Abstract
Cdc42p is a member of the RAS superfamily of GTPases and plays an essential role in polarized growth in many eukaryotic cells. We cloned the Candida albicans CaCDC42 by functional complementation in Saccharomyces cerevisiae and analyzed its function in C. albicans. A double deletion of CaCDC42 was made in a C. albicans strain containing CaCDC42 under the control of the PCK1 promoter. When expression of the heterologous copy of CaCDC42 was repressed in this strain, the cells ceased proliferation. These arrested cells were large, round, and unbudded and contained predominantly two nuclei. The PCK1-mediated overexpression of wild-type CaCdc42p had no effect on cells. However, in cells overexpressing CaCdc42p containing the dominant-negative D118A substitution, proliferation was blocked and the arrested cells were large, round, unbudded, and multinucleated, similar to the phenotype of the cdc42 double-deletion strain. Cells overexpressing CaCdc42p containing the hyperactive G12V substitution also ceased proliferation in yeast growth medium; in this case the arrested cells were multinucleated and multibudded. An intact CAAX box is essential for the phenotypes associated with either CaCdc42pG12V or CaCdc42pD118A ectopic expression, suggesting that membrane attachment is involved in CaCdc42p function. In addition, the lethality caused by ectopic expression of CaCdc42pG12V was suppressed by deletion of CST20 but not by deletion of CaCLA4. CaCdc42p function was also examined under hypha-inducing conditions. Cdc42p depletion prior to hyphal induction trapped cells in a round, unbudded state, while depletion triggered at the same time as hyphal induction permitted the initiation of germ tubes that failed to be extended. Ectopic expression of either the G12V or D118A substitution protein modified hyphal formation in a CAAX box-dependent manner. Thus, CaCdc42p function appears important for polarized growth of both the yeast and hyphal forms of C. albicans.
Cdc42p is an important member of the Rho family of Ras-like small GTPases. The Rho branch controls both the organization of the actin-based cytoskeleton and gene expression in eukaryotic cells in response to various signals (22). Cdc42p regulates its effects through a variety of proteins. These “effectors” include kinases, such as PAK (21) and ACK (20) family members, polarity regulators such as PAR6 (18), actin cytoskeleton regulators such as WASP (34), and formins such as Bni1p (8). Regulation of many of these effectors has been shown to occur through direct interaction between the GTPase and its target protein. Among the best studied of these interactions is the association between the PAK family kinases and Cdc42p (26). This interaction involves a binding motif on the kinase known as a CRIB domain and a relatively large surface of the GTPase (10).
The biological function of Cdc42p has been extensively studied in Saccharomyces cerevisiae. In this budding yeast, Cdc42p activity has been implicated in polarized growth and bud emergence (11, 29). Cdc42p also functions in regulating a mitogen-activated protein (MAP) kinase cascade controlling pseudohyphal formation. Intriguingly, many of the kinases involved in this pathway also function in the pheromone response pathway (30). The regulation of pseudohyphal growth requires the PAK kinase family member Ste20p (19) and involves the association of Cdc42p with a CRIB domain of the kinase (15, 28). The role of Cdc42p in the pheromone response has been controversial (15, 28, 32), but recent evidence suggests a minor but direct effect of Cdc42p interaction with Ste20p in the mating process (27).
Pseudohyphal growth is the most extreme form of polarized growth that has been described for S. cerevisiae, but many other fungi are able to form true hyphae. In response to external signals, the fungal pathogen Candida albicans can even undergo a switch from yeast-like growth to true hyphal growth (25, 39). Many of the C. albicans hyphal growth pathway genes that have been identified thus far are homologous to genes involved in the S. cerevisiae MAP kinase pathways controlling pheromone response and pseudohyphal growth (6, 13, 14). Disruption of a subset of these genes in C. albicans created strains with decreased virulence, implicating this MAP kinase module in the pathogenicity of the organism (6, 13, 14). Recently, evidence that C. albicans also contains a homolog of the CDC42 gene has been presented (24). To further investigate the molecular basis of the yeast-to-hyphal morphogenetic transition, we have created a strain where the only copy of CDC42 is under the control of a regulatable promoter. We have also studied the consequences of a series of mutations that create constitutive and dominant-negative forms of Cdc42p. Our results show that Cdc42p function is required for cellular proliferation and polarized growth in C. albicans.
MATERIALS AND METHODS
Strains and plasmids.
CaCDC42 was isolated by complementation in the S. cerevisiae CDC42-ts strain DJTD2-16A (12) (MATa his4 leu2 trp1 ura3 cdc42-1) (kindly provided by D. Johnson, University of Vermont), which was transformed with a genomic C. albicans library cloned into the S. cerevisiae vector YEp352 (2). More than 2,000 transformants were plated at room temperature and replica plated to test for growth at 34°C, and plasmids were isolated from surviving colonies. A 2.8-kb SacI-EcoRI complementing fragment from one plasmid was subcloned into pBSKS+ (Stratagene) to generate pDH208, which contains the entire open reading frame of CaCDC42 with 300 bp of upstream noncoding sequence and 1.9 kb of downstream noncoding sequence. Our sequence is identical to that previously reported for CaCDC42 (24). A BamHI site was introduced 5 bp upstream of the ATG by using primers OJA12 and OJA13 (sequences listed in Table 1) to yield pJA19. Site-directed mutagenesis was carried out with the Quickchange Site-Directed Mutagenesis kit from Stratagene to generate the following single and double point mutations in CaCDC42 in pJA19 (with the resulting plasmids and the primers used given in parentheses): G12V (pSU7, using oligonucleotides OSU5 and OSU6), D118A (pSU9, using oligonucleotides OSU7 and OSU8), C188S (pSU13, using oligonucleotides OSU9 and OSU64), G12V C188S (pSU16), and D118A C188S (pSU18). All constructs were verified by sequence analysis. To overexpress and integrate wild-type CaCDC42 and the five mutant CaCDC42 versions in C. albicans, a cassette was made containing 3 kb of hisG URA3 hisG sequences (providing an excisable C. albicans selectable marker) isolated from pCUB− (9) and the 1.4-kb PCK1 promoter (a C. albicans regulatable promoter) isolated from pCA01 (17), yielding pJA24. PCK1 encodes phosphoenolpyruvate (PEP) carboxykinase, an enzyme strongly repressed in glucose-containing media (17). PCK1 expression can be induced in media containing a gluconeogenic carbon source such as 2% Casamino Acids (CAA) medium (17). This cassette was cloned into the unique BamHI site in pJA19 and pSU7, -9, -13, -16, and -18, generating pJA28 and pSU48, -50, -51, -52, and -45, respectively. Each plasmid was linearized using a unique HpaI site within the PCK1 promoter and was transformed into strain CAI4 or CaDH85 (Table 2) by either the lithium acetate procedure (31) or the one-step transformation protocol (4). In addition, pJA28 and pSU48 were integrated into the doubly deleted CST20 and CaCLA4 strains CaDH25 and CaLJ5, respectively, to create CaSU112 (CST20 double deletion with PCK1 CDC42) and CaSU116 (CST20 double deletion with PCK1 CDC42G12V) as well as CaSU138 (CaCLA4 double deletion with PCK1 CDC42) and CaSU142 (CaCLA4 double deletion with PCK1 CDC42G12V). Correct integration was verified by isolating genomic DNA and using PCR with primers OSU43 and OSU45. Integration of PCK1 CaCDC42 derivatives was further verified by Southern analysis using the digoxigenin (DIG) kit from Boehringer Mannheim.
TABLE 1.
Oligonucleotides
| Primer | Sequencea |
|---|---|
| OJA12 | CGGGATCCATGAGCTTGAATACTTTTCTCTTGTGC |
| OJA13 | CGGGATCCCCACATATCATGCAAACTATAAAATGT |
| OEL112 | CGGGATCCTCTGTCGTAATCTTCTTGACC |
| OEL113 | CGGGATCCGTACGTGTTTCAAAAGTGATA |
| OSU5 | GTGTTGGTTGTCGGTGATGtTGCCGTTGGTAAAACTTGC |
| OSU6 | GCAAGTTTTACCAACGGCAaCATCACCGACAACAACAC |
| OSU7 | GTCGGTACCCAAACTGcTTTACGAAACGATGATG |
| OSU8 | CATCATCGTTTCGTAAAgCAGTTTGGGTACCGAC |
| OSU9 | CCTGTAATTAAAAAATCGAAAAAGTcTACTATTTTATAGGTCGGCG |
| OSU64 | CGACGACCTATAAAATAGTAgACTTTTTCGATTTTTTAATTACAGG |
| OSU33 | CATCCTTCTACCAATATCTTC |
| OSU34 | CTAGAGTCTTACACACATC |
| OSU43 | GGAGCATTGTGTCTGATC |
| OSU44 | CTGAGAGTGATAGATATCTTAGCAAGAG |
| OSU45 | GATAATACCGAGATCGAC |
| ODH103 | GTGTTACGAATCAATGGCACT |
The introduced BamHI site is underlined; changed nucleotides are lowercased.
TABLE 2.
Strains
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| SC5314 | URA3/URA3 | 9 |
| CAI4 | ura3/ura3 | 9 |
| CaDH50 | ura3/ura3 CaCDC42/cacdc42::hisG-URA3-hisG | This study |
| CaDH85 | ura3/ura3 CaCDC42/cacdc42::hisG | This study |
| CaSU64 | CaDH85 PCK1-CaCDC42G12V::hisG-URA3-hisG | This study |
| CaSU69 | CaDH85 PCK1-CaCDC42D118A::hisG-URA3-hisG | This study |
| CaSU71 | CaDH85 PCK1-CaCDC42C188S::hisG-URA3-hisG | This study |
| CaSU76 | CaDH85 PCK1-CaCDC42G12V C188S::hisG-URA3-hisG | This study |
| CaSU79 | CaDH85 PCK1-CaCDC42D118A C188S::hisG-URA3-hisG | This study |
| CaSU84 | CaDH85 PCK1-CaCDC42::hisG-URA3-hisG | This study |
| CaSU92 | CaDH85 PCK1-CaCDC42::hisG | This study |
| CaSU96-98 | PCK1-CaCDC42::hisG/cacdc42::hisG/cacdc42::hisG-URA3-hisG | This study |
| CaDH22 | ura3/ura3 cst20::hisG/cst20::hisG-URA3-hisG | 14 |
| CaDH25 | ura3/ura3 cst20::hisG/cst20::hisG | 14 |
| CaSU112 | CaDH25 PCK1-CaCDC42::hisG-URA3-hisG | This study |
| CaSU116 | CaDH25 PCK1-CaCDC42G12V::hisG-URA3-hisG | This study |
| CaLJ3 | ura3/ura3 cacla4::hisG/cacla4::hisG-URA3-hisG | 16 |
| CaLJ5 | ura3/ura3 cacla4::hisG/cacla4::hisG | 16 |
| CaSU138 | CaLJ5 PCK1-CaCDC42::hisG-URA3-hisG | This study |
| CaSU142 | CaLJ5 PCK1-CaCDC42G12V::hisG-URA3-hisG | This study |
Construction and analysis of a CaCDC42 double deletion in a PCK1-CDC42-containing strain.
A deletion cassette was constructed by removing 640 bp between the KpnI and XbaI sites in pDH208, replacing this with a BamHI site by using oligonucleotides OEL112 and OEL113, and inserting the hisG URA3 hisG cassette to yield pDH212. C. albicans strains CaDH85 and CaSU92 (PCK1 CaCDC42) were transformed either by the lithium acetate procedure or by a one-step protocol using the 5.9-kb SacI-HindIII fragment from pDH212 that contains 478 bp of CaCDC42 upstream sequences, the URA blaster cassette, and 1.8 kb of downstream sequences. Deletion of the second CaCDC42 allele was screened by PCR (three independent products from OSU44 plus OSU8, OSU33 plus OSU34, and ODH103 plus OSU44) and confirmed by Southern analysis; three independent knockouts were found among the first 30 transformants screened in CaSU92 (PCK1 CaCDC42), yielding CaSU96 to -98. No double knockouts were obtained among the 150 CaDH85 transformants screened.
Phenotypic analysis.
To assess the effect of Cdc42p depletion on vegetatively grown C. albicans cells, all three of strains CaSU96 to -98 were grown overnight in 2% CAA-yeast nitrogen base (YNB; Difco)-Ura medium at 30°C. After 18 h of growth, cells were subcultured in either 2% glucose-YNB-Ura or 2% CAA-YNB-Ura, and aliquots were stained with 4",6"-diamidino-2-phenylindole (DAPI) and examined microscopically at various time points up to 10 h. To assess the effect of CaCdc42p depletion on hyphal induction, cells were grown for 18 h in 2% CAA-Ura and were then subcultured under the following conditions: 2% CAA-Ura plus 10% serum at 37°C, 2% glucose plus 10% serum at 37°C, or 2% glucose for 4 h at 30°C followed by addition of 10% serum and incubation at 37°C. Aliquots of each culture were stained with DAPI and examined microscopically every 2 to 3 h.
FACS analysis.
Cells were grown in the specified medium and fixed with 70% ethanol for 20 min. They were then resuspended in 1 ml of phosphate-buffered saline (PBS) containing 10 μg of propidium iodide (Molecular Probes, Eugene, Oreg.)/ml. Fluorescence-activated cell sorter (FACS) analysis was performed on an EPICS XL-MCL flow cytometer (Beckman-Coulter) using a 488-nm dichroic filter for side scattering (SS) detection and a 620-nm band-pass filter for propidium iodide fluorescence detection. Forward scattering (FS) and SS data were expressed in mean relative units, and propidium iodide data were expressed in mean fluorescence units, both on a linear scale. Approximately 30,000 cells were analyzed per experiment to monitor the size of CaSU96 and CaSU97 cells either 6 or 18 h after subculture into 2% glucose-Ura or 2% CAA-Ura.
PCK1 CaCDC42 point mutant overexpression.
A series of point mutants in CaCDC42 were made in order to assess the functions of these residues in C. albicans morphogenesis. The point mutations chosen are well defined, and the effects have been investigated in other systems (11). These included the G12V, D118A, and C188S changes, as well as the G12V C188S and D118A C188S double mutants. To assess the effect of the ectopic expression of the PCK1 CaCDC42 wild-type strain and mutants, each strain was grown overnight in liquid YNB-2% glucose-Ura or YNB-2% CAA-Ura. For solid plating, cells were grown overnight in liquid YNB-2% glucose-Ura, then washed and diluted 10−5, and 20-μl aliquots were plated onto YNB-2% glucose-Ura or YNB-2% CAA-Ura and either incubated at 23 or 30°C . For liquid hyphal induction, cells were grown overnight at 30°C in either YNB-2% glucose-Ura or YNB-2% CAA-Ura, then subcultured in the same medium with the addition of 10% fetal bovine serum (FBS), and transferred to 37°C. Hyphal inductions were monitored for as long as 6 h.
Cell staining (DAPI and Calcofluor) and microscopy (Nomarski/fluorescence).
Cells were first fixed in 70% ethanol for 20 min, rinsed twice in 10 mM phosphate buffer (pH 7.4)-150 mM NaCl (PBS), and resuspended in 500 μl of PBS before addition of a 1:1,000 dilution of either DAPI at 1 mg/ml or Calcofluor White at 1 mg/ml. Cells were stained either with DAPI for 12 min or with Calcofluor for 5 min; then they were rinsed twice in PBS, examined on a Zeiss Axiophot microscope with a 40× objective under Nomarski optics or under fluorescence conditions, and photographed.
RESULTS
CaCdc42p is required for vegetative growth.
To investigate CaCDC42 function in the yeast form of C. albicans, both copies of CaCDC42 were deleted in a strain containing a copy of CaCDC42 under the control of the PCK1 promoter. This strain was subcultured in a liquid medium that represses PCK1 expression (2% glucose-Ura) and monitored microscopically at various time points after subculture. By 6 h the majority of the culture had arrested as large, round, unbudded cells, suggesting that CaCdc42p is necessary for bud formation and for polarized growth. By FACS analysis, the average size of CaSU96 or CASU97 after 6 h in 2% glucose-Ura was increased 2.3- or 2.1-fold, respectively. DAPI staining was used to determine the nuclear content of the CaCdc42p-depleted cells. The majority of the arrested cells contained two nuclei, and the proportion of binucleate cells remained essentially constant upon continued inhibition of CaCdc42p function (Fig. 1). Monitoring of the cultures by FACS analysis showed that the size of CaSU96 or CASU97 cells continued to increase to 3.4- and 4.7-fold, respectively, in glucose medium compared to cells grown in CAA medium after 18 h. In addition, cellular polarity was investigated by staining cells with Calcofluor to monitor chitin distribution. In contrast to wild-type strains, where chitin staining was primarily localized to bud sites, CaCdc42p-depleted cells were round and showed delocalized Calcofluor staining (Fig. 2).
FIG. 1.
Requirement for CaCdc42p function in yeast growth. (a) Vegetatively growing cells of strain CaSU96 (ΔΔCaCDC42 with PCK1 CaCDC42) were switched to 2% glucose medium for 18 h to repress PCK1 CDC42 expression. (Left) Many of the cells are enlarged and unbudded. (Right) A majority of the enlarged cells are binucleate. (b) Vegetatively growing cells of strain CaSU96 (ΔΔCaCDC42 with PCK1 CaCDC42) were cultured in 2% CAA medium for 18 h to maintain PCK1 CDC42 expression. Under these conditions, the cells show normal morphology and 1 DAPI-staining body per cell.
FIG. 2.
Calcofluor staining of vegetatively growing cells. (a) Strain CaSU96 (ΔΔCaCDC42 with PCK1 CaCDC42) was cultured for 6 h in 2% CAA medium to maintain CaCDC42 expression prior to staining with Calcofluor to determine chitin localization. The chitin staining is predominantly concentrated in the bud scars. (b) Strain CaSU96 (ΔΔCaCDC42 with PCK1 CaCDC42) was cultured for 6 h in 2% glucose medium to repress CaCDC42 expression prior to staining with Calcofluor. These cells are large and round, and they exhibit delocalized chitin deposition.
Overexpression of Cdc42 point mutants in vegetatively grown cells.
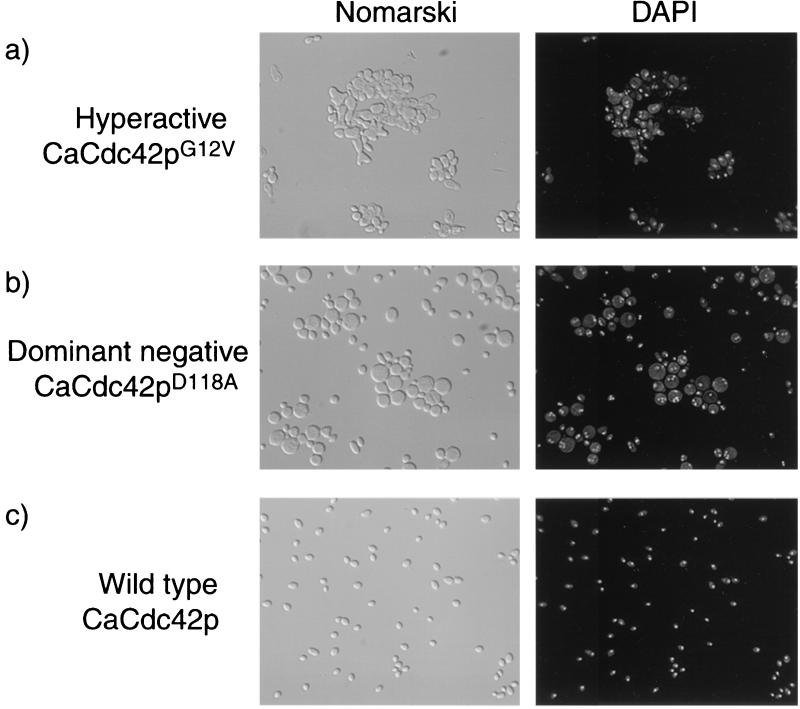
As described in Materials and Methods, wild-type CDC42 and a series of point mutant derivatives were fused to the PCK1 promoter and integrated either into strain CAI4, which contains both wild-type copies of CaCDC42, or into strain CaDH85, which contains one copy of disrupted CaCDC42 and one copy of the wild-type gene. The G12V mutant has decreased intrinsic GTPase activity, resulting in a mutant protein locked in an activated GTP-bound state (42), while the D118A mutant protein does not undergo GDP-to-GTP exchange, resulting in a protein locked in the inactive GDP-bound state (42). The C188S mutant protein cannot be isoprenylated and therefore is not localized to the membrane (42). Western analysis performed on SC5314, CaDH50, CaSU64, CaSU69, and CaSU84 showed significantly higher CaCdc42p levels in CaSU64, CaSU69, and CaSU84 (data not shown). These cells were grown overnight in a PCK1-inducing medium (2% CAA-Ura), stained with DAPI, and examined microscopically. Ectopic expression of the wild-type CaCdc42p in strain CaDH85 (CaSU84) had no effect on cell proliferation or cell morphology; cells formed colonies after overnight growth on solid medium (data not shown) and appeared normal (Fig. 3). In contrast, ectopic expression of either the hyperactive CaCdc42G12V protein or the dominant-negative D118A protein in strain CaDH85 (CaSU64 and CaSU69, respectively) blocked cell proliferation, showing no growth on solid medium (data not shown). PCK1-mediated expression of the G12V allele generated aberrant multibudded cells (Fig. 3), while ectopic expression of the dominant-negative D118A protein resulted in large, round cells that accumulated nuclei (Fig. 3). The proliferation arrest and the cell morphology changes caused by overexpression of the hyperactive and dominant-negative alleles require a functional CaCdc42p CAAX box. The C188S mutation rescued the inviability caused by ectopic expression of the G12V and D118A allele proteins and generated morphologically normal cells (data not shown). In strain CAI4, which contains the two wild-type copies of CaCDC42 in addition to the PCK1-driven gene, the phenotypic effects of the mutant proteins are similar but not as severe (data not shown).
FIG. 3.
Effects of ectopic expression of hyperactive (G12V) and dominant-negative (D118A) CaCdc42p alleles on vegetatively growing cells. (a) Strain CaSU64 (ectopic expression of CaCdc42pG12Vin the heterozygous disruptant strain CaDH85) was grown in 2% CAA medium for 6 h to induce CaCdc42p expression. Under these conditions the cells are clumped and often misshapen, and they can show more than 1 DAPI staining body per cell. (b) Strain CaSU69 (ectopic expression of CaCdc42pD118A in the heterozygous disruptant strain CaDH85) was grown in 2% CAA medium for 6 h to induce CaCdc42p expression. Under these conditions the cells are large and round and can show more than 1 DAPI staining body per cell. (c) Strain CaSU84 (ectopic expression of CaCdc42p in the heterozygous disruptant strain CaDH85) was grown in 2% CAA medium for 6 h to induce CaCdc42p expression. Under these conditions the cells have normal morphology and have 1 DAPI staining body per cell.
Overexpression of CaCdc42pG12V in a CST20 deletion strain or a CaCLA4 deletion strain.
PCK1 CaCDC42 (pJA28) and PCK1 CaCDC42G12V (pSU48) were integrated into strains with double deletions of either CST20 (14) or CaCLA4 (16), and phenotypes were examined under PCK1-inducing or non-PCK1-inducing conditions. Ectopic expression of CaCdc42pG12V in a CaCLA4-deficient strain resulted in inviable cells that were severely elongated and multibudded; prolonged incubation led to extensive lysis of the cells. This was not observed in the CaSTE20 deletion strain overexpressing CaCdc42pG12V. A minor growth defect was observed on solid medium, but the cells appeared normal upon microscopic analysis. There was no significant effect of overexpressing CaCDC42 in either of these strains (Fig. 4). The influence of the PCK1 CaCDC42G12V allele on cell growth was temperature dependent; room temperature expression had a more detrimental effect on proliferation than expression at 30°C, although at either temperature the CST20 deletion strain grew better than either the control or the CaCLA4 deletion strain.
FIG. 4.
PAK kinase requirement for hyperactive CaCDC42-mediated lethality. Cells were grown for 3 days on solid medium containing 2% CAA to induce expression of the wild-type or hyperactive CaCDC42 gene, and colonies were photographed with darkfield lighting using a Nikon TMS inverted microscope with a 2× objective. (a) (Left) Strain CaSU84 (CaDH85 transformed with PCK1 CaCDC42). (Right) CaSU64 (CaDH85 transformed with PCK1 CaCDC42G12V). Ectopic expression of wild-type CaCdc42p has no effect on the growth of the strain, but overproduction of the hyperactive allele greatly reduces proliferation and leads to the formation of microcolonies. (b) (Left) Strain CaSU112 (CaDH85 transformed with PCK1 CaCDC42). (Right) CaSU116 (CaDH25 transformed with PCK1 CaCDC42G12V). Growth of the cst20 disruptant strain was not affected by ectopic expression of wild-type CaCdc42p. Overexpression of the hyperactive CaCdc42p reduced proliferation of the strain, but macroscopic colonies formed. (c) (Left) Strain CaSU138 (CaLJ5 transformed with PCK1CaCDC42). (Right) CaSU142 (CaLJ5 transformed with PCK1CaCDC42G12V). Growth of the cacla4 mutant strain was not influenced by ectopic expression of wild-type CaCdc42p, but the strain was unable to form macroscopic colonies in the presence of hyperactive CaCdc42p.
CaCdc42p involvement in hyphal formation.
A time course experiment was done to examine the effect of depletion of CaCdc42p on hyphal formation (as described in Materials and Methods). After CaCdc42p is largely depleted (by incubation for 4 h in glucose-Ura and subsequent induction of hyphal formation with 10% FBS for 2 h), the cells arrest and are large, round, and primarily binucleate. If the cells are immediately switched to 2% glucose-Ura plus 10% serum, short germ tubes are initiated but then stop as CaCdc42p is depleted. These cells also increase in size and arrest with primarily two nuclei (Fig. 5). Intriguingly, cells with only the ectopically expressed CDC42 gene are more rod-shaped than cells also expressing a normal allele of CDC42.
FIG. 5.
CaCdc42p involvement in hyphal formation. (a) Strain CaSU96 (ΔΔCaCDC42 with PCK1 CaCDC42) was grown for 4 h in 2% glucose to repress CaCdc42p expression and then was switched to the hypha-inducing conditions of 2% glucose plus 10% FBS at 37°C, while CaCDC42 repression was maintained. The cells are large, round, and predominantly binucleate, and there is no evidence for germ tube formation. (b) Strain CaSU96 (ΔΔCaCDC42 with PCK1 CaCDC42) was grown at 37°C for 3 h in a hypha-inducing medium (2% CAA plus 10% FBS) that permits CaCDC42 expression. Germ tube formation is evident for the majority of cells, and there is 1 DAPI-staining body per cell.
Overexpression of Cdc42 point mutants under hypha-inducing conditions.
The effects of overexpressing the point mutants in CAI4 (data not shown) or CaDH85 were also examined under hypha-inducing conditions. Hyperactive CaCdc42pG12V expression under hypha-inducing conditions resulted in cells with large, aberrant, branched hypha-like structures (Fig. 6), suggesting that unregulated polarized growth was occurring. Surprisingly, ectopic expression of CaCdc42pD118A under these conditions resulted in cells with typically two hyphae containing many nuclei (Fig. 6). This experiment was repeated using a lower incubation temperature of 30°C and the hypha-inducing conditions of Soll's medium, and the same effect was observed (data not shown). Ectopic expression of either wild-type CaCdc42p (Fig. 6), CaCdc42pC188S, CaCdc42pG12V C188S, or CaCdc42pD118A C188S (data not shown) had no effect, suggesting that simple increased levels of wild-type CaCdc42p are not stimulatory for hyphal formation and that isoprenylation and membrane localization are still critical for CaCdc42p function under these conditions.
FIG. 6.
Effects of overexpression of hyperactive (G12V) and dominant-negative (D118A) CaCdc42p alleles under hypha-inducing conditions. (a) Strain CaSU64 (ectopic expression of CaCdc42pG12Vin the heterozygous disruptant strain CaDH85) was grown in 2% CAA plus 10% FBS medium for 3 h at 37°C to induce CaCdc42p expression under hyphal growth conditions. The cells are clumped and form aberrant germ tubes, and they can show more than 1 DAPI-staining body per cell. (b) Strain CaSU69 (ectopic expression of CaCdc42pD118A in the heterozygous disruptant strain CaDH85) was grown in 2% CAA plus 10% FBS medium for 3 h at 37°C to induce CaCdc42p expression. The cells form large, aberrant hyphae and often extend two hyphae from the same starting cell. (c) Strain CaSU84 (ectopic expression of CaCdc42p in the heterozygous disruptant strain CaDH85) was grown in 2% CAA plus 10% FBS medium for 3 h at 37°C to induce CaCdc42p expression. Under these conditions the cells form normal germ tubes.
DISCUSSION
In C. albicans the homolog of the Cdc42p GTPase, CaCdc42p, is required for proper proliferation and cellular morphogenesis. Construction of a strain with the only functional copy of the CaCDC42 gene under control of the regulated PCK1 promoter permitted the controlled shutoff of CaCDC42 expression. Vegetatively growing cells that were no longer expressing CaCDC42 ceased proliferation within several hours of promoter shutoff. Continued inhibition of CaCDC42 expression resulted in accumulation of large, round, unbudded cells. These cells showed delocalized chitin staining, in contrast to wild-type cells, which localized chitin to the bud necks and bud scars. A block in proliferation and budding, coupled with an apparent block in polarized growth resulting in round cells, was also exhibited by S. cerevisiae strains containing conditional mutations in CDC42 (1, 12).
As was also noted for S. cerevisiae (1), the arrested C. albicans cells were multinucleate, with most of the cells containing two nuclei. However, continued maintenance of the arrested state did not lead to highly multinucleated cells. FACS analysis suggests that DNA replication is continuing in these cells; this replication could be occurring with limited segregation of the DNA, thus maintaining the low number of DAPI-staining bodies per cell. Alternatively, the reduction of CaCdc42p function may not completely uncouple DNA replication from bud emergence, and so the cells are able to recognize the block in bud formation and shut off DNA replication after one or two rounds of DNA synthesis. C. albicans cells may thus contain a G2 checkpoint that monitors a block in bud formation or actin cytoskeleton organization. We obtained identical phenotypes for strains containing the only functional CaCDC42 gene under the control of the MET3 promoter (data not shown) and cells that had the CDC42 gene removed by regulated excision (S. Michel et al., submitted for publication), establishing that the terminal phenotype was not dependent on the physiology of the promoter shutoff.
Overexpression of mutant versions of CaCdc42p were used to further investigate the role of CaCDC42 in morphogenetic control in C. albicans. Simple ectopic expression of wild-type CaCdc42p under the control of the PCK1 promoter had no effect on cell growth and morphogenesis. The glycine 12-to-valine (G12V) mutation, initially identified in oncogenic versions of mammalian Ras (35), activates these GTPases by locking them in a form that mimics the GTP-bound protein (33). This activating mutation was introduced into CaCDC42 expressed under the control of the PCK1 promoter, and the mutant CaCdc42p was overexpressed in both wild-type and CaCDC42/ΔCaCDC42 heterozygous cells. Ectopic expression of the activated allele was highly detrimental to the cells, and this was somewhat dependent on the dosage of the wild-type allele, as the heterozygote died more rapidly than the homozygous wild type. In addition, the influence of the activated allele was temperature sensitive; lower temperatures accentuated the influence of the overexpressed G12V mutant protein. The equivalent mutation in S. cerevisiae (42) also caused lethality, suggesting that deregulated Cdc42p activity cannot be tolerated in a variety of cells, although it does not cause lethality in all cells (23).
We investigated the requirements for this hyperactive CaCdc42p-mediated lethality. Membrane association appeared essential, because mutation of the prenylated cysteine of the CAAX box to a serine completely suppressed the phenotype of cells expressing G12V mutated CaCdc42p. It has been suggested that the lethality of Cdc42pG12V in S. cerevisiae is due to the constitutive interaction with its downstream effectors (7). These effectors include members of the PAK kinase family, STE20 and CLA4. Homologs of both STE20 (CST20) and CLA4 (CaCla4) have been identified and characterized in C. albicans (14, 16). We tested whether either of these kinases was essential for G12V-mediated growth inhibition. Ectopic expression of the G12V version of CaCdc42p was not able to kill cells lacking the CST20 gene. In contrast, deletion of CaCla4 did not block the inviability of cells containing the hyperactive CaCdc42p, and in fact, cells with CaCLA4 deleted were killed more rapidly than wild-type cells in the presence of CaCdc42pG12V. This suggests that the lethal effects of hyperactive CaCdc42p are generated primarily through the action of Cst20p and that the Cla4p and Cdc42p effects may be in parallel. This result is in contrast to the situation in S. cerevisiae, where deletions of CLA4 or SKM1 but not STE20 suppressed Cdc42pG12V-mediated lethality (7). In the C. albicans CST20 deletion strain, there are still effects on cell proliferation, showing that hyperactive CaCdc42p has other targets that play a less important role in proliferative control. This observation that Cst20p is a lethal “effector” of CaCdc42pG12V is somewhat surprising in light of the apparently minor role of Cst20p in morphogenetic control. While cells lacking CaCla4p are morphologically aberrant, are defective in hyphal formation, and are avirulent (16), cells lacking Cst20p are morphologically normal, form hyphae under many conditions including the standard serum-plus-37°C regimen, and are virulent in systemic infections in mice (14).
Ectopic expression of the D118A version of CaCdc42p also caused cellular inviability. The morphology of the arrested cells was similar to that of the CaCDC42 double-deletion mutant, consistent with the D118A allele acting as a dominant negative. Analysis in S. cerevisiae has suggested that the D118A allele acts to sequester the exchange factor Cdc24p, thus limiting activation of the endogenous wild-type Cdc42p (7). It is somewhat surprising that both in S. cerevisiae and in C. albicans this dominant-negative phenotype requires a functional CAAX box and thus, potentially, membrane association of the mutant GTPase. In other systems CAAX box-defective mutant proteins themselves are capable of acting as dominant negatives (40). It is possible that Cdc24p is itself membrane associated and that Cdc42p mutants have to be able to attach to the membrane in order to efficiently inactivate Cdc24p. Alternatively, it may be that prenylation is involved in the association of Cdc42p with Cdc24p and that mutation of the CAAX box affects this interaction. Although CaCdc42pD118A expression produces a phenotype similar to that of the CaCDC42 double-deletion mutant, there are some distinctions. In particular, under yeast growth conditions, the D118A mutant appears to generate cells with a higher frequency of multiple nuclei than the CaCDC42 double-deletion mutant. It is possible that the uncoupling of bud emergence and DNA segregation is more complete in the dominant-negative situation.
Because of the importance of the Rho family GTPases in the general regulation of polarized morphogenesis in eukaryotic cells (5, 36, 37), we further investigated the involvement of CaCDC42 in the control of hyphal growth in C. albicans. There are some differences in the role of Cdc42p in the establishment and maintenance of hyphal growth in other filamentous fungi. In Wangiella dermatitidis, deletion of CDC42 does not prevent initiation of apical growth or elongation, suggesting that in this organism CDC42 is not required for hyphal growth (41). However, in Ashbya gossypii, spores lacking CDC42 were able to induce the isotropic growth phase but could not induce the isotropic-to-polar switch, and thus no hyphae were formed (38).
In C. albicans, specific external signals such as high temperature and serum are capable of inducing an essentially quantitative shift of yeast-like cells into hyphal forms (3, 39). When C. albicans cells were depleted of CaCdc42p prior to triggering the yeast-to-hypha switch, the cells arrested with phenotypes similar to those of the yeast form mutants, namely large, round, unbudded cells. When the yeast-to-hypha switch was triggered at the same time as repression of CaCDC42 expression, the cells formed abortive germ tubes but then arrested as primarily binucleate, unbudded cells. This result suggests that the germ tube initiates before the CaCdc42p is depleted, but that in the absence of continuing CaCdc42p function, the germ tube cannot be extended. It is unlikely that the failure to extend the germ tube is simply caused by the failure to proliferate; nuclear division occurs without germ tube initiation, and depletion of Cdc42p blocks germ tube growth after initiation. Thus, CaCDC42 appears essential for proper polarized growth of C. albicans cells growing under both yeast and hypha-inducing conditions. In addition, proper regulation of CaCDC42 is essential for normal hyphal formation, as the germ tubes generated in CaSU96 under PCK1-inducing conditions are clearly not wild type (Fig. 5b versus Fig. 6c).
As was also noted in the yeast cell growth situation, the phenotype of the dominant-negative D118A allele was not identical to that of the CaCDC42 double-deletion mutant under hyphal conditions. The D118A mutant generates aberrant hyphal structures in the presence of serum, while the CaCDC42 double-deletion mutant blocks hyphal formation under these conditions. It is possible that the growth conditions for stimulating hyphal formation are somewhat incompatible with PCK1 expression and that therefore the expression levels of CaCdc42pD118A are insufficient to block endogenous GTPase function when cells are grown in the presence of serum. Alternatively, it may be that the target of the dominant-negative protein plays a different role in yeast and hyphal growing cells.
Previous work on CaCDC42 suggested that the encoded protein may regulate hyphal formation, because the expression of the gene increased under hypha-inducing conditions (24). The present work shows that CaCdc42p is required for both yeast and hyphal proliferation. In addition, this work establishes that simple overexpression of CaCDC42 has little effect on the cells, while modification of CaCdc42p activity through activating and dominant-negative mutations has profound cellular effects. In the case of the hyperactive G12V allele, it is evident that a primary effector is the Cst20p kinase. Thus, it is likely that the activity of the CaCdc42p GTPase, rather than its expression, is the primary means through which CaCdc42p influences cellular morphogenesis.
Acknowledgments
S.C.U. thanks George Szatmari for critical reading of the manuscript. We thank the members of the BRI Genetics group for comments and support.
Footnotes
Dedicated to the memory of Teresa Keng. National Research Council of Canada publication 44793.
REFERENCES
- 1.Adams, A. E., D. I. Johnson, R. M. Longnecker, B. F. Sloat, and J. R. Pringle. 1990. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J. Cell Biol. 111:131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boone, C., A. Sdicu, M. Laroche, and H. Bussey. 1991. Isolation from Candida albicans of a functional homolog of the Saccharomyces cerevisiae KRE1 gene, which is involved in cell wall beta-glucan synthesis. J. Bacteriol. 173:6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, A. J., and N. A. Gow. 1999. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 7:333-338. [DOI] [PubMed] [Google Scholar]
- 4.Chen, D. C., B. C. Yang, and T. T. Kuo. 1992. One-step transformation of yeast in stationary phase. Curr. Genet. 21:83-84. [DOI] [PubMed] [Google Scholar]
- 5.Chimini, G., and P. Chavrier. 2000. Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat. Cell Biol. 2:E191-E196. [DOI] [PubMed]
- 6.Csank, C., K. Schroppel, E. Leberer, D. Harcus, O. Mohamed, S. Meloche, D. Y. Thomas, and M. Whiteway. 1998. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect. Immun. 66:2713-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, C. R., T. J. Richman, S. B. Deliduka, J. O. Blaisdell, C. C. Collins, and D. I. Johnson. 1998. Analysis of the mechanisms of action of the Saccharomyces cerevisiae dominant lethal cdc42G12V and dominant negative cdc42D118A mutations. J. Biol. Chem. 273:849-858. [DOI] [PubMed] [Google Scholar]
- 8.Evangelista, M., K. Blundell, M. S. Longtine, C. J. Chow, N. Adames, J. R. Pringle, M. Peter, and C. Boone. 1997. Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science 276:118-122. [DOI] [PubMed] [Google Scholar]
- 9.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo, W., M. J. Sutcliffe, R. A. Cerione, and R. E. Oswald. 1998. Identification of the binding surface on Cdc42Hs for p21-activated kinase. Biochemistry 37:14030-14037. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, D. I. 1999. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63:54-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, D. I., and J. R. Pringle. 1990. Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J. Cell Biol. 111:143-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohler, J. R., and G. R. Fink. 1996. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc. Natl. Acad. Sci. USA 93:13223-13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leberer, E., D. Harcus, I. D. Broadbent, K. L. Clark, D. Dignard, K. Ziegelbauer, A. Schmidt, N. A. Gow, A. J. Brown, and D. Y. Thomas. 1996. Signal transduction through homologs of the Ste20p and Ste7p protein kinases can trigger hyphal formation in the pathogenic fungus Candida albicans. Proc. Natl. Acad. Sci. USA 93:13217-13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leberer, E., C. Wu, T. Leeuw, A. Fourest-Lieuvin, J. E. Segall, and D. Y. Thomas. 1997. Functional characterization of the Cdc42p binding domain of yeast Ste20p protein kinase. EMBO J. 16:83-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leberer, E., K. Ziegelbauer, A. Schmidt, D. Harcus, D. Dignard, J. Ash, L. Johnson, and D. Y. Thomas. 1997. Virulence and hyphal formation of Candida albicans require the Ste20p-like protein kinase CaCla4p. Curr. Biol. 7:539-546. [DOI] [PubMed] [Google Scholar]
- 17.Leuker, C. E., A. Sonneborn, S. Delbruck, and J. F. Ernst. 1997. Sequence and promoter regulation of the PCK1 gene encoding phosphoenolpyruvate carboxykinase of the fungal pathogen Candida albicans. Gene 192:235-240. [DOI] [PubMed] [Google Scholar]
- 18.Lin, D., A. S. Edwards, J. P. Fawcett, G. Mbamalu, J. D. Scott, and T. Pawson. 2000. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat. Cell Biol. 2:540-547. [DOI] [PubMed] [Google Scholar]
- 19.Liu, H., C. A. Styles, and G. R. Fink. 1993. Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science 262:1741-1744. [DOI] [PubMed] [Google Scholar]
- 20.Manser, E., T. Leung, H. Salihuddin, L. Tan, and L. Lim. 1993. A non-receptor tyrosine kinase that inhibits the GTPase activity of p21cdc42. Nature 363:364-367. [DOI] [PubMed] [Google Scholar]
- 21.Manser, E., T. Leung, H. Salihuddin, Z. S. Zhao, and L. Lim. 1994. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367:40-46. [DOI] [PubMed] [Google Scholar]
- 22.Matozaki, T., H. Nakanishi, and Y. Takai. 2000. Small G-protein networks: their crosstalk and signal cascades. Cell Signal 12:515-524. [DOI] [PubMed] [Google Scholar]
- 23.Miller, P. J., and D. I. Johnson. 1994. Cdc42p GTPase is involved in controlling polarized cell growth in Schizosaccharomyces pombe. Mol. Cell. Biol. 14:1075-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirbod, F., S. Nakashima, Y. Kitajima, R. D. Cannon, and Y. Nozawa. 1997. Molecular cloning of a Rho family, CDC42Ca gene from Candida albicans and its mRNA expression changes during morphogenesis. J. Med. Vet. Mycol. 35:173-179. [PubMed] [Google Scholar]
- 25.Mitchell, A. P. 1998. Dimorphism and virulence in Candida albicans. Curr. Opin. Microbiol. 1:687-692. [DOI] [PubMed] [Google Scholar]
- 26.Morreale, A., M. Venkatesan, H. R. Mott, D. Owen, D. Nietlispach, P. N. Lowe, and E. D. Laue. 2000. Structure of Cdc42 bound to the GTPase binding domain of PAK. Nat. Struct. Biol. 7:384-388. [DOI] [PubMed] [Google Scholar]
- 27.Moskow, J. J., A. S. Gladfelter, R. E. Lamson, P. M. Pryciak, and D. J. Lew. 2000. Role of Cdc42p in pheromone-stimulated signal transduction in Saccharomyces cerevisiae. Mol. Cell. Biol. 20:7559-7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peter, M., A. M. Neiman, H. O. Park, M. van Lohuizen, and I. Herskowitz. 1996. Functional analysis of the interaction between the small GTP binding protein Cdc42 and the Ste20 protein kinase in yeast. EMBO J. 15:7046-7059. [PMC free article] [PubMed] [Google Scholar]
- 29.Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J. Cell Sci. 113:365-375. [DOI] [PubMed] [Google Scholar]
- 30.Roberts, R. L., and G. R. Fink. 1994. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 8:2974-2985. [DOI] [PubMed] [Google Scholar]
- 31.Schiestl, R. H., M. Dominska, and T. D. Petes. 1993. Transformation of Saccharomyces cerevisiae with nonhomologous DNA: illegitimate integration of transforming DNA into yeast chromosomes and in vivo ligation of transforming DNA to mitochondrial DNA sequences. Mol. Cell. Biol. 13:2697-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon, M. N., C. De Virgilio, B. Souza, J. R. Pringle, A. Abo, and S. I. Reed. 1995. Role for the Rho-family GTPase Cdc42 in yeast mating-pheromone signal pathway. Nature 376:702-705. [DOI] [PubMed] [Google Scholar]
- 33.Sweet, R. W., S. Yokoyama, T. Kamata, J. R. Feramisco, M. Rosenberg, and M. Gross. 1984. The product of ras is a GTPase, and the T24 oncogenic mutant is deficient in this activity. Nature 311:273-275. [DOI] [PubMed] [Google Scholar]
- 34.Symons, M., J. M. Derry, B. Karlak, S. Jiang, V. Lemahieu, F. McCormick, U. Francke, and A. Abo. 1996. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell 84:723-734. [DOI] [PubMed] [Google Scholar]
- 35.Tabin, C. J., S. M. Bradley, C. I. Bargmann, R. A. Weinberg, A. G. Papageorge, E. M. Scolnick, R. Dhar, D. R. Lowy, and E. H. Chang. 1982. Mechanism of activation of a human oncogene. Nature 300:143-149. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka, K., and Y. Takai. 1998. Control of reorganization of the actin cytoskeleton by Rho family small GTP-binding proteins in yeast. Curr. Opin. Cell Biol. 10:112-116. [DOI] [PubMed] [Google Scholar]
- 37.Valster, A. H., P. K. Hepler, and J. Chernoff. 2000. Plant GTPases: the Rhos in bloom. Trends Cell Biol. 10:141-146. [DOI] [PubMed] [Google Scholar]
- 38.Wendland, J., and P. Philippsen. 2001. Cell polarity and hyphal morphogenesis are controlled by multiple Rho-protein modules in the filamentous ascomycete Ashbya gossypii. Genetics 157:601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whiteway, M. 2000. Transcriptional control of cell type and morphogenesis in Candida albicans. Curr. Opin. Microbiol. 3:582-588. [DOI] [PubMed] [Google Scholar]
- 40.Whiteway, M. S., and D. Y. Thomas. 1994. Site-directed mutations altering the CAAX box of Ste18, the yeast pheromone-response pathway G gamma subunit. Genetics 137:967-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye, X., and P. J. Szaniszlo. 2000. Expression of a constitutively active Cdc42 homologue promotes development of sclerotic bodies but represses hyphal growth in the zoopathogenic fungus Wangiella (Exophiala) dermatitidis. J. Bacteriol. 182:4941-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ziman, M., J. M. O'Brien, L. A. Ouellette, W. R. Church, and D. I. Johnson. 1991. Mutational analysis of CDC42Sc, a Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol. Cell. Biol. 11:3537-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]