Abstract
Background
Despite great advances in its early diagnosis and treatment, lung cancer is still an intractable disease and the second leading cause of cancer-related deaths and morbidity in the world. The family of Polo-like kinases (PLKs) consists of five serine/threonine kinases, which have been reported to participate in various human diseases. However, the expression and prognostic value of each PLK in human lung cancer have not been fully understood. This study analyzed mRNA expression and prognostic value of different PLKs in human non-small cell lung cancer (NSCLC).
Methods
First, mRNA expression of PLKs in patients with NSCLC from the Oncomine and the Gene Expression Profiling Interactive Analysis (GEPIA) database was investigated. Then, a Kaplan–Meier plotter was employed for survival analysis. The sequence alteration for PLKs was analyzed using The Cancer Genome Atlas (TCGA) and the cBioPortal database. Additionally, we analyzed the association among different PLKs using the LinkedOmics database. Finally, the enrichment analysis of PLKs was achieved using the DAVID database.
Results
The mRNA expression levels of PLK1 and PLK4 were significantly overexpressed, while mRNA expression level of PLK3 was underexpressed in patients with NSCLC. mRNA expressions of PLK1 and PLK4 were significantly and positively related to the tumor stage of NSCLC. Increased expressions of PLK1, PLK4, and PLK5 and decreased expression of PLK2 were attributed to limited overall survival time in NSCLC. PLK1 was positively correlated with PLK4 via the LinkedOmics database.
Conclusions
PLKs are relevant targets for NSCLC treatment, especially PLK1 and PLK4.
Keywords: Lung cancer, Polo-like kinases, Prognosis, Oncomine, Kaplan–Meier plotter
Introduction
Lung cancer is an intractable disease and the second leading cause of cancer-related deaths and morbidity (Siegel et al. 2019). The main pathological subtypes of lung cancer include lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), and small cell lung cancer (SCLC) (Bray et al. 2018). Despite advances in understanding of the potential molecular mechanisms in the development of non-small cell lung cancer (NSCLC) and the development of new therapeutic strategies, the 5-year survival rates for lung cancer remain low. The poor prognosis of NSCLC is largely due to the delayed diagnosis, the rapid progression, and the frequent drug resistance. Recently, immunotherapy and oncogene-targeted therapy, including the use of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs), have revolutionized the treatment of NSCLC. Studies of tumor molecular mechanism have accelerated the development of targeted therapy and immunotherapy and these new therapies have provided prospects for individualized treatment and improved prognosis for lung cancer patients. In the post-genome era, massive biological information data were discovered. Biological information scientists have developed novel algorithms and software to establish databases for systematic management and generate enough sequence data. In this way, the molecular mechanisms of cancer development can be better understood. Due to the heterogeneity of tumors, the current biomarkers for prognosis have certain limitations. Nevertheless, the development of tumor bioinformatics provides a reference for identification of appropriate biomarkers for tumor prognosis and improvement of tumor prognosis.
The family of Polo-like kinases (PLKs) consists of five serine/threonine kinases (PLK1, PLK2, PLK3, PLK4, PLK5) and plays an important role in multiple cellular processes, including DNA replication and mitosis in both normal and cancer cells (Liu 2015; Strebhardt 2010). PLKs have complicated, yet unique effects on human cancers. Abnormal expression of different PLK family members has been demonstrated in many cancer types and has been related to poor prognosis. Among the five mammalian PLKs, PLK1 (also known as STPK13) is closely related to cellular proliferation and has been thoroughly studied. The expression of PLK1 is dysregulated in several human cancers, including melanomas, breast cancer, colorectal cancer, gastric cancer, and lung cancer (Strebhardt 2010). The tumor suppressor p53 is destroyed in more than 50% of human cancers. It is reported that overexpression of PLK1 may lead to proliferation and metastasis of cancer cells by accelerating the destruction of p53 (Strebhardt 2010). PLK2 (also known as SNK) is an early growth response gene (Simmons et al. 1992) and its expression is significantly reduced in many hematologic malignancies, including multiple myeloma and Burkitt lymphomas (Syed et al. 2006). This may be attributed to methylation of the CpG islands in the PLK2 gene in these malignancies (Benetatos et al. 2011). It is suggested that PLK2 may be a tumor suppressor. In contrast to PLK1, PLK3 is underexpressed in various human cancers, including lung cancer, liver cancer, and head and neck cancer (Dai et al. 2000; Li et al. 1996; Pellegrino et al. 2010). Western blot analysis revealed underexpression of PLK3 in hepatocellular carcinoma patients with less survival time (Pellegrino et al. 2010). It is also suggested that PLK3 may be a tumor suppressor in human cancers. PLK4 is upregulated in cancers such as colon cancer, breast cancer, and lung cancer (Kawakami et al. 2018; Li et al. 2016; Marina and Mihaela 2014). Zhou et al. reported that PLK4 was associated with poor survival in NSCLC (Zhou et al. 2020). In addition, it is reported that inhibition of PLK4 expression was associated with increased sensitization of cancer treatment (Mason et al. 2014). However, few studies on the role of PLK5 in human cancers have been reported. Indeed, PLK5 is significantly downregulated in human brain tumors and may have tumor suppressor activity in brain cancer (de Cárcer et al. 2011).
The dysregulated expression levels of PLKs and their relationship with clinicopathological features and prognosis in human NSCLC have been partly reported in the past. To the best of our knowledge, bioinformatics analysis has yet to be applied to explore the role of PLKs in NSCLC. In this study, we analyzed the expression and mutations of different PLKs in patients with lung cancer to determine the distinct patterns of expression and significance of five PLKs for survival prognosis in patients with NSCLC.
Materials and methods
Oncomine analysis
Based on the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) and The Cancer Genome Atlas (TCGA, https://cancergenome.nih.gov/) (Rhodes et al. 2004), Oncomine (https://www.oncomine.org/) is a cancer microarray database and integrated data-mining platform for DNA and RNA sequence analysis. In this study, the mRNA expression levels of different PLKs of lung cancer and control samples were compared using the Oncomine database. Student’s t test with the thresholds set was as follows: P value = 0.01; fold change = 2; gene rank: 10%; data type: mRNA.
GEPIA analysis
Based on the TCGA and Genotype-Tissue Expression (GTEx) databases (Tang et al. 2017), the Gene Expression Profiling Interactive Analysis (GEPIA) database (https://gepia.cancer-pku.cn/) is a public one that could be used to analyze gene expression profiles. GEPIA can be employed for multidimensional analysis of cancers, including cancer or normal differential expression analysis, cancer type/pathological stage analysis, patient survival analysis, and similar gene testing (Tang et al. 2017).
UALCAN analysis
UALCAN (https://ualcan.path.uab.edu/) is a website for effective analysis of cancer data based on relevant cancer data in the TCGA database (Chandrashekar et al. 2017). The website can be used to analyze genes correlated with cancer and para cancer staging, and prognostic factors using TCGA database samples. We further analyzed the relationship between the expression levels of different PLKs and the individual cancer stages via UALCAN database.
The Kaplan–Meier plotter analysis
Kaplan–Meier plotter (https://kmplot.com/) is an open-access online survival analysis tool based on EGA (European Genome-phenome Archive), TCGA, and GEO databases. It contains gene expression data and survival information of lung cancer patients and could be used for analysis of prognostic value of mRNA expression of genes (Győrffy et al. 2013). To analyze the overall survival (OS), first progression (FP), and post-progression survival (PPS) of NSCLC patients, patient samples were divided into the high expression and the low expression groups according to the median expression of each PLK and assessed via K–M survival plot. The number-at-risk cases, hazard ratio (HR) with 95% confidence intervals (CIs), and log-rank P values were displayed on the plot. A log-rank P value < 0.05 was considered statistically significant.
TCGA and cBioPortal analysis
TCGA is a project overseen by the National Cancer Institute and the National Human Genome Research Institute. It aims to employ genome analysis techniques to improve cancer awareness, thereby facilitating the development of prevention, diagnosis, and treatment of cancers (Tomczak et al. 2015). As the largest cancer information database, TCGA is characterized by not only various cancer types, but also multiple omics data, including mRNA expression data, miRNA expression data, copy number variation, and DNA methylation. cBioPortal (www.cbioportal.org) is an open-access online tool for multidimensional analysis of cancer data, including DNA copy number analysis, mRNA and microRNA expression analysis, non-synonymous mutation, and DNA methylation analysis (Gao et al. 2013). In this study, the lung squamous cell carcinoma (TCGA, Firehose Legacy) dataset, including 511 patients with NSCLC diagnosed by pathology, was chosen for exploration of genetic alterations of PLKs by the cBioPortal database. The genomic profiles included mutations, putative copy number alterations (CNAs) from GISTIC, and mRNA expression Z scores (RNA Seq V2 RSEM) with a z score threshold of ± 2.0.
LinkedOmics analysis
LinkedOmics (https://www.linkedomics.orglogin.php) is a public portal that provides multi-omics data of cancers within and across tumor types from all 32 TCGA cancer types (Vasaikar et al. 2018). It uses preprocessed and normalized data from the Broad TCGA Firehose and Clinical Proteomic Tumor Analysis (CPTAC) data portal to reduce redundant efforts, and focused on the discovery and interpretation of attribute associations, and thus complements existing cancer data portals (Vasaikar et al. 2018). We investigated the associations among expression levels of different PLKs based on the LinkedOmics database.
Functional enrichment and bioinformatics analysis
The DAVID database (https://david.ncifcrf.gov/) is an online bioinformatics database, from which biological functional annotation information can be extracted for large-scale genes. The DAVID database was employed to perform the Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of PLKs. The GO enrichment analysis consisted of cellular component (CC) analysis, biological process (BP) analysis, and molecule function (MF) analysis.
Results
Transcriptional expression of different PLKs in patients with lung cancer
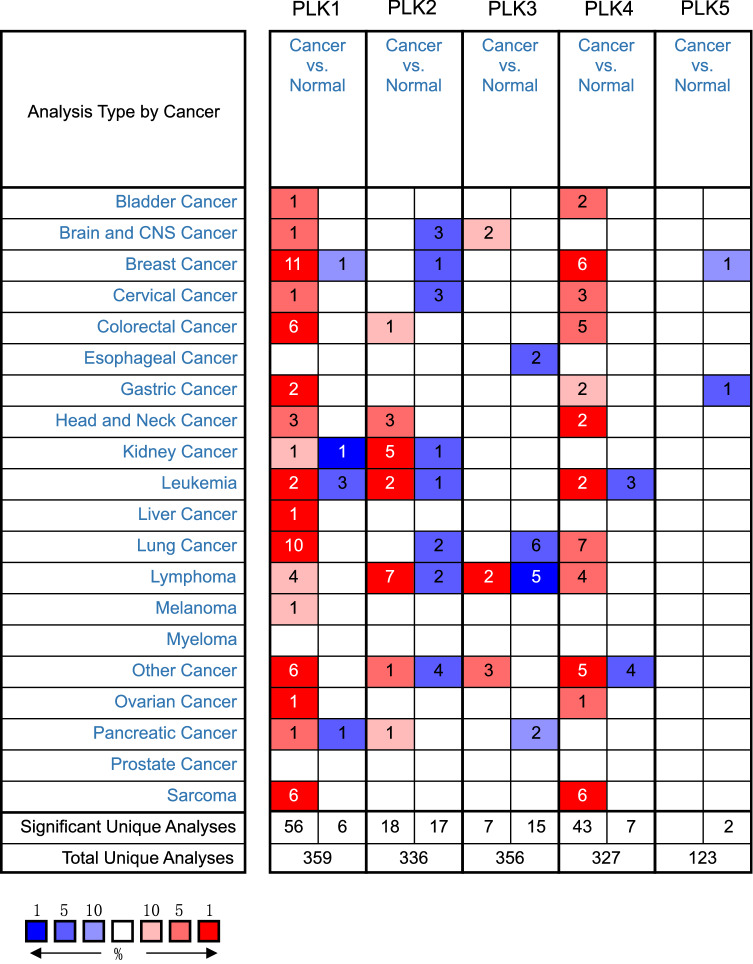
To explore the expression of the families of PLKs in lung cancer, we use the Oncomine database to compare the mRNA differential expression of PLKs in human lung cancer samples with those of control samples. As shown in Fig. 1 and Table 1, compared to control samples, ten datasets showed that the mRNA expression level of PLK1 was significantly upregulated in lung cancer patients, while seven datasets showed PLK4 high expression. These different datasets from the Oncomine database include the Hou Lung’s dataset, the Garber Lung’s dataset, the Bhattacharjee Lung’s dataset, the Su Lung’s dataset and the Stearman Lung’s dataset. According to the Hou Lung’s dataset (Hou et al. 2010), PLK1 was overexpressed in LUAD, squamous cell lung carcinoma and large cell lung carcinoma vs. normal tissue, with a fold change (FC) of 2.115, 2.741, and 3.624, respectively. According to the Garber Lung’s dataset (Garber et al. 2001), PLK1 was overexpressed in large cell lung carcinoma (FC = 6.837), small cell lung carcinoma (FC = 5.960), squamous cell lung carcinoma (FC = 5.076), and LUAD (FC = 4.120). According to the Bhattacharjee Lung’s dataset (Bhattacharjee et al. 2001), PLK1 was overexpressed in squamous cell lung carcinoma vs. normal samples, with an FC of 3.135. In addition, Su et al. (2007) and Stearman et al. (2005) reported that PLK1 was overexpressed in LUAD vs. normal tissues, with an FC of 2.348 and 3.127, respectively. According to the Garber Lung dataset (Garber et al. 2001), PLK4 was overexpressed in LUAD (FC = 2.020), large cell lung carcinoma (FC = 2.363), and small cell lung carcinoma (FC = 2.955), compared to control samples. Bhattacharjee et al. reported overexpression of PLK4 in patients with lung cancers (FC = 3.316), compared to normal samples (Bhattacharjee et al. 2001). According to the Hou Lung’s dataset (Hou et al. 2010), PLK4 was overexpressed in large cell lung carcinoma (FC = 3.104) and squamous cell lung carcinoma (FC = 2.415). Su et al. reported overexpression of PLK1 in LUAD (FC = 2.240), compared to normal tissues (Su et al. 2007).
Fig. 1.
The mRNA expression levels of PLKs in different cancers (Oncomine). The graphic demonstrated the numbers of datasets with statistically significant mRNA overexpression (red) or down-expression (blue) of the target gene. The P value threshold is 0.01. The number in each cell represents the number of analyses that meet the threshold within those analysis and cancer types. The gene rank was analyzed by percentile of target gene in the top of all genes measured in each research. Cell color is determined by the best gene rank percentile for the analyses within the cell
Table 1.
Significant upregulated expression in mRNA level of PLKs between lung cancer and normal tissues by Oncomine database
| Gene | Types of lung cancer vs. normal | P value | t test | FC | Cancer tissue | Normal tissue | References |
|---|---|---|---|---|---|---|---|
| PLK1 | Lung adenocarcinoma vs. normal | 1.05E−16 | 11.397 | 2.115 | 45 | 65 | Hou Lung |
| Squamous cell lung carcinoma vs. normal | 4.75E−15 | 13.452 | 2.741 | 27 | 65 | Hou Lung | |
| Large cell lung carcinoma vs. normal | 1.80E−07 | 7.640 | 3.624 | 19 | 65 | Hou Lung | |
| Large cell lung carcinoma vs. normal | 2.95E−04 | 6.235 | 6.837 | 4 | 6 | Garber Lung | |
| Small cell lung carcinoma vs. normal | 2.24E−04 | 6.184 | 5.960 | 4 | 6 | Garber Lung | |
| Squamous cell lung carcinoma vs. normal | 4.27E−05 | 7.556 | 5.076 | 13 | 6 | Garber Lung | |
| Lung adenocarcinoma vs. normal | 1.32E−04 | 6.764 | 4.120 | 40 | 6 | Garber Lung | |
| Squamous cell lung carcinoma vs. normal | 5.18E−04 | 3.570 | 3.135 | 21 | 17 | Bhattacharjee Lung | |
| Lung adenocarcinoma vs. normal | 4.99E−06 | 4.873 | 2.348 | 27 | 30 | Su Lung | |
| Lung adenocarcinoma vs. normal | 2.63E−05 | 4.593 | 3.127 | 20 | 19 | Stearman Lung | |
| PLK4 | Lung adenocarcinoma vs. normal | 0.001 | 4.472 | 2.020 | 41 | 6 | Garber Lung |
| Large cell lung carcinoma vs. normal | 5.78E−04 | 5.652 | 2.363 | 5 | 6 | Garber Lung | |
| Small cell lung carcinoma vs. normal | 7.81E−04 | 5.016 | 2.955 | 5 | 6 | Garber Lung | |
| Small cell lung carcinoma vs. normal | 8.34E−04 | 3.607 | 3.316 | 21 | 17 | Bhattacharjee Lung | |
| Large cell lung carcinoma vs. normal | 4.42E−07 | 7.096 | 3.104 | 19 | 65 | Hou Lung | |
| Squamous cell lung carcinoma vs. normal | 1.64E−12 | 10.595 | 2.415 | 27 | 65 | Hou Lung | |
| Lung adenocarcinoma vs. normal | 1.65E−04 | 3.839 | 2.240 | 27 | 30 | Su Lung |
Relationship between the mRNA expression levels of PLKs and the clinicopathological parameters of lung cancer patients
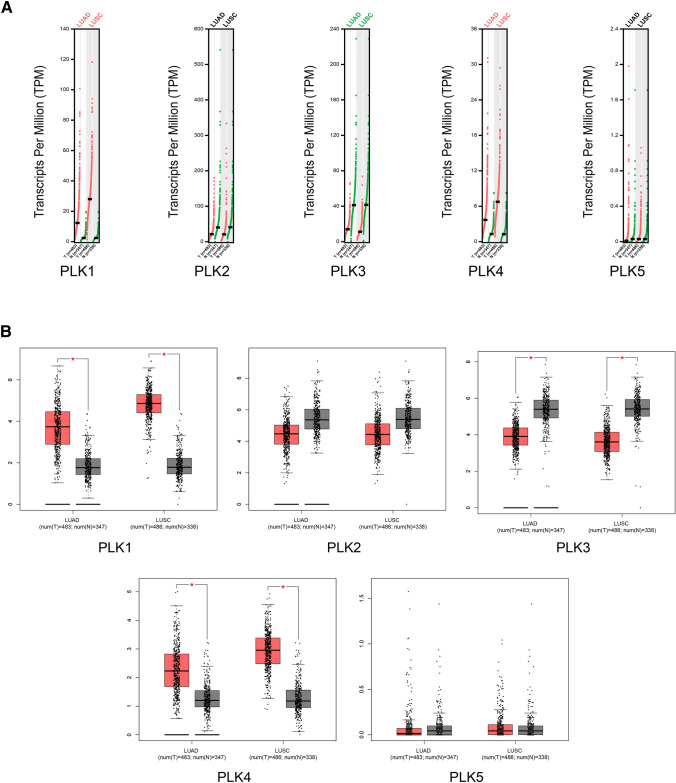
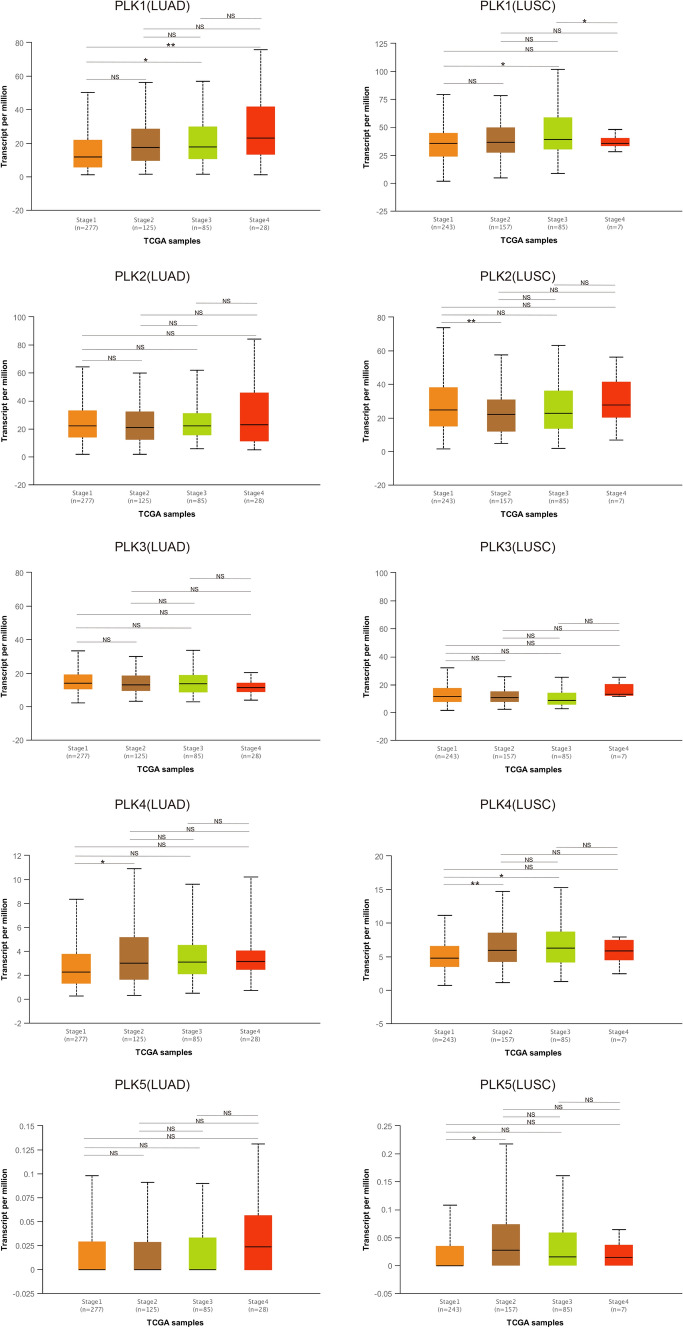
The mRNA expression of each PLK in lung cancer and control samples was compared based on the GEPIA database. The results showed that the mRNA expression levels of PLK1 and PLK4 in lung cancer samples were higher than those in normal samples; the mRNA expression level of PLK3 in lung cancer samples was lower than those in normal samples (Fig. 2a, b); the mRNA expression level of PLK2 in lung cancer tissues was lower than that in normal tissues, although the difference was not statistically significant. Additionally, the relationship between the mRNA expression levels of PLKs and the tumor stage of NSCLC patients was analyzed based on the UALCAN database. As shown in Fig. 3, the mRNA expressions of PLK1 and PLK4 changed significantly across the tumor stage of NSCLC, while the expression levels of the rest of the PLK family members in various tumor stages were not different.
Fig. 2.
Expressions of PLKs in lung cancer and correlation with tumor stage in NSCLC patients (GEPIA). a Expression of PLKs in lung cancer (scatter diagram). b Expression of PLKs in lung cancer (box plot). LUAD lung adenocarcinoma, LUSC lung squamous cell carcinoma, NSCLC non-small cell lung cancer
Fig. 3.
The relationship between PLK mRNA expression and individual cancer stages (UALCAN). Individual cancer stages include NSCLC from stage 1 to stage 4. LUAD lung adenocarcinoma, LUSC lung squamous cell carcinoma, NSCLC non-small cell lung cancer, NS no significant; *P < 0.05; **P < 0.01
The prognostic value of mRNA level of PLKs in NSCLC patients
The prognosis of each PLK in NSCLC samples was analyzed using Kaplan–Meier plotter. As shown in Fig. 4a, overexpressions of PLK1, PLK4, and PLK5 and underexpression of PLK2 were associated with limited OS time in lung cancer. The overexpressions of PLK1 and PLK4 were related to reduced FP in NSCLC, while the overexpression of PLK2 was related to increased FP (Fig. 4b). Low mRNA expression level of PLK1 was predicted to have high PPS (Fig. 4c). Hence, highly expressed PLK1 and PLK4 may be a worse prognostic factor for NSCLC patients.
Fig. 4.
The prognostic value of mRNA expression level of PLKs in NSCLC patients (Kaplan–Meier plotter). a The relationship between PLKs expression and OS in NSCLC patients. b The relationship between PLKs expression and FP in NSCLC patients. c The relationship between PLKs expression and PPS in NSCLC patients. NSCLC non-small cell lung cancer
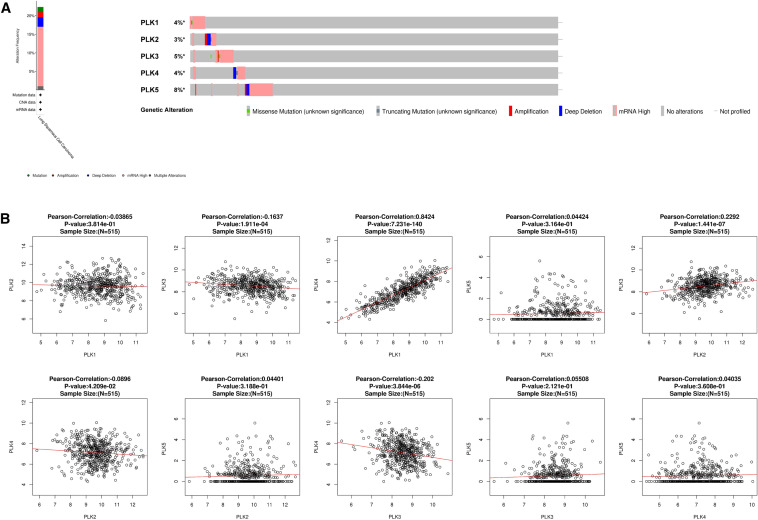
PLKs genetic alterations in NSCLC
In this study, the lung squamous cell carcinoma (TCGA, Firehose Legacy) dataset, including 511 patients with NSCLC diagnosed by pathology, was chosen for exploration of genetic alterations of PLKs based on the cBioPortal database. PLKs were altered in 113 (22%) samples with lung squamous cell carcinoma. The frequency of mutations, amplifications, deep deletions, mRNA high, and multiple alterations was 1.39%, 1.39%, 2.57%, 16.04%, and 0.99%, respectively, and mRNA high had the highest frequency in the above genetic alterations (Fig. 5a). The percentage of genetic alterations of PLK1 to PLK5 in lung squamous cell carcinoma was 4%, 3%, 5%, 4%, and 8%, respectively (Fig. 5a).
Fig. 5.
Mutations of PLKs and the correlation between PLKs family members in NSCLC (cBioPortal and LinkedOmics). a PLKs gene expression and mutation analysis in NSCLC (cBioPortal). b The correlation between PLKs in NSCLC, analyzed by LinkedOmics database. NSCLC non-small cell lung cancer
Correlation between PLKs in NSCLC
The LinkedOmics database was employed to investigate the associations among PLK1, PLK2, PLK3, PLK4, and PLK5. As shown in Fig. 5b, PLK1 was positively and negatively correlated with PLK4 (R = 0.8424, P < 0.05) and PLK3 (R = − 0.1637, P < 0.05), respectively. PLK2 was positively and negatively correlated with PLK3 (R = 0.8424, P < 0.05) and PLK4 (R = − 0.08896, P < 0.05), respectively. PLK3 was negatively correlated with PLK4 (R = − 0.202, P < 0.05). No significant correlation between PLK5 and PLK1-4 was observed.
Enrichment analysis of PLKs in lung cancer
To further investigate the role of PLKs in lung cancer, we applied a functional enrichment analysis for PLKs based on the DAVID database. As shown in Table 2, 15 GO terms were enriched. PLKs were enriched in the following biological processes: protein phosphorylation, G2/M transition of mitotic cell cycle, negative regulation of apoptotic process, G2 DNA damage checkpoint, mitotic cell cycle checkpoint, DNA damage response, signal transduction by p53 class mediator resulting in cell cycle arrest, positive regulation of proteasomal ubiquitin-dependent protein catabolic process, G1/S transition of mitotic cell cycle, and peptidyl-serine phosphorylation. For the CC category, PLKs were correlated with centrosome, nucleolus, chromatin, and centriole. For the MF category, PLKs were enriched in protein serine/threonine kinase activity and ATP binding. Only one KEGG pathway was enriched for PLKs, that is, forkhead box transcription factors (FoxO) family signaling pathway.
Table 2.
The GO function and KEGG enrichment analysis of PLKs by DAVID database
| Category | Term | Name | Count | P value |
|---|---|---|---|---|
| BP | GO:0006468 | Protein phosphorylation | 5 | 5.37E−07 |
| BP | GO:0000086 | G2/M transition of mitotic cell cycle | 3 | 3.92E−04 |
| BP | GO:0043066 | Negative regulation of apoptotic process | 3 | 4.24E−03 |
| BP | GO:0031572 | G2 DNA damage checkpoint | 2 | 4.76E−03 |
| BP | GO:0007093 | Mitotic cell cycle checkpoint | 2 | 7.60E−03 |
| BP | GO:0006977 | DNA damage response, signal transduction by p53 | 2 | 1.47E−02 |
| BP | GO:0032436 | Positive regulation of proteasomal ubiquitin-dependent protein catabolic process | 2 | 1.52E−02 |
| BP | GO:0000082 | G1/S transition of mitotic cell cycle | 2 | 2.41E−02 |
| BP | GO:0018105 | peptidyl-serine phosphorylation | 2 | 2.94E−02 |
| CC | GO:0005813 | Centrosome | 4 | 4.99E−05 |
| CC | GO:0005730 | Nucleolus | 4 | 4.00E−04 |
| CC | GO:0000785 | Chromatin | 2 | 1.94E−02 |
| CC | GO:0005814 | Centriole | 2 | 2.46E−02 |
| MF | GO:0004674 | Protein serine/threonine kinase activity | 4 | 4.31E−05 |
| MF | GO:0005524 | ATP binding | 5 | 6.13E−05 |
| KEGG-pathway | hsa04068 | FoxO signaling pathway | 4 | 7.23E−06 |
Discussion
Although PLK1 has been considered as a promising target for NSCLC, the specific roles of all PLK family members in the development, metastasis, and prognostication of NSCLC remain to be clarified. According to currently available limited studies, only one member of PLKs (PLK1) was reported that it involves in the development of lung cancer and has prognostic values for patients with lung cancer. In this study, we are the first to use various web visual analysis tools based on TCGA database to, respectively, analyze the expression and prognostic values of all the PLK family members in NSCLC. In addition, we explored the expression correlation with each other of the five PLKs in human NSCLC. We found PLK family members could play an oncogenic or tumor-suppressive role in NSCLC development. Compared to normal tissue, PLK1 and PLK4 are high expressed and suggested a worse prognosis for patients with NSCLC, while PLK2 has the opposite performance. PLK3 and PLK5 had no differential expression and remains unaffected in prognosis except that PLK5 high expression is involved in shorter OS. Moreover, the expression of PLK1 and PLK4 has a strong positive correlation, while the others are almost irrelevant.
PLK1, the most well-studied member of the PLK family, plays a critical role in the regulation of the cell cycle upon DNA damage. As the previous researches reported, PLK1 is considered an oncogene, poor prognostic biomarker and an attractive cancer target for many cancers, including lung cancer (Li et al. 2017a; Mao et al. 2018; Yang et al. 2016). Consistent with these results, our research showed that PLK1 is overexpressed in NSCLC tissue and prognose worse OS, FP and PPS in lung cancer patients. The overexpression of PLK1 breaks out the balance regulation of cell cycle and finally promotes chromosome instability and aneuploidy (de Cárcer et al. 2018; Smits et al. 2000). Intriguingly, researchers found that PLK1 involves in the regulation of invasiveness and anti-apoptosis for cancer cells (Rizki et al. 2007). PLK1 may promote migration of lung cancer cells via regulation of STAT3 signaling pathway (Yan et al. 2018). What is more important, it was reported that the overexpression of PLK1 is closely related to worse clinicopathological parameters in cancers such as lung cancer, including smoke state, differentiation, TNM classification, clinical stage, lymph node and distant metastasis (Li et al. 2017a; Takai et al. 2001; Van den Bossche et al. 2017; Zhang et al. 2013).
Upon cell stress such as DNA damage, PLK1 is one of the p53 downstream cell cycle-promoting genes and p53 indirectly represses the expression of PLK1 via the p53-p21-DREAM-CDE/CHR pathway (Fischer et al. 2015). TP53 (p53) is a tumor suppressor gene, which promotes apoptosis and prevents tumors when DNA is severely damaged. The TP53 gene mutation occurs in more than 50% of human tumor tissues, and is considered to be the most common genetic change and the main pathogenic factor in tumors (Duffy et al. 2017). Previous studies revealed that PLK1 plays a key role in the negative regulation of p53 activity through two new targets: G2 and S phase-expressed protein 1 (GTSE1) and DNA topoisomerase 1 binding protein (Topors) (Liu et al. 2010). The negative regulation of overexpressed PLK1 on p53 activity may provide a potential mechanism for the tumorigenic potential of PLK1. Intriguingly, it was demonstrated that the therapeutic effect of NSCLC by the PLK1 inhibitor is dependent on the p53 status (Van den Bossche et al. 2019). Compared with p53 knockdown/mutant NSCLC cells, the Plk1 inhibitor volasertib and radiotherapy induced senescence in wild-type p53 cells (Smith et al. 2017; Van den Bossche et al. 2019). Also, Wang and Simon put forward that PLK1 has a potential synthetic lethality relationship with TP53 (Wang and Simon 2013). Therefore, the Plk1 inhibitor has been considered as a promising therapy for TP53-mutated cancers. In addition, PLK1 inhibitor also showed therapeutic effect for K-ras-mutant tumors, which may solve a big puzzle whether effective targeted therapies exist for tumor entities with K-ras mutations (Wang et al. 2016). After all, there are 25% of NSCLC patients’ tumor tissue harboring K-ras mutations, which suggests that PLK1 might be an amazing therapeutic target (Román et al. 2018).
Volasertib, one of the most effective in vitro and in vivo Plk1 inhibitors, has entered phase III clinical trial and showed a promising therapeutic value (Gutteridge et al. 2016). However, there are many challenges to prevent PLK1 inhibitors from entering clinical application, such as low drug specificity, strong toxic reaction, induction of chemotherapy resistance and so on (Elsayed and Wang 2019). Additionally, it is worth noting that the role of PLK1 overexpression in cancers has the other layer of function, tumor-suppressive activities, which is contrary to generally consideration as an oncogene. This opposite conclusion was demonstrated by Guillermo de Cárcer et al. that the overexpression of PLK1 induces chromosomal instability and prevents the development and malignant transformation of solid tumor with K-ras or Her2 oncogenes (de Cárcer et al. 2018). Besides, a study reported that PLK1 overexpressed in mouse did not cause tumor formation and show any significant phenotype either (de Cárcer et al. 2018; Li et al. 2017b; Raab et al. 2011). The role of PLK1, an oncogene or tumor-suppressive gene, has been made a profound discussion in Guillermo de Cárcer’s review. However, the mechanism has not been investigated. According to the contradiction and excellent therapeutic potential, it is still worthy of more vigorous researches about PLK1 and its inhibitor in lung cancer.
PLK2, which is the first member of the PLK family to be identified in mammals, is also a transcriptional target of p53 and the expression of PLK2 increases with the DNA-damaging agent (Burns et al. 2003). Studies revealed that PLK2 is an independent prognostic marker that regulates the growth and apoptosis of colorectal cancer (CRC) cells by targeting the Fbxw7/cyclin E pathway, suggesting that PLK2 is a potential therapeutic target (Ou et al. 2016). Xie et al. reported that PLK2 was related to drug resistance of CRC via inhibiting apoptosis and suggested that PLK2 could be used as a predictor of chemotherapy resistance and a new target for treatment of CRC (Xie et al. 2018). In this study, we found that the expression of PLK2 was lower in NSCLC tissues than that in normal tissues. The expression of PLK2 was negatively correlated with the expression of PLK1. Additionally, decreased PLK2 was related to limited OS time. In summary, PLK2 plays a negative regulatory role in NSCLC development.
PLK3, which is also known as CNK, appears to be most similar to PLK2 (Yang et al. 2008). The expression of PLK3 is reduced in many tumor tissues in cases of lung cancer, liver cancer, colon cancer, melanoma, and kidney cancer (Helmke et al. 2016; Xu et al. 2012). In contrast to PLK1, the expression of PLK3 exhibited a negative correlation with the development of cancers such as lung cancer (Li et al. 1996). This study revealed that PLK3 was significantly underexpressed in NSCLC tissues, which is consistent with the results of previous studies. Meanwhile, the expression of PLK3 was positively correlated with that of PLK2. It seems that decreased expression level of PLK3 may be related to tumor development. Angiogenesis is a key factor to guarantee tumor blood supply and solid tumor growth and metastasis. One of the key events that triggers tumor angiogenesis is cellular hypoxia. It is reported that PLK3 regulates tumor angiogenesis by regulating degradation and nuclear export of hypoxia-inducible factor-1a (HIF-1a) (Xu et al. 2017, 2012). Seven in absentia homologue 2 (SIAH2) is regarded as an oncogene overexpressed in a variety of cancer tissues, including lung cancer (Wong and Möller 2013). PLK3 regulates tumor development by disrupting the stability of SIAH2 via a kinase-dependent manner highlight (Xu et al. 2017). In this study, however, no significant correlation between PLK3 expression and OS time of NSCLC patients was observed. Therefore, the role of PLK3 in lung cancer needs further investigations.
PLK4, which is also known as SAK or STK18, encodes a member of the polo family of serine/threonine protein kinases. Its expression in human cancer is not consistent. PLK4 is overexpressed in colon cancer and breast cancer, while it is significantly underexpressed in liver cancer (Liu et al. 2012b; Macmillan et al. 2001; Mason et al. 2011). It is reported that the overexpression of PLK4 is related to poor prognosis in breast cancer patients, while downregulation of PLK4 is associated with poor prognosis in hepatocellular carcinoma (Liu et al. 2012a; Van De Vijver et al. 2002). Kawakami et al. reported that PLK4 is overexpressed in lung cancer patients (Kawakami et al. 2018). This is consistent with our study. PLK4 plays an important role in regulation of centrosome functionality. PLK4 promotes centrosome amplification, leads to chromosome instability and finally results in increasing the activity and invasion ability of cancer cells in NSCLC (Kawakami et al. 2018; Koutsami et al. 2006; Press et al. 2019; Shinmura et al. 2014; Zhou et al. 2020). It was reported that PLK4 dysregulation is involved in many critical clinical paraments including solid tumor size, metastasis and stage (Zhou et al. 2020). Our results found that the increased expression of PLK4 is correlated with poor OS and FP time in NSCLC patients, while without any influence on PPS time, and the expression of PLK4 was positively correlated with that of PLK1, which further indicates the carcinogenic effect of PLK4. Also, PLK4, a highly selective inhibitor CFI-400945, has been undergoing phase I clinical trial testing to study its therapeutic effects on advanced cancer patients (NCT01954316). However, the detailed mechanisms of PLK4 in lung cancer is rare and worth further exploration.
PLK5, which is also known as PLK5P, does not appear to be involved in the regulation of cell cycle processes. Indeed, it is downregulated in proliferating cells and accumulated in resting cells (de Cárcer et al. 2011; Goroshchuk et al. 2019). PLK5 is a DNA damage-inducing gene, and cycle arrest and apoptosis of cells in mice are the results of heterotopic expression of PLK5 (Andrysik et al. 2010). PLK5 is found almost exclusively in brain tissues and it is involved in neuronal formation (de Cárcer et al. 2011). PLK5 may induce apoptosis of glioblastoma multiforme (GBM) cells by re-expression, suggesting that it may be a therapeutic target for GBM patients (de Cárcer et al. 2011). In this study, expressions of PLK5 in NSCLC and normal lung tissues were not significantly different, while increased PLK5 was related to limited OS time in NSCLC. Therefore, we speculate that PLK5 may be associated with the development of lung cancer. Besides, no evidence showing involvement of PLK5 in lung cancer pathogenesis has been reported by other studies. The mechanism of PLK5 in the development of cancer needs further investigations.
We firstly explored the expression relative between all the PLK family members. The result showed us that the mRNA expression of PLK1 and PLK4 has a strong positive correlation in NSCLC, which suggests that they might be a cooperative relationship in tumor progression. Whether there is an interaction between PLK1 and PLK4 need experiments to be proved. KEGG pathway enrichment analysis for PLKs reveals that the FoxO signaling pathway had the highest number of PLK family members. The FoxO signaling pathway has cross talk with many vital cancer-relative pathways including the PI3K/AKT pathway, the Ras-MEK-ERK, IKK and AMPK pathways (Farhan et al. 2017). These association suggests that the FoxO signaling pathway might relate PLKs to tumorigenesis, progression, and metastasis in lung cancer. However, there are rare studies about their complicative regulation relationship, which might open a new molecular mechanism in targeting therapy in PLKs for NSCLC.
In this study, expression and prognostic value of five PLKs in NSCLC was investigated. The results showed that PLKs may be relevant targets for NSCLC treatment. Nowadays, PLK inhibitors have been thoroughly investigated and rapidly developed. Identification and development of biomarkers related to sensitivity and clinical response of PLK inhibitors could not only improve patient selection and provide means to monitor treatment outcomes, but also open the door to new combination strategies.
Conclusions
The mRNA expression level, mutation, and prognostic values of different PLKs in lung cancer patients were systemically analyzed. The results showed that the overexpressions of PLK1 and PLK4 were associated with poor prognosis in lung cancer patients. The expression of PLK1 was positively correlated with that of PLK4 in lung cancer. PLK1 and PLK4 may be employed as prognostic targets for comprehensive treatment of lung cancer. PLK2 and PLK3 may affect the development of lung cancer as tumor suppressors. In summary, PLKs are relevant targets for NSCLC treatment especially PLK1 and PLK4.
Funding
The work was supported by the National Natural Science Foundation of China (81572610) and the “Yangfan Plan” for Outstanding Scholars in Guangdong Province (4YF16002G).
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yu Zeng and Nanhong Li contributed equally to this work.
Contributor Information
Junfen Cheng, Email: 13729063939@139.com.
Jian Huang, Email: 18665763598@163.com.
References
- Andrysik Z et al (2010) The novel mouse Polo-like kinase 5 responds to DNA damage and localizes in the nucleolus. Nucleic Acids Res 38:2931–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetatos L et al (2011) Polo-like kinase 2 (SNK/PLK2) is a novel epigenetically regulated gene in acute myeloid leukemia and myelodysplastic syndromes: genetic and epigenetic interactions. Ann Hematol 90:1037–1045 [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A et al (2001) Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci 98:13790–13795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424 [DOI] [PubMed] [Google Scholar]
- Burns TF, Fei P, Scata KA, Dicker DT, El-Deiry WS (2003) Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (taxol)-exposed cells. Mol Cell Biol 23:5556–5571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BV, Varambally S (2017) UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 19:649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W et al (2000) PRK, a cell cycle gene localized to 8p21, is downregulated in head and neck cancer. Genes Chromosom Cancer 27:332–336 [DOI] [PubMed] [Google Scholar]
- de Cárcer G et al (2018) Plk1 overexpression induces chromosomal instability and suppresses tumor development. Nat Commun 9:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cárcer G et al (2011) Plk5, a polo box domain-only protein with specific roles in neuron differentiation and glioblastoma suppression. Mol Cell Biol 31:1225–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MJ, Synnott NC, Crown JJ (2017) Mutant p53 as a target for cancer treatment. Eur J Cancer 83:258–265 [DOI] [PubMed] [Google Scholar]
- Elsayed I, Wang X (2019) PLK1 inhibition in cancer therapy: potentials and challenges. Future Med Chem 11:1383–1386 [DOI] [PubMed] [Google Scholar]
- Farhan M, Wang H, Gaur U, Little PJ, Xu J, Zheng WJ (2017) FOXO signaling pathways as therapeutic targets in cancer. Int J Biol Sci 13:815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Quaas M, Nickel A, Engeland KJO (2015) Indirect p53-dependent transcriptional repression of Survivin, CDC25C, and PLK1 genes requires the cyclin-dependent kinase inhibitor p21/CDKN1A and CDE/CHR promoter sites binding the DREAM complex. Oncotarget 6:41402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber ME et al (2001) Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci 98:13784–13789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goroshchuk O, Kolosenko I, Vidarsdottir L, Azimi A, Palm-Apergi C (2019) Polo-like kinases and acute leukemia. Oncogene 38:1–16 [DOI] [PubMed] [Google Scholar]
- Gutteridge REA, Ndiaye MA, Liu X, Ahmad NJ (2016) Plk1 inhibitors in cancer therapy: from laboratory to clinics. Mol Cancer Ther 15:1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Győrffy B, Surowiak P, Budczies J, Lanczky A (2013) Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS ONE 8:82241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmke C, Becker S, Strebhardt K (2016) The role of Plk3 in oncogenesis. Oncogene 35:135–147 [DOI] [PubMed] [Google Scholar]
- Hou J et al (2010) Gene expression-based classification of non-small cell lung carcinomas and survival prediction. PLoS One 5:e10312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M et al (2018) Polo-like kinase 4 inhibition produces polyploidy and apoptotic death of lung cancers. Proc Natl Acad Sci 115:1913–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsami M et al (2006) Centrosome abnormalities are frequently observed in non-small-cell lung cancer and are associated with aneuploidy and cyclin E overexpression. J Pathol J Pathol Soc G B Irel 209:512–521 [DOI] [PubMed] [Google Scholar]
- Li B et al (1996) Prk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be down-regulated in lung carcinomas. J Biol Chem 271:19402–19408 [DOI] [PubMed] [Google Scholar]
- Li Z, Dai K, Wang C, Song Y, Gu F, Liu F, Fu L (2016) Expression of Polo-like kinase 4(PLK4) in breast cancer and its response to taxane-based neoadjuvant chemotherapy. J Cancer 7:1125–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang H, Sun Z, Guo Q, Shi H, Jia Y (2017a) The clinical and prognostic value of polo-like kinase 1 in lung squamous cell carcinoma patients: immunohistochemical analysis. Biosci Rep 37:BSR20170852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z et al (2017) Polo-like kinase 1 (Plk1) overexpression enhances ionizing radiation-induced cancer formation in mice. J Biol Chem 292:17461–17472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X (2015) Targeting Polo-like kinases: a promising therapeutic approach for cancer treatment translational. Oncology 8:185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Li H, Song B, Liu X (2010) Polo-like kinase 1 phosphorylation of G2 and S-phase-expressed 1 protein is essential for p53 inactivation during G2 checkpoint recovery. EMBO Rep 11:626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang CZ, Cai M, Fu J, Chen GG, Yun J (2012a) Downregulation of polo-like kinase 4 in hepatocellular carcinoma associates with poor prognosis. PLoS One 7:e41293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Song B, Tang J, Liu W, Kuang S, Liu X (2012) Plk1 phosphorylates Sgt1 at the kinetochores to promote timely kinetochore-microtubule attachment. Mol Cell Biol 32:4053–4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan JC, Hudson JW, Bull S, Dennis JW, Swallow CJ (2001) Comparative expression of the mitotic regulators SAK and PLK in colorectal cancer. Ann Surg Oncol 8:729–740 [DOI] [PubMed] [Google Scholar]
- Mao F et al (2018) Plk1 inhibition enhances the efficacy of BET epigenetic reader blockade in castration-resistant prostate cancer. Mol Caner Ther 17:1554–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina M, Mihaela HI (2014) Nek2 and Plk4: prognostic markers, drivers of breast tumorigenesis and drug resistance. Front Biosc 19:352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J et al. (2011) Abstract LB-215: inhibition of Polo-like kinase 4 as an anti-cancer strategy. AACR
- Mason JM et al (2014) Functional characterization of CFI-400945, a Polo-like kinase 4 inhibitor, as a potential anticancer agent. Cancer Cell 26:163–176 [DOI] [PubMed] [Google Scholar]
- Ou B et al (2016) Plk2 promotes tumor growth and inhibits apoptosis by targeting Fbxw7/Cyclin E in colorectal cancer. Cancer Lett 380:457–466 [DOI] [PubMed] [Google Scholar]
- Pellegrino R et al (2010) Oncogenic and tumor suppressive roles of polo-like kinases in human hepatocellular carcinoma. Hepatology 51:857–868 [DOI] [PubMed] [Google Scholar]
- Press MF et al (2019) Role for polo-like kinase 4 in mediation of cytokinesis. Proc Natl Acad Sci 116:11309–11318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab M et al (2011) Toxicity modelling of Plk1-targeted therapies in genetically engineered mice and cultured primary mammalian cells. Nat Commun 2:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DR et al (2004) ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 6:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki A, Mott JD, Bissell MJJCR (2007) Polo-like kinase 1 is involved in invasion through extracellular matrix. Cancer Res 67:11106–11110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román M, Baraibar I, López I, Nadal E, Rolfo C, Vicent S, Gil-Bazo IJ (2018) KRAS oncogene in non-small cell lung cancer: clinical perspectives on the treatment of an old target. Mol Cancer 17:33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Kurabe N, Goto M, Yamada H, Natsume H, Konno H, Sugimura H (2014) PLK4 overexpression and its effect on centrosome regulation and chromosome stability in human gastric cancer. Mol Biol Rep 41:6635–6644 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34 [DOI] [PubMed] [Google Scholar]
- Simmons D, Neel B, Stevens R, Evett G, Erikson R (1992) Identification of an early-growth-response gene encoding a novel putative protein kinase. Mol Cell Biol 12:4164–4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Farzan R, Ali S, Buluwela L, Saurin AT, Meek DWJ (2017) The responses of cancer cells to PLK1 inhibitors reveal a novel protective role for p53 in maintaining centrosome separation. Sci Res 7:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RHJ (2000) Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell Biol 2:672–676 [DOI] [PubMed] [Google Scholar]
- Stearman RS et al (2005) Analysis of orthologous gene expression between human pulmonary adenocarcinoma and a carcinogen-induced murine model. Am J Pathol 167:1763–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebhardt K (2010) Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov 9:643–660 [DOI] [PubMed] [Google Scholar]
- Su L-J et al (2007) Selection of DDX5 as a novel internal control for Q-RT-PCR from microarray data using a block bootstrap re-sampling scheme. BMC Genom 8:140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed N et al (2006) Transcriptional silencing of Polo-like kinase 2 (SNK/PLK2) is a frequent event in B-cell malignancies. Blood 107:250–256 [DOI] [PubMed] [Google Scholar]
- Takai N, Miyazaki T, Fujisawa K, Nasu K, Hamanaka R, Miyakawa IJ (2001) Expression of polo-like kinase in ovarian cancer is associated with histological grade and clinical stage. Cancer Lett 164:41–49 [DOI] [PubMed] [Google Scholar]
- Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45:W98–W102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomczak K, Czerwińska P, Wiznerowicz M (2015) The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol 19:A68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Vijver MJ et al (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009 [DOI] [PubMed] [Google Scholar]
- Van den Bossche J et al (2017) Towards prognostic profiling of non-small cell lung cancer: new perspectives on the relevance of polo-like kinase 1 expression, the TP53 mutation status and hypoxia. J Cancer 8:1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche J et al (2019) Radiosensitization of non-small cell lung cancer cells by the Plk1 inhibitor volasertib is dependent on the p53 status. Cancers 11:1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasaikar SV, Straub P, Wang J, Zhang B (2018) LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res 46:D956–D963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Simon RJ (2013) Identification of potential synthetic lethal genes to p53 using a computational biology approach. BMC Med Genom 6:30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J et al (2016) Suppression of KRas-mutant cancer through the combined inhibition of KRAS with PLK1 and ROCK. Nat Commun 7:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CS, Möller A (2013) Siah: a promising anticancer target. Cancer Res 73:2400–2406 [DOI] [PubMed] [Google Scholar]
- Xie Y, Liu Y, Li Q, Chen J (2018) Polo-like kinase 2 promotes chemoresistance and predicts limited survival benefit from adjuvant chemotherapy in colorectal cancer. Int J Oncol 52:1401–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Wang Q, Jiang Y, Zhang Y, Vega-SaenzdeMiera E, Osman I, Dai W (2012) Roles of Polo-like kinase 3 in suppressing tumor angiogenesis. Exp Hematol Oncol 1:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Dai W, Li C (2017) Polo-like kinase 3, hypoxic responses, and tumorigenesis. Cell Cycle 16:2032–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Yu H, Li W, Li F, Wang S, Yu N, Jiang Q (2018) Plk1 promotes the migration of human lung adenocarcinoma epithelial cells via STAT3 signaling. Oncol Lett 16:6801–6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y et al (2008) Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1α under hypoxic conditions. Cancer Res 68:4077–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Chen G, Li W, Peng C, Pei HJ (2016) Cervical cancer growth is regulated by a c-ABL-PLK1 signaling axis. Cancer Res 77:1142–1154 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang G, Kong C (2013) High expression of polo-like kinase 1 is associated with the metastasis and recurrence in urothelial carcinoma of bladder. Urol Oncol 31:1222–1230 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Fan G, Dong Y (2020) Polo-like kinase 4 correlates with greater tumor size, lymph node metastasis and confers poor survival in non-small cell lung cancer. J Clin Lab Anal 34:e23152 [DOI] [PMC free article] [PubMed] [Google Scholar]