrain M et al (2019) Integrative approach to sporadic Alzheimer’s disease: deficiency of TYROBP in cerebral Abeta amyloidosis mouse normalizes clinical phenotype and complement subnetwork molecular pathology without reducing Abeta burden. Mol Psychiatry 24(3):431–446. 10.1038/s41380-018-0255-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wetzel-Smith MK, Hunkapiller J, Bhangale TR et al (2014) A rare mutation in UNC5C predisposes to late-onset Alzheimer’s disease and increases neuronal cell death. Nat Med 20(12):1452–1457. 10.1038/nm.3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cao Q, Wang W, Williams JB et al (2020) Targeting histone K4 trimethylation for treatment of cognitive and synaptic deficits in mouse models of Alzheimer’s disease. Sci Adv. 10.1126/sciadv.abc8096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zheng Y, Liu A, Wang ZJ et al (2019) Inhibition of EHMT1/2 rescues synaptic and cognitive functions for Alzheimer’s disease. Brain 142(3):787–807. 10.1093/brain/awy354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Long JM, Maloney B, Rogers JT et al (2019) Novel upregulation of amyloid-beta precursor protein (APP) by microRNA-346 via targeting of APP mRNA 5’-untranslated region: Implications in Alzheimer’s disease. Mol Psychiatry 24(3):345–363. 10.1038/s41380-018-0266-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yue D, Guanqun G, Jingxin L et al (2020) Silencing of long noncoding RNA XIST attenuated Alzheimer’s disease-related BACE1 alteration through miR-124. Cell Biol Int 44(2):630–636. 10.1002/cbin.11263 [DOI] [PubMed] [Google Scholar]
- 205.Zimmermann HR, Yang W, Kasica NP et al (2020) Brain-specific repression of AMPKalpha1 alleviates pathophysiology in Alzheimer’s model mice. J Clin Invest 130(7):3511–3527. 10.1172/JCI133982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nagahara AH, Merrill DA, Coppola G et al (2009) Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med 15(3):331–337. 10.1038/nm.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sosna J, Philipp S, Albay R 3rd et al (2018) Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol Neurodegener 13(1):11. 10.1186/s13024-018-0244-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mancuso R, Fryatt G, Cleal M et al (2019) CSF1R inhibitor JNJ-40346527 attenuates microglial proliferation and neurodegeneration in P301S mice. Brain 142(10):3243–3264. 10.1093/brain/awz241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lalli MA, Bettcher BM, Arcila ML et al (2015) Whole-genome sequencing suggests a chemokine gene cluster that modifies age at onset in familial Alzheimer’s disease. Mol Psychiatry 20(11):1294–1300. 10.1038/mp.2015.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nygaard HB (2018) Targeting Fyn kinase in Alzheimer’s disease. Biol Psychiatry 83(4):369–376. 10.1016/j.biopsych.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li S, Qu L, Wang X et al (2022) Novel insights into RIPK1 as a promising target for future Alzheimer’s disease treatment. Pharmacol Ther. 10.1016/j.pharmthera.2021.107979 [DOI] [PubMed] [Google Scholar]
- 212.Huang D, Cao Y, Yang X et al (2021) A nanoformulation-mediated multifunctional stem cell therapy with improved beta-amyloid clearance and neural regeneration for Alzheimer’s disease. Adv Mater 33(13):e2006357. 10.1002/adma.202006357 [DOI] [PubMed] [Google Scholar]
- 213.Rafii MS, Tuszynski MH, Thomas RG et al (2018) Adeno-associated viral vector (Serotype 2)-nerve growth factor for patients with Alzheimer disease: a randomized clinical trial. JAMA Neurol 75(7):834–841. 10.1001/jamaneurol.2018.0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Alam JJ (2015) Selective brain-targeted antagonism of p38 MAPKalpha reduces hippocampal IL-1beta levels and improves Morris water maze performance in aged rats. J Alzheimers Dis 48(1):219–227. 10.3233/JAD-150277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Aisen PS, Schneider LS, Sano M et al (2008) High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA 300(15):1774–1783. 10.1001/jama.300.15.1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Plascencia-Villa G, Perry G (2021) Preventive and therapeutic strategies in Alzheimer’s disease: focus on oxidative stress, redox metals, and ferroptosis. Antioxid Redox Signal 34(8):591–610. 10.1089/ars.2020.8134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gu XH, Xu LJ, Liu ZQ et al (2016) The flavonoid baicalein rescues synaptic plasticity and memory deficits in a mouse model of Alzheimer’s disease. Behav Brain Res. 10.1016/j.bbr.2016.05.052 [DOI] [PubMed] [Google Scholar]
- 218.Stoiljkovic M, Horvath TL, Hajos M (2021) Therapy for Alzheimer’s disease: missing targets and functional markers? Ageing Res Rev. 10.1016/j.arr.2021.101318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Peña-Bautista C, Álvarez L, Durand T et al (2020) Clinical utility of plasma lipid peroxidation biomarkers in Alzheimer’s disease differential diagnosis. Antioxidants (Basel, Switzerland). 10.3390/antiox9080649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Knopman DS, Jones DT (2019) Greicius MD (2021) Failure to demonstrate efficacy of aducanumab: an analysis of the EMERGE and ENGAGE trials as reported by Biogen. Alzheimer’s Dementia 17(4):696–701. 10.1002/alz.12213 [DOI] [PubMed] [Google Scholar]
- 221.Bourdenx M, Martín-Segura A, Scrivo A et al (2021) Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome. Cell. 10.1016/j.cell.2021.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mullard A (2021) Failure of first anti-tau antibody in Alzheimer disease highlights risks of history repeating. Nat Rev Drug Discovery 20(1):3–5. 10.1038/d41573-020-00217-7 [DOI] [PubMed] [Google Scholar]
- 223.Jack CR Jr, Bennett DA, Blennow K et al (2018) NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14(4):535–562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shen XN, Li JQ, Wang HF et al (2020) Plasma amyloid, tau, and neurodegeneration biomarker profiles predict Alzheimer’s disease pathology and clinical progression in older adults without dementia. Alzheimers Dement (Amst) 12(1):e12104. 10.1002/dad2.12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Alawode DOT, Heslegrave AJ, Ashton NJ et al (2021) Transitioning from cerebrospinal fluid to blood tests to facilitate diagnosis and disease monitoring in Alzheimer’s disease. J Intern Med 290(3):583–601. 10.1111/joim.13332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Shi L, Winchester LM, Westwood S et al (2021) Replication study of plasma proteins relating to Alzheimer’s pathology. Alzheimer’s Dementia. 10.1002/alz.12322 [DOI] [PubMed] [Google Scholar]
- 227.O’Bryant SE, Zhang F, Petersen M et al (2022) Proteomic profiles of neurodegeneration among Mexican Americans and non-hispanic whites in the HABS-HD study. J Alzheimers Dis 86(3):1243–1254. 10.3233/JAD-210543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cummings J (2019) The National Institute on Aging-Alzheimer’s association framework on Alzheimer’s disease: application to clinical trials. Alzheimers Dement 15(1):172–178. 10.1016/j.jalz.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Knopman DS, Haeberlein SB, Carrillo MC et al (2018) The National Institute on Aging and the Alzheimer’s Association Research Framework for Alzheimer’s disease: perspectives from the research roundtable. Alzheimers Dement 14(4):563–575. 10.1016/j.jalz.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Preuss C, Pandey R, Piazza E et al (2020) A novel systems biology approach to evaluate mouse models of late-onset Alzheimer’s disease. Mol Neurodegener 15(1):67. 10.1186/s13024-020-00412-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang P, Xu S, Zhu Z et al (2019) Multi-target design strategies for the improved treatment of Alzheimer’s disease. Eur J Med Chem. 10.1016/j.ejmech.2019.05.020 [DOI] [PubMed] [Google Scholar]
- 232.Benek O, Korabecny J, Soukup O (2020) A perspective on multi-target drugs for Alzheimer’s disease. Trends Pharmacol Sci 41(7):434–445. 10.1016/j.tips.2020.04.008 [DOI] [PubMed] [Google Scholar]
- 233.Liu K, Lin H-H, Pi R et al (2018) Research and development of anti-Alzheimer’s disease drugs: an update from the perspective of technology flows. Expert Opin Ther Pat 28(4):341–350. 10.1080/13543776.2018.1439475 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.