Abstract
The role of immune system in carcinogenesis represents fundamental events associated with cancer eradication; however, tumor evolution is connected with various mechanisms of tumor evasion and progression of cancer. Based on recent evidence, phytochemicals are directly associated with immunomodulation of the innate and adaptive immunity via different mechanisms of action including stimulation and amplification of immune cells, humoral compartments, and associated molecules. This comprehensive study focuses on immunomodulating potential of phytochemicals (mixture in plants or separately such as individual phytochemical) and their impact on regulation of immune response during cancer development, immune tolerance, and immune escape. Clinical application of phytochemicals as modulators of host immunity against cancer may represent perspective approach in anticancer therapy.
Keywords: Cancer, Immunomodulation, Cytokines, Phytochemicals, Preclinical and clinical research
Introduction
Cancer, representing a heterogeneous group of the tumor types, still remains one of the major problems of global health affecting both sexes equally (Bray et al. 2018). As a consequence of the heterogeneity, tumor mass represents a various collection of cells with distinct molecular signatures manifesting differential sensitivity for the therapy and prognosis for patients (Dagogo-Jack and Shaw 2018). An immune system that comprises cellular and humoral compartments of adaptive and innate immunity plays a crucial role in the protection against cancer initiation and promotion. On the other hand, the protective system of the host can promote tumor development via mechanisms of immunoediting of the tumor in which malignant cells generate immunosuppressive microenvironments (Vinay et al. 2015). Consequently, the cells and molecules (such as cytokines and growth factors) of immune system play a dual role during carcinogenesis associated with the promotion of or discouraging cancer (Dranoff 2004). It has been well established that alterations in immune cells and responses are signatures of cancer development and thus they could serve as prognostic markers in cancer diseases (Whiteside 2013). There is strong evidence that immune cells associated with tumor could induce chemoresistance via regulation of signaling pathways associated with chemo-drug sensitivity of malignant cells (Whiteside 2013; Yin et al. 2017). Cytokines produced by tumor cells and immune microenvironment could provide anti-apoptotic and proliferative signals helping cancer cells to evade the chemo-drug intervention (Levina et al. 2008).
Phytochemicals are naturally occurring compounds of the plants with a beneficial impact on health through different mechanisms of action. Evidence accumulated over the last decades indicates an interaction between phytochemicals in isolated form or in mixture and suppression of cancer development (Kapinova et al. 2019). Currently, cross talk between phytochemicals and immune system demonstrates a promising approach in cancer prevention a treatment via suppression, amplification or stimulation of cells and components of the innate and adaptive immunity. There are many mechanisms contributing to the immunomodulation via phytochemicals including regulation of the balance of pro- and anti-inflammatory cytokines and potential stimulatory activity of cellular compounds of host immunity (Venkatalakshmi et al. 2016).
Aim of the study
The review focuses on the anticancer effectiveness of naturally occurring compounds of plants via modulation of adaptive and innate immune system cells and their interconnection with cancer. Firstly, it discusses the basics of the immune system, mechanism of cancer immunosurveillance and immunoediting also cytokines that play a vital role in the antitumoral response but can induce cell transformation during chronic inflammation. The core of the review is focused on both preclinical and clinical research and assessment of whether and how naturally occurring compounds of plants modulated the balance of pro- and anti-inflammatory cytokines in the cancer microenvironment.
Source of data
Data from the available biomedical English language literature were reviewed in the PubMed database. Relevant articles were retrieved by using the following keywords:”cytokines” and”cancer” or”immunomodulation” or”phytochemicals” or”functional foods” or”plants” or “vegetable” or “fruit” or “spices” or “grains”. The database was accessed between July 2019 and August 2019 with the focus placed on the most recent scientific papers from the years 2015 to 2019.
General overview of immune system
The human immune system plays a vital role in the protection of the host from pathological agents including viruses, bacteria, fungi or their biological products. Moreover, the protective mechanism of immune response involves defense actions against transformed cell during carcinogenesis (Calder 2013). Overall, fundamental mechanisms of immunity function consist of events such as the resolution of infectious diseases, detection and elimination of tumor cells, and organ acceptation or rejection. Moreover, defects in the above-mentioned mechanisms lead to autoimmune action against own healthy cells or allergy development. There are two subtypes of the immune system with general function: the innate and adaptive immune system. Both systems comprise humoral and cellular immunity including numerous specific cells and macromolecules, respectively (Medina 2016). Adaptive immunity demonstrates a slower immune response; on the other hand, it is more specific when compared to innate (acquainted) immunity (Fig. 1).
Fig. 1.
General overview of the immune system. The major cells of adaptive immunity are B and T cells with specific antigen receptors. T cells are divided into two populations by differentiation in the expression of CD4 (T-helper cells) and CD8 (cytotoxic T cells) receptors. CD4+ T-helper cells are further subdivided into a population of Th1 (IL-2, IFN-gamma), Th2 (IL-4, IL-5, IL-6, and IL-10) Th17 (IL-17A and IL-17F), Tr1 (IL-10) and Treg (IL-2) (Bessler and Djaldetti 2017). B-lymphocytes have a role in adaptive immunity via recognition of foreign antigen through various B cell receptors (Bessler and Djaldetti 2018). Antigen-presenting cells (APC) are essential for adaptive immunity via interaction with Th and cytotoxic T cells. The innate immune response represents non-specific but faster immune action of the body as a consequence of exposure to environmental pathogens or other harmful insults. Macrophages produce inflammatory mediators including IL-1 and IL-6 that lead to the recruitment of neutrophils into the infection site (Boubaker et al. 2018). Neutrophils play a key role during inflammation via phagocytosis and secretion of antimicrobial products stored in specialized granules with effector function (Bray et al. 2018). Basophils and eosinophils represent granulocytes with non-redundant effector function associated with the promotion of Th2 cytokine-mediated inflammation (Bruno et al. 2017) and antiparasitic function, respectively (Calder 2013). NK cells (natural killer) are crucial for the elimination of infected host cells (viral infection or tumor transformation). Activated NK cells produce the number of cytokines such as IFN-γ, TNF-α, granulocyte–macrophage colony-stimulating factor (GM-CSF) and chemokines including CCL1, CCL2, CCL3, CCL4, CCL5, and CXCL8 for the modulation of innate and adaptive immune response (Calì et al. 2017). Dendritic cells (DC) represent central regulators of specific immune responses via presenting antigen to T-helper cells and B cells-mediated immunity (Candeias and Gaipl 2015). The main component of innate humoral immunity is complex of numerous soluble and membrane proteins called the complement system with a dominant role aimed at lysis and eradication of bacterial pathogens in the host (Cao et al. 2019)
Cancer immunosurveillance and immunoediting
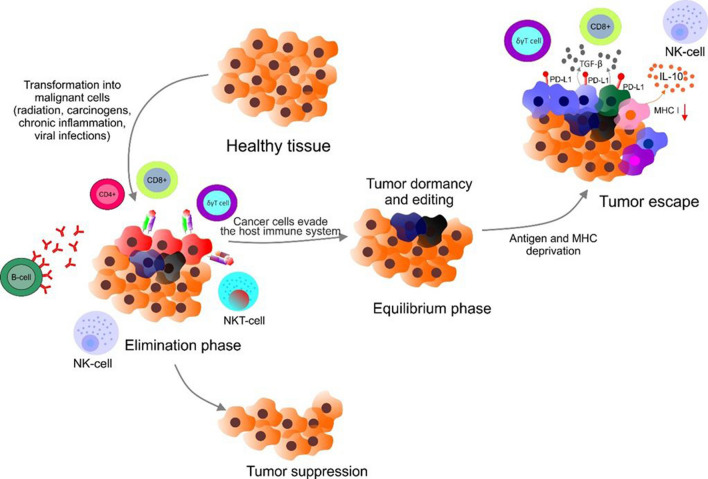
As mentioned above, the immune system plays a crucial role in the host defense and inflammation via the cooperation of specific cells and macromolecules of innate and adaptive immunity (Gagliani et al. 2017; Sodergren et al. 2020; Hoffman et al. 2016; Gubernatorova et al. 2018; Gonzalez et al. 2018; Siracusa et al. 2013; Wen and Rothenberg 2016; Paul and Lal 2017; Gardner and Ruffell 2016; Merle et al. 2015; Carrillo et al. 2017). Importantly, both major components of immunity are associated with protection of the host organism against cancer development (Candeias and Gaipl 2015). Immune response aimed at cancer prevention comprises three dominant mechanisms including the defense against viruses associated with cancer (Epstein–Barr virus, human papillomavirus) by suppression of viral replication and infection (Cao et al. 2019), the prevention of tumorigenesis through the prompt eradication of pathogens from the host and thus prevention of inflammation events leading to environment promoting cancer development (Todoric et al. 2016) or via recognition of specific receptors on cell surface inducing cellular stress by immune system leading to the elimination of tumor (Lakshmi Narendra et al. 2013). The process of cancer development controlling in the host organism was formulated as the hypothesis of cancer immunosurveillance. Cancer cells dispose different strategies to avoid the attack of the immune system (Ribatti 2017). Immunoediting demonstrates a process in which the immunity system operates against cancer, but modifies tumor immunogenicity. There are three phases of immunoediting including elimination, equilibrium, and escape (Bell et al. 2018). Briefly, immunoediting begins by the elimination phase in which transformed cells are eliminated by the host innate and adaptive immune system including the secretion of perforin via T cells, NK cells, NK-T cells, CD8+ T cells, γδ T cells or via action of humoral effectors such as antibody-dependent complement cytotoxicity and complement-dependent cytotoxicity, respectively (Calì et al. 2017).
After the first elimination phase, several tumor cells that evade the immune machinery can enter the second, equilibrium, phase. During the second phase, the longest, in the surviving tumor cells editing occurs. Heterogeneity and various genetic alterations lead to the acquisition of ability that allows the cancer cell to become immune-evasive. The last, third, phase represents the population of immunologically sculpted tumor cells with specific immunosuppressive mechanisms that grow rapidly such as a consequence of selection pressure in the chronic inflammatory microenvironment (Pandya et al. 2016). Mechanisms connected to immune evasion include the downregulation or alteration in expression of MHC class I on the surface of transformed cells, leading to protection against effector cells of immunity (Colegio 2018). Furthermore, another way to avoid the immune system is associated with the expression of PD-L1 or secretion of cytokines with suppressive abilities such as IL-10 or TGF-β, leading to reduction of cancer cells’ immunogenicity (Beatty and Gladney 2015) (Fig. 2).
Fig. 2.
Mechanism of immune evasion by tumors. Transformed cells are eliminated in the first phase by the host immune system (CD4+, CD8+, NK cells, B cells, NK-T cells, γδ T cells, etc.). Equilibrium phase represents surviving tumor cells in dormancy stage. During the second phase, the tumor cells occur editing. Escape phase represents the population of immunologically sculpted tumor cells with specific immunosuppressive mechanisms, including overexpression of PD-L1, production of TGFβ and IL-10, and decreased levels of MHC-I expression
Immune response associated with cancer
Cancer is a genetically heterogeneous disease associated with numerous alterations in DNA sequence, leading to changes in proteins and other molecules that are recognized by compartments of adaptive as well as innate immune system (Li et al. 2016). Furthermore, epigenetic modifications have a direct impact on cellular immunogenicity and viability and can promote immune evasion (Kartikasari et al. 2019) via regulation of expression of the key receptors including dominant pro-apoptotic Fas, the member of TNF superfamily transmembrane proteins (Chen et al. 2014; Manoocheri et al. 2016), or overexpression of PD-L1 proteins (Bally et al. 2016; Kumar and Sharawat 2018). As mentioned above, the immune system can eliminate malignant cells from the organism, if not it is established protracted equilibrium phase of cancer persistence leading to the escape phase and cancer progression (Bates et al. 2018).
The cells and cellular factors such as cytokines associated with inflammation or immunosuppression have a dual role during carcinogenesis. This dual role of immune system consists either of promotion or suppression of events leading to cancer development (Lakshmi Narendra et al. 2013; Calì et al. 2017).
The cross-link of innate immunity and cancer development
The first section of the review discussed the general overview of the immune system with their cell elements. Each cell plays an important protecting role; on the other hand, they can promote immune evasion and cancer progression in the later phase of carcinogenesis (Li et al. 2019; Upadhyay et al. 2018). Macrophages are derived from monocyte lineage and have a crucial role in both types of immune response against cancer. Characteristic hallmarks of this type of cells are heterogeneity and genome plasticity defining numerous subtypes of macrophages with unique phenotypes depending on signals from the microenvironment under homeostatic or pathological conditions (Mantovani and Locati 2013; Ostuni et al. 2015; Zhao et al. 2019). According to stimulating signals (cytokines, the difference in oxygen tension) in the tumor microenvironment, macrophages are polarized into M1 cells (classically activated) associated with anti-tumor function cells or into M2 cells (alternatively activated) connected with pro-tumor features (Orecchioni et al. 2019). Tumor-associated macrophages (TAMs) are dominant elements (up to 50%) of the tumor mass and most of the TAMs have M2 phenotype (Guo et al. 2016). Experimental data suggest the difference between M1 and M2 activated macrophages and their roles in cancer promotion. The first aspect of divergence demonstrates alteration in cytokine production. M1 produces a high level of pro-inflammatory cytokines connected with inflammation processes in tumor including TNF-α, IL-1β, IL-6, IL-12, IL-18, and IL-23 (Atri et al. 2018). Moreover, M1-activated macrophages release high levels of reactive oxygen intermediates, reactive nitrogen spices, and nitric oxide with cytostatic and cytotoxic features against tumor cells (Shapouri-Moghaddam et al. 2018).
Furthermore, M1 are characterized by the production of chemokines associated with the inflammatory microenvironment, such as CCL5, CXCL9, CXCL10, CXCL11, and CXCL16 mediating recruitment of NK cells, Th1 and cytotoxic T cells (Koh et al. 2018). Differently, alternatively activated macrophages release anti-inflammatory cytokines (IL-10, TGF-β, IL-4, and IL-13) and chemokines (CCL-17, CCL-18, CCL-22, and CCL-24) favoring the recruitment of regulatory T cells and TH2 cells (Ruytinx et al. 2018; Lee and Lee 2019). Moreover, M2 TAMs produce numerous cancer-promoting factors (VEGF, EGF, FGF, MMP-2) leading to cancer growth, invasion, and metastasis (Koh et al. 2018; Ruytinx et al. 2018) and predict worse prognosis.
Neutrophils represent the first defense mechanism of the host immune system against pathogens. These neutrophil granulocytes also play an essential role during carcinogenesis including pro-tumor and anti-tumor activity which fluctuate among different cancer stage and type (Treffers et al. 2016). According to cell polarization, tumor-associated neutrophils are differentiated into two phenotypes: N1 (anti-tumor) and N2 (pro-tumor) phenotypes, respectively (Grecian et al. 2018). They are involved in the development of cancer initiation, growth, and metastasis and each step is modulated by various signals from the tumor microenvironment. There is evidence that numerous cytokines including GM-CSF (granulocyte–macrophage colony-stimulating factor) CXCR2 and their ligands CXCL1,CXCL2, CXCL5, TNF-α, TGF-β, IL-6, IL-8, IL-17, and IL-35 play a crucial role in the recruitment and polarization of neutrophil cells during carcinogenesis (Comen et al. 2016; Wu et al. 2019; Zou et al. 2017; Shaul et al. 2016). Furthermore, the mechanism of cancer initiation mediated by neutrophils is c
onnected with secretion of ROS, RNS, and proteases leading to epithelial damage and generate pro-tumor inflammation (Wang et al. 2018). Moreover, the production of neutrophil elastase (NE) by TANs is directly associated with activation of PI3K signaling which is mediated by the degradation of insulin receptor substrate 1 (IRS1) and so promotes cell invasion and proliferation of the tumor (Uribe-Querol and Rosales 2015). Interestingly, TANs regulate angiogenesis via prokineticin 2 (PROK2) and MMP-9 which activate VEGF, leading to the development of new blood vessels in carcinogenesis (Albini et al. 2018). Pro-angiogenic stimulation via MMP-9 and BV8 induces the generation of leaky vasculature, leading to extravasation of cancer cells from the primary tumor and support metastasis development (Coffelt et al. 2016).
As previously discussed, dendritic cells (DC) are specialized antigen-presenting cells, critical components of the antic
ancer immune response, representing the cross-connection between innate and adaptive immunity. Their ultimate ability is in the presentation of antigens to T cells via MHC molecules (Pinho et al. 2016). Recent evidence suggests a linkage between cancer and DC dysfunction in TME. resulting in the failure of immune surveillance. Hypoxia in TME leads to the accumulation of adenosine which is connected to the elevated level of lactate and reduction of pH, resulting in an imbalance of the DC function (Li et al. 2018a, b; Veglia and Gabrilovich 2017). Specifically, cytokine IL-10 affects DCs differentiation generating subsets of deficient cells with lower expression of MHC and costimulatory molecules including CD80 and CD88, respectively (Llopiz et al. 2018). Moreover, tolerogenic DCs expose higher expression of IL-10. Further failure in DCs mechanisms indicates the participation of IL-12 resulting in defects in T cell activation, the expression of PD-L1 on the cell surface, and secretion of IL-6 and galectin-1 regulating pro-tumor and immunosuppressive activity, respectively (Veglia and Gabrilovich 2017). Furthermore, a dysfunction in antigen presentation is mediated by abnormal accumulation of lipids in DCs, and higher expression of markers including ICOS and OX40L linked with the production of pro-tumor cytokines by T cells (Jenkins et al. 2007). Lastly, DCs produce indolamine 2,3-dioxygenase (IDO) that regulates differentiation of Treg cells (Veglia and Gabrilovich 2017; Tesone et al. 2016).
Natural killer (NK) cells have stimulation and effector function and are able to destroy infected or transformed cells that are recognized via sophisticated mechanisms through the synergy of costimulatory and inhibitory receptors on NK cell surface with a broad diversity in function (Miller and Lanier 2019). Like the other cells of the innate immune system, NK cells undergo various dysfunction events connected to cancer development resulting in the escape of tumor cells from cell-mediated immune surveillance (Market et al. 2019). Among the mechanisms associated with escaping from detection of immunity, expression of ligand programmed cell death (PDL-1) results in the suppression of normal function of PD1 receptors on the NK surface. Moreover, tumor cells produce soluble forms of ligands of family MHC class I polypeptide-related sequence A and B (MICA/MICB), B7 homolog 6, and UL16 binding proteins in an advanced stage as means of immune evasion (Dhar and Wu 2018). Additionally, soluble ligands PDGF-DD act as stimulatory factors of the NKp44 receptor leading to tumor growth (Barrow et al. 2018). Importantly, permanent production of immunosuppressive factors including IL-10 and prostaglandin E2 by cancer cells promotes tumorigenesis and regulates immunomodulation of cells such as Treg and MDSC (myeloid-derived suppressor cells) (Chiossone et al. 2018). Interestingly, hypoxia is one of the crucial signs of cancer development and this low oxygen condition stimulates secretion of a soluble form of VEGF receptor 1 (sVEGFR1) by NK cells in a HIF1α-dependent manner, allowing the formation of new blood vessels in tumor progression (Chiossone et al. 2018). All the above-mentioned mechanisms contribute to the immunosuppression of tumor microenvironment that suppresses the activity of NK cells and supports carcinogenesis.
Relationship between adaptive immunity and cancer
It is important to remind that innate and adaptive immunity cooperate in the process of eradication of the transformed cells from the organism. The synergy of both immune mechanisms includes the interaction of adaptive immune cells with APC and other effector cells which are necessary for a complete and effective antitumor response. During cancer promotion, tumor cells begin proliferation and growth through the specific abilities of immune evasion, leading to accumulation of immunosuppressive immune cell subpopulations (Calì et al. 2017; Gaudino and Kumar 2019; Yang et al. 2019a, b).
The presence of B cells in the tumor microenvironment has been described in numerous types of cancer (Shen et al. 2018; Lechner et al. 2019; Bruno et al. 2017; Mion et al. 2017). In contrast to T cells, our knowledge about the participation of B cells in TME is limited. Besides typical features of B cells including immune regulation via antibodies, these types of cells demonstrate abilities such as presenting antigens, co-stimulation events and producing cytokines. Globally, B cells represent compartments of adaptive immunity with various cellular subtypes including phenotype suppressing or promoting cancer development (Tsou et al. 2016). There is evidence suggesting miscellaneous effects contributing to the suppression of antitumor mechanisms by B cells. These pro-tumorigenic features display an association with angiogenesis in tumor environment that is mediated by secretion of lymphotoxin-α (a member of TNF family) by tumor-infiltrating B cells, leading to tumor growth and metastasis (Yang et al. 2019a, b). Interestingly, extracellular vesicles produced by tumor cells stimulate B cells that are further connected to the production of specific antibody. The complex antigen–antibody activates the receptor (Fc-γ) and promotes transformation of myeloid cells into myeloid-derived suppressor cells directly suppressing T cell response (Yuen et al. 2016). Cytokines play a dominant role in TME and tumor progression. TNFα secretion by naïve B cells leads to the generation of regulatory B cells (Bregs). Regulatory B cells have secretory activity including the production of TGFβ supporting Fox3+ iTreg cells generation that suppresses the activity of NK and CD8+ cells, and IL-10 directly associated with suppression of the cytolytic activity of T cells and NK cells, respectively (Olkhanud et al. 2011; Hoffman et al. 2016; Yang et al. 2019a, b).
T cells act as regulatory and effector compartments of adaptive immunity. According to the phenotypes of cells, they can exhibit pro- and anti-inflammatory potential. These immune cells contain low-affinity T receptors (TCR) recognizing antigens presented by molecules of MHC I/II on the APC surface (Martin-Blanco et al. 2018). During cancer development, two important points provide effector activity of T cells: firstly, induction of immune response by tumor antigen and, secondly, presence or absence of signals that can suppress T cell function as effector machinery of adaptive immunity. The tumor microenvironment secretes numerous molecules able to inhibit effector activity of T cells including TGFβ or IL-10 (Dennis et al. 2013). Moreover, myeloid-derived suppressor cells produce suppressive factors such as arginine 1 (Arg1), reactive oxygen species (ROS), and inducible nitric oxide synthase (iNOS) regulating activation of CD8+ cells (Gabrilovich and Nagaraj 2009). The appropriate effective way to inactivate CD8+ is demonstrated by the expression of PD-L1 and CD80 receptors on the surface of tumor cells binding to their partners on T cell surface (PD1 and CTLA4, respectively) (Maimela et al. 2018). Tumor progression and growing are accompanied by the production of novel antigens that support the generation of new population, specifically T cells. The tumor infiltrated regulatory CD4+ T cells (Tregs) can also act as the suppressors of the activity of immune cells (NKs, macrophages, CD8+, and Th1 CD4+) through the contact-dependent manner including factors such as CTLA,4, PD1, PD-L1, TIM-3 or LAG-3 (Chaudhary and Elkord 2016). Additionally, Tregs secrete immunosuppressive cytokines including IL-10, TGFβ or IL-35 (Wan 2010; Li et al. 2017; Whiteside 2012).
Naturally occurring compounds modulating immune responses during carcinogenesis
Substances derived from plants represent important elements with significant antioxidant, anti-inflammatory, and antineoplastic properties in a different types of cancer (Kapinova et al. 2018; Abdel-Rahman et al. 2020; Chen et al. 2020; Nalini et al. 2020). Recent evidence suggests a strong positive correlation between natural compounds of the plants and suppression of cancer development which is mediated via various mechanisms of action including epigenetic regulation mediated by miRNAs (Samec et al. 2019), modulation of the methylation status of genes associated with cancer (Jasek et al. 2019), and targeting aberrantly regulated signaling of cancer stem cells (Liskova et al. 2019). The immune system plays a crucial role in the maintenance of host organism integrity against pathogens and cell transformation leading to cancer development. Various mechanisms to escape from surveillance of host adaptive and innate immunity are considered to be characteristic features of cancer cells (Venkatalakshmi et al. 2016). A promising approach in the field of cancer prevention and treatment is immunomodulation that regulates immune response against cancer via mechanisms including suppression, stimulation, and amplification of the components of immune system according to the therapeutic purpose (Trung and An 2018). Phytochemicals and complex plant foods are proposed to possess immunomodulatory functions in numerous cancers (Kapinova et al. 2018).
Plant modulators of immune response in preclinical studies
Acidic polysaccharide IAPS-2 from Chinese plant Ilex asprella demonstrated immunomodulation effect via modulation of tumor-associated macrophage polarity in sarcoma S180 tumor-bearing mice model. Application of the IAPS-2 showed an ability to change polarity of macrophages via regulation of expression of IL-12 and IL-10. Antitumor activity of extract from Ilex asprella led to the activation of NF-κB and STAT signaling, which plays a crucial role in the polarization of macrophages. In animal model of sarcoma, antitumor activity was demonstrated through inhibition of tumor growth as a consequence of modulation of macrophages function, leading to increased survival of mice (Li et al. 2018a, b). Furthermore, the aqueous extract from Daphne gnidium showed antitumor and immunomodulatory effects in mice-bearing melanoma. Animal models were implanted by B16-F10 cells and treated by an extract from the plant. After decapitation and tumor autopsy, lymphocyte proliferation, cytotoxic T lymphocyte, and NK cell activity were evaluated. The results of the study suggested the immunomodulatory effect via induction of splenocytes proliferation and subsequent activation of NK and cytotoxic T lymphocytes in the murine model (Chaabane et al. 2016). Additionally, the treatment with Daphne gnidium was associated with the promoted lysosomal activity of host macrophages. Identically, aqueous gall extract from Limoniastrum guyonianum demonstrated anti-tumor effects via inhibition of the tumor growth in animal model of melanoma cancer. Mice were inoculated with B16-F10 melanoma cells and then an extract from the plant was applied intraperitoneally. Aqueous gall extract from Limoniastrum guyonianum significantly increased proliferation of splenocytes and activities of both NK and CTL (cytotoxic T lymphocytes) in animal model. Similar to Daphne gnidium extract, Limoniastrum guyonianum extract promoted lysosomal activity of macrophages in tumor (Krifa et al. 2014). Antitumor potential of the leaves of Nitraria retusa, a Tunisian traditional herb, was demonstrated via mechanisms of immunomodulation. Major compounds of the plant are β-sitosterols and palmitic acid, associated with anticancer properties. Intra-peritoneal application of the chloroform extract of Nitraria retusa into BALB/c mice led to an increased level of splenocytes proliferation and CTL activity. As a consequence of the presence of β-sitosterols and palmitic acid, the increased activity of lysosomal activity of host macrophages was detected (Boubaker et al. 2018). In another study, Yue et al. focused on the immunomodulatory function of the extract from traditional Chinese medicine plant Andrographis paniculate in esophageal cancer in vitro and in vivo experiments. The results showed the significant modulatory effects of plant extract in vitro on peripheral blood mononuclear cells characterized by the proliferation of immune cells and stimulation of the production of TNF-α, and IFN-γ, respectively. In vivo experiment using the mice model demonstrated a decreased level of epithelial anomalies in the esophagus and modulatory effect on regulatory T cells. Moreover, the levels of cytokines produced by mice spleens were normalized and their productions were in equilibrium with control subjects (Yue et al. 2019). The beneficial potential of blueberries (BB) against cancer and other inflammatory diseases is well known (Johnson and Arjmandi 2013; Kristo et al. 2016). BB were observed to exert anticancer effects through modulation of the immune response in the mouse xenograft model of triple-negative breast cancer. Feeding with 5% whole BB powder led to better prognosis connected to smaller tumors and less ulceration when compared to control groups. Moreover, evaluation of serum cytokines in the treated mice indicated an increased level of anti-inflammatory cytokines, including monokine induced by interferon-γ (MIG), IP-10, IL-2, IL-12, and TNF-α (Kanaya et al. 2014). The direct impact on regulation and progression of cancer cells via immunomodulation is linked to curcumin.
Microglia are important components of the keeping of homeostasis in CNS (central nervous system) and their dysfunction leads to the development of neurological abnormalities or cancer. Moreover, they represent cell population of innate immune system localized in CNS (Nayak et al. 2014). Rutin (their aglycone is quercetin) is a member of flavonoids that demonstrates anti-inflammatory potential. Interestingly, da Silva et al. focused on the anti-inflammatory features of rutin in C6 rat glioma cells, and U251 and TG1 human glioblastoma cells. Application of rutin in co-cultures (glioma cells and microglia) reduced the migration and proliferation of cancer cells, upregulated protein expression of TNF, downregulated protein level of IL-10, and downregulated mRNA expression for chemokines including CCL2, CCL5, CX3CL1, HDGF (heparin-binding growth factor), IGF, and GDNF (glial cell-derived neurotrophic factor). Similarly, the decreased level of IL-6 and IL-10 mRNA expression and upregulated expression of TNF mRNA were evaluated in human glioblastoma cells U251 and TG1. In co-cultured C6 and microglia cells, the increased expression of IL-1β, IL-6, and IL-18 was detected while the level of IGF, TGF-β, nitric oxide synthase 2 (NOS2), and prostaglandin-endoperoxide synthase 2 (PTGS2) was decreased. The relationship between curcumin application and tumor growth was demonstrated on MDA-MB-231 cells. Curcumin significantly reduced expression of the inflammatory cytokines CXCL1 and CXCL2 via the silencing of NF-κβ. Moreover, a decreased level of these two cytokines led to the downregulation of the expression of the genes associated with metastasis development (Bachmeier et al. 2008). Interestingly, recent evidence suggested a cross-connection between miRNAs and immunomodulation representing the epigenetic way of regulation of the tumor microenvironment. Epigallocatechin-3-gallate (EGCG) is a dominant compound in green tea exhibiting numerous abilities associated with inhibition of cancer development (Wei et al. 2018). The experiment using breast cancer cell lines 4T1 demonstrated a suppressed effect on tumor growth in the murine model. The results from the study showed a protective effect of EGCG via decreased TAM infiltration and M2 macrophage polarization through upregulation of miR-16 in ex vivo and in vivo experiments (Jang et al. 2013). Analogically, study focusing on EGCG and their role in immunomodulation of cancer showed a crucial function of the polyphenols in the inhibition of tumor initiation and progression in leukemia murine model using WEHI-3 cell line. Researchers mainly evaluated clusters of differentiations and identified increased percentage of CD3 (T cells), CD19 (B ells), and Macrophage-3 antigen (macrophage). The percentage of the CD11b (monocyte) was significantly reduced when compared to non-treated animals. Additionally, in contrast with the control group, EGCG treatment led to the promotion of phagocytosis of macrophages (Huang et al. 2013). Quercetin is a phytochemical that possesses anti-inflammatory features that were confirmed in many preclinical experiments (Lesjak et al. 2018; Lee et al. 2018; Güran et al. 2019). (Igbe et al. 2017) evaluated the anti-inflammatory effect of quercetin in hepatocellular cancer using HepG2 and Huh-7 cell lines. Application of the phytochemical led to the activation of JAK/STAT signaling pathway resulting in inhibition of Src homology domain 2 containing tyrosine phosphatase-2 (SHP-2), leading to upregulation of IFN-α with anti-proliferative and anti-tumor activity. Direct impact on IFN-α/β production in Huh7 hepatocellular carcinoma-bearing mice and HeLa cells demonstrated emodin (natural anthraquinone). Emodin inhibited 26S proteasome activity via activation JAK/STAT signaling pathway and potentiated IFN-α anti-proliferate activity (He et al. 2016). HepG2 cells of human hepatoma were used as in vitro model studying the function 6-hydroxy-3-O-methyl-kaempferol 6-O-glucopyranoside (K6G) on the anti-proliferate effect of IFN-α/β. The results of the study suggested the promotion of IFN-α via inhibition of suppressor of cytokine signaling 3 (SOCS3) by K6G (Wonganan et al. 2017). Resveratrol, naturally occurring polyphenol, has enormous health benefits (Salehi et al. 2018; Berman et al. 2017). Animal model of mammary carcinogenesis was analyzed after nocturnal administration of the high dose of resveratrol and then evaluated biomarkers including IL-1 and IL-2. Experimental data suggested an increased level of plasma IL-1 and IL-2 that could be associated with the suppression of the tumor development (Kiskova et al. 2017). Furthermore, another in vitro study of immunomodulatory potential of resveratrol focused on the evaluation of the macrophage inhibitory cytokine (MIC-1) expression in human pancreatic cell lines (S2-013 and CD18). There was a significant correlation between resveratrol administration and upregulation of MIC-1 connected with inhibition of proliferation of human pancreatic cells via transcriptional regulation of the gene expression (Golkar et al. 2007). The isocyanate sulforaphane is a compound of vegetables such as broccoli has proven immunomodulatory potential in the cross talk between human peripheral blood mononuclear cells (PBMS), and colon cancer cell lines (HT-29 and RKO). Acquired data showed alteration of the production of several cytokines such as TNF α, IL1-β and IL-6 directly affected by sulforaphane (Bessler and Djaldetti 2018). Furthermore, Bessler et al. suggested an active role of capsaicin in the immunomodulation of colon cancer line HT-29 or RKO interconnected with PBMS. The data evaluating the effects of capsaicin indicated concentration-dependent changes associated with the decrease of inflammatory cytokines including IL-1 β, IFN-γ, IL-10, and IL-1ra (Bessler and Djaldetti 2017). Table 1 summarizes the preclinical studies evaluating the impact of phytochemicals in vitro and in vivo.
Table 1.
The immunomodulatory aspect of phytochemicals in preclinical research
| Phytochemical/plant | Cell line/animal model | Mechanism of regulation | References |
|---|---|---|---|
| Ilex asprella | C57BL/6J mice | Regulation of macrophages polarity via activation of NF-κB and STAT signaling | Nowicky et al. (1991) |
| Daphne gnidium | Balb/c mice | Splenocytes proliferation and activation of NK and cytotoxic T lymphocytes; promotion of lysosomal activity of host macrophages | Olkhanud e al. (2011) |
| Limoniastrum guyonianum | BALB /c mice | Splenocytes proliferation and activation of NK and cytotoxic T lymphocytes; promotion of lysosomal activity of host macrophages | Orecchioni et al. (2019) |
| Nitraria retusa | BALB/c mice | Splenocytes proliferation and cytotoxic T lymphocytes activity; promotion of lysosomal activity of host macrophages | Ostuni et al. (2015) |
| Andrographis paniculate | C57BL/6 mice | Stimulation of the production of TNF-α, and IFN-γ, decreased level of esophagus dysplasia; modulatory effect on regulatory T cells; normalization of the cytokines production | Paixão et al. (2017) |
| Blueberries | BALB/c Nu-Nu athymic mice | ↑ level of MIG, IP-10, IL-2, IL-12, and TNF-α | Paul and Lal (2017) |
| Rutin | C6 rat glioma cells; U251 cells; TG1 cells |
↓ CCL2; CCL5; CX3CL1; HDGF; IGF GDNF; IL-10; IGF, TGF-β, NOS2; PTGS2 ↑TNF; IL-1β; IL-6; IL-18 |
Upadhyay et al. (2018) |
| Curcumin | MDA-MB-231 cells | ↓ expression of CXCL1 and CXCL2 | Pongnikorn et al. (2003) |
| Capsaicin | HT-29/ RKO cells | ↓ level of IL-1 β, IFN-γ, IL-10, and IL-1ra | Tsou et al. (2016) |
| Sulforaphane | HT-29/ RKO cells | ↓ level of TNF α, IL1- β and IL-6 | Trung and An (2018) |
| Resveratrol | S2-013 /CD18 cells | ↑ of MIC-1 connected with inhibition of proliferation of the cancer cells | Treffers et al. (2016) |
| Sprague–Dawley rats | ↑ level of plasma IL-1 and IL-2 | Todoric et al. (2016) | |
| Quercetin | HepG2 /Huh-7 | ↑ of IFN-α via inhibition of Src homology domain 2 containing tyrosine phosphatase-2 | Shen et al. (2018) |
| Emodin | Huh7 hepatocellular carcinoma-bearing mice/HeLa cells | ↑ of IFN-α via inhibition 26S proteasome activity | Siracusa et al. (2013) |
| K6G | HepG2 cells | ↑ of IFN-α via inhibition of suppressor of cytokine signaling 3 | Sodergren et al. (2020) |
| EGCG |
BALB/c mice 4T1 cells |
Inhibition of TAM polarization and M2 infiltration | Ruytinx et al. (2018) |
| BALB/c mice | Increased percentage of CD3 (T cells), CD19, and macrophage-3 antigen; reduced the percentage of CD11b; promotion of the phagocytosis of macrophages | Salehi et al. (2018) |
Explanatory notes: ↑ increase, ↓ decrease
EGCG, epigallocatechin-3-gallate; K6G, 6-hydroxy-3-O-methyl-kaempferol 6-O-glucopyranoside
Phytochemicals modulating the immune response in clinical studies
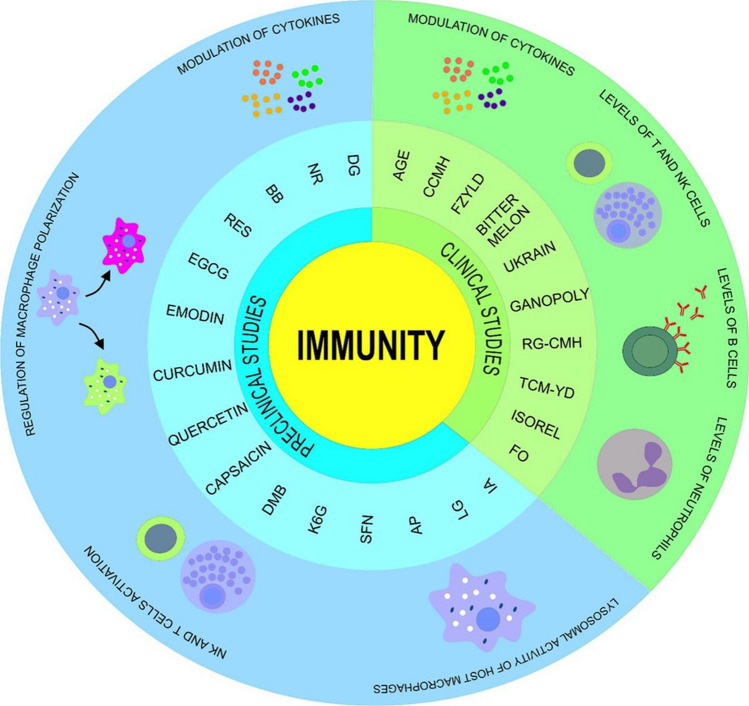
It is known that cancer as well as cancer therapy is associated with impairment of the immune system (Silva et al. 2020; Zhuang et al. 2009; Pongnikorn et al. 2003). The potential immunomodulatory efficiency of natural substances was the focus of the clinical studies already at the end of the twentieth century. The immunopotentiating ability of the preparation named “Ukrain”, containing a thiophosphoric acid alkaloid derivative from the plant Chelidonium majus L., administrated to patients with various malignancies, was demonstrated by the study which revealed significant increase in total T cells, T-helper lymphocytes, erythrocyte-rosette-forming T cells, and NK cells. Moreover, the decrease in T-suppressor cells and normalization of the helper/suppressor ratio was also observed (Nowicky et al. 1991). Importantly, an interest in this research area has persisted. Bitter melon (Momordica charantia Linn) is a Thai herb whose administration was investigated in the study with subjects divided into three groups, including patient control and patient treatment both treated with radiotherapy, and normal control. Importantly, the results demonstrated a significant increase in NK cells in patient control and patient treatment group in comparison with sampling after radiation with or without the intake of bitter melon for 45 and 90 days (second and third sampling time) with sampling before treatment (first sampling time). Moreover, the level of P-glycoprotein significantly decreased in the second and third sampling time in comparison with the first sampling of the patient treatment group, but no significant difference was observed in the patient control group. Accordingly, the bitter melon did not change the level of NK cells; however, the decrease in P-gp level on NK membrane was affected (Pongnikorn et al. 2003). Interestingly, Ganoderma lucidum, a mushroom widely used in Asia, is considered to possess antitumor and immune system-enhancing activities (Chen et al. 2006). An ability of Ganoderma lucidum polysaccharide fractions (Ganopoly) to enhance the immune responses was demonstrated in the study conducted on advanced-stage cancer patients. The results revealed significant increase in the mean plasma concentrations of IL-2, IL-6, and IFN-ℽ and significant decrease in the level of IL-1 and TNF-α. Moreover, the Ganopoly treatment increased the mean absolute number of CD56+ cells, while CD3+, CD4+, and CD8+ increased marginally when compared with baseline levels. Additionally, the treatment enhanced phytohemagglutinin (PHA) response in most patients in comparison with pretreatment baseline and increased the mean NK activity when compared to baselines (Gao et al. 2003). Despite that some patients demonstrated modulation of immune function, the administration of Ganopoly did not lead to significant changes in the mean counts of CD3, CD4, CD8, and CD56, mean plasma concentrations of IL-2, IL-6, IFN-ℽ, NK activity, and mean mitogenic reactivity to PHA in advanced lung cancer patients. Actually, the results were significantly variable; therefore, patient subgroups might respond to the Ganopoly treatment combined with chemo- or radiotherapy (Gao et al. 2005). In addition, Fuzheng Yiliu decoction (FZYLD), made of medicinal Chinese herbs, in combination with chemotherapy was applied in the study in which patients with intermediate and late malignant tumors of digestive tract were included. FZYLD increased CD3+, CD4+, CD4+/CD8+ and decreased CD8+ and sIL-2R in the treatment group with opposite results in the control group consisting of patients receiving chemotherapy alone. Actually, the results of the study suggested an ability of FZYLD to improve chemotherapy-induced immunosuppression (Pan et al. 2005). Interestingly, the perioperative use of Viscum album extract Isorel weakened the immunosuppressive impact of surgery in digestive tract cancer patients which was demonstrated by an increase in the number of T and B cells, particularly the T-helper subset, complement, IgA, IgG, and IgM when compared with the respective values before the treatment. Furthermore, the authors of the trial considered an increase in the number of NK cells as the most important result. Basically, due to the decrease in T-suppressor cells, the Th/Ts ratio tends to normalize in patients when compared with the control group (Enesel et al. 2005). Notably, an ability of aged garlic extract (AGE) to prevent the decline of cell number and activity of NK was evaluated in patients with advanced colorectal, liver, or pancreatic cancer characterized by immunity decline and decrease in quality of life. When compared with the placebo group, the administration of AGE led to the increase in NK cells number and activity in these patients with inoperable advanced cancer of the digestive system (Ishikawa et al. 2006). Moreover, the immunomodulatory capability of traditional Chinese medicine (TCM) Yunzhi-Danshen (YD) capsules of the reduction of lymphopenia in nasopharyngeal carcinoma patients treated with radiotherapy was observed in a 16-week study in which subjects were divided into the TCM and placebo group. After all, the results demonstrated lesser decrease in the percentage and absolute count of T lymphocytes and significantly lower decrease in the absolute count of T-suppressor cells plus cytotoxic T lymphocytes, and T-helper cells in the TCM group when compared with the placebo group (Bao et al. 2006). The effectiveness of Chinese medicinal herb complex, consisting of a mixture of citronellol, an essential oil found in Pelargonium graveolens, and extracts of Angelica sinensis, Ganoderma lucidum, and Codonopsis pilosula, (CCMH) in the improvement of the immune function was evaluated in the study conducted on cancer patients receiving chemo- and/or radiotherapy. More than a hundred patients enrolled in the study received either CCMH or placebo. Eventually, CCMH administration led to the significant reduction of the depletion of leukocytes (14.2% compared with 28.2%) and neutrophils (11% compared with 29.1%). Moreover, the placebo group was associated with the reduction of CD4 lymphocytes and NK cells when compared with the CCMH group; therefore, the delay of the CD4 lymphocytes and NK cells could be attributed to the CCMH. Accordingly, the administration of CCMH to patients receiving chemo- or radiotherapy may be beneficial for the immune function, thus enhancing the ability to fight off the malignant disease or secondary infections (Zhuang et al. 2009). Interestingly, administration of Chinese medicinal herb complex (RG-CMH, a mixture of Rose geranium and extracts of Ganoderma tsugae, Codonopsis pilosula, and Angelica sinensis) led to the significant reduction of the decrease of leucocytes and neutrophils levels in the RG-CMH when compared with the placebo group. Specifically, level of leucocytes decreased from 31.5% for the placebo group to 13.4% for the RG-CMH group as well as neutrophils from 35, 6% for the placebo group to 11, 0% for the RG-CMH group. Conclusively, the decrease in the levels of leucocytes and neutrophils in cancer patients during therapy may be delayed by the administration of RG-CMH (Zhuang et al. 2012). The beneficial effects of n-3 fatty acids (eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids)-enriched fish oil (FO) on the immunity and less active inflammatory response were evaluated in a study conducted on newly diagnosed breast cancer patients. Maintenance of the peripheral blood CD4+ T lymphocytes and serum level of hsCRP were observed in patients supplemented with FO, while the control group was associated with significant increase in hsCRP and decrease in the percentage of CD4+ lymphocytes (Paixão et al. 2017). A summary of the clinical studies evaluating the immunomodulating effects of natural products is shown in Table 2. A detailed overview of phytochemicals as immunity modulators against cancer in clinical and preclinical research is shown in Fig. 3.
Table 2.
Immunomodulatory effectiveness of natural products in clinical research
| Intervention (natural product) | Dosage | Cancer type | Study design (duration) | Distribution of patients (n) | Results | Ref |
|---|---|---|---|---|---|---|
| "Ukrain" | 10 mg/every three days | Various malignancies | – | n = 27 |
↑ T cells and T-helper lymphocytes, erythrocyte-rosette-forming T cells, NK cells ↓ T-suppressor cells, Normalization of the helper/suppressor ratio |
Veglia and Gabrilovich (2017) |
| Bitter melon | – | Cervical cancer patients | – | Normal control (n = 35), patient control (n = 30), patient treatment (n = 30) |
↑ percentage of NK (patient control, patient treatment group) ↓ P-glycoprotein (P-gp) (patient treatment group) |
Uribe-Querol and Rosales (2015) |
| Ganopoly | 1800 mg/3 times daily | Advanced stage | Open-label study (12 weeks) | n = 34 |
↑ IL-2, IL-6, IFN-ℽ ↓ IL-1, TNF-α ↑ NK activity ↑ CD56 + cells Enhancement of PHA response |
Vinay et al. (2015) |
| 5.4 g/daily | 12 weeks | Advanced lung cancer patients (n = 30) | No significant changes | Wan (2010) | ||
| FZYLD | – | Gastrointestinal malignant tumors | – | Treatment group (chemotherapy + FZYLD, n = 30), control group (chemotherapy alone, n = 30), healthy control (n = 15) |
↑ CD3+, CD4+, CD4+/CD8+ ↓ CD8+, sIL-2R (treatment group) |
Wang et al. (2018)) |
| Isorel | 60 mg plant/ml extract s.c. for 2 weeks preoperatively and 2 weeks postoperatively (6 vials per week) | Digestive tract cancer patients | Prospective and randomized clinically controlled study | Isorel treated group (n = 40), control group (n = 30) |
↑ T and B cells (T-helper subset) ↑ IgA, IgG, IgM, complement ↑ NK cells ↓ T-suppressor cells (isorel treated group) |
Wei et al. (2018) |
| AGE | Trial capsules 4/daily (500 mg of AGE, 727 mg of crystalline cellulose, and 11 mg of sucrose fatty acid ester/daily), | Advanced colon, liver or pancreatic cancer patients | Randomized double-blind trial (6 months) | AGE group (n = 25) and control group (n = 25) | ↑ NK cells number and activity (AGE group) | [Wen and Rothenberg (2016) |
| TCM-YD | Yunzhi (3.6 g/daily) and Dangshem (1.4 g/ daily) | Nasopharyngeal cancer patients | Randomized, double-blind, placebo-controlled study (16 weeks) | TCM-YD group (n = 14), placebo group (n = 13) | Less decrease in percentage, absolute count of T lymphocyte and absolute count of T-suppressor cells + cytotoxic T lymphocytes and T-helper cells (TCM-YD group) | Whiteside (2012) |
| CCMH | 9 capsules/daily (capsule: G. lucidum extract 3 mg, C. pilosula extract 27.1 mg, A. sinensis extract 64.5 mg, citronellol powder 273.6 mg | Cancer patients receiving chemo- or radiotherapy | Randomized, double-blind, placebo-controlled study (6 weeks) | CCMH group (n = 55); control group (n = 50) |
↓ the loss of leukocytes and neutrophils (CCMH group) ↓ CD4 lymphocytes and NK cells (placebo group) |
Uramova et al. (2018) |
| RG-CMH | - | Breast cancer patients (surgery followed by chemo- or radiotherapy) | Randomized, placebo-controlled study (treatment period 6 weeks) | Experimental group (n = 31), control group (n = 27) |
↓ leucocytes (control group 31,5%, RG-CMH group 13.4%) ↓ neutrophils (control group 35, 6%, RG-CMH group 11, 0%) |
Whiteside (2013) |
| FO | 2 g/day | Newly diagnosed breast cancer patients prior to treatment | Randomized double blind controlled trial (30 days) | FO group (n = 18), placebo group (n = 19) | Maintenance of CD4+ T cells hsCRP (FO group) | Wonganan et al. (2017) |
Explanatory notes: ↑ increase, ↓ decrease
AGE, aged garlic extract; CCMH, Chinese medicinal herb complex (citronellol and extracts of Ganoderma lucidum, Codonopsis pilosula and Angelica sinensis); FO, eicosapentaenoic and docosahexaenoic acids enriched fish oil; FZYLD, Fuzheng Yiliu decoction; RG-CMH, Chinese medicinal herb complex—a mixture of Rose geranium and extracts of Ganoderma tsugae, Codonopsis pilosula and Angelica sinensis; TCM-YD, traditional Chinese medicine (TCM) Yunzhi-Danshen (YD) capsules
Fig. 3.
Anticancer activities of phytochemicals mediated via immunomodulatory mechanisms in clinical (green) and preclinical (blue) research. Acquired data suggests positive correlation between phytochemicals and immune system including modulation of the lysosomal activity of host macrophages, regulation of NK cells and T cells activation, regulation of macrophage polarization, regulation of cytokines production, regulation of NK cell and T cell counts, regulation of B cell count, and regulation of neutrophil count. AGE, aged garlic extract; AP, Andrographis paniculate; BB, Blueberries; CCMH, Chinese medicinal herb complex (citronellol and extracts of Ganoderma lucidum, Codonopsis pilosula and Angelica sinensis); DG, Daphne gnidium; DMB, Dimorphandra Mollis Benth; EGCG, Epigallocatechin-3-gallate; FO, Eicosapentaenoic and docosahexaenoic acids enriched fish oil; FZYLD, Fuzheng Yiliu decoction; IA, Ilex asprella; K6G, 6-hydroxy-3-O-methyl-kaempferol 6-O-glucopyranoside; LG, Limoniastrum guyonianum; NR, Nitraria retusa; RES, Resveratrol; RG-CMH, Chinese medicinal herb complex—a mixture of Rose geranium and extracts of Ganoderma tsugae, Codonopsis pilosula and Angelica sinensis; SFN, Sulforaphane; TCM-YD, traditional Chinese medicine (TCM) Yunzhi-Danshen (YD) capsules
Conclusion and future directions
There is a growing experimental evidence that dietary phytochemicals via their immunomodulation activities may play important roles in the prevention and therapy of many diseases, including cancer. A broad spectrum of anti-inflammatory or immunomodulatory plant natural compounds and products has been found and described in traditional herbal medicines, fruits, vegetables, and spices. Extensive research in this area suggests that phytochemicals administered as whole foods or isolated molecules demonstrate clear potential in the treatment of immune imbalances in cancer disease and provide several benefits over synthetic agents. In this regard, they offer a convenient, inexpensive, readily applicable, and accessible clinical approach for cancer management and control, including chemoprevention and therapy approach (Aravindaram and Yang 2010). Precise understanding of their mechanism of action will provide the rationale for their using in the combination with the conventional anticancer drugs within modern therapeutic clinical strategies. A number of potential immunoenhancing molecular targets for phytochemicals like Th1, NK cell, γδ T cell responses, or suppressing Treg functionality and induction of IL-12 have been evaluated and designed to suppress tumor progression with much success (Vinay et al. 2015). Moreover, there are well-described data demonstrating that immunostimulating activities of phytochemical correlates with their antioxidant properties. Therefore, targeting the oxidative stress on the cellular level in vivo by the administration of potent plant-derived antioxidants may play crucial role in the development of immunity response of the organism against cancer cells (Thyagarajan and Sahu 2018). However, there is still a lack of clinical data validating the results from preclinical mechanistic studies. For this reason, precisely designed systematic clinical trials, especially in terms of translational research, are needed to confirm their benefits for cancer patients as potent immunomodulatory agents.
The applicability of major phytochemicals/whole plant foods as immunomodulatory agents in controlling cancer has great potential to open a new area of individualized healthcare in clinical medicine (Golubnitschaja et al. 2016). Plant-derived bioactive molecules offer great promise as anticancer therapeutics or chemopreventive agents; however, in terms of personalized clinical approach in cancer patients, much better understanding of the molecular target genes, specific signaling pathways and transcription factors and other mechanisms affected by anti-inflammatory or immunomodulatory plant compounds is necessary. In addition, it is clear that some data of preclinical nature cannot be easily translated to clinical practice. For this reason, more detailed research and insight are needed on the determination of effective (individual) doses, improving the bioavailability of phytochemicals in the organism, clinical approaches using isolated phytochemicals and fractionated or crude plant extracts and multiple plant formulations, or assessing the combined administration of plant molecules targeting several relevant immunomodulation mechanisms or combinations with the conventional drugs; also, the individual characteristics concerning the personalized approach warrant further considerations (Uramova et al. 2018).
Funding
This work was supported by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic under the contracts no. VEGA 1/0136/19, 1/0124/17.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Not applicable (this is a review paper).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Peter Kruzliak, Email: kruzliakpeter@gmail.com.
Peter Kubatka, Email: peter.kubatka@uniba.sk.
References
- Abdel-Rahman O, Spratlin J, Koski S (2020) Vitamin and herbal supplements’ use among patients with advanced gastrointestinal cancers included in eight clinical trials. J Cancer Res Clin Oncol 146:2089–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini A, Bruno A, Noonan DM, Mortara L (2018) Contribution to tumor angiogenesis from innate immune cells within the tumor microenvironment: implications for immunotherapy. Front Immunol 9:527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravindaram K, Yang NS (2010) Anti-inflammatory plant natural products for cancer therapy. Planta Med 76:1103–1117 [DOI] [PubMed] [Google Scholar]
- Atri C, Guerfali FZ, Laouini D (2018) Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci 19:1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmeier BE, Mohrenz IV, Mirisola V et al (2008) Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis 29:779–789 [DOI] [PubMed]
- Bally APR, Austin JW, Boss JM (2016) Genetic and epigenetic regulation of PD-1 expression. J Immunol 196:2431–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao YX, Wong CK, Leung SF, Chan ATC, Li PW, Wong ELY, Leung PC, Fung KP, Yin YB, Lam CWK (2006) Clinical studies of immunomodulatory activities of Yunzhi-Danshen in patients with nasopharyngeal carcinoma. J Altern Complement Med 12:771–776 [DOI] [PubMed] [Google Scholar]
- Barrow AD, Edeling MA, Trifonov V, Luo J, Goyal P, Bohl B, Bando JK, Kim AH, Walker J, Andahazy M, Bugatti M, Melocchi L, Vermi W, Fremont DH, Cox S, Cella M, Schmedt C, Colonna M (2018) Natural Killer cells control tumor growth by sensing a growth factor. Cell 172:534–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JP, Derakhshandeh R, Jones L, Webb TJ (2018) Mechanisms of immune evasion in breastcancer. BMC Cancer 18:556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Gladney WL (2015) Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res 21:687–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RB, Feng Z, Bifulco CB et al (2018) Immunotherapy. In: Oral, head and neck oncology and reconstructive surgery. Elsevier, pp 314–340, ISBN: 9780323265683
- Berman AY, Motechin RA, Wiesenfeld MY, Holz MK (2017) The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol 1:35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessler H, Djaldetti M (2017) Capsaicin modulates the immune cross talk between human mononuclears and cells from two colon carcinoma lines. Nutr Cancer 69:14–20 [DOI] [PubMed] [Google Scholar]
- Bessler H, Djaldetti M (2018) Broccoli and human health: immunomodulatory effect of sulforaphane in a model of colon cancer. Int J Food Sci Nutr 69:946–953 [DOI] [PubMed] [Google Scholar]
- Boubaker J, Toumia IB, Sassi A, Bzouich-Mokded I, Mazgar SG, Sioud F, Bedoui A, Skhiri SS, Ghedira K, Chekir-Ghedira L (2018) Antitumoral potency by immunomodulation of chloroform extract from leaves of Nitraria retusa, Tunisian medicinal plant, via its major compounds β-sitosterol and palmitic acid in BALB/c mice bearing induced tumor. Nutr Cancer 70:650–662 [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424 [DOI] [PubMed] [Google Scholar]
- Bruno TC, Ebner PJ, Moore BL, Squalls OG, Waugh KA, Eruslanov EB, Singhal S, Mitchell JD, Franklin WA, Merrick DT, McCarter MD, Palmer BE, Kern JA, Slansky JE (2017) Antigen-presenting intratumoral B cells affect CD4+ TIL phenotypes in non-small cell lung cancer patients. Cancer Immunol Res 5:898–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC (2013) Feeding the immune system. Proc Nutr Soc 72:299–309 [DOI] [PubMed] [Google Scholar]
- Calì B, Molon B, Viola A (2017) Tuning cancer fate: the unremitting role of host immunity. Open Biol 7:170006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candeias S, Gaipl U (2015) The immune system in cancer prevention, development and therapy. Anticancer Agents Med Chem 16:101–107 [DOI] [PubMed] [Google Scholar]
- Cao S, Wylie KM, Wyczalkowski MA, Karpova A, Ley J, Sun S, Mashl RJ, Liang WW, Wang X, Johnson K, DiPersio JF, Gay H, Ratner L, Chen F, Adkins DR, Ding L (2019) Dynamic host immune response in virus-associated cancers. Commun Biol 2:109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo JLM, Rodríguez FPC, Coronado OG, García MAM, Cordero JFC (2017) Physiology and pathology of innate immune response against pathogens. In: Rezaei N (ed) Physiology and pathology of immunology. InTech, Rijeka, pp 275–293 [Google Scholar]
- Chaabane F, Mustapha N, Mokdad-Bzeouich I, Sassi A, Kilani-Jaziri S, Franca MGD, Michalet S, Fathallah M, Krifa M, Ghedira K, Chekir-Ghedira L (2016) In vitro and in vivo anti-melanoma effects of Daphne gnidium aqueous extract via activation of the immune system. Tumor Biol 37:6511–6517 [DOI] [PubMed] [Google Scholar]
- Chaudhary B, Elkord E (2016) Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines 4:28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Hu ZP, Yang XX, Huang M, Gao Y, Tang W, Chan SY, Dai X, Ye J, Ho PCL, Duan W, Yang HY, Zhu YZ, Zhou SF (2006) Monitoring of immune responses to a herbal immuno-modulator in patients with advanced colorectal cancer. Int Immunopharmacol 6:499–508 [DOI] [PubMed] [Google Scholar]
- Chen Y, Li H, Zhang G, Wu Y, Xiao J, Liu J, Qiu P, Liu X, Sun L, Du B, Tan Y (2020) Synergistic inhibitory effect of resveratrol and TK/GCV therapy on melanoma cells. J Cancer Res Clin Oncol 146:1489–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QW, Zhu XY, Li YY, Meng ZQ (2014) Epigenetic regulation and cancer (Review). Oncol Rep 31:523–532 [DOI] [PubMed] [Google Scholar]
- Chiossone L, Dumas PY, Vienne M, Vivier E (2018) Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol 18:671–688 [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Wellenstein MD, de Visser KE (2016) Neutrophils in cancer: neutral no more. Nat Rev Cancer 16:431–446 [DOI] [PubMed] [Google Scholar]
- Colegio OR (2018) Skin diseases in the immunosuppressed. InTech, Rijeka [Google Scholar]
- Comen E, Wojnarowicz P, Seshan VE, Shah R, Coker C, Norton L, Benezra R (2016) TNF is a key cytokine mediating neutrophil cytotoxic activity in breast cancer patients. NPJ Breast Cancer 2:16009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagogo-Jack I, Shaw AT (2018) Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 15:81–94 [DOI] [PubMed] [Google Scholar]
- Da Silva AB, Cerqueira Coelho PL, Das MNO et al (2020) The flavonoid rutin and its aglycone quercetin modulate the microglia inflammatory profile improving antiglioma activity. Brain Behav Immun 85:170–185 [DOI] [PubMed] [Google Scholar]
- Dennis KL, Blatner NR, Gounari F, Khazaie K (2013) Current status of IL-10 and regulatory T-cells in cancer. Curr Opin Oncol 25:637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar P, Wu JD (2018) NKG2D and its ligands in cancer. Curr Opin Immunol 51:55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G (2004) Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer 4:11–22 [DOI] [PubMed] [Google Scholar]
- Enesel MB, Acalovschi I, Grosu V, Sbarcea A, Rusu C, Dobre A, Weiss T, Zarkovic N (2005) Perioperative application of the Viscum album extract Isorel in digestive tract cancer patients. Anticancer Res 25:4583–4590 [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S (2009) Myeloid-derived-suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Huber S (2017) Basic aspects of T helper cell differentiation. In: Lugli E (ed) T-cell differentiation: methods and protocols. Springer, New York, pp 19–30 [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhou S, Jiang W, Huang M, Dai X (2003) Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol Investig 2:201–215 [DOI] [PubMed] [Google Scholar]
- Gao Y, Tang W, Dai X, Gao H, Chen G, Ye J, Chan E, Koh HL, Li X, Zhou S (2005) Effects of water-soluble Ganoderma lucidum polysaccharides on the immune functions of patients with advanced lung cancer. J Med Food 8:159–168 [DOI] [PubMed] [Google Scholar]
- Gardner A, Ruffell B (2016) Dendritic cells and cancer immunity. Trends Immunol 37:855–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudino SJ, Kumar P (2019) Cross-talk between antigen presenting cells and t cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front Immunol 10:360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar L, Ding XZ, Ujiki MB, Salabat MR, Kelly DL, Scholtens D, Fought AJ, Bentrem DJ, Talamonti MS, Bell RH, Adrian TE (2007) Resveratrol inhibits pancreatic cancer cell proliferation through transcriptional induction of macrophage inhibitory cytokine-1. J Surg Res 138:163–169 [DOI] [PubMed] [Google Scholar]
- Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, Krapfenbauer K, Mozaffari MS, Costigliola V (2016) Medicine in the early twenty-first century: paradigm and anticipation—EPMA position paper 2016. EPMA J 7:23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H, Hagerling C, Werb Z (2018) Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 32:1267–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecian R, Whyte MKB, Walmsley SR (2018) The role of neutrophils in cancer. Br Med Bull 128:5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubernatorova EO, Gorshkova EA, Namakanova OA, Zvartsev RA, Hidalgo J, Drutskaya MS, Tumanov AV, Nedospasov SA (2018) Non-redundant functions of IL-6 produced by macrophages and dendritic cells in allergic airway inflammation. Front Immunol 9:2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Jin Z, Yuan Y, Liu R, Xu T, Wei H, Xu X, He S, Chen S, Shi Z, Hou W, Hua B (2016) New mechanisms of tumor-associated macrophages on promoting tumor progression: recent research advances and potential targets for tumor immunotherapy. J Immunol Res 2016:9720912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güran M, Şanlıtürk G, Kerküklü NR, Altundağ EM, Süha Yalçın A (2019) Combined effects of quercetin and curcumin on anti-inflammatory and antimicrobial parameters in vitro. Eur J Pharmacol 859:172486 [DOI] [PubMed] [Google Scholar]
- He Y, Huang J, Wang P, Shen X, Li S, Yang L, Liu W, Suksamrarn A, Zhang G, Wang F (2016) Emodin potentiates the antiproliferative effect of interferon α/β by activation of JAK/STAT pathway signaling through inhibition of the 26S proteasome. Oncotarget 7:4664–4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman W, Lakkis FG, Chalasani G (2016) B cells, antibodies, and more. Clin J Am Soc Nephrol 11:137–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AC, Cheng HY, Lin TS, Chen WH, Lin JH, Lin JJ, Lu CC, Chiang JH, Hsu SC, Wu PP, Huang YP, Chung JG (2013) Epigallocatechin gallate (EGCG), influences a murine WEHI-3 leukemia model in vivo through enhancing phagocytosis of macrophages and populations of T- and B-cells. In Vivo 27:627–634 [PubMed] [Google Scholar]
- Igbe I, Shen XF, Jiao W, Qiang Z, Deng T, Li S, Liu WL, Liu HW, Zhang GL, Wang F (2017) Dietary quercetin potentiates the antiproliferative effect of interferon-α in hepatocellular carcinoma cells through activation of JAK/STAT pathway signaling by inhibition of SHP2 phosphatase. Oncotarget 8:113734–113748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Saeki T, Otani T, Suzuki T, Shimozuma K, Nishino H, Fukuda S, Morimoto K (2006) Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J Nutr 136(3 Suppl):816S-S820 [DOI] [PubMed] [Google Scholar]
- Jang JY, Lee JK, Jeon YK, Kim CW (2013) Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer 13:421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasek K, Kubatka P, Samec M, Liskova A, Smejkal K, Vybohova D, Bugos O, Biskupska-Bodova K, Bielik T, Zubor P, Danko J, Adamkov M, Kwon TK, Büsselberg D (2019) DNA methylation status in cancer disease: modulations by plant-derived natural compounds and dietary interventions. Biomolecules 9:289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SJ, Perona-Wright G, Worsley AGF, Ishii N, MacDonald AS (2007) Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal Th2 priming and memory induction in vivo. J Immunol 179:3515–3523 [DOI] [PubMed] [Google Scholar]
- Johnson SA, Arjmandi BH (2013) Evidence for anti-cancer properties of blueberries: a mini-review. Anticancer Agents Med Chem 13:1142–1148 [DOI] [PubMed] [Google Scholar]
- Kanaya N, Adams L, Takasaki A, Chen S (2014) Whole blueberry powder inhibits metastasis of triple negative breast cancer in a xenograft mouse model through modulation of inflammatory cytokines. Nutr Cancer 66:242–248 [DOI] [PubMed] [Google Scholar]
- Kapinova A, Kubatka P, Golubnitschaja O, Kello M, Zubor P, Solar P, Pec M (2018) Dietary phytochemicals in breast cancer research: anticancer effects and potential utility for effective chemoprevention. Environ Health Prev Med 23:36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapinova A, Kubatka P, Liskova A, Baranenko D, Kruzliak P, Matta M, Büsselberg D, Malicherova B, Zulli A, Kwon TK, Jezkova E, Blahutova D, Zubor P, Danko J (2019) Controlling metastatic cancer: the role of phytochemicals in cell signaling. J Cancer Res Clin Oncol 145:1087–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartikasari AER, Prakash MD, Cox M, Wilson K, Boer JC, Cauchi JA, Plebanski M (2019) Therapeutic cancer vaccines—T cell responses and epigenetic modulation. Front Immunol 9:3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskova T, Demeckova V, Jendzelovska Z, Kiktava M, Venglovska K, Bohmdorfer M, Jager W, Thalhammer T (2017) Nocturnal resveratrol administration inhibits chemically induced breast cancer formation in rats. J Physiol Pharmacol 68:867–875 [PubMed] [Google Scholar]
- Koh YC, Yang G, Lai CS, Weerawatanakorn M, Pan MH (2018) Chemopreventive effects of phytochemicals and medicines on M1/M2 polarized macrophage role in inflammation-related diseases. Int J Mol Sci 19:2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krifa M, Skandrani I, Pizzi A, Nasr N, Ghedira Z, Mustapha N, Ghedira K, Chekir-Ghedira L (2014) An aqueous extract of Limoniastrum guyonianum gall induces anti-tumor effects in melanoma-injected mice via modulation of the immune response. Food Chem Toxicol 69:76–85 [DOI] [PubMed] [Google Scholar]
- Kristo AS, Klimis-Zacas D, Sikalidis AK (2016) Protective role of dietary berries in cancer. Antioxidants 5:37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Sharawat SK (2018) Epigenetic regulators of programmed death-ligand 1 expression in human cancers. Transl Res 202:129–145 [DOI] [PubMed] [Google Scholar]
- Lakshmi Narendra B, Eshvendar Reddy K, Shantikumar S, Ramakrishna S (2013) Immune system: a double-edged sword in cancer. Inflamm Res 62:823–834 [DOI] [PubMed] [Google Scholar]
- Lechner A, Schlößer HA, Thelen M et al (2019) Tumor-associated B cells and humoral immune response in head and neck squamous cell carcinoma. Oncoimmunology 8:1535293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lee JY (2019) Blackcurrant (Ribes nigrum) extract exerts an anti-inflammatory action by modulating macrophage phenotypes. Nutrients 11:975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HN, Shin SA, Choo GS, Kim HJ, Park YS, Kim BS, Kim SK, Cho SD, Nam JS, Choi CS, Che JH, Park BK, Jung JY (2018) Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB-induced atopic dermatitis animal models. Int J Mol Med 41:888–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesjak M, Beara I, Simin N, Pintác D, Majkić T, Bekvalac K, Orčić D, Mimica-Dukić N (2018) Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J Funct Foods 40:68–75 [Google Scholar]
- Levina V, Su Y, Nolen B, Liu X, Gordin Y, Lee M, Lokshin A, Gorelik E (2008) Chemotherapeutic drugs and human tumor cells cytokine network. Int J Cancer 123:2031–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen HY, Liu WX, Jia XX, Zhang JG, Ma CL, Zhang XJ, Yu F, Cong B (2017) Prostaglandin E2 restrains human Treg cell differentiation via E prostanoid receptor 2-protein kinase A signaling. Immunol Lett 191:63–72 [DOI] [PubMed] [Google Scholar]
- Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J, Jiang P, Shen H, Aster JC, Rodig S, Signoretti S, Liu JS, Liu XS (2016) Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol 17:174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Hao Z, Hong Y, He W, Zhao W (2018a) Reprogramming tumor associated macrophage phenotype by a polysaccharide from Ilex asprella for sarcoma immunotherapy. Int J Mol Sci 19:3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Patel SP, Roszik J, Qin Y (2018b) Hypoxia-driven immunosuppressive metabolites in the tumor microenvironment: new approaches for combinational immunotherapy. Front Immunol 9:1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Spakowicz D, Burkart J, Patel S, Husain M, He K, Bertino EM, Shields PG, Carbone DP, Verschraegen CF, Presley CJ, Otterson GA, Kendra K, Owen DH (2019) Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J Cancer Res Clin Oncol 145:2541–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskova A, Kubatka P, Samec M, Zubor P, Mlyncek M, Bielik T, Samuel SM, Zulli A, Kwon TK, Büsselberg D (2019) Dietary phytochemicals targeting cancer stem cells. Molecules 24:899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llopiz D, Ruiz M, Silva L, Sarobe P (2018) Enhancement of antitumor vaccination by targeting dendritic cell-related IL-10. Front Immunol 9:1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimela NR, Liu S, Zhang Y (2018) Fates of CD8+ T cells in tumor microenvironment. Comput Struct Biotechnol J 17:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoocheri M, Borhani N, Karbasi A, Koochaki A, Kazemi B (2016) Promoter hypermethylation and downregulation of the FAS gene may be involved in colorectal carcinogenesis. Oncol Lett 12:285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Locati M (2013) Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization. Arterioscler Thromb Vasc Biol 33:1478–1483 [DOI] [PubMed] [Google Scholar]
- Market M, Baxter KE, Angka L, Kennedy MA, Auer RC (2019) The potential for cancer immunotherapy in targeting surgery-induced natural killer cell dysfunction. Cancers (Basel) 11:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blanco N, Blanco R, Alda-Catalinas C, Bovolenta ER, Oeste CL, Palmer E, Schamel WW, Lythe G, Molina-París C, Castro M, Alarcon B (2018) A window of opportunity for cooperativity in the T cell receptor. Nat Commun 9:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL (2016) Overview of the immune system. Handb Clin Neurol 133:61–76 [DOI] [PubMed] [Google Scholar]
- Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT (2015) Complement system part II: role in immunity. Front Immunol 6:257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Lanier LL (2019) Natural killer cells in cancer immunotherapy. Annu Rev Cancer Biol 3:77–103 [Google Scholar]
- Mion F, Vetrano S, Tonon S, Valeri V, Piontini A, Burocchi A, Petti L, Frossi B, Gulino A, Tripodo C, Colombo MP, Pucillo CE (2017) Reciprocal influence of B cells and tumor macro and microenvironments in the ApcMin/+ model of colorectal cancer. Oncoimmunology 6:e1336593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalini D, Selvaraj J, Kumar GS (2020) Herbal nutraceuticals: safe and potent therapeutics to battle tumor hypoxia. J Cancer Res Clin Oncol 146:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D, Roth TL, McGavern DB (2014) Microglia development and function. Annu Rev Immunol 32:367–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicky JW, Staniszewski A, Zbroja-Sontag W, Slesak B, Nowicky W, Hiesmayr W (1991) Evaluation of thiophosphoric acid alkaloid derivatives from Chelidonium majus L. (“Ukrain”) as an immunostimulant in patients with various carcinomas. Drugs Exp Clin Res 17:139–143 [PubMed] [Google Scholar]
- Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A (2011) Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T regulatory cells. Cancer Res 71:3505–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orecchioni M, Ghosheh Y, Pramod AB, Ley K (2019) Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS–) vs. alternatively activated macrophages. Front Immunol 10:1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostuni R, Kratochvill F, Murray PJ, Natoli G (2015) Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol 36:229–239 [DOI] [PubMed] [Google Scholar]
- Paixão EMDS, Oliveira ACM, Pizato N, Muniz-Junqueira MI, Magalhães KG, Nakano EY, Ito MK (2017) The effects of EPA and DHA enriched fish oil on nutritional and immunological markers of treatment naïve breast cancer patients: a randomized double-blind controlled trial. Nutr J 16:71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Cheng T, Nan KJ, Qiu GQ, Sun XC (2005) Effect of Fuzheng Yiliu decoction combined with chemotherapy on patients with intermediate and late stage gastrointestinal cancer. World J Gastroenterol 11:439–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya PH, Murray ME, Pollok KE, Renbarger JL (2016) The immune system in cancer pathogenesis: potential therapeutic approaches. J Immunol Res 2016:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Lal G (2017) The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol 8:1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho MP, Sundarasetty BS, Bergami-Santos PC, Steponavicius-Cruz K, Ferreira AK, Stripecke R, Barbuto JAM (2016) Dendritic-tumor cell hybrids induce tumor-specific immune responses more effectively than the simple mixture of dendritic and tumor cells. Cytotherapy 18:570–580 [DOI] [PubMed] [Google Scholar]
- Pongnikorn S, Fongmoon D, Kasinrerk W, Limtrakul PN (2003) Effect of bitter melon (Momordica charantia Linn) on level and function of natural killer cells in cervical cancer patients with radiotherapy. J Med Assoc Thai 86:61–68 [PubMed] [Google Scholar]
- Ribatti D (2017) The concept of immune surveillance against tumors: the first theories. Oncotarget 8:7175–7180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruytinx P, Proost P, Van Damme J, Struyf S (2018) Chemokine-induced macrophage polarization in inflammatory conditions. Front Immunol 9:1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B, Mishra AP, Nigam M, Sener B, Kilic M, Sharifi-Rad M, Fokou PVT, Martins N, Sharifi-Rad J (2018) Resveratrol: a double-edged sword in health benefits. Biomedicines 6:91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samec M, Liskova A, Kubatka P et al (2019) The role of dietary phytochemicals in the carcinogenesis via the modulation of miRNA expression. J Cancer Res Clin Oncol 145:1665–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapouri-Moghaddam A, Mohammadian S, Vazini H et al (2018) Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 233:6425–6440 [DOI] [PubMed] [Google Scholar]
- Shaul ME, Levy L, Sun J et al (2016) Tumor-associated neutrophils display a distinct N1 profile following TGFβ modulation: a transcriptomics analysis of pro- vs. antitumor TANs. Oncoimmunology 5:e1232221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Wang J, Ren X (2018) New insights into tumor-infiltrating B lymphocytes in breast cancer: clinical impacts and regulatory mechanisms. Front Immunol 9:470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa MC, Kim BS, Spergel JM, Artis D (2013) Basophils and allergic inflammation. J Allergy Clin Immunol 132:789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodergren MH, Mangal N, Wasan H, Sadanandam A, Balachandran VP, Jiao LR, Habib N (2020) Immunological combination treatment holds the key to improving survival in pancreatic cancer. J Cancer Res Clin Oncol 146:2897–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesone AJ, Rutkowski MR, Brencicova E et al (2016) Satb1 overexpression drives tumor-promoting activities in cancer-associated dendritic cells. Cell Rep 14:1774–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan A, Sahu RP (2018) Potential contributions of antioxidants to cancer therapy: immunomodulation and radiosensitization. Integr Cancer Ther 17:210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todoric J, Antonucci L, Karin M (2016) Targeting inflammation in cancer prevention and therapy. Cancer Prev Res (Phila Pa) 9:895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treffers LW, Hiemstra IH, Kuijpers TW, van den Berg TK, Matlung HL (2016) Neutrophils in cancer. Immunol Rev 273:312–328 [DOI] [PubMed] [Google Scholar]
- Trung LQ, An DTT (2018) Is resveratrol a cancer immunomodulatory molecule? Front Pharmacol 9:1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou P, Katayama H, Ostrin EJ, Hanash SM (2016) The emerging role of B cells in tumor immunity. Cancer Res 76:5597–5601 [DOI] [PubMed] [Google Scholar]
- Upadhyay S, Sharma N, Gupta KB, Dhiman M (2018) Role of immune system in tumor progression and carcinogenesis. J Cell Biochem 119:5028–5042 [DOI] [PubMed] [Google Scholar]
- Uramova S, Kubatka P, Dankova Z et al (2018) Plant natural modulators in breast cancer prevention: status quo and future perspectives reinforced by predictive, preventive, and personalized medical approach. EPMA J 9:403–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe-Querol E, Rosales C (2015) Neutrophils in cancer: two sides of the same coin [Internet]. J Immunol Res 2015:983698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veglia F, Gabrilovich DI (2017) Dendritic cells in cancer: the role revisited. Curr Opin Immunol 45:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatalakshmi P, Vadivel V, Brindha PP (2016) Role of phytochemicals as immunomodulatory agents: a review. Int J Green Pharm 10:1 [Google Scholar]
- Vinay DS, Ryan EP, Pawelec G et al (2015) Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol 35(Suppl):S185–S198 [DOI] [PubMed] [Google Scholar]
- Wan YY (2010) Regulatory T cells: immune suppression and beyond. Cell Mol Immunol 7:204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Qiu L, Li Z, Wang XY, Yi H (2018) Understanding the multifaceted role of neutrophilsin cancer and autoimmune diseases. Front Immunol 9:2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R, Mao L, Xu P et al (2018) Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food Funct 9:5682–5696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen T, Rothenberg ME (2016) The regulatory function of eosinophils. Microbiol Spectr 4:10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL (2012) What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol 22:327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL (2013) Immune responses to cancer: are they potential biomarkers of prognosis? Front Oncol 3:107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonganan O, He YJ, Shen XF et al (2017) 6-Hydroxy-3-O-methyl-kaempferol 6-O-glucopyranoside potentiates the anti-proliferative effect of interferon α/β by promoting activation of the JAK/STAT signaling by inhibiting SOCS3 in hepatocellular carcinoma cells. Toxicol Appl Pharmacol 336:31–39 [DOI] [PubMed] [Google Scholar]
- Wu L, Saxena S, Awaji M, Singh RK (2019) Tumor-associated neutrophils in cancer: going pro. Cancers (Basel) 11:564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JG, Wang WM, Xia HF et al (2019a) Lymphotoxin-α promotes tumor angiogenesis in HNSCC by modulating glycolysis in a PFKFB3-dependent manner. Int J Cancer 145:1358–1370 [DOI] [PubMed] [Google Scholar]
- Yang G, Zheng RY, Jin ZS (2019b) Correlations between microsatellite instability and the biological behaviour of tumours. J Cancer Res Clin Oncol 145:2891–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Yao S, Hu Y et al (2017) The immune-microenvironment confers chemoresistance of colorectal cancer through macrophage-derived IL6. Clin Cancer Res 23:7375–7387 [DOI] [PubMed] [Google Scholar]
- Yue GGL, Li L, Lee JKM et al (2019) Multiple modulatory activities of Andrographis paniculata on immune responses and xenograft growth in esophageal cancer preclinical models. Phytomedicine 60:152886 [DOI] [PubMed] [Google Scholar]
- Yuen GJ, Demissie E, Pillai S (2016) B lymphocytes and cancer: a love-hate relationship. Trends Cancer 2:747–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ge X, Xu X, Yu S, Wang J, Sun L (2019) Prognostic value and clinicopathological roles of phenotypes of tumour-associated macrophages in colorectal cancer. J Cancer Res Clin Oncol 145:3005–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang SR, Chen SL, Tsai JH et al (2009) Effect of citronellol and the Chinese medical herb complex on cellular immunity of cancer patients receiving chemotherapy/radiotherapy. Phytother Res 23:785–790 [DOI] [PubMed] [Google Scholar]
- Zhuang SR, Chiu HF, Chen SL et al (2012) Effects of a Chinese medical herbs complex on cellular immunity and toxicity-related conditions of breast cancer patients. Br J Nutr 107:712–718 [DOI] [PubMed] [Google Scholar]
- Zou JM, Qin J, Li YC et al (2017) IL-35 induces N2 phenotype of neutrophils to promote tumor growth. Oncotarget 8:33501–33514 [DOI] [PMC free article] [PubMed] [Google Scholar]