Abstract
The Saudi Thoracic Society (STS) developed an updated evidence-based guideline for diagnosing and managing chronic obstructive pulmonary disease (COPD) in Saudi Arabia. This guideline aims to provide a comprehensive and unbiased review of current evidence for assessing, diagnosing, and treating COPD. While epidemiological data on COPD in Saudi Arabia are limited, the STS panel believes that the prevalence is increasing due to rising rates of tobacco smoking. The key objectives of the guidelines are to facilitate accurate diagnosis of COPD, identify the risk for COPD exacerbations, and provide recommendations for relieving and reducing COPD symptoms in stable patients and during exacerbations. A unique aspect of this guideline is its simplified, practical approach to classifying patients into three classes based on symptom severity using the COPD Assessment Test and the risk of exacerbations and hospitalizations. The guideline provides the reader with an executive summary of recommended COPD treatments based on the best available evidence and also addresses other major aspects of COPD management and comorbidities. This guideline is primarily intended for use by internists and general practitioners in Saudi Arabia.
Keywords: Chronic bronchitis, chronic obstructive pulmonary disease, emphysema, guidelines, Saudi Arabia
Executive summary
Question 1- should a long-acting muscarinic antagonist (LAMA) plus a long-acting beta2-adrenoceptor agonist (LABA) vs. LAMA alone be used in patients with stable chronic obstructive pulmonary disease (COPD)?
Recommendation:
In patients with stable COPD, the COPD Task Force suggests using LAMA plus LABA) over LAMA alone (Conditional recommendation, favours the intervention. Moderate certainty of the evidence)
Additional observation: The COPD Task Force suggests considering dual therapy with LAMA plus LABA for patients classified under COPD Class 2 and 3 (groups B and E).
Question 2: Should LAMA plus LABA plus an inhaled corticosteroid (ICS) vs. LABA plus LAMA be used in patients with stable COPD?
Recommendation:
In patients with stable COPD, the COPD Task Force suggests using triple therapy with LAMA plus LABA plus ICS over dual therapy with LABA plus LAMA (Conditional recommendation, favors the intervention. Moderate certainty of the evidence)
Additional observations: The COPD Task Force suggests using triple therapy with LAMA plus LABA plus ICS in patients with stable COPD and frequent exacerbations (2 or more exacerbation episodes, or one episode of hospitalization in the last year) (Class 3)(Group-E) or with elevated eosinophil counts (≥300 cells/μL)
In contrast, the COPD Task Force suggests using dual therapy with LABA plus LAMA in patients with stable COPD and low eosinophil counts.
Question 3: Should phosphodiesterase-4 (PDE4) inhibitors vs. no PDE4 inhibitors be used in patients with stable COPD?
Recommendation:
In patients with stable COPD, the COPD Task Force suggests using phosphodiesterase-4 (PDE4) inhibitors (Conditional recommendation, favours the intervention. Low certainty of the evidence)
Additional observation: The COPD Task Force suggests considering PDE4 inhibitors in particular in patients with chronic bronchitis or severe to very severe COPD with a history of exacerbations.
Question 4: Should mucolytic agents vs. placebo be used in patients with stable COPD?
Recommendation:
In patients with stable COPD, the COPD Task Force suggests adding mucolytic agents (Conditional recommendation, favours the intervention. Low certainty of the evidence)
Additional observation: The COPD Task Force suggests not using mucolytics as monotherapy.
Question 5: Should long-term oxygen therapy vs. no long-term oxygen therapy be used in subgroups of patients with stable COPD with moderate desaturation: (Not meeting the known criteria for oxygen indication):
Recommendation:
In subgroups of patients with stable COPD, particularly those who are mild to moderate hypoxemic or non-hypoxemic at rest, or have exercise-induced moderate desaturation, the COPD Task Force suggests not using long-term oxygen therapy (Conditional recommendation, favours the comparison. Very low certainty of the evidence)
Additional observations: Individual patient’s factors including comorbidities should be considered when evaluating the patient’s need for supplemental oxygen.
Question 6: Should shorter durations of ≤7 days of systemic corticosteroid treatment vs. longer treatments of >7 days be used in patients with an acute exacerbation of COPD (AECOPD)??
Recommendation:
In patients with an AECOPD, the COPD Task Force suggests using shorter durations of ≤7 days of systemic corticosteroid treatment over longer treatments of >7 days (Conditional recommendation, favours the intervention. Very low certainty of the evidence)
Additional observation: Long-term systemic corticosteroid use may be associated with serious side effects and/or sequelae (for example weight gain, increased risk of infection, adrenal insufficiency, osteoporosis, cataract, and/or aseptic joint necrosis).
Question 7: Should antimicrobial interventions vs. no antimicrobial interventions be used in patients with an acute exacerbation of COPD
Recommendation:
In patients with an AECOPD, the COPD Task Force suggests using antimicrobial interventions (Conditional recommendation, favours the intervention. Low certainty of the evidence)
Question 8: Should non-invasive ventilation vs. usual medical care be used in patients with an AECOPD and hypercapnic respiratory failure in the ward, High-Dependency Unit, or Intensive Therapy Unit?
Recommendation:
In patients with an acute exacerbation of COPD and hypercapnic respiratory failure, the COPD Task Force suggests using non-invasive ventilation in the ward, High-Dependency Unit, or Intensive Therapy Unit over usual care (Conditional recommendation, favours the intervention. Very low certainty of the evidence).
Question 9: Should certain criteria to assess the suitability of and planning of home treatment or early discharge vs. no such criteria be used in patients with an acute exacerbation COPD?
Recommendation:
In patients with an AECOPD, the COPD Task Force suggests using certain criteria to assess the suitability of and planning of home treatment or early discharge (Conditional recommendation, favors the intervention. Very low certainty of the evidence).
-
Additional observations: The COPD Task Force suggests that before discharge the treating team should:
Ensure that the patient is in a stable condition to be discharged home, with all comorbidities adequately managed and any laboratory and/or metabolic abnormalities corrected
assess the patient’s need for long-term oxygen therapy.
Review with the patient all medication doses and durations, and the proper technique for using inhaler devices
Give the patient a follow-up appointment within a few weeks
Consider referring the patient to pulmonary rehabilitation.
Question 10: Should pulmonary rehabilitation vs. no pulmonary rehabilitation be used in patients with stable COPD?
Recommendation:
In patients with stable COPD, the COPD Task Force recommends using pulmonary rehabilitation (Strong recommendation, favors the intervention. Moderate certainty of the evidence).
Question 11: Should non-invasive ventilation vs. usual care be used in patients with stable COPD who have remained hypercapnic after an exacerbation?
Recommendation:
In patients with stable COPD who have remained hypercapnic after an exacerbation, the COPD Task Force suggests using non-invasive ventilation over usual care (Conditional recommendation, favours the intervention. Very low certainty of the evidence).
-
Additional observations:
The COPD Task Force suggests considering non-invasive ventilation in patients who continue to experience hypercapnia two weeks after an exacerbation.
While non-invasive ventilation is not appropriate in all patients, the Task Force highlights its potential to reduce hospital readmissions and mortality in this specific group of COPD patients.
Question 12: Should long-term prophylactic antibiotic therapy vs. no long-term prophylactic antibiotic therapy be used in patients with recurrent exacerbations of COPD?
Recommendation
In patients with recurrent exacerbations of COPD, the COPD Task Force suggests using long-term prophylactic antibiotic therapy (Conditional recommendation, favours the intervention. Low certainty of the evidence).
Additional observation: The COPD Task Force suggests considering an intermittent antibiotic approach (azithromycin 250 mg daily or 500 mg three times per week) in populations who have experienced one or more exacerbations and at least one hospitalization in the past year. Class 3 (Group E)
Introduction:
Chronic obstructive pulmonary disease (COPD) is a common noncommunicable disease characterized by persistent respiratory symptoms and airflow limitation, with a global prevalence ranging from 10.3% to 12.8%.[1]
COPD is among the leading causes of mortality worldwide and poses a significant economic burden.[2,3] The prevalence of COPD is projected to rise due to increasing smoking rates in low- and middle-income countries and aging populations in high-income countries.[2]
In Saudi Arabia, the overall prevalence of COPD is estimated at 4.2%, with a higher prevalence of 14.2% among current and former smokers.[4,5] Enhancing awareness, knowledge, and adherence to COPD guidelines among health-care providers in Saudi Arabia could improve the management and outcomes for COPD patients.[6,7,8]
Significant advancements have been made in COPD research and management since the publication of the previous Saudi Initiative for Chronic Airway Disease (SICAD) guidelines in 2014.[9] The current COPD guidelines aim to provide updated, evidence-based recommendations for managing stable and acute COPD exacerbations, reflecting the latest research and clinical practices.
Scope and Purpose
The Saudi Thoracic Society (STS) guidelines for the diagnosis and management of COPD aim to support pulmonologists, internists, family medicine practitioners, emergency medicine specialists, general practitioners, and their health-care teams in providing optimal care for patients with COPD. In addition, these guidelines seek to assist policymakers in clinical decision-making, improving patient outcomes, and efficiently allocating resources.
The current guidelines are designed to reflect the best practices in COPD management. However, they do not replace the clinician’s decision-making capabilities in unique clinical situations involving COPD patients. Recognizing the importance of individualized care and shared decision-making, health-care professionals are encouraged to consider patients’ preferences, values, and goals when implementing these recommendations. This approach ensures a patient-centered strategy in managing COPD.
Methods
A crucial aspect of the Saudi Ministry of Health (MoH) goal to achieve Vision 2030 is establishing a National Guidelines Program to ensure evidence-based clinical care for all diseases, including COPD. This guideline was developed through a collaborative effort between the MoH and the STS, involving a multidisciplinary group of 13 local experts. These experts were led by specialists who had previously contributed to the publication of the 2014 Saudi COPD guidelines.[9] The Elsevier Guidelines International team provided support to the COPD Task Force by gathering evidence and conducting a literature review.
At the outset of the guideline development process, the COPD Task Force members formulated a series of key questions to address various aspects of COPD diagnosis and treatment. Initially, 33 clinical questions were proposed, focusing on different facets of COPD management. These were then prioritized to 12 key questions, which served as a roadmap for the subsequent evidence search and synthesis. The expert panel thoroughly searched for relevant evidence from various sources, including published literature, existing guidelines, clinical trials, systematic reviews, and meta-analyses. The search process utilized established databases and incorporated input from experts in evidence grading.
The identified evidence was critically appraised and evaluated for its relevance, quality, and applicability to the clinical context. The expert panel carefully reviewed and analyzed the evidence, considering its strengths, limitations, and potential biases. Through a consensus-based approach, the panel synthesized the available evidence and formulated evidence-based recommendations. The discussions within the expert panel were structured and focused, with a balanced consideration of the available evidence, clinical expertise, and patient perspectives. Deliberations involved thorough evaluations of the benefits, risks, feasibility, and equity of various diagnostic and treatment approaches. The panel members engaged in constructive debates, allowing for the exploration of different viewpoints and the resolution of any discrepancies. The discussion also acknowledged the limitations of the evidence and potential areas for further research and refinement. Overall, the stringent review process, including the systematic evidence search and the collaborative efforts of the expert panel, ensures that these COPD guidelines are based on the best available evidence and expert consensus. To leverage recent high-quality efforts locally and internationally, the guideline development followed the Grading of Recommendations Assessment, Development, and Evaluation-ADOLOPMENT methodology, an internationally accepted approach for adopting, adapting, and creating new guidelines.[10]
In addition to the internal review process conducted by the expert panel, the guidelines underwent a crucial external review by three independent experts in COPD management. These reviewers were selected for their expertise and experience in the field. They were provided with the draft of the management guidelines and tasked with evaluating its content, methodology, and recommendations. The feedback and comments from these external reviewers were carefully considered and incorporated into the final version of the guidelines. This independent evaluation enhanced the overall robustness of the recommendations, increased the confidence in their applicability and relevance to clinical practice, and bolstered the credibility and reliability of the guidelines.
Moreover, the expert panel thoroughly reviewed and updated sections from the previous SICAD guidelines[9] that needed to be addressed by the current focused 12 questions on COPD management. These sections are epidemiology, diagnosis, clinical assessment of stable COPD patients, COPD exacerbation, addressing comorbidities, and other treatment strategies. The recommendations are summarized in the experts’ executive summary.
The COPD Task Force adopted the same evidence criteria used in previous SICAD guidelines:
Evidence Category A: Randomized controlled trials (RCTs) with a rich body of evidence
Evidence Category B: RCTs with a limited body of evidence
Evidence Category C: Nonrandomized trials and observational studies
Evidence Category D: COPD Task Force consensus judgment.
Epidemiology
The pooled prevalence of COPD in the Eastern Mediterranean Region, based on a total of 92 studies, was 5.39%.[11] In the Middle East and North Africa (MENA) region, the pooled prevalence of COPD was 2.7% in the general population and 2.8% in hospital-based studies, with a higher frequency in men (5.2%) compared to women (1.8%).[12] COPD is one of the leading causes of death and disability in the MENA region, with hospitalizations being the most utilized health-care resource for this patient group.[13]
The pooled prevalence of COPD in the Eastern Mediterranean Region, based on a total of 92 studies, was 5.39%.[11] In the MENA region, the pooled prevalence of COPD was 2.7% in the general population and 2.8% in hospital-based studies, with a higher frequency in men (5.2%) compared to women (1.8%).[12] COPD is one of the leading causes of death and disability in the MENA region, with hospitalizations being the most utilized health-care resource for this patient group.[12]
The overall prevalence of COPD in Saudi Arabia among patients aged 40 years and older is 4.2%, with a higher prevalence in men (5.7%) compared to women (2.5%). The prevalence increases with age, reaching 11.4% in individuals aged 60 years and above, and 10.5% in those with a smoking history of over 20 pack-years.[4] In addition, the COPD prevalence among current or ex-smokers attending private primary health-care facilities in 2011 was reported to be 14.2%.[14] The intensive care unit (ICU) mortality rate for patients admitted with COPD exacerbation was 6%, while the hospital mortality rate was 11% in 2012.[15]
In general, the management of COPD faces several significant challenges:
Early diagnosis is crucial, as COPD often goes undetected in its initial stages
Appropriate treatment should be provided, including the proper use of medication devices
Patients’ adherence to the prescribed COPD therapies must be ensured
Adherence to guidelines and access to specialized health care in COPD.
A study on managing COPD in the MENA region revealed that 31.8% of participants had received a COPD diagnosis from a physician, and only 20.6% had undergone spirometry in the previous year.[16] Furthermore, 15.7% reported receiving specific treatments for respiratory symptoms, 3.8% used inhaled long-acting bronchodilators together with corticosteroids, and 20.4% had been hospitalized overnight for their COPD, with a mean of 2.3 ± 3.7 hospitalizations per year.[16] A cross-sectional study in patients with severe COPD in the MENA region reported that 81.6% and 83.4% of patients experienced weekly and daily symptom variability, respectively. The number of exacerbations in the previous year, smoking cessation, and COPD Global Initiative for Chronic Obstructive Lung Disease (GOLD) D status were the most consistent factors associated with symptom variability.[17]
Patient adherence to COPD medication and inhaler technique has also been found to be suboptimal, with over 60% of Saudi patients reporting low medication adherence.[8] Over 70% of COPD patients make at least one critical error when demonstrating their inhaler technique, mainly when using dry powder inhalers.[7,18] A study on COPD medication adherence among Saudi and Turkish patients found an overall reported adherence of only 49%; of those, 74.7% reported high disease impact (COPD Assessment Test [CAT] >15) compared to 58.4% reporting medium–high adherence.[19]
Despite numerous high-quality, evidence-based guidelines published over the last decade, awareness of COPD guideline recommendations in Saudi Arabia remains insufficient.[8,20] In addition, the current secondary care services for COPD patients are limited, with many hospitals lacking respiratory departments, spirometry facilities, ICUs, and pulmonary rehabilitation programs.
Definitions of Chronic Obstructive Pulmonary Disease
COPD is a chronic lung disease that encompasses emphysema, chronic bronchitis, or a combination of both. Chronic bronchitis is defined by a chronic cough or expectoration lasting at least 3 months per year for 2 consecutive years. Emphysema, on the other hand, is a pathological condition marked by the permanent, destructive enlargement of airspaces distal to the terminal bronchioles, without prominent fibrosis.[9] COPD is characterized by chronic respiratory symptoms resulting from abnormalities in the airways or alveoli, leading to persistent and often progressive airflow obstruction. Patients with COPD commonly experience symptoms such as dyspnea, wheezing, chest tightness, fatigue, and cough, and they may suffer exacerbations marked by worsening symptoms triggered by factors such as respiratory infections and pollutants.[21] It is crucial to exclude other causes of chronic cough and expectoration, such as asthma or bronchiectasis.
Risk Factors
Cigarette smoking is by far the most significant risk factor for COPD.[21,22] Passive smoking and shisha (water pipe) smoking are other recognized risk factors for COPD.[23] Occupational exposure to organic or inorganic dust and heavy outdoor pollution are also notable risk factors.[22] In developing countries, the use of biomass fuel for indoor cooking with poor ventilation significantly exacerbates the risk of COPD.[24] Other major risk factors include male gender, advancing age, a history of airway diseases such as asthma and tuberculosis, HIV infection, recurrent childhood infections, and premature birth with underdeveloped lungs volumes.[21,25,26]
Epidemiological studies have demonstrated that lower socioeconomic status significantly increases the risk of developing COPD.[27] Furthermore, genetic and environmental factors may play a role in determining who develops the disease.[28]
Clinical Presentation
COPD should be considered in individuals aged 40 years and above who present with chronic cough, sputum production, or dyspnea during physical exertion, especially if they have a history of smoking, are ex-smokers, or have been exposed to harmful particles. However, these symptoms alone are insufficient for accurately diagnosing airway obstruction; therefore, spirometry is essential for a definitive COPD diagnosis. The severity of symptoms directly correlates with the extent of smoking and lung function impairment, as measured by forced expiratory volume in 1 s (FEV1). Initially, shortness of breath occurs only during physical exertion, but as the disease progresses, it may be present even at rest. Coughing, typically accompanied by scanty sputum in the early stages, may become more frequent and produce larger volumes of sputum over time.[29,30]
Careful evaluation of COPD presentations is crucial to differentiate them from bronchiectasis, a common condition in Saudi Arabia.[31] Any changes in the amount or color of sputum in COPD patients may indicate an acute exacerbation and should be promptly recognized and treated to prevent a decline in clinical condition and lung function. Unlike asthma, audible wheezing is not a common presenting symptom in COPD.
Other symptoms of COPD include fatigue, loss of appetite, and weight loss, which typically manifest in the late stages of the disease and are associated with a poor prognosis, and increased risk of anxiety, depression, and disability.[32] Depression and anxiety are common in COPD patients and are linked to poor health status, more frequent exacerbations, and higher hospitalization rates.[33] Importantly, a normal physical examination does not exclude the presence of COPD, underscoring the need for thorough assessment and diagnostic testing.
In advanced cases of COPD, signs of lung hyperinflation and wheezing may only be audible during forced expiration. Severe and very severe chronic bronchitis can lead to ankle swelling due to right heart failure. Lower limb edema may indicate cor pulmonale, a recognized complication of severe COPD and chronic hypoxia. Clubbing is not typically associated with COPD, and its presence should raise suspicion for other conditions such as bronchiectasis, lung fibrosis, or lung cancer.
Recommendations
The presence of cough, sputum production, dyspnea, and wheezing in an individual who is over 40 years of age and has a smoking history of more than 20 pack-years indicates a higher probability of having COPD (Evidence C).
Diagnostic Tools
Spirometry
Spirometry is essential for diagnosing COPD. It can be conveniently conducted in a clinic or as part of formal pulmonary function testing (PFT) in a laboratory. Handheld spirometry devices can serve as effective screening tools to measure spirometry parameters. However, if the results are abnormal, it is recommended to refer the patient for formal spirometry to confirm the diagnosis.
When spirometry is performed in a clinic, it is crucial to use a validated machine and ensure proper calibration according to the manufacturer’s specifications when adhering to established procedural standards.[34] Given that spirometry results are effort-dependent, the best outcome from a minimum of three attempts should be selected to ensure accuracy.
It is recommended to evaluate reversibility by assessing the response to short-acting bronchodilators, if spirometry reveals a FEV1 to forced vital capacity (FVC) (FEV1/FVC ratio) of <70%. This can be achieved by administering a short-acting β2-adrenoceptor agonist, such as salbutamol (2 puffs of 100 μg/dose), or an anticholinergic, such as ipratropium bromide (2 puffs of 40 μg/dose), using a spacer device.
After administering salbutamol, spirometry should be performed 15–20 min later, while spirometry should be conducted 30 min after administering ipratropium bromide. Reversibility is defined as an improvement in FEV1 of at least 10% from the predose value, accompanied by an absolute increase in FEV1 of more than 200 ml.[34,35]
In COPD, PFT typically reveals airflow limitation, defined as a postbronchodilator FEV1/FVC ratio of <70%.[36] PFT also shows signs of air trapping and hyperinflation, indicated by elevated residual volume (RV) and total lung capacity (TLC), along with an increased RV/TLC ratio. Emphysema, characterized by the destruction of the lung parenchyma, is commonly associated with a reduction in diffusion capacity (DLco).[34]
Screening spirometry is not recommended for the general population, especially for individuals without symptoms or significant exposure to tobacco or other risk factors.[37,38,39,40] However, for those exhibiting symptoms or having risk factors such as a smoking history exceeding 20 pack-years, recurrent chest infections, or adverse early life events, spirometry is a valuable tool for early COPD detection.[21]
Recommendations
Symptomatic individuals with risk factors such as a history of smoking and exposure to harmful particles should undergo spirometry testing (Evidence A)
Handheld spirometry, which measures the ratio of FEV1 to FVC, can be utilized as a preliminary screening tool. If the results are abnormal, patients should be referred for formal PFT (Evidence D)
In the appropriate clinical context and with exposure to risk factors, the presence of airflow limitation (FEV1/FVC ratio <0.7 after bronchodilator use) confirms the diagnosis of COPD (Evidence A).
Screening and symptoms scoring questionnaires
Screening questionnaires can be valuable tools in diagnosing COPD, although they are not considered definitive diagnostic tests. They are typically designed to help identify individuals who are at a higher risk of having COPD and should be followed by additional diagnostic testing for confirmation.
There are a few commonly used self-administered screening questionnaires to detect COPD in primary and secondary care and the population for COPD. These questionnaires include CAT, COPD Diagnostic Questionnaire, International Primary Care Airways Group questionnaire, COPD Population Screener questionnaire, Lung Function Questionnaire, COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk, Dyspnea-12 (D-12) questionnaire, and PUMA questionnaire.[40,41,42,43,44,45,46,47,48] The PUMA questionnaire was developed for COPD detection in the primary care setting (PUMA Study) of four Latin American countries and has increased in popularity over the past few years. A PUMA score of ≥5 has a sensitivity of 74.2% and a specificity of 64.8% for diagnosing COPD.[46,47] All COPD screening questionnaires are easy to use; however, sensitivity and specificity values vary across populations, and the positive result needs to be confirmed by spirometry. Only CAT and D-12 are validated in the Arabic language.[45,49]
Recommendations
Screening questionnaires for COPD are not definitive diagnostic tests. They can be valuable tools for screening and identifying individuals at a higher risk of COPD (Evidence C).
Chest radiography
The absence of abnormalities on a normal chest X-ray does not rule out the possibility of COPD. In more advanced cases, characteristic findings may include hyperinflation, diaphragmatic flattening, enlarged retrosternal airspace observed on the lateral view, and a tubular appearance of the heart. Occasionally, areas of increased lung transparency may be present. In addition, a chest X-ray can be valuable in excluding alternative conditions such as bronchiectasis, heart failure, lung fibrosis, and lung cancer.[21]
Recommendations
Chest X-ray is not required for the diagnosis of COPD; however, it is valuable in excluding alternative conditions (Evidence C).
Chest computed tomography
Routine use of chest computed tomography (CT) is not recommended due to its cost and radiation hazards. However, high-resolution CT can be valuable in cases where there is uncertainty about the diagnosis of COPD, as it can confirm the condition or rule out other diseases like bronchiectasis, lung fibrosis, or lung cancer. CT imaging should be considered for patients with persistent exacerbations and symptoms that are disproportionate to the severity of the disease, especially if their FEV1 is below 45% of the predicted value and they exhibit significant hyperinflation and air trapping. In addition, patients who meet the criteria for lung cancer screening should also be considered for CT imaging.[21]
Recommendations
CT chest should be considered in patients experiencing frequent exacerbations, exhibiting symptoms that are disproportionate to the severity of the disease, or meet the criteria for lung cancer screening (Evidence C).
Laboratory
Clinical evidence indicates that a higher eosinophil count in the blood is associated with an increased risk of future exacerbations in patients with a history of COPD exacerbations. These patients tend to respond better to treatment with inhaled corticosteroids (ICSs) in combination with long-acting bronchodilators. Therefore, blood eosinophil count serves as a potential biomarker for phenotyping COPD patients.[35,50] In addition, measuring alpha-1 antitrypsin (AAT) levels and conducting genetic testing should be considered for relatively young COPD patients with low smoking exposure to exclude the diagnosis of AAT deficiency.[51]
Recommendations
Blood eosinophil count should be obtained as a biomarker for phenotyping COPD patients with exacerbations (Evidence B).
Chronic Obstructive Pulmonary Disease Assessment
COPD is a heterogeneous disease with diverse phenotypes, resulting in a wide range of clinical presentations. Some patients may predominantly experience symptoms such as dyspnea or a productive cough, while others face a higher risk of exacerbations. Therefore, it is crucial to conduct an individualized assessment of each patient’s symptoms and identify those at greater risk of exacerbations during the initial evaluation. By considering these critical aspects, health-care providers can develop an effective management plan tailored to each patient’s specific needs.
The comprehensive and personalized assessment for COPD should be based on the following components:
Confirming the diagnosis
Assessment of symptoms
Assessment of exacerbation risk
Classification of COPD based on the symptoms and risk of future exacerbations.
Confirming the diagnosis
Individuals aged 40 years and above who exhibit symptoms such as chronic cough, sputum production, or dyspnea during physical exertion should be evaluated for COPD, especially if they have a history of present or past smoking or exposure to harmful particles. The presence of these symptoms alone is not sufficient for a definitive diagnosis; therefore, spirometry is essential to accurately diagnose COPD and document its severity [Table 1].
Table 1.
Spirometry criteria for chronic obstructive pulmonary disease severity
| Severity | Criteria |
|---|---|
| Mild | FEV1≥80% predicted |
| Moderate | FEV1≥50% and <80% predicted |
| Severe | FEV1≥30% and <50% predicted |
| Very severe | FEV1<30% predicted |
FEV1=Forced expiratory volume in 1 s
Recommendations
Spirometry is recommended to confirm the diagnosis and to assess severity of COPD (Evidence A)
Airflow limitation (FEV1/FVC) ratio of <70% after bronchodilator confirms the diagnosis of COPD (Evidence A).
Assessment of symptoms
The cardinal symptoms of COPD, including cough, sputum production, and dyspnea during exertion, should be thoroughly evaluated in all patients. It is recommended to use a validated tool for assessing the severity of dyspnea in patients with respiratory conditions, such as the COPD Modified Medical Research Council (mMRC) dyspnea scale [Table 2] or the CAT [Table 3] for an objective assessment of these symptoms.[48,52,53]
Table 2.
Modified Medical Research Council dyspnea scale
| mMRC dyspnea scale | Description |
|---|---|
| 0 | I only get breathless with strenuous exercise |
| 1 | I get short of breath when hurrying on the level or walking up a slight hill |
| 2 | I walk slower than people of the same age on the level because of breathlessness or have to stop for breath when walking at my own pace on the level |
| 3 | I stop for breath after walking about 100 yards or after a few minutes on the level |
| 4 | I am too breathless to leave the house, “or” I am breathless when dressing |
mMRC=Modified Medical Research Council
Table 3.
Interpretation of the Chronic Obstructive Pulmonary Disease Assessment Test
| Score of symptoms | Severity of symptoms |
|---|---|
| <10 | Low |
| 10–20 | Medium |
| >20 | High |
| >30 | Very high |
The CAT questionnaire comprehensively evaluates dyspnea and health status impairment in COPD patients and has been translated and validated in Arabic[49] [Figures 1 and 2]. Consisting of eight items rated on a Likert scale from 0 to 5, the CAT strongly correlates with the St. George’s Respiratory Questionnaire, which measures health status. The CAT score ranges from 0 (completely asymptomatic) to 40 (extremely symptomatic) [Table 3]. A CAT score of ≥10 is associated with significantly impaired health status.[54]
Figure 1.

English version of Chronic Obstructive Pulmonary Disease Assessment Test score[49]
Figure 2.
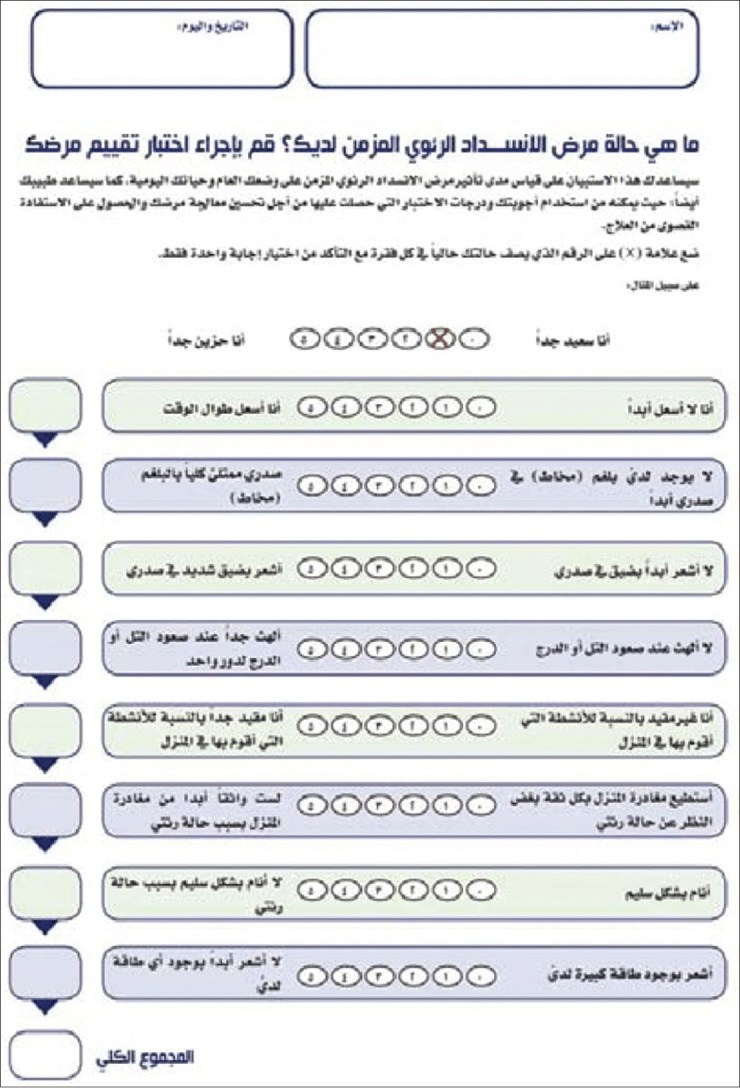
Arabic version of Chronic Obstructive Pulmonary Disease Assessment Test score[49]
Recommendations
The severity of the symptoms based on CAT or mMRC score should be documented in patients with COPD (Evidence B).
Assessment of exacerbation risk
Exacerbations of COPD are marked by heightened inflammation in the airways and throughout the body, increased air trapping, hyperinflation, and reduced expiratory flow, leading to increased breathlessness and a decline in lung function.[55]
Various factors can trigger COPD exacerbations, including respiratory infections (both viral and bacterial), air pollution, exposure to irritants, and nonadherence to prescribed medications or treatment regimens. The definition of an exacerbation is complex; however, the most widely accepted criteria involve subjective elements such as increased dyspnea, cough, and sputum production beyond the patient’s usual day-to-day variations.[56,57,58] These symptoms are nonspecific, making it essential to exclude other causes of acute dyspnea during the initial diagnosis of COPD exacerbations, as many COPD patients may have other chronic concomitant respiratory and nonrespiratory diseases.[58]
To assess the severity of COPD exacerbations, a recent proposal known as ROME recommends using specific measures such as a dyspnea visual score, respiratory rate, heart rate, resting room air oxygen saturation, and C-reactive protein (CRP) levels.[59] These objective criteria are crucial for evaluating the severity of exacerbations.[59]
An eosinophil count of more than 300 cells/μL in the blood is associated with an elevated risk of future exacerbations.[60,61,62,63,64,65,66,67] Recurrent COPD exacerbation is associated with a progressive decline in lung functions in approximately 25% of patients.[61,62] The average rate of recurrent exacerbation ranges from 0.5 to 3.5 per year.[63] Patients with two or more exacerbations per year tend to experience a more rapid decline in lung function, increased hospitalization, and increased risk of morbidity and mortality compared to those with infrequent exacerbations of <2 per year.[62,64] Studies have indicated that patients hospitalized for acute exacerbations of COPD (AECOPD) have a mortality rate of 25% and 65% at 1 year and 5 years, respectively.[62,65]
Furthermore, approximately 2%–19% of hospitalized patients with AECOPD require transfer to the ICU, with in-hospital mortality ranging from 12% to 25% and a readmission rate of 35% within 3 months.[62,66,67] Most of the patients admitted for COPD exacerbation typically have additional comorbidities. About 70% of readmissions following a hospitalization for exacerbation are due to the decompensation of these other medical conditions.[65]
Recommendations
COPD exacerbation symptoms are nonspecific symptoms, and the initial diagnosis of COPD exacerbations should exclude other causes of acute symptoms as most of COPD patients have other chronic concomitant respiratory and nonrespiratory diseases (Evidence C)
Modified ROME [Figure 3] provides an objective criterion in evaluating the seriousness of COPD exacerbation (Evidence C)
Eosinophil count should be obtained in patients with COPD exacerbation. It is helpful to phenotype patients who may respond to inhaled steroids (Evidence A)
Risk of exacerbation should be documented in COPD patients based on the history of exacerbation or hospitalization due to COPD in the past year and symptoms severity CAT score (Evidence C)
-
The risk of COPD exacerbation is defined as (Evidence A)
-
Low exacerbation risk (all the following should be fulfilled)
At the most, one exacerbation in the past year
No hospitalization due to COPD in the past year
-
High exacerbation risk (one of the following should at least be fulfilled)
Two or more exacerbations in the past year
History of hospitalization due to COPD in the past year.
-
Figure 3.

Diagnostic approach to a patient suspected of an exacerbation of chronic obstructive pulmonary disease. Modified from MacLeod et al.[59] VAS = Visual Assessment Score, CRP = C-reactive protein, ECOPD = Exacerbation of chronic obstructive pulmonary disease, HR = Hear rate, RR = Respiratory rate
Chronic obstructive pulmonary disease classification
In 2014, the SICAD recommended three clinical classes for COPD by combining GOLD groups C and D.[9] The GOLD 2023 Report has further refined the ABCD assessment framework, introducing the ABE tool. This new approach merges the former C and D groups and places greater emphasis on the clinical impact of exacerbations, irrespective of symptom levels.[21,35]
In the current guidelines, the authors recommend three clinical classes for assessing symptoms and exacerbations in COPD patients [Table 4].
Table 4.
Saudi Thoracic Society guidelines chronic obstructive pulmonary disease classification: Assessment of symptoms and exacerbations
| Class | Characteristics | CAT or mMRC score | Exacerbation in the past year | GOLD 2024 equivalent |
|---|---|---|---|---|
| I | Less symptoms: At low risk of exacerbation | CAT <10 or mMRC 1–2 | 0–1 and no hospitalization | Group A |
| II | More symptoms: At low risk of exacerbation | CAT ≥10 or mMRC≥2 | 0–1 and no hospitalization | Group B |
| III | At high risk of exacerbation regardless of symptoms | Any score | ≥2 or ≥1 hospitalizations | Group E (C and D) |
CAT=Chronic Obstructive Pulmonary Disease Assessment Test, mMRC=Modified Medical Research Council, GOLD=Global Initiative for Chronic Obstructive Lung Disease
Recommendation
-
Assessment of symptoms and exacerbations for COPD patients (Evidence D)
-
Class I (GOLD A)
Less symptoms (CAT <10) or (mMRC 0–1)
0–1 exacerbation in the past year and no hospitalization
-
Class II (GOLD B)
More symptoms (CAT ≥10) or mMRC ≥2
0–1 exacerbation in the past year and no hospitalization
-
Class III (GOLD E)
At high risk of exacerbations regardless of symptoms
≥2 exacerbations in the past year and/or hospitalization.
-
Management of Chronic Obstructive Pulmonary Disease
Management of COPD is divided broadly into pharmacological and nonpharmacological treatment.
Pharmacological Therapy of Chronic Obstructive Pulmonary Disease
Pharmacological treatment for COPD focuses primarily on managing symptoms and preventing acute exacerbations. This approach aims to slow disease progression and reduce mortality. Inhaled therapy is the cornerstone of maintenance treatment for COPD and plays a crucial role in preventing exacerbations. These therapies improve airflow by altering smooth muscle tone, which enhances lung emptying and reduces hyperinflation at rest and during exercise. However, despite the symptomatic improvement, they do not change the progressive decline in FEV1 observed in COPD patients.[68,69,70,71]
Inhalation is the preferred route of administration, with various inhaler device options available in Saudi Arabia, including metered-dose inhalers, Evohaler, breath-actuated inhalers, and dry-powder inhalers. Nebulized bronchodilators may be beneficial for patients with poor inspiratory force and physical limitations that make them unable to use inhalers. However, due to cost and other practical concerns, regular use of nebulizers is generally not recommended. The medications available for treating COPD in the Saudi market are listed in Table 5.
Table 5.
Medications used in the treatment of chronic obstructive pulmonary disease
| Drug | Dose | Mode of administration |
|---|---|---|
| b2-agonists | ||
| Short-acting b2-agonists | ||
| Salbutamol | 100, 200 μg 5 mg/mL |
MDI Nebulizer solutation |
| Long-acting b2-agonists | ||
| Unavailable as solo therapy in Saudi Arabia | ||
| Anticholinergics | ||
| Short acting anticholinergics | ||
| Ipratropium bromide | 20, 40 μg 0.25–0.5 μg |
MDI Nebulizer solution |
| Long-acting anticholinergics | ||
| Tiotropium | 18 μg 5 μg |
DPI DPI |
| Inhaled steroids | ||
| Beclomethasone | 50–400 μg | MDI, DPI |
| Budesonide | 100, 200 μg 0.25, 0.5 mg/mL |
DPI Nebulizer solution |
| Fluticasone propionate | 50–500 μg | MDI, DPI |
| Ciclesonide | 80–320 μg | MDI |
| Combination of long-acting b2-agonist and inhaled steroid | ||
| Formoterol/budesonide | 4.5/160 μg | DPI |
| Salmeterol/fluticasone | 25/50, 25/125, 25/250 μg 50/100, 50/250, 50/500 μg |
MDI DPI |
| Fluticasone furoate/vilanterol | 100/25 μg | DPI |
| Beclometasone/formoterol | 100/6 μg | MDI |
| Fluticasone propionate/formoterol fumerate | 250/10 μg | MDI |
| Combination of long-acting b2-agonist and long acting anticholinergics | ||
| Umeclidinium bromide and vilanterol | 62.5/25 μg | DPI |
| Tiotropium/olodaterol | 2.5/2.5 μg | Respimat |
| Glycopyrronium/indacaterol | 110/50 μg | |
| Single inhaler triple therapy with ICSs, long-acting β2 agonist, and long-acting antimuscarinic agent | ||
| Fluticasone furoate/umeclidinium bromide/vilanterol | 100/62.5/25 μg | DPI |
| Formoterol fumarate/glycopyrronium bromide/budesonide | 5/7.2/160 μg | MDI |
| Beclomethasone dipropionate/formoterol/glycopyrronium | 87/5/9 μg | DPI |
| Methylxanthines | ||
| Aminophylline | 200–600 mg | Oral |
| Theophylline (SR) | 100–600 mg | Oral |
| PDE4 inhibitors | ||
| Roflumilast | 500 μg | Oral |
| Systemic steroids | ||
| Prednisolone/prednisone | 5–60 mg | Oral |
DPI=Dry powder inhaler, MDI=Metered-dose inhaler, ICSs=Inhaled corticosteroids, PDE4=Phosphodiesterase-4
Beta-2 agonists
The primary action of β2-agonists is to induce relaxation of airway smooth muscle by stimulating β2-adrenergic receptors and increasing the production of cyclic adenosine monophosphate (cAMP). Inhaled β2-agonists have a rapid onset of action, though this effect may be slower in COPD compared to asthma. These agents improve symptoms and FEV1.
The short-acting β2-agonists (SABAs) work within a few minutes, but the effects usually wear off within 4–6 h. SABA can be used on a regular and as-needed basis in COPD.[72,73,74] In contrast, LABAs have an effect lasting 12–24 h, allowing for once-daily or twice-daily dosing. LABA significantly improved FEV1, lung volumes, dyspnea, quality of life (QoL), decreased exacerbation rate, and number of hospitalizations.[75,76,77,78,79] However, β2-agnostic therapies have not demonstrated an effect on mortality or the rate of decline in lung function over time.[78]
LABAs are recommended as maintenance therapy for COPD, as they are more effective and convenient than SABA treatment. The use of LABA should not preclude additional benefits from as-needed SABA.
The side effects are related to the stimulation of β2-adrenergic receptors that cause sinus tachycardia and rarely other cardiac rhythm abnormalities in susceptible patients. Symptomatic tremors may be troublesome in some older patients treated with high doses of β2-agonists. Hypokalemia can also occur, especially when treatment is combined with diuretics, and should be monitored in susceptible patients.[80] In general, β2-agonists are safe and well tolerated. The side effects are dose dependent and often resolve after treatment discontinuation.[81] However, COPD patients who are typically elderly and have cardiovascular comorbidities may be at a greater risk of developing clinically significant side effects.
Muscarinic antagonists
Antimuscarinic drugs, also known as anticholinergic medications, work by blocking the bronchoconstrictor effects of acetylcholine on M3 muscarinic receptors in airway smooth muscle.[82] The bronchodilation effect of a short-acting muscarinic antagonist (SAMA), such as inhaled ipratropium, can last up to 8 h. Long-acting muscarinic antagonists (LAMAs) have prolonged binding to M3 muscarinic receptors and faster dissociation from M2 muscarinic receptors, thereby extending the bronchodilator effect. LAMAs are administered once or twice daily.[83,84,85]
Treatment with LAMA effectively reduces symptoms, improves QoL, and decreases exacerbations and hospitalizations at any stage in COPD patients.[85] Treatment with tiotropium is associated with reduced in mortality of COPD patients.[86] Moreover, treatment with LAMA improves the effectiveness of pulmonary rehabilitation. It may also reduce the rate of decline in lung function compared with a placebo in patients not on other maintenance drugs.[85,86,87,88,89,90,91] Clinical trials have shown greater protection from exacerbations for LAMA versus LABA treatment.[79,90,91]
A systematic review of RCTs concluded that ipratropium, a SAMA, provides the modest benefits over SABA in terms of lung function, health status, and the requirement for oral steroids.[89]
Anticholinergic drugs are generally safe, with dry mouth being the most common side effect. Although mild prostatic symptoms have been reported, no clear causal relationship has been established. In rare instances, the use of nebulized ipratropium with a facemask has been linked to the development of acute glaucoma.[92]
Recommendations
The central role of bronchodilators in COPD is for symptom relief, rather than improving FEV1 (Evidence A)
Regular or as-needed SABA or SAMA provides relief of acute symptoms of COPD related to bronchospasm and improves FEV1 (Evidence A)
Regular treatment with LABAs is more effective and convenient compared to SABAs and has been shown to improve health status, QoL, and exercise tolerance in COPD patients (Evidence A)
Regular treatment with LAMA as a monotherapy is better than LABA (Evidence A).
Combined bronchodilators
Combining bronchodilators with different mechanisms of action can provide greater bronchodilation than simply increasing the dose of a single bronchodilator.[93,94,95] Combinations of SABAs and SAMAs are superior to either medication alone in improving FEV1 and symptoms.[95,96,97,98] In addition, using a single inhaler therapy improves treatment adherence.[96]
Combining bronchodilators with different mechanisms of action can provide greater bronchodilation than simply increasing the dose of a single bronchodilator.[92,93,94] Combinations of SABAs and SAMAs are superior to either medication alone in improving FEV1 and symptoms.[95,96,97,98] In addition, using a single inhaler therapy improves treatment adherence.[98]
The COPD Task Force reviewed the evidence to determine whether combination therapy with LABAs and LAMAs is preferred over LAMA alone in patients with stable COPD. The review found little or no difference in the frequency of moderate or severe exacerbations (odds ratio [OR], 0.96; 95% confidence interval [CI], 0.75–1.23) and (OR, 0.90; 95% CI, 0.59–1.36), respectively.[96,97,98,99,100,101,102,103,104] However, there were improvements in QoL (OR, 1.19; 95% CI, 1.04–1.35) and dyspnea index (OR, 0.21; 95% CI, 0.1–0.33).[92,97] LABA alone is not marketed in Saudi Arabia but is available in combination with LAMA.
Recommendations
A combination of SABA and SAMA is superior to either in improving the symptoms and FEV1 (Evidence A)
Combined LABA/LAMA better than LABA or LAMA alone or LABA/ICS (Evidence A)
A combination of LAMA and LABA preferably as a single inhaler is superior for symptomatic COPD patients (Evidence A).
Inhaled corticosteroids
The effects of oral and ICS in COPD are less well defined compared to their use in asthma. However, ICS may reduce COPD exacerbations, particularly in patients with high blood eosinophil count (>150 ells/μL) or overlap syndrome (OVS).[105] The use of ICS can increase the risk of pneumonia, so the benefits must be carefully weighed against risks such as pneumonia and fractures.[106,107,108]
Treatment with ICS alone does not significantly alter the long-term decline in FEV1 or mortality in patients with COPD.[109,110,111,112]
Inhaled corticosteroid and LABA combination
ICS and LABA combination reduces exacerbations compared to LABA alone, but not compared to LAMA. Furthermore, the LABA/LAMA combination significantly reduced exacerbations compared to ICS/LABA.[112,113,114] Clinical trials investigating ICS/LABA combinations in COPD patients failed to show a significant reduction in mortality as the primary outcome.[115,116,117]
Inhaled corticosteroid/LABA/long-acting muscarinic antagonist Triple Therapy
Triple therapy with ICS/LABA/LAMA is indicated for reducing exacerbations in patients not optimally controlled on dual bronchodilator therapy. The COPD Task Force reviewed the evidence and evaluated the effect of triple therapy in COPD. The rate of moderate-to-severe exacerbation reduction favored triple therapy (rate ratio [RR], 1.16; 95% CI, 1.11–1.20).[117,118,119,120,121,122,123] There was high certainty in the evidence regarding the rate of severe exacerbations (RR, 1.22; 95% CI, 1.11–1.34).[121,122]
While the lack of a mortality benefit as the primary endpoint is an important consideration, two landmark studies showed a mortality advantage of triple therapy as a secondary endpoint.[121,122] A recent meta-analysis of 60 RCTs involving 103,034 COPD patients treated for more than 6 months concluded that inhaled therapy containing ICS, particularly triple therapy, was associated with reducing the all-cause mortality risk. Predictors of this association included eosinophil counts of ≥200/μL or a percentage of ≥2%, a history of ≥2 moderate or severe exacerbations in the previous year, COPD stages III or IV, age younger than 65 years, and a body mass index (BMI) of ≥25 kg/m2.[117,122,123]
Recommendations
Triple therapy (ICS/LABA/LAMA) is recommended for patients with a high risk of COPD exacerbation Class 3 (Group E) (Evidence A).
Phosphodiesterase-4 inhibitors (roflumilast)
Roflumilast, an oral PDE-4 inhibitor, is an anti-inflammatory drug that specifically inhibits the phosphodiesterase-4 enzyme responsible for breaking down intracellular cAMP. It has been approved for COPD patients with a chronic bronchitis phenotype who suffer from frequent exacerbations.
The COPD Task Force reviewed the evidence to determine the effects of phosphodiesterase-4 (PDE4) inhibitors in patients with stable COPD. Treatment with roflumilast was associated with a lower incidence of COPD exacerbations (OR, 0.78; 95% CI, 0.73–0.84), corresponding to 52 fewer events per 1000 patients. However, there was no significant effect on mortality (OR, 0.98; 95% CI, 0.77–1.24).[118,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143]
The most common side effects of roflumilast include nausea, abdominal pain, diarrhea, reduced appetite, headache, and sleep disturbances. Most of these adverse effects improve over time. Mild weight reduction has also been reported.[144]
Recommendations
Roflumilast is indicated in Class III COPD patients with chronic bronchitis phenotype (Evidence A).
Mucolytics
The regular use of mucolytics in COPD remains controversial. Mucolytics are often considered an “add-on” therapy for patients with severe COPD who have recurrent exacerbations and remain symptomatic despite maximum standard therapy.
The COPD Task Force reviewed the evidence to determine the efficacy of mucolytic agents in stable COPD patients. There was no effect on mortality (RR, 1.10; 95% CI, 0.56–2.16), FEV1 (MD, 0.05; 95% CI, 0.01–0.08), or St. George’s Respiratory Questionnaire (−1.37; 95% CI, −2.85–0.11).[145,146,147,148,149,150,151,152,153,154,155,156] However, in a randomized, double-blind, placebo-controlled study, 1-year treatment with high-dose N-acetylcysteine (1200 mg/day) significantly improved small airway function and decreased frequency of exacerbations in stable COPD patients.[145]
Given that mucolytic agents are generally safe and well tolerated, the COPD Task Force concluded that, overall, the resources required for implementing the intervention of mucolytic agents in stable COPD resulted in negligible costs and potential savings and that cost-effectiveness probably favors the intervention.
Recommendations
Mucolytics are not to be prescribed routinely for all patients with COPD (Evidence A)
Mucolytics may be considered an “add-on” therapy in some selected patients of COPD who remain symptomatic despite maximum standard therapy (Evidence C).
Long-term prophylactic antibiotics
The prophylactic use of antibiotics is not routinely recommended for all patients with stable COPD, as the benefits do not always outweigh the risks. However, patients with severe COPD and frequent exacerbations, despite optimal treatment regimens, may benefit from macrolide prophylaxis.
The COPD Task Force reviewed the evidence on long-term prophylactic macrolide antibiotic therapy in patients with stable COPD and recurrent exacerbations. The review found a significant reduction in the number of exacerbations (OR, 0.57; 95% CI, 0.42–0.78), corresponding to 139 fewer events per 1000 patients.[157,158,159,160,161,162,163,164] Similarly, a Cochrane meta-analysis review of 2732 patients showed a reduction with macrolide exacerbations compared to placebo (OR 1.34, 95% CI, 1.19–1.50) with 127 fewer per 1000.[165]
For macrolide prophylaxis, azithromycin at a dose of 250–500 mg three times per week is recommended.[161,164] However, chronic use of azithromycin has been associated with adverse effects such as cardiovascular events, hearing loss, gastrointestinal symptoms, and mycobacterial resistance.[166,167] For macrolide prophylaxis, azithromycin at a dose of 250 daily or 500 mg three times per week is recommended.[160,163] However, chronic use of azithromycin has been associated with adverse effects such as cardiovascular events, hearing loss, gastrointestinal symptoms, and mycobacterial resistance.[165,166] Therefore, the benefits and risks of long-term azithromycin therapy must be carefully considered for each patient.
Recommendations
Long-term prophylactic antibiotics are not recommended for all stable COPD patients (Evidence C)
Consider an intermittent antibiotic approach (azithromycin 250 mg daily or 500 mg three times per week) in populations who have experienced one or more exacerbations and at least one hospitalization in the past year despite optimum therapy Class II (Group E) (Evidence A).
Biological therapies
Biological therapy has shown effectiveness in asthma management.[168] However, the impact of biologics on COPD outcomes has been inconsistent. Mepolizumab was evaluated in eosinophilic COPD patients in two trials.[169] Mepolizumab showed a slight reduction in moderate-to-severe exacerbations compared to a placebo. Benralizumab did not significantly reduce the COPD exacerbation rate compared to placebo.[170] On the other hand, dupilumab demonstrated a reduction in the annual rate of moderate-to-severe exacerbations and improved FEV1 in COPD patients with eosinophil counts >300 cells/μL and symptoms of chronic bronchitis.[171]
Recommendations
Biological therapy may have potential benefits for a subset of COPD patients, but there is currently insufficient evidence to recommend their routine use (Evidence C)
Dupilumab may have a potential role in eosinophilic COPD patients with chronic bronchitis phenotype and frequent exacerbations (Evidence B).
Nonpharmacological Therapy of Chronic Obstructive Pulmonary Disease
Smoking cessation
Smoking cessation has the most significant impact on the natural progression of COPD, slowing the accelerated decline in lung function and reducing mortality risk.[172,173,174] A study on smoking cessation among US adult smokers with and without COPD highlighted the essential benefits of reducing various health hazards associated with smoking.[173] The younger a smoker is when they quit, the more likely their rate of lung function decline will parallel that of nonsmokers.[175]
Quitting smoking is recommended at any age, as it can decrease COPD symptoms, reduce the number of exacerbations, and improve overall health status and exercise tolerance. Smoking cessation is one of the few interventions proven to decrease mortality in COPD patients, due to its beneficial effects on reducing the risks of lung cancer, cardiovascular disease, and other comorbid conditions.[176] The following strategies can help patients achieve their goal of quitting smoking.
Counseling
Obtaining a thorough smoking history from the patient and providing brief counseling by a health-care professional can result in quit rates ranging from 5% to 10%.[177] Tobacco dependence is a chronic condition, often requiring multiple attempts to quit successfully. More intensive interventions, however, can achieve long-term quit rates as high as 25%.[177] Combining a detailed smoking history with brief counseling, mindfulness-based therapy, and cognitive behavioral therapy acknowledges the chronic nature of tobacco dependence and can significantly support patients in their efforts to quit smoking.[178]
Pharmacological interventions
Pharmacological interventions are essential for smoking cessation in COPD patients. Nicotine replacement therapy (NRT) increases long-term quit rates and is more effective than placebo. However, NRT is contraindicated in patients with recent myocardial infarction, unstable angina, stroke, or untreated peptic ulcer disease. The most commonly used drugs for smoking cessation are varenicline and bupropion SR.[179,180] Varenicline is an effective partial agonist at nicotinic receptors,[181] while bupropion SR is an antidepressant that reduces withdrawal symptoms and cravings.[179] Combining different NRT forms or using varenicline with NRT can increase cessation rates.[179,180,182,183] Furthermore, professional counseling, group support, and pharmacotherapy significantly improve the success rate of smoking cessation efforts and slow the rate of decline of FEV1.[174,176,184]
Recommendations
Smoking cessation is recommended at any age, as it improves health status and exercise tolerance and decreases COPD symptoms, exacerbations, and mortality. Quitting smoking is a crucial intervention that can provide significant benefits for COPD patients (Evidence A).
Long-term oxygen therapy
Approximately 7% of patients with moderate-to-severe COPD develop resting hypoxemia within 5 years.[185] The primary mechanisms leading to hypoxemia in COPD are ventilation–perfusion mismatch and destruction of the alveolar–capillary membrane due to emphysema. Pulmonary vasoconstriction occurs as a compensatory response to alveolar hypoxia. However, this ongoing vasoconstriction can result in pulmonary remodeling and pulmonary hypertension (PH), ultimately leading to right heart failure. Furthermore, persistent hypoxemia causes peripheral vasodilation, which triggers compensatory tachycardia and increased cardiac output to improve oxygen delivery. In addition, it may induce secondary erythrocytosis, increasing blood viscosity, leading to muscle dysfunction, and causing neurocognitive impairment.[186]
These consequences contribute to increased hospitalization and increased mortality observed in COPD patients.[187] Strong evidence demonstrates that long-term oxygen therapy (LTOT) can improve survival and the QoL in COPD patients with significant hypoxemia.[188,189] LTOT is recommended when the patient’s partial pressure of arterial oxygen (PaO2) is <55 mmHg (<7.3 kPa), or the arterial oxygen saturation is <88% while breathing room air and in a stable clinical condition for at least 2 months with optimized medical therapy. LTOT is also indicated if the PaO2 is between 55 and 60 mmHg (7.4–8 kPa) in a COPD patient with associated conditions such as cor pulmonale, peripheral edema, or a hematocrit of 55% or greater. In a randomized study, LTOT did not result in a longer time to death or first hospitalization or provided any sustained benefit concerning any of the other measured outcomes in stable COPD patients with moderate resting hypoxemia (arterial oxygen saturation 89%–93%).[190]
Although the supporting evidence is less robust, LTOT may also be considered for COPD patients with PaO2 >60 mmHg (>8kPa) or arterial oxygen saturation > 88% who experience nocturnal hypoxemia or exercise-induced hypoxemia or in those with coexisting cardiovascular comorbidities.[186]
Once LTOT is initiated, it is recommended to regularly evaluate the patient at 3-month and 1-year intervals to optimize the oxygen prescription. The goal is maintaining a resting PaO2 between 60 and 65 mmHg or an arterial oxygen saturation range of 88%–94%. The typical oxygen flow rate is 1–2.5 L/min, usually administered via nasal cannula, for a minimum of 18 h per day to achieve survival benefits. Home-based oxygen can be conveniently provided using an oxygen concentrator, and portable oxygen delivery systems are available for outdoor use.
Recommendations
LTOT is recommended when the patient’s PaO2 is <55 mmHg (or <7.3 kPa) or PaO2 is between 55 and 60 mmHg (7.4–8 kPa) and the patient has evidence of cor pulmonale, peripheral edema, or a hematocrit of 55% or higher in a stable clinical condition for at least 2 months with optimized medical therapy (Evidence A).
Noninvasive Ventilation
Noninvasive ventilation (NIV) provides ventilatory support to patients using a nasal or full facemask, eliminating the need for an endotracheal tube. The benefits of NIV in treating acute respiratory failure (ARF) due to COPD exacerbations and improving survival in COPD patients are well documented.[191,192,193,194,195,196]
A Cochrane review and meta-analysis did not find clear evidence of benefit from the long-term use of noninvasive positive pressure ventilation stable COPD patients.[191] However, more recent studies suggest that NIV may improve survival and QoL and reduce dyspnea in hypercapnic COPD patients, particularly when combined with pulmonary rehabilitation.[197] Despite these potential benefits, the overall certainty of the evidence on the outcomes of NIV for stable COPD patients with chronic respiratory failure remains low.[197]
The latest European Respiratory Society guidelines conclude that NIV may improve health outcomes in a subset of severe COPD patients with hypercapnia on maximal medical therapy, by targeting a reduction in carbon dioxide levels (PCO2).[198] However, it is also important to consider the potential barriers and costs associated with NIV. Many insurers may not fully cover the cost of NIV, leading to high out-of-pocket costs for patients. This financial burden can limit accessibility. In addition, some patients struggle to tolerate NIV due to issues like claustrophobia or poor synchronization with the ventilation.[199]
High-flow nasal cannula (HFNC) is a respiratory support device used during the early, noninvasive management of ARF. Physiologically, HFNC has been shown to enhance oxygenation, promote alveolar recruitment, provide effective humidification and heating, facilitate secretion clearance, reduce dead space ventilation, and improve hypercapnia.[200,201] These combined benefits of HFNC can help prevent further deterioration of lung function and potentially avoid the need for endotracheal intubation in patients with ARF. Despite these results, little and limited evidence for improved clinical outcomes is currently available.[201,202]
Compared to NIV, HFNC has been associated with a higher reintubation rate and minimal impact on mortality.[201,202] While HFNC can slightly reduce the length of stay in the ICU and hospital, it offers only a modest increase in patient comfort compared to NIV. In addition, there is no significant difference between HFNC and NIV in terms of respiratory rate and gas exchange parameters.[202]
Recommendations
NIV in the treatment of ARF due to COPD exacerbation is recommended (Evidence A)
Based on the current evidence, the role of long-term NIV in stable COPD with chronic hypercapnic respiratory failure remains uncertain. NIV may be considered in a select group with severe COPD patients (Evidence C)
Consider NIV in patients who experience hypercapnia 2 weeks after an exacerbation (Evidence B).
Vaccination
COPD exacerbations are associated with significant morbidity and mortality, leading to worsening airflow obstruction, increased hospitalizations, reduced QoL, accelerated disease progression, and even death. Notably, more than 70% of COPD exacerbations are triggered by infectious causes, with respiratory viruses identified in approximately 30% of cases.[203] Despite well-established recommendations for influenza and pneumococcal vaccinations in COPD patients, vaccination rates in this population remain suboptimal.[204]
Influenza vaccination has been shown to clearly reduce the risk of acute COPD exacerbations and may also help decrease hospitalizations and mortality in this patient population. Therefore, it is recommended that all COPD patients receive an annual influenza vaccination.[205]
The pneumococcal polysaccharide vaccine (PPSV23) has been shown to reduce the risk of pneumococcal pneumonia and invasive pneumococcal disease in COPD patients, particularly among smokers.[206,207] The pneumococcal 13-valent polysaccharide conjugate vaccine (PCV13) has proven effective in preventing vaccine-type pneumococcal bacteremia and nonbacteremic community-acquired pneumonia.[208] In one study, the protective effect of PCV13 was shown to last for 5 years.[207]
COVID-19 infection significantly increases the risk of COPD exacerbation, leading to higher complication rates and mortality.[209] Vaccination against COVID-19 is highly effective in preventing hospitalization and respiratory failure among COPD patients.[210]
Adults with chronic heart or lung disease compromised immune systems, and those living in long-term care facilities are at the highest risk for severe respiratory syncytial virus (RSV) illness.[211,212,213] Following the approval of RSV vaccines in the European Union, Canada, United States, United Kingdom, and Japan, the MoH in Saudi Arabia has approved the use of RSV vaccines for all individuals aged 60 years and older.
Herpes zoster (HZ) is a vaccine-preventable disease caused by the reactivation of latent varicella-zoster virus, which is present in >95% of adults ≥40 years of age.[214] In asthma and COPD, the risk of HZ infection increases by 24%–41% compared to the normal population.[215]
Recommendations
All COPD patients should receive the annual influenza vaccine (Evidence A)
All COPD patients should receive a pneumococcal vaccine (Evidence B)
The RSV vaccine is recommended for all COPD patients >60 years of age (Evidence A)
The COVID-19 vaccine is recommended for all COPD patients (Evidence C)
The HZ vaccine is recommended for all COPD patients (Evidence C).
Pulmonary rehabilitation
Pulmonary rehabilitation is a multidisciplinary, individualized, and comprehensive intervention for patients with chronic respiratory diseases who are symptomatic and often have decreased daily life activities.[216,217]
Pulmonary rehabilitation is an essential component of the comprehensive management strategy for patients with chronic lung diseases.[218] By addressing the functional and psychological challenges faced by these individuals, pulmonary rehabilitation aims to improve their overall well-being and QoL. The key objectives of pulmonary rehabilitation are to reduce symptoms, optimize functional status, increase participation in daily activities, and ultimately lower health-care costs by stabilizing or reversing the systemic effects of the chronic lung condition. Pulmonary rehabilitation, delivered as a supervised multidisciplinary program including exercise training, is one of the cornerstones in COPD management.[219,220]
Patients participating in pulmonary rehabilitation programs lasting 6–8 weeks experience significant improvements in symptoms, functional status, and QoL. Available evidence indicates that there are no additional benefits gained by extending the duration of these programs beyond 12 weeks.[221]
Pulmonary rehabilitation has demonstrated improvements in health-related QoL (HRQoL) and exercise capacity that are maintained for at least 12 months. However, there are no significant long-term effects on mortality.[222] Early pulmonary rehabilitation in patients hospitalized with a COPD exacerbation reduces the mortality, number of days in hospital, and number of readmissions.[223,224]
Patients with a high symptom burden and risk of COPD exacerbations (Class II and III Group B and E) should be referred to a well-structured pulmonary rehabilitation program. This evidence-based intervention should be tailored to the individual’s specific COPD characteristics and any coexisting medical conditions. The rehabilitation program should incorporate patient-centered goal setting to ensure that it addresses each patient’s unique needs and priorities.
Pulmonary rehabilitation remains significantly underresourced and underutilized across health-care systems. Despite its substantial clinical benefits, <5% of individuals with COPD who could potentially benefit from this intervention receive it.[225,226] In Saudi Arabia, the availability of pulmonary rehabilitation programs is limited in most hospitals. This lack of access to evidence-based, multidisciplinary therapy represents a significant gap and challenges in the comprehensive management of chronic respiratory diseases, which must be addressed to optimize patient outcomes.
Recommendations
Pulmonary rehabilitation is recommended for COPD patients with an FEV1 of at least 50% of the predicted value and those with moderate disease who have an FEV1 between 50% and 80% of the predicted value (Evidence B)
A typical pulmonary rehabilitation protocol is recommended to be at least three supervised visits per week over 8–12 weeks for approximately 20 visits (Evidence B)
The COPD Task Force recommends early supervised pulmonary rehabilitation for patients hospitalized with COPD exacerbations (Evidence A)
Pulmonary rehabilitation should be initiated during the hospital stay or within 4 weeks of discharge (Evidence A).
Lung volume reduction
Lung volume reduction surgery (LVRS) and bronchoscopic lung volume reduction (BLVR) with endobronchial valves can improve outcomes in appropriately selected COPD patients with emphysema phenotype. LVRS is reserved for highly selected patients with upper lobe predominant heterogeneous emphysema who do not improve significantly with a pulmonary rehabilitation program. It is contraindicated in very severely ill patients with either FEV1 or diffusion lung capacity (DLCO) <20% of the predicted value.[227] Bronchoscopic techniques were introduced aiming at lung volume reduction by causing distal lung collapse via unidirectional valves or endobronchial glue.[228] Both techniques for lung volume reduction resulted in a clinically meaningful reduction in hyperinflation and similar improvements in patients with intact fissures.[229]
Recommendations
LVRS is reserved for a highly selected small group of patients with upper lobe predominant heterogeneous emphysema who do not improve significantly from a pulmonary rehabilitation program (Evidence B)
BLVR is an alternative procedure in patients without interlobar collateral ventilation (Evidence C).
Lung transplant
Lung transplantation is an option to increase the QoL and survival for highly selected patients with end-stage COPD, without obvious contraindications, when all possible treatments are optimized including optimal medication, O2 therapy, NIV, controlling medical comorbidities, and pulmonary rehabilitation.[217]
COPD patients with clinical deterioration despite maximal treatment should be referred to a lung transplant center when the BODE score (BMI, obstruction, dyspnea, and exercise capacity) is 5 or higher, especially in the presence of frequent exacerbations, increase in BODE score >1 in the past 2 years, elevated pulmonary artery pressure, and an FEV1 <25% predicted.[230]
The outcome of lung transplant for COPD is certainly acceptable and provides a better HRQoL and longer survival in most patients with a 5-year survival after transplantation of 70.4%.[231]
Recommendations
Lung transplant should be considered for those with a high risk of death (>50%) due to COPD within 2 years if lung transplant is not performed and who have an FEV1 <25% of the predicted value, hypercapnia, associated PH, progressive deterioration, and frequent exacerbations (Evidence C).
Treatment for stable chronic obstructive pulmonary disease
The current management strategy for COPD primarily focuses on four key objectives:
Controlling symptoms
Preventing exacerbations
Improving patients’ QoL
Reducing mortality.
In addition, health-care providers are recommended to focus on prevention, diagnosis, and appropriate treatment of any comorbidities associated with COPD, as these comorbidities are associated with higher risks of hospitalization and mortality.
Assessment of COPD patient includes using symptom scores (CAT or mMRC) to evaluate the severity of the patient’s symptoms and inquiring about the history of exacerbations or hospitalizations in the past year, as shown in Table 4 and Figure 4. The treatment of COPD is stratified based on the COPD classification, as shown in Figure 5.
Figure 4.

Classification based on diagnosis, assessment, and patient stratification
Figure 5.

Algorithm for pharmacological treatment of stable chronic obstructive pulmonary disease. COPD = Chronic obstructive pulmonary disease, GOLD = Global Initiative for Chronic Obstructive Lung Disease, CAT = COPD Assessment Test, SABA = Short-acting β2-agonist, SAMA = Short-acting muscarinic antagonist, LABA = Long-acting beta agonist, LAMA = Long-acting muscarinic antagonist, mMRC = Modified Medical Research Council
Class I (Group A)
Patients in this class are less symptomatic (CAT <10) and are at low risk of acute exacerbations or hospitalizations.
Recommendations
First choice for COPD Class I: A short-acting bronchodilator such as SAMA or SABA on an as-needed basis (Evidence A)
Second choice for COPD Class I: Combination of the two short-acting bronchodilators on as-needed basis (Evidence A)
A long-acting bronchodilator is the preferred choice except in patients with very occasional breathlessness since LABA is not available in the Saudi market, we recommend LAMA (Evidence A)
Short-acting bronchodilators on an as-needed basis are recommended with LAMA (Evidence B).
Class II (Group B)
Patients in this group are symptomatic with minimal exacerbations over the last year. In such individuals, symptom control is more appropriate and hence they would need more potent long-acting bronchodilators. It has been shown that LABA/LAMA is superior to LAMA in controlling the patient’s symptoms.[232]
Recommendations
Dual therapy with LAMA/LABA for Class II (Group B) (Evidence A)
Other options include LAMA alone in COPD with no exacerbation. LABA alone is not available in Saudi Arabia and hence the task force cannot recommend it (Evidence A)
Short-acting bronchodilators on an as-needed basis are recommended (Evidence B).
Class III (Group E)
In this group, patients have a high risk for recurrent exacerbation with 2 or more moderate exacerbations over the past year or one hospitalization due to AECOPD regardless of their symptom burden. The treatment strategy here is focused primarily on reducing exacerbation risk.
Recommendations
Triple therapy with LAMA/LABA/ICS is recommended in COPD patients with eosinophil counts ≥300 cells/μL
Dual therapy with LABA/LAMA is recommended in COPD patients with eosinophil counts <300 cells/μL (Evidence A)
Phosphodiesterase-4 inhibitor (roflumilast) is recommended as an add-on therapy for patients who still have exacerbations despite triple therapy or eosinophil count <300 cells/μL and have chronic bronchitis phenotype (Evidence A)
Maintenance azithromycin (250 mg daily or 500 mg three times per week) may be considered an add-on therapy for patients who still have exacerbations despite maximum therapy and low risk of side effects (Evidence A)
Dupilumab may have potential benefits in a subset of COPD patients, but there is currently insufficient evidence to routinely recommend its use (Evidence C).
Management of Acute Exacerbations of Chronic Obstructive Pulmonary Disease
The goals of treatment for AECOPD are to minimize the negative impact of the current exacerbation and prevent the development of future exacerbation. Management of the patient as an outpatient or need for hospitalization depends on the severity of the exacerbation and the severity of the underlying COPD and coexisting comorbidities.[233]
The main causes of hospital admission secondary to AECOPD are ARF with respiratory acidosis and hypercapnia, severe hypoxia, confusion or drowsiness, failure to respond to initial therapy, or uncontrolled coexisting comorbidities such as pneumonia, sepsis or heart failure.
Systemic steroids
Intravenous methylprednisolone or oral prednisolone should be routinely used for moderately severe or severe AECOPD. Studies have confirmed their efficacy in acute exacerbations, with a more rapid improvement of FEV1 and shorter hospital stays. Oral corticosteroids are equally efficacious as intravenous corticosteroids for the treatment of most exacerbations of COPD.[234] The dose of systemic corticosteroids for treating a COPD exacerbation ranges from prednisone 30–60 mg once daily to methylprednisolone 60–125 mg 2–4 times daily.[235,236,237,238]
The COPD Task Force reviewed the evidence to determine the efficacy of shorter duration (i.e., ≤7 days) of corticosteroid treatment. Short duration of corticosteroid treatment has shown a noninferiority compared to longer duration (>7 days) in moderate-to-severe AECOPD and may lead to considerably better outcomes in milder exacerbations.[239,240,241,242] Long-term corticosteroid use may be associated with serious side effects, such as weight gain, hyperglycemia, increased risk of infection, adrenal insufficiency, osteoporosis, cataract, and/or aseptic joint necrosis.[243]
Recommendations
Systemic steroids should be routinely used for moderately severe or severe AECOPD (Evidence A)
The COPD Task Force suggests using shorter durations (5–7 days) of corticosteroid over a longer treatment (>7 days) (Evidence B)
Antimicrobial therapies
Approximately one-third of the exacerbations are associated with a viral infection. Bacterial colonization is reported in 20%–30% of cases during remissions and increases to 30%–50% during exacerbations. The COPD Task Force reviewed the evidence for antimicrobial intervention in AECOPD. The use of antibiotics for AECOPD is associated with a statistically significant[244,245] reduction in all-cause mortality, symptoms (dyspnea and cough), time to clinical cure, and improvement in infection cure rate.[246,247,248,249,250,251,252,253]
The usual organisms are Haemophilus influenzae, Streptococcus pneumoniae, and Branhamella catarrhalis. Pseudomonas aeruginosa is occasionally present in severe or long-standing COPD. The following antibiotics are useful in COPD exacerbation: second-generation cephalosporins, amoxicillin–clavulanate, macrolide, quinolones such as moxifloxacin or levofloxacin, and doxycycline.[244,245,254,255,256]
Recommendations
In patients with AECOPD, the COPD Task Force suggests using antimicrobial interventions (Evidence B).
Noninvasive ventilation
The COPD Task Force reviewed the evidence for utilization of NIV in patients with AECOPD. NIV is associated with a significant reduction in mortality, the need for endotracheal intubation, and hospital length of stay.[192,193,194,195,257,258,259]
Recommendations
In patients with AECOPD and hypercapnic respiratory failure, the COPD Task Force suggests using NIV in the ward, high-dependency unit, or intensive therapy unit (Evidence B).
Discharge postacute exacerbations of chronic obstructive pulmonary disease
Four RCTs, one systematic review, and one meta-analysis were identified that compared the effects of using specific criteria to assess the suitability of patients for early discharge and planning for home treatment versus not using such criteria in patients with acute COPD exacerbations.[260,261,262,263,264,265]
The COPD Task Force reviewed the evidence using certain criteria to assess the suitability of and planning for home treatment or early discharge after AECOPD. The evidence indicates that using such criteria can impact mortality (RR, 0.76; 95% CI, 0.39–1.49) and reduce readmissions, including patients returning to the hospital within 2–6 months (RR, 0.72; 95% CI, 0.56–0.94).[260,262,263,264,265]
After stabilization of a patient post-AECOPD, the treating team should ensure the following:
The patient is in a stable condition to be discharged home
All comorbidities are adequately managed
All laboratory and/or metabolic abnormalities corrected
Assess the patient’s need for long-term oxygen
Review with the patient all medication doses and durations and the proper technique for using inhaler devices
Give the patient a follow-up appointment within 2–4 weeks
Consider referring the patient to pulmonary rehabilitation
Assess the patient’s need for home NIV.
Recommendations
The COPD Task Force suggests using certain criteria to assess the suitability of and planning of home treatment or early discharge after AECOPD (Evidence C).
Systemic Effects of Chronic Obstructive Pulmonary Disease and Comorbidities
COPD is associated with low-grade, systemic inflammation with neutrophil elastase believed to be a key mediator of this process. This is evidenced by increased levels of various systemic inflammatory markers, such as leukocytosis, increased fibrinogen, and elevated CRP. Higher levels of inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) are also recognized in COPD patients.[266] The intensity of this systemic inflammation tends to increase during COPD exacerbations.[267]
Emerging evidence suggests that proinflammatory cytokines, particularly TNF-α, may be a driving force behind the pathogenesis of COPD.[268] However, the roles of inflammation and these proinflammatory cytokines may extend beyond the lungs, potentially contributing to the systemic effects of the disease and its associated comorbidities. This chronic, low-grade inflammation has also been implicated in contributing to several complications and comorbidities related to COPD, including weight loss, skeletal muscle dysfunction and wasting, cardiovascular disease, and depression.[267,269,270]
Sleep and chronic obstructive pulmonary disease
The interaction between sleep disorders and COPD is multifactorial and has a complex pathogenesis.[271,272] Epidemiological studies have estimated that the prevalence of various sleep disorders, including insomnia, restless leg syndrome, and sleep-disordered breathing, ranges from 34% to 78% among patients diagnosed with COPD.[273,274] The wide range of reported prevalence rates may reflect differences in study methodologies, diagnostic criteria, and patient populations examined. Nonetheless, these findings underscore the high comorbidity of sleep disorders in the context of COPD, which has important implications for the comprehensive management of this patient group. Furthermore, the presence of sleep disorders in COPD patients may have a more deleterious impact on their health outcomes.
Physicians should be aware of the comorbid relationship between those two conditions and develop targeted interventions to optimize the management of patients afflicted by both sleep disorders and COPD.[275]
Overlap syndrome
OVS is characterized by the coexistence of obstructive sleep apnea (OSA) and COPD. In the general population, the prevalence of OVS falls within the range of 1%–3.6%. In patients with OSA, the prevalence of OVS falls between 7% and 55%.[276]
Patients with OVS experience more significant oxygen desaturation and are more prone to developing hypercapnia and PH, compared to COPD patients without concurrent OSA, despite similar degrees of airflow obstruction.[268,269] Furthermore, OVS is associated with an increased risk of COPD exacerbations, hospitalizations, and mortality.[277] Importantly, the treatment with continuous positive airway pressure (CPAP) has been shown to improve survival and decrease hospitalizations in patients with OVS and COPD.[278]
Clinicians should have a high level of suspicion for the coexistence of OSA (OVS) or obesity hypoventilation syndrome in COPD patients who exhibit the following characteristics:
Significant hypoxemia or hypercapnia disproportionate to the degree of airflow limitation
Presence of PH
Complaints of daytime sleepiness
Symptoms of sleep-disordered breathing, such as snoring, in the presence of a high BMI, need referral for a sleep study to evaluate for the presence of OSA syndrome (OVS).
Recommendations
A comprehensive and multidisciplinary approach to the screening, diagnosis, and management of the OVS syndrome is recommended to optimize clinical outcomes for this high-risk patient group (Evidence B).
Lung cancer
Lung cancer is a major cause of death in COPD patients; COPD patients have a 3–4-fold increased risk of developing lung cancer when compared to smokers without COPD. The presence of COPD, especially severe COPD, is an independent risk factor for lung cancer.[279,280,281] Smoking cessation is the most effective measure to reduce lung cancer mortality.[176] Several studies have shown that lung cancer screening with low-dose CT scans can detect early-stage lung cancers in COPD patients and reduce lung cancer mortality in this high-risk group.[282,283,284] The GOLD guidelines recommend annual lung cancer screening with low-dose CT for COPD patients aged 55–80 years who have a ≥30 pack-years smoking history.[285] The US Preventive Services Task Force also recommends annual lung cancer screening with low-dose CT for adults aged 50–80 years who have a ≥20 pack-year smoking history and currently smoke or have quit within the past 15 years.[286] Many national and international guidelines specifically call out COPD as a high-risk condition that warrants lung cancer screening, often with eligibility criteria that are less stringent compared to the general population.[287,288,289,290,291]
Recommendations
Annual lung cancer screening with low-dose CT for COPD for patients aged 55–80 years who have a ≥ 30 pack-year smoking history (Evidence B).
Cardiovascular diseases
COPD and heart failure share certain common risk factors, including cigarette smoking, advanced age, and systemic inflammation.[292]
The prevalence of COPD among individuals with heart failure ranges from 20 to 32%, and approximately 10% of hospitalized heart failure patients have concurrent COPD. Furthermore, heart failure is prevalent in more than 20% of patients with COPD and the risk of developing heart failure is 4.5 times higher in COPD patients compared to individuals without COPD. Finally, the prevalence of heart failure is three times greater among patients discharged from the hospital with COPD.[292,293,294,295,296,297]
The coexistence of heart failure and COPD in the same patient can present a significant diagnostic challenge, as the clinical symptoms and signs of these two common conditions often overlap. The use of biomarkers, like B-type natriuretic peptide (BNP), can help differentiate COPD exacerbations from heart failure episodes.[295,298]
Clinicians are encouraged to employ a combination of clinical assessment, spirometry, and echocardiography findings to accurately distinguish between these two conditions in patients suspected of having both.
Importantly, the use of beta-blockers for the treatment of heart failure appears to be safe and well tolerated in patients with coexisting COPD. Studies have suggested that beta-blocker therapy in this patient population may decrease the risk of COPD exacerbations, hospitalizations, and all-cause mortality.[299,300]
COPD patients have a significantly increased risk of ischemic heart disease given the common risk factors both conditions share. The mortality from myocardial infarction is more common in patients with frequent COPD exacerbations and worse lung function.[296,301,302,303]
PH in COPD is primarily driven by chronic hypoxia, leading to vasoconstriction, vascular remodeling, and increased pulmonary artery pressure. The incidence of PH in COPD patients varies widely, ranging from 6% to 60% of individuals, depending on the severity of their COPD and the diagnostic criteria used. However, most cases involve mild-to-moderate PH, with only 1%–3.7% experiencing severe PH.[304,305,306,307] COPD patients with hypoxia that is disproportionate to the degree of their airway obstruction should be screened for PH using transthoracic echocardiogram. Treatment for PH primarily involves managing COPD, including the use of oxygen supplementation.
COPD patients with severe PH should be referred to expert centers for further assessment, as routine use of PH target therapy in this group of patients is discouraged because of lack of efficacy and potential side effects.[308,309]
Finally, COPD patients have a higher risk of arrhythmias, particularly atrial fibrillation which is associated with lower FEV1.[310] Arrhythmias do not alter the treatment of COPD, though caution is advised when high-dose B2 agonist is used, especially during AECOPD.[311]
Recommendations
Clinicians are encouraged to use a combination of clinical assessment, spirometry, and echocardiography findings to accurately distinguish between COPD and heart failure, as the symptoms and signs of these two conditions often overlap (Evidence C)
The use of biomarkers like BNP can help differentiate COPD exacerbations from heart failure episodes (Evidence B)
The use of beta-blockers for the treatment of heart failure appears to be safe and well tolerated in patients with coexisting COPD (Evidence C)
COPD patients with hypoxia out of proportion to the degree of their airway obstruction should be screened for PH (Evidence B).
Nutrition
Malnutrition is a significant problem in COPD patients. The estimated prevalence of malnutrition and underweight is around 25%–40%, and 35% have a severely low fat-free mass index.[312] The studies revealed that patients who are underweight or lose weight during hospitalization due to COPD exacerbation are at high risk for future COPD exacerbations.[313,314] Studies also found a strong association between body weight, COPD events, and risk of death.[315,316] Malnourished COPD patients who received nutritional supplementation had significantly better respiratory muscle strength, but there was no significant improvement in the HRQoL or dyspnea.[312,317] The long-term benefit of supplementary nutrition needs further study, addressing underweight and weight loss in this patient population, and is yet to be determined.[317,318,319]
Recommendations
Regular nutritional screening and supplementation for COPD patients are recommended, as malnutrition is prevalent and can increase the risk of exacerbations (Evidence C).
Vitamin D and osteoporosis
Vitamin D insufficiency and osteoporosis appear to be common problems affecting populations globally, even in sunny regions like Saudi Arabia.[320,321,322,323] Studies have linked Vitamin D deficiency to increased respiratory symptoms, decreased functional status, increased frequency of severe exacerbations, airway wall thickening on chest CT scans, and declines in lung function in COPD patients.[324,325] Vitamin D deficiency is also closely associated with the high prevalence of osteoporosis, which is particularly common in older COPD patients and those on chronic steroid therapy.[326] The factors leading to osteoporosis in COPD patients include systemic inflammation, corticosteroid use, Vitamin D deficiency, physical inactivity, tobacco exposure, lower bone mineral density, hypogonadism, hypoxia, sedentary lifestyle, and weight loss.[327,328,329]
Osteoporosis is considered one of the major systemic comorbidities of COPD. While the causal relationship and molecular link between COPD and osteoporosis are not fully established, recent epidemiological data found that osteoporosis is highly prevalent in COPD patients.[327,330] There is no clear evidence that Vitamin D supplementation has a beneficial impact on exacerbations in unselected patient populations.[331] However, studies about Vitamin D supplementation showed conflicting results in reducing COPD exacerbation rates in patients with low baseline Vitamin D levels.[332,333]
Recent endocrine society recommends against routine 25(OH)D testing for the general population aged 50–74 years.[334] Given the higher prevalence of osteoporosis in the Saudi population compared to Western countries and the increased risk of osteoporosis and osteopenia in COPD patients, Vitamin D level measurements and screening for osteoporosis should be considered in high-risk patients.
Recommendations
For COPD patients with risk factors for osteoporosis, we recommend measuring 25-hydroxyvitamin D levels and performing dual-energy X-ray absorptiometry scans (Evidence C)
Vitamin D supplementation is not routinely recommended for patient with COPD; however, Vitamin D deficiency should be identified and treated (Evidence C).
Depression and anxiety
Depression and anxiety are common comorbidities experienced by COPD patients. Studies estimate that up to 40% of COPD patients also suffer from clinical depression,[335,336] and a similar proportion experience clinically significant anxiety.[337,338] The burden of COPD, including breathlessness, activity limitations, and fear of exacerbations, can contribute to the development of mood disorders in this population.[339,340,341] Furthermore, depression and anxiety in COPD patients are associated with poorer treatment adherence, more frequent hospitalizations, and decreased QoL.[339,342]
Effectively screening for and managing these mental health conditions is an important part of comprehensive COPD care. Strategies may include counseling, support groups, and appropriate pharmacological interventions.[343] Addressing the psychological impacts of COPD can help improve overall health outcomes for this vulnerable patient population.
Recommendation
COPD Task Force recommends regular screening and management of depression and anxiety should be an integral part of comprehensive COPD care (Evidence B).
Surgery in chronic obstructive pulmonary disease
Surgery and general anesthesia are generally not contraindicated in patients with COPD. However, the risk of postoperative complications is higher in COPD patients compared to healthy individuals, and the specific surgical site plays a significant role during the postoperative period. Upper abdominal and thoracic surgeries have more postoperative complications compared to lower abdominal surgeries.[344,345,346] Other important risk factors for postoperative complications include active smoking, the presence of bronchospasm, excessive secretions, and severity and class of COPD.
Patients with COPD undergoing surgery must be carefully assessed for symptoms of ongoing infection, bronchospasm, smoking, and the site of surgery. Comorbidity risks that need to be assessed include hypoxia, hypercapnia, risk for thromboembolism, PH, and associated cardiac comorbidities, such as coronary artery disease and heart failure.
Postlung resection pulmonary complications are unpredictable.[347] The individual patient’s risk factors should be identified for lung resection procedures through a comprehensive clinical history, physical examination, chest imaging, and pulmonary function tests.[348,349]
COPD patients at high risk for surgical complications due to poor lung function should undergo further assessment, such as diffusion capacity (DLco), regional perfusion distribution using ventilation-perfusion scans, and evaluation of exercise capacity.[348,349]
Before any elective surgery, COPD patients should be advised to stop smoking for at least 6–8 weeks and receive maximal therapy for their COPD, including pulmonary rehabilitation.[350] Furthermore, early mobilization, chest physiotherapy, and incentive spirometry may help reduce postoperative complications in this patient population.[351]
Recommendations
-
The COPD Task Force recommends the following measures for COPD patients undergoing elective surgery
Comprehensive evaluation before surgery including clinical history, physical examination, chest radiography, and pulmonary function tests (Evidence C)
COPD patients should be advised to abstain from smoking for at least 6–8 weeks and receive optimal treatment for their COPD, including pulmonary rehabilitation (Evidence C)
Quantitative ventilation–perfusion lung scan to assess predicted postoperative lung function should be considered in COPD patients going for lung resection (Evidence C)
Early mobilization, chest physiotherapy, and incentive spirometry may help reduce the risk of postoperative complications in this patient population (Evidence C).
Air travel
Compared to healthy individuals, patients with moderate-to-severe COPD patients are at risk of developing potentially serious oxygen desaturation during long commercial flights.[352] However, air travel is generally safe for most patients with chronic respiratory failure who are provided with oxygen supplementation during travel.[353] It is recommended to maintain a PaO2 above 50 mmHg (6.7 kPa) in COPD patients during flight.[354]
Studies indicate that this can be achieved in those with moderate-to-severe hypoxemia at sea level by supplementary oxygen at 3 liters by nasal mask or 32% venturi mask.[354] Patients with oxygen saturation of more than 95% or oxygen saturation during a 6-min walking test of more than 84% do not need oxygen supplements during travel.[354,355,356] Patients with COPD are recommended to ask their doctors to fill out the oxygen supplement request form provided by airlines. Attention also should be paid to coexisting conditions that could preclude air travel like unstable angina, severe anemia, uncontrolled heart failure, large emphysematous bullae, or pneumothorax.
Recommendations
COPD management should be optimized before air travel (Evidence C)
Oxygen supplements should be considered in COPD patients with oxygen saturation <95% or <84% during a 6-min walking test (Evidence C).
Conflicts of interest
There are no conflicts of interest.
Funding Statement
Nil.
References
- 1.Al Wachami N, Guennouni M, Iderdar Y, Boumendil K, Arraji M, Mourajid Y, et al. Estimating the global prevalence of chronic obstructive pulmonary disease (COPD): A systematic review and meta-analysis. BMC Public Health. 2024;24:297. doi: 10.1186/s12889-024-17686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo B, Gan H, Xue M, Huang Z, Lin Z, Li S, et al. The changing and predicted trends in chronic obstructive pulmonary disease burden in China, the United States, and India from 1990 to 2030. Int J Chron Obstruct Pulmon Dis. 2024;19:695–706. doi: 10.2147/COPD.S448770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iheanacho I, Zhang S, King D, Rizzo M, Ismaila AS. Economic Burden of chronic obstructive pulmonary disease (COPD): A systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:439–60. doi: 10.2147/COPD.S234942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Ghobain M, Alhamad EH, Alorainy HS, Al Kassimi F, Lababidi H, Al-Hajjaj MS. The prevalence of chronic obstructive pulmonary disease in Riyadh, Saudi Arabia: A BOLD study. Int J Tuberc Lung Dis. 2015;19:1252–7. doi: 10.5588/ijtld.14.0939. [DOI] [PubMed] [Google Scholar]
- 5.Al Ghobain M, Al-Hajjaj MS, Wali SO. Prevalence of chronic obstructive pulmonary disease among smokers attending primary healthcare clinics in Saudi Arabia. Ann Saudi Med. 2011;31:129–33. doi: 10.4103/0256-4947.77485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldhahir AM, Alghamdi SM, Alqahtani JS, Alqahtani KA, Al Rajah AM, Alkhathlan BS, et al. Pulmonary rehabilitation for COPD: A narrative review and call for further implementation in Saudi Arabia. Ann Thorac Med. 2021;16:299–305. doi: 10.4103/atm.atm_639_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alsubaiei ME, Cafarella PA, Frith PA, McEvoy RD, Effing TW. Factors influencing management of chronic respiratory diseases in general and chronic obstructive pulmonary disease in particular in Saudi Arabia: An overview. Ann Thorac Med. 2018;13:144–9. doi: 10.4103/atm.ATM_293_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alsubaiei ME, Cafarella PA, Frith PA, McEvoy RD, Effing TW. Current care services provided for patients with COPD in the Eastern Province in Saudi Arabia: A descriptive study. Int J Chron Obstruct Pulmon Dis. 2015;10:2379–91. doi: 10.2147/COPD.S89456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan JH, Lababidi HM, Al-Moamary MS, Zeitouni MO, Al-Jahdali HH, Al-Amoudi OS, et al. The Saudi guidelines for the diagnosis and management of COPD. Ann Thorac Med. 2014;9:55–76. doi: 10.4103/1817-1737.128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schünemann HJ, Wiercioch W, Brozek J, Etxeandia-Ikobaltzeta I, Mustafa RA, Manja V, et al. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81:101–10. doi: 10.1016/j.jclinepi.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Masjedi M, Ainy E, Zayeri F, Paydar R. Assessing the prevalence and incidence of asthma and chronic obstructive pulmonary disease in the Eastern Mediterranean Region. Turk Thorac J. 2018;19:56–60. doi: 10.5152/TurkThoracJ.2018.17051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tageldin MA, Nafti S, Khan JA, Nejjari C, Beji M, Mahboub B, et al. Distribution of COPD-related symptoms in the Middle East and North Africa: Results of the BREATHE study. Respir Med. 2012;106(Suppl 2):S25–32. doi: 10.1016/S0954-6111(12)70012-4. [DOI] [PubMed] [Google Scholar]
- 13.Feizi H, Alizadeh M, Nejadghaderi SA, Noori M, Sullman MJ, Ahmadian Heris J, et al. The burden of chronic obstructive pulmonary disease and its attributable risk factors in the Middle East and North Africa Region, 1990-2019. Respir Res. 2022;23:319. doi: 10.1186/s12931-022-02242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wali SO, Idrees MM, Alamoudi OS, Aboulfarag AM, Salem AD, Aljohaney AA, et al. Prevalence of chronic obstructive pulmonary disease in Saudi Arabia. Saudi Med J. 2014;35:684–90. [PubMed] [Google Scholar]
- 15.Alaithan AM, Memon JI, Rehmani RS, Qureshi AA, Salam A. Chronic obstructive pulmonary disease: Hospital and intensive care unit outcomes in the Kingdom of Saudi Arabia. Int J Chron Obstruct Pulmon Dis. 2012;7:819–23. doi: 10.2147/COPD.S37611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Idrees M, Koniski ML, Taright S, Shahrour N, Polatli M, Ben Kheder A, et al. Management of chronic obstructive pulmonary disease in the Middle East and North Africa: Results of the BREATHE study. Respir Med. 2012;106(Suppl 2):S33–44. doi: 10.1016/S0954-6111(12)70013-6. [DOI] [PubMed] [Google Scholar]
- 17.Kokturk N, Abuharbid W, Albanna AS, Gunen H, Gurgun A, Khadadah M, et al. A cross-sectional study in patients with severe COPD to assess the perception of symptom variability (COPVAR) in the Middle East and Africa. Int J Chron Obstruct Pulmon Dis. 2019;14:2959–70. doi: 10.2147/COPD.S215859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al Ammari M, Sultana K, Yunus F, Al Ghobain M, Al Halwan SM. A cross-sectional observational study to assess inhaler technique in Saudi hospitalized patients with asthma and chronic obstructive pulmonary disease. Saudi Med J. 2016;37:570–4. doi: 10.15537/smj.2016.5.14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokturk N, Polatli M, Oguzulgen IK, Saleemi S, Al Ghobain M, Khan J, et al. Adherence to COPD treatment in Turkey and Saudi Arabia: Results of the ADCARE study. Int J Chron Obstruct Pulmon Dis. 2018;13:1377–88. doi: 10.2147/COPD.S150411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsubaiei ME, Frith PA, Cafarella PA, Quinn S, Al Moamary MS, McEvoy RD, et al. COPD care in Saudi Arabia: Physicians’ awareness and knowledge of guidelines and barriers to implementation. Int J Tuberc Lung Dis. 2017;21:592–5. doi: 10.5588/ijtld.16.0656. [DOI] [PubMed] [Google Scholar]
- 21.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Bethesda: Global Initiative for Chronic Obstructive Lung Disease; 2024. Global Strategy for Prevention, Diagnosis and Management of COPD: 2024 Report. Available from: https://goldcopd.org/2024-gold-report . [Last accessed on 2024 May12] [Google Scholar]
- 22.Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, et al. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 23.Eisner MD, Balmes J, Katz PP, Trupin L, Yelin EH, Blanc PD. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health. 2005;4:7. doi: 10.1186/1476-069X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orozco-Levi M, Garcia-Aymerich J, Villar J, Ramírez-Sarmiento A, Antó JM, Gea J. Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:542–6. doi: 10.1183/09031936.06.00052705. [DOI] [PubMed] [Google Scholar]
- 25.Holtjer JC, Bloemsma LD, Beijers RJ, Cornelissen ME, Hilvering B, Houweling L, et al. Identifying risk factors for COPD and adult-onset asthma: An umbrella review. Eur Respir Rev. 2023;32:230009. doi: 10.1183/16000617.0009-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang IA, Jenkins CR, Salvi SS. Chronic obstructive pulmonary disease in never-smokers: Risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir Med. 2022;10:497–511. doi: 10.1016/S2213-2600(21)00506-3. [DOI] [PubMed] [Google Scholar]
- 27.Prescott E, Lange P, Vestbo J. Socioeconomic status, lung function and admission to hospital for COPD: Results from the Copenhagen City Heart Study. Eur Respir J. 1999;13:1109–14. doi: 10.1034/j.1399-3003.1999.13e28.x. [DOI] [PubMed] [Google Scholar]
- 28.Silverman EK. Genetics of COPD. Annu Rev Physiol. 2020;82:413–31. doi: 10.1146/annurev-physiol-021317-121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riley CM, Sciurba FC. Diagnosis and outpatient management of chronic obstructive pulmonary disease: A review. JAMA. 2019;321:786–97. doi: 10.1001/jama.2019.0131. [DOI] [PubMed] [Google Scholar]
- 30.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: A clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–91. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 31.Al-Jahdali H, Alshimemeri A, Mobeireek A, Albanna AS, Al Shirawi NN, Wali S, et al. The Saudi Thoracic Society guidelines for diagnosis and management of noncystic fibrosis bronchiectasis. Ann Thorac Med. 2017;12:135–61. doi: 10.4103/atm.ATM_171_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:871–88. doi: 10.2147/COPD.S49621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blakemore A, Dickens C, Chew-Graham CA, Afzal CW, Tomenson B, Coventry PA, et al. Depression predicts emergency care use in people with chronic obstructive pulmonary disease: A large cohort study in primary care. Int J Chron Obstruct Pulmon Dis. 2019;14:1343–53. doi: 10.2147/COPD.S179109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60:2101499. doi: 10.1183/13993003.01499-2021. [DOI] [PubMed] [Google Scholar]
- 35.Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023;61:2300239. doi: 10.1183/13993003.00239-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–82. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 37.US Preventive Services Task Force. Mangione CM, Barry MJ, Nicholson WK, Cabana M, Caughey AB, et al. Screening for chronic obstructive pulmonary disease: US preventive services task force reaffirmation recommendation statement. JAMA. 2022;327:1806–11. doi: 10.1001/jama.2022.5692. [DOI] [PubMed] [Google Scholar]
- 38.Petrie K, Abramson MJ, George J. Case-finding and treatment effects in COPD: Secondary analysis of an interdisciplinary intervention trial. Int J Chron Obstruct Pulmon Dis. 2024;19:451–8. doi: 10.2147/COPD.S436690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan A, Thomas M. Screening for COPD: The gap between logic and evidence. Eur Respir Rev. 2017;26:160113. doi: 10.1183/16000617.0113-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaezi A, Mirsaeidi M. Proposing the potential of utilizing the CAT score for early detection of COPD in asymptomatic patients, shifting towards a patient-centered approach: A review. Medicine (Baltimore) 2024;103:e37715. doi: 10.1097/MD.0000000000037715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yawn BP, Mapel DW, Mannino DM, Martinez FJ, Donohue JF, Hanania NA, et al. Development of the Lung Function Questionnaire (LFQ) to identify airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2010;5:1–10. [PMC free article] [PubMed] [Google Scholar]
- 42.Spyratos D, Haidich AB, Chloros D, Michalopoulou D, Sichletidis L. Comparison of three screening questionnaires for chronic obstructive pulmonary disease in the primary care. Respiration. 2017;93:83–9. doi: 10.1159/000453586. [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, Yu N, Li X, Wang W. Accuracy of six chronic obstructive pulmonary disease screening questionnaires in the Chinese population. Int J Chron Obstruct Pulmon Dis. 2022;17:317–27. doi: 10.2147/COPD.S341648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dragonieri S, Galloway S, Quaranta VN, Portacci A, Vulpi MR, Santomasi C, et al. Assessment of five questionnaires for chronic obstructive pulmonary disease in a Southern Italian population: A proof-of-concept study. Medicina (Kaunas) 2023;59:1252. doi: 10.3390/medicina59071252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alyami MM, Jenkins SC, Lababidi H, Hill K. Reliability and validity of an Arabic version of the dyspnea-12 questionnaire for Saudi nationals with chronic obstructive pulmonary disease. Ann Thorac Med. 2015;10:112–7. doi: 10.4103/1817-1737.150730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bastidas AR, Tuta-Quintero E, Arias JS, Cufiño D, Moya D, Martin D, et al. Comparison of the diagnostic performance of five clinical questionnaires for chronic obstructive pulmonary disease. Can Respir J. 2023;2023:2821056. doi: 10.1155/2023/2821056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez Varela MV, Montes de Oca M, Wehrmeister FC, Rodriguez C, Ramirez L, Menezes A. External validation of the PUMA COPD diagnostic questionnaire in a general practice sample and the PLATINO study population. Int J Chron Obstruct Pulmon Dis. 2019;14:1901–11. doi: 10.2147/COPD.S206250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al Wachami N, Boumendil K, Arraji M, Iderdar Y, Mourajid Y, Ghosne N, et al. Evaluating the effectiveness of the COPD Assessment Test (CAT) in screening for chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2024;19:1623–33. doi: 10.2147/COPD.S460649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Moamary MS, Al-Hajjaj MS, Tamim HM, Al-Ghobain MO, Al-Qahtani HA, Al-Kassimi FA. The reliability of an Arabic translation of the chronic obstructive pulmonary disease assessment test. Saudi Med J. 2011;32:1028–33. [PubMed] [Google Scholar]
- 50.Brusselle G, Pavord ID, Landis S, Pascoe S, Lettis S, Morjaria N, et al. Blood eosinophil levels as a biomarker in COPD. Respir Med. 2018;138:21–31. doi: 10.1016/j.rmed.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 51.de Serres FJ, Blanco I, Fernández-Bustillo E. Estimating the risk for alpha-1 antitrypsin deficiency among COPD patients: Evidence supporting targeted screening. COPD. 2006;3:133–9. doi: 10.1080/15412550600829257. [DOI] [PubMed] [Google Scholar]
- 52.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–6. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 53.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–54. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 54.Jones PW, Tabberer M, Chen WH. Creating scenarios of the impact of COPD and their relationship to COPD Assessment Test (CAT™) scores. BMC Pulm Med. 2011;11:42. doi: 10.1186/1471-2466-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parker CM, Voduc N, Aaron SD, Webb KA, O’Donnell DE. Physiological changes during symptom recovery from moderate exacerbations of COPD. Eur Respir J. 2005;26:420–8. doi: 10.1183/09031936.05.00136304. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117:398S–401S. doi: 10.1378/chest.117.5_suppl_2.398s. [DOI] [PubMed] [Google Scholar]
- 57.Wedzicha JA, Ers Co-Chair, Miravitlles M, Hurst JR, Calverley PM, Albert RK, Anzueto A, et al. Management of COPD exacerbations: A European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49:1600791. doi: 10.1183/13993003.00791-2016. [DOI] [PubMed] [Google Scholar]
- 58.MacLeod M, Papi A, Contoli M, Beghé B, Celli BR, Wedzicha JA, et al. Chronic obstructive pulmonary disease exacerbation fundamentals: Diagnosis, treatment, prevention and disease impact. Respirology. 2021;26:532–51. doi: 10.1111/resp.14041. [DOI] [PubMed] [Google Scholar]
- 59.Celli BR, Fabbri LM, Aaron SD, Agusti A, Brook R, Criner GJ, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: The Rome proposal. Am J Respir Crit Care Med. 2021;204:1251–8. doi: 10.1164/rccm.202108-1819PP. [DOI] [PubMed] [Google Scholar]
- 60.Yun JH, Lamb A, Chase R, Singh D, Parker MM, Saferali A, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037–47. doi: 10.1016/j.jaci.2018.04.010. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ko FW, Chan KP, Hui DS, Goddard JR, Shaw JG, Reid DW, et al. Acute exacerbation of COPD. Respirology. 2016;21:1152–65. doi: 10.1111/resp.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qian Y, Cai C, Sun M, Lv D, Zhao Y. Analyses of factors associated with acute exacerbations of chronic obstructive pulmonary disease: A review. Int J Chron Obstruct Pulmon Dis. 2023;18:2707–23. doi: 10.2147/COPD.S433183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han MK, Quibrera PM, Carretta EE, Barr RG, Bleecker ER, Bowler RP, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: An analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5:619–26. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181. doi: 10.1186/1741-7015-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Celli BR, Fabbri LM, Aaron SD, Agusti A, Brook RD, Criner GJ, et al. Differential diagnosis of suspected chronic obstructive pulmonary disease exacerbations in the acute care setting: Best practice. Am J Respir Crit Care Med. 2023;207:1134–44. doi: 10.1164/rccm.202209-1795CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoogendoorn M, Hoogenveen RT, Rutten-van Mölken MP, Vestbo J, Feenstra TL. Case fatality of COPD exacerbations: A meta-analysis and statistical modelling approach. Eur Respir J. 2011;37:508–15. doi: 10.1183/09031936.00043710. [DOI] [PubMed] [Google Scholar]
- 67.Hartl S, Lopez-Campos JL, Pozo-Rodriguez F, Castro-Acosta A, Studnicka M, Kaiser B, et al. Risk of death and readmission of hospital-admitted COPD exacerbations: European COPD audit. Eur Respir J. 2016;47:113–21. doi: 10.1183/13993003.01391-2014. [DOI] [PubMed] [Google Scholar]
- 68.O’Donnell DE, Voduc N, Fitzpatrick M, Webb KA. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur Respir J. 2004;24:86–94. doi: 10.1183/09031936.04.00072703. [DOI] [PubMed] [Google Scholar]
- 69.O’Donnell DE, Flüge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23:832–40. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 70.O’Donnell DE, Sciurba F, Celli B, Mahler DA, Webb KA, Kalberg CJ, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest. 2006;130:647–56. doi: 10.1378/chest.130.3.647. [DOI] [PubMed] [Google Scholar]
- 71.Hay JG, Stone P, Carter J, Church S, Eyre-Brook A, Pearson MG, et al. Bronchodilator reversibility, exercise performance and breathlessness in stable chronic obstructive pulmonary disease. Eur Respir J. 1992;5:659–64. [PubMed] [Google Scholar]
- 72.Higgins BG, Powell RM, Cooper S, Tattersfield AE. Effect of salbutamol and ipratropium bromide on airway calibre and bronchial reactivity in asthma and chronic bronchitis. Eur Respir J. 1991;4:415–20. [PubMed] [Google Scholar]
- 73.Vathenen AS, Britton JR, Ebden P, Cookson JB, Wharrad HJ, Tattersfield AE. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850–5. doi: 10.1164/ajrccm/138.4.850. [DOI] [PubMed] [Google Scholar]
- 74.Sestini P, Renzoni E, Robinson S, Poole P, Ram FS. Short-acting beta 2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002:CD001495. doi: 10.1002/14651858.CD001495. doi: 10.1002/14651858.CD001495. [DOI] [PubMed] [Google Scholar]
- 75.Cazzola M, Rogliani P, Ruggeri P, Segreti A, Proietto A, Picciolo S, et al. Chronic treatment with indacaterol and airway response to salbutamol in stable COPD. Respir Med. 2013;107:848–53. doi: 10.1016/j.rmed.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 76.Kew KM, Mavergames C, Walters JA. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013:CD010177. doi: 10.1002/14651858.CD010177.pub2. doi: 10.1002/14651858.CD010177.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geake JB, Dabscheck EJ, Wood-Baker R, Cates CJ. Indacaterol, a once-daily beta2-agonist, versus twice-daily beta2-agonists or placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;1:CD010139. doi: 10.1002/14651858.CD010139.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han J, Dai L, Zhong N. Indacaterol on dyspnea in chronic obstructive pulmonary disease: A systematic review and meta-analysis of randomized placebo-controlled trials. BMC Pulm Med. 2013;13:26. doi: 10.1186/1471-2466-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness of initial LAMA versus LABA in COPD: Real-world cohort study. COPD. 2021;18:1–8. doi: 10.1080/15412555.2021.1877649. [DOI] [PubMed] [Google Scholar]
- 80.Lipworth BJ, McDevitt DG, Struthers AD. Hypokalemic and ECG sequelae of combined beta-agonist/diuretic therapy. Protection by conventional doses of spironolactone but not triamterene. Chest. 1990;98:811–5. doi: 10.1378/chest.98.4.811. [DOI] [PubMed] [Google Scholar]
- 81.Kempsford R, Norris V, Siederer S. Vilanterol trifenatate, a novel inhaled long-acting beta2 adrenoceptor agonist, is well tolerated in healthy subjects and demonstrates prolonged bronchodilation in subjects with asthma and COPD. Pulm Pharmacol Ther. 2013;26:256–64. doi: 10.1016/j.pupt.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 82.Montastruc JL, Durrieu G, Sommet A, Damase-Michel C, Lapeyre-Mestre M. Anticholinergics, antimuscarinics or atropinics? About the words in pharmacology. Br J Clin Pharmacol. 2010;69:561–2. doi: 10.1111/j.1365-2125.2010.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones PW, Singh D, Bateman ED, Agusti A, Lamarca R, de Miquel G, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: The ATTAIN study. Eur Respir J. 2012;40:830–6. doi: 10.1183/09031936.00225511. [DOI] [PubMed] [Google Scholar]
- 84.Melani AS. Long-acting muscarinic antagonists. Expert Rev Clin Pharmacol. 2015;8:479–501. doi: 10.1586/17512433.2015.1058154. [DOI] [PubMed] [Google Scholar]
- 85.Cheyne L, Irvin-Sellers MJ, White J. Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2015:CD009552. doi: 10.1002/14651858.CD009552.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vincken W, van Noord JA, Greefhorst AP, Bantje TA, Kesten S, Korducki L, et al. Improved health outcomes in patients with COPD during 1 yr’s treatment with tiotropium. Eur Respir J. 2002;19:209–16. doi: 10.1183/09031936.02.00238702. [DOI] [PubMed] [Google Scholar]
- 87.Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP, et al. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:948–55. doi: 10.1164/rccm.200906-0876OC. [DOI] [PubMed] [Google Scholar]
- 88.Suzuki Y, Sato S, Sato K, Inoue S, Shibata Y. Treatment efficacy of LAMA versus placebo for stable chronic obstructive pulmonary disease: A systematic review and meta-analysis. Respir Investig. 2022;60:108–18. doi: 10.1016/j.resinv.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 89.Appleton S, Jones T, Poole P, Pilotto L, Adams R, Lasserson TJ, et al. Ipratropium bromide versus long-acting beta-2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;2006:CD006101. doi: 10.1002/14651858.CD006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Mölken MP, Beeh KM, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093–103. doi: 10.1056/NEJMoa1008378. [DOI] [PubMed] [Google Scholar]
- 91.Decramer ML, Chapman KR, Dahl R, Frith P, Devouassoux G, Fritscher C, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): A randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1:524–33. doi: 10.1016/S2213-2600(13)70158-9. [DOI] [PubMed] [Google Scholar]
- 92.Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 microg)/salmeterol (50 microg) combined in the Diskus inhaler for the treatment of COPD. Chest. 2003;124:834–43. doi: 10.1378/chest.124.3.834. [DOI] [PubMed] [Google Scholar]
- 93.Cazzola M, Molimard M. The scientific rationale for combining long-acting beta2-agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther. 2010;23:257–67. doi: 10.1016/j.pupt.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 94.Calzetta L, Matera MG, Cazzola M. Pharmacological interaction between LABAs and LAMAs in the airways: Optimizing synergy. Eur J Pharmacol. 2015;761:168–73. doi: 10.1016/j.ejphar.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 95.Ray R, Tombs L, Naya I, Compton C, Lipson DA, Boucot I. Efficacy and safety of the dual bronchodilator combination umeclidinium/vilanterol in COPD by age and airflow limitation severity: A pooled post hoc analysis of seven clinical trials. Pulm Pharmacol Ther. 2019;57:101802. doi: 10.1016/j.pupt.2019.101802. [DOI] [PubMed] [Google Scholar]
- 96.Kesten S, Casaburi R, Kukafka D, Cooper CB. Improvement in self-reported exercise participation with the combination of tiotropium and rehabilitative exercise training in COPD patients. Int J Chron Obstruct Pulmon Dis. 2008;3:127–36. doi: 10.2147/copd.s2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandström T, Taylor AF, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): A randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1:199–209. doi: 10.1016/S2213-2600(13)70052-3. [DOI] [PubMed] [Google Scholar]
- 98.Kerwin EM, Kalberg CJ, Galkin DV, Zhu CQ, Church A, Riley JH, et al. Umeclidinium/vilanterol as step-up therapy from tiotropium in patients with moderate COPD: A randomized, parallel-group, 12-week study. Int J Chron Obstruct Pulmon Dis. 2017;12:745–55. doi: 10.2147/COPD.S119032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vogelmeier C, Kardos P, Harari S, Gans SJ, Stenglein S, Thirlwell J. Formoterol mono- and combination therapy with tiotropium in patients with COPD: A 6-month study. Respir Med. 2008;102:1511–20. doi: 10.1016/j.rmed.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 100.Tashkin DP, Pearle J, Iezzoni D, Varghese ST. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD. 2009;6:17–25. doi: 10.1080/15412550902724073. [DOI] [PubMed] [Google Scholar]
- 101.Bateman ED, Ferguson GT, Barnes N, Gallagher N, Green Y, Henley M, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: The SHINE study. Eur Respir J. 2013;42:1484–94. doi: 10.1183/09031936.00200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maleki-Yazdi MR, Kaelin T, Richard N, Zvarich M, Church A. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: Results of a 24-week, randomized, controlled trial. Respir Med. 2014;108:1752–60. doi: 10.1016/j.rmed.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 103.Decramer M, Anzueto A, Kerwin E, Kaelin T, Richard N, Crater G, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: Results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med. 2014;2:472–86. doi: 10.1016/S2213-2600(14)70065-7. [DOI] [PubMed] [Google Scholar]
- 104.Gross N, Tashkin D, Miller R, Oren J, Coleman W, Linberg S. Inhalation by nebulization of albuterol-ipratropium combination (dey combination) is superior to either agent alone in the treatment of chronic obstructive pulmonary disease. Dey Combination Solution Study Group. Respiration. 1998;65:354–62. doi: 10.1159/000029295. [DOI] [PubMed] [Google Scholar]
- 105.Dalin DA, Løkke A, Kristiansen P, Jensen C, Birkefoss K, Christensen HR, et al. A systematic review of blood eosinophils and continued treatment with inhaled corticosteroids in patients with COPD. Respir Med. 2022;198:106880. doi: 10.1016/j.rmed.2022.106880. [DOI] [PubMed] [Google Scholar]
- 106.Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness and safety of LABA-LAMA versus LABA-ICS treatment of COPD in real-world clinical practice. Chest. 2019;155:1158–65. doi: 10.1016/j.chest.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 107.Tashkin DP, Miravitlles M, Celli BR, Metzdorf N, Mueller A, Halpin DM, et al. Concomitant inhaled corticosteroid use and the risk of pneumonia in COPD: A matched-subgroup post hoc analysis of the UPLIFT® trial. Respir Res. 2018;19:196. doi: 10.1186/s12931-018-0874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liapikou A, Toumbis M, Torres A. Managing the safety of inhaled corticosteroids in COPD and the risk of pneumonia. Expert Opin Drug Saf. 2015;14:1237–47. doi: 10.1517/14740338.2015.1057494. [DOI] [PubMed] [Google Scholar]
- 109.Pauwels RA, Löfdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society study on chronic obstructive pulmonary disease. N Engl J Med. 1999;340:1948–53. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 110.Vestbo J, Sørensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: A randomised controlled trial. Lancet. 1999;353:1819–23. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- 111.Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD002991. doi: 10.1002/14651858.CD002991.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Horita N, Goto A, Shibata Y, Ota E, Nakashima K, Nagai K, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD) Cochrane Database Syst Rev. 2017;2:CD012066. doi: 10.1002/14651858.CD012066.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fukuda N, Horita N, Kaneko A, Goto A, Kaneko T, Ota E, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2023;6:CD012066. doi: 10.1002/14651858.CD012066.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oba Y, Keeney E, Ghatehorde N, Dias S. Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): A systematic review and network meta-analysis. Cochrane Database Syst Rev. 2018;12:CD012620. doi: 10.1002/14651858.CD012620.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 116.Vestbo J, Anderson J, Brook RD, Calverley PM, Celli BR, Crim C, et al. The study to understand mortality and morbidity in COPD (SUMMIT) study protocol. Eur Respir J. 2013;41:1017–22. doi: 10.1183/09031936.00087312. [DOI] [PubMed] [Google Scholar]
- 117.Chen H, Deng ZX, Sun J, Huang Q, Huang L, He YH, et al. Association of inhaled corticosteroids with all-cause mortality risk in patients with COPD: A meta-analysis of 60 randomized controlled trials. Chest. 2023;163:100–14. doi: 10.1016/j.chest.2022.07.015. [DOI] [PubMed] [Google Scholar]
- 118.Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: A randomized trial. Ann Intern Med. 2007;146:545–55. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- 119.Ferguson GT, Rabe KF, Martinez FJ, Fabbri LM, Wang C, Ichinose M, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): A double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6:747–58. doi: 10.1016/S2213-2600(18)30327-8. [DOI] [PubMed] [Google Scholar]
- 120.Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–80. doi: 10.1056/NEJMoa1713901. [DOI] [PubMed] [Google Scholar]
- 121.Papi A, Vestbo J, Fabbri L, Corradi M, Prunier H, Cohuet G, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): A double-blind, parallel group, randomised controlled trial. Lancet. 2018;391:1076–84. doi: 10.1016/S0140-6736(18)30206-X. [DOI] [PubMed] [Google Scholar]
- 122.Lipson DA, Crim C, Criner GJ, Day NC, Dransfield MT, Halpin DM, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201:1508–16. doi: 10.1164/rccm.201911-2207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35–48. doi: 10.1056/NEJMoa1916046. [DOI] [PubMed] [Google Scholar]
- 124.Martinez FJ, Rabe KF, Sethi S, Pizzichini E, McIvor A, Anzueto A, et al. Effect of roflumilast and inhaled corticosteroid/long-acting β2-agonist on chronic obstructive pulmonary disease exacerbations (RE (2) SPOND). A randomized clinical trial. Am J Respir Crit Care Med. 2016;194:559–67. doi: 10.1164/rccm.201607-1349OC. [DOI] [PubMed] [Google Scholar]
- 125.Rennard SI, Martinez FJ, Rabe KF, Sethi S, Pizzichini E, McIvor A, et al. Effects of roflumilast in COPD patients receiving inhaled corticosteroid/long-acting β2-agonist fixed-dose combination: RE (2) SPOND rationale and study design. Int J Chron Obstruct Pulmon Dis. 2016;11:1921–8. doi: 10.2147/COPD.S109661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013:CD002309. doi: 10.1002/14651858.CD002309.pub4. doi: 10.1002/14651858.CD002309.pub5. [DOI] [PubMed] [Google Scholar]
- 127.Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;9:CD002309. doi: 10.1002/14651858.CD002309.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicentre randomised controlled trial. Lancet. 2015;385:857–66. doi: 10.1016/S0140-6736(14)62410-7. [DOI] [PubMed] [Google Scholar]
- 129.Mackay AJ, Patel AR, Singh R, Sapsford RJ, Donaldson GC, Prasad N, et al. Randomized double-blind controlled trial of roflumilast at acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196:656–9. doi: 10.1164/rccm.201612-2518LE. [DOI] [PubMed] [Google Scholar]
- 130.Vos W, Hajian B, De Backer J, Van Holsbeke C, Vinchurkar S, Claes R, et al. Functional respiratory imaging to assess the interaction between systemic roflumilast and inhaled ICS/LABA/LAMA. Int J Chron Obstruct Pulmon Dis. 2016;11:263–71. doi: 10.2147/COPD.S93830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Franciosi LG, Diamant Z, Banner KH, Zuiker R, Morelli N, Kamerling IM, et al. Efficacy and safety of RPL554, a dual PDE3 and PDE4 inhibitor, in healthy volunteers and in patients with asthma or chronic obstructive pulmonary disease: Findings from four clinical trials. Lancet Respir Med. 2013;1:714–27. doi: 10.1016/S2213-2600(13)70187-5. [DOI] [PubMed] [Google Scholar]
- 132.Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: Two randomised clinical trials. Lancet. 2009;374:695–703. doi: 10.1016/S0140-6736(09)61252-6. [DOI] [PubMed] [Google Scholar]
- 133.Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: Two randomised clinical trials. Lancet. 2009;374:685–94. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 134.Pinner NA, Hamilton LA, Hughes A. Roflumilast: A phosphodiesterase-4 inhibitor for the treatment of severe chronic obstructive pulmonary disease. Clin Ther. 2012;34:56–66. doi: 10.1016/j.clinthera.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 135.Mariotti F, Govoni M, Lucci G, Santoro D, Nandeuil MA. Safety, tolerability, and pharmacokinetics of single and repeat ascending doses of CHF6001, a novel inhaled phosphodiesterase-4 inhibitor: Two randomized trials in healthy volunteers. Int J Chron Obstruct Pulmon Dis. 2018;13:3399–410. doi: 10.2147/COPD.S174156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rabe KF, Watz H, Baraldo S, Pedersen F, Biondini D, Bagul N, et al. Anti-inflammatory effects of roflumilast in chronic obstructive pulmonary disease (ROBERT): A 16-week, randomised, placebo-controlled trial. Lancet Respir Med. 2018;6:827–36. doi: 10.1016/S2213-2600(18)30331-X. [DOI] [PubMed] [Google Scholar]
- 137.Martinez FJ, Rabe KF, Calverley PM, Fabbri LM, Sethi S, Pizzichini E, et al. Determinants of response to roflumilast in severe chronic obstructive pulmonary disease. Pooled analysis of two randomized trials. Am J Respir Crit Care Med. 2018;198:1268–78. doi: 10.1164/rccm.201712-2493OC. [DOI] [PubMed] [Google Scholar]
- 138.Watz H, Bagul N, Rabe KF, Rennard S, Alagappan VK, Román J, et al. Use of a 4-week up-titration regimen of roflumilast in patients with severe COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:813–22. doi: 10.2147/COPD.S154012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nowak D, Ehlken B, Kotchie R, Wecht S, Magnussen H. Roflumilast in combination with long-acting bronchodilators in the management of patients with severe and very severe COPD. A cost-effectiveness analysis for Germany. Dtsch Med Wochenschr. 2013;138:119–25. doi: 10.1055/s-0032-1327416. [DOI] [PubMed] [Google Scholar]
- 140.Wedzicha JA, Rabe KF, Martinez FJ, Bredenbröker D, Brose M, Goehring UM, et al. Efficacy of roflumilast in the COPD frequent exacerbator phenotype. Chest. 2013;143:1302–11. doi: 10.1378/chest.12-1489. [DOI] [PubMed] [Google Scholar]
- 141.Rennard SI, Schachter N, Strek M, Rickard K, Amit O. Cilomilast for COPD: Results of a 6-month, placebo-controlled study of a potent, selective inhibitor of phosphodiesterase 4. Chest. 2006;129:56–66. doi: 10.1378/chest.129.1.56. [DOI] [PubMed] [Google Scholar]
- 142.O’Donnell DE, Bredenbröker D, Brose M, Webb KA. Physiological effects of roflumilast at rest and during exercise in COPD. Eur Respir J. 2012;39:1104–12. doi: 10.1183/09031936.00096511. [DOI] [PubMed] [Google Scholar]
- 143.Rutten-van Mölken MP, van Nooten FE, Lindemann M, Caeser M, Calverley PM. A 1-year prospective cost-effectiveness analysis of roflumilast for the treatment of patients with severe chronic obstructive pulmonary disease. Pharmacoeconomics. 2007;25:695–711. doi: 10.2165/00019053-200725080-00007. [DOI] [PubMed] [Google Scholar]
- 144.Stolfa I, Page C. Phosphodiesterase inhibitors and lung diseases. Adv Pharmacol. 2023;98:55–81. doi: 10.1016/bs.apha.2023.05.001. [DOI] [PubMed] [Google Scholar]
- 145.Tse HN, Raiteri L, Wong KY, Yee KS, Ng LY, Wai KY, et al. High-dose N-acetylcysteine in stable COPD: The 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest. 2013;144:106–18. doi: 10.1378/chest.12-2357. [DOI] [PubMed] [Google Scholar]
- 146.Moretti M, Bottrighi P, Dallari R, Da Porto R, Dolcetti A, Grandi P, et al. The effect of long-term treatment with erdosteine on chronic obstructive pulmonary disease: The EQUALIFE study. Drugs Exp Clin Res. 2004;30:143–52. [PubMed] [Google Scholar]
- 147.Zheng JP, Wen FQ, Bai CX, Wan HY, Kang J, Chen P, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (pantheon): A randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2:187–94. doi: 10.1016/S2213-2600(13)70286-8. [DOI] [PubMed] [Google Scholar]
- 148.Worth H, Schacher C, Dethlefsen U. Concomitant therapy with cineole (eucalyptole) reduces exacerbations in COPD: A placebo-controlled double-blind trial. Respir Res. 2009;10:69. doi: 10.1186/1465-9921-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dal Negro RW, Wedzicha JA, Iversen M, Fontana G, Page C, Cicero AF, et al. Effect of erdosteine on the rate and duration of COPD exacerbations: The RESTORE study. Eur Respir J. 2017;50:1700711. doi: 10.1183/13993003.00711-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Malerba M, Ponticiello A, Radaeli A, Bensi G, Grassi V. Effect of twelve-months therapy with oral ambroxol in preventing exacerbations in patients with COPD. Double-blind, randomized, multicenter, placebo-controlled study (the AMETHIST trial) Pulm Pharmacol Ther. 2004;17:27–34. doi: 10.1016/j.pupt.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 151.Johnson K, McEvoy CE, Naqvi S, Wendt C, Reilkoff RA, Kunisaki KM, et al. High-dose oral N-acetylcysteine fails to improve respiratory health status in patients with chronic obstructive pulmonary disease and chronic bronchitis: A randomized, placebo-controlled trial. Int J Chron Obstruct Pulmon Dis. 2016;11:799–807. doi: 10.2147/COPD.S102375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Decramer M, Rutten-van Mölken M, Dekhuijzen PN, Troosters T, van Herwaarden C, Pellegrino R, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomised placebo-controlled trial. Lancet. 2005;365:1552–60. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 153.Fukuchi Y, Tatsumi K, Inoue H, Sakata Y, Shibata K, Miyagishi H, et al. Prevention of COPD exacerbation by lysozyme: A double-blind, randomized, placebo-controlled study. Int J Chron Obstruct Pulmon Dis. 2016;11:831–8. doi: 10.2147/COPD.S103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grillage M, Barnard-Jones K. Long-term oral carbocisteine therapy in patients with chronic bronchitis. A double blind trial with placebo control. Br J Clin Pract. 1985;39:395–8. [PubMed] [Google Scholar]
- 155.Zheng JP, Kang J, Huang SG, Chen P, Yao WZ, Yang L, et al. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE study): A randomised placebo-controlled study. Lancet. 2008;371:2013–8. doi: 10.1016/S0140-6736(08)60869-7. [DOI] [PubMed] [Google Scholar]
- 156.Schermer T, Chavannes N, Dekhuijzen R, Wouters E, Muris J, Akkermans R, et al. Fluticasone and N-acetylcysteine in primary care patients with COPD or chronic bronchitis. Respir Med. 2009;103:542–51. doi: 10.1016/j.rmed.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 157.Simpson JL, Powell H, Baines KJ, Milne D, Coxson HO, Hansbro PM, et al. The effect of azithromycin in adults with stable neutrophilic COPD: A double blind randomised, placebo controlled trial. PLoS One. 2014;9:e105609. doi: 10.1371/journal.pone.0105609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139–47. doi: 10.1164/rccm.200801-145OC. [DOI] [PubMed] [Google Scholar]
- 159.He ZY, Ou LM, Zhang JQ, Bai J, Liu GN, Li MH, et al. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. Respiration. 2010;80:445–52. doi: 10.1159/000321374. [DOI] [PubMed] [Google Scholar]
- 160.Berkhof FF, Doornewaard-ten Hertog NE, Uil SM, Kerstjens HA, van den Berg JW. Azithromycin and cough-specific health status in patients with chronic obstructive pulmonary disease and chronic cough: A randomised controlled trial. Respir Res. 2013;14:125. doi: 10.1186/1465-9921-14-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Jr., Criner GJ, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689–98. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sethi S, Jones PW, Theron MS, Miravitlles M, Rubinstein E, Wedzicha JA, et al. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: A randomized controlled trial. Respir Res. 2010;11:10. doi: 10.1186/1465-9921-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shafuddin E, Mills GD, Holmes MD, Poole PJ, Mullins PR, Black PN. A double-blind, randomised, placebo-controlled study of roxithromycin and doxycycline combination, roxithromycin alone, or matching placebo for 12 weeks in adults with frequent exacerbations of chronic obstructive pulmonary disease. J Negat Results Biomed. 2015;14:15. doi: 10.1186/s12952-015-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Uzun S, Djamin RS, Kluytmans JA, Mulder PG, van’t Veer NE, Ermens AA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): A randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361–8. doi: 10.1016/S2213-2600(14)70019-0. [DOI] [PubMed] [Google Scholar]
- 165.Janjua S, Mathioudakis AG, Fortescue R, Walker RA, Sharif S, Threapleton CJ, et al. Prophylactic antibiotics for adults with chronic obstructive pulmonary disease: A network meta-analysis. Cochrane Database Syst Rev. 2021;1:CD013198. doi: 10.1002/14651858.CD013198.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Taylor SP, Sellers E, Taylor BT. Azithromycin for the prevention of COPD exacerbations: The good, bad, and ugly. Am J Med. 2015;128:1362. doi: 10.1016/j.amjmed.2015.07.032. e1-6. [DOI] [PubMed] [Google Scholar]
- 167.Ni W, Shao X, Cai X, Wei C, Cui J, Wang R, et al. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: A meta-analysis. PLoS One. 2015;10:e0121257. doi: 10.1371/journal.pone.0121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rogers L, Jesenak M, Bjermer L, Hanania NA, Seys SF, Diamant Z. Biologics in severe asthma: A pragmatic approach for choosing the right treatment for the right patient. Respir Med. 2023;218:107414. doi: 10.1016/j.rmed.2023.107414. [DOI] [PubMed] [Google Scholar]
- 169.Pavord ID, Chapman KR, Bafadhel M, Sciurba FC, Bradford ES, Schweiker Harris S, et al. Mepolizumab for eosinophil-associated COPD: Analysis of METREX and METREO. Int J Chron Obstruct Pulmon Dis. 2021;16:1755–70. doi: 10.2147/COPD.S294333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Criner GJ, Celli BR, Brightling CE, Agusti A, Papi A, Singh D, et al. Benralizumab for the prevention of COPD exacerbations. N Engl J Med. 2019;381:1023–34. doi: 10.1056/NEJMoa1905248. [DOI] [PubMed] [Google Scholar]
- 171.Bhatt SP, Rabe KF, Hanania NA, Vogelmeier CF, Bafadhel M, Christenson SA, et al. Dupilumab for COPD with blood eosinophil evidence of type 2 inflammation. N Engl J Med. 2024;390:2274–83. doi: 10.1056/NEJMoa2401304. [DOI] [PubMed] [Google Scholar]
- 172.Doo JH, Kim SM, Park YJ, Kim KH, Oh YH, Kim JS, et al. Smoking cessation after diagnosis of COPD is associated with lower all-cause and cause-specific mortality: A nationwide population-based cohort study of South Korean men. BMC Pulm Med. 2023;23:237. doi: 10.1186/s12890-023-02533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu Y, Greenlund KJ, VanFrank B, Xu F, Lu H, Croft JB. Smoking cessation among U.S. Adult smokers with and without chronic obstructive pulmonary disease, 2018. Am J Prev Med. 2022;62:492–502. doi: 10.1016/j.amepre.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of lung health study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675–9. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
- 175.Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: An analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180:3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 176.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE, et al. The effects of a smoking cessation intervention on 14.5-year mortality: A randomized clinical trial. Ann Intern Med. 2005;142:233–9. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 177.Williams C. Dissemination of the agency for health care policy and research guideline. Tob Control. 1998;7:S17–8. doi: 10.1136/tc.7.2008.s17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Roos CR, Harp NR, Vafaie N, Gueorguieva R, Frankforter T, Carroll KM, et al. Randomized trial of mindfulness- and reappraisal-based regulation of craving training among daily cigarette smokers. Psychol Addict Behav. 2023;37:829–40. doi: 10.1037/adb0000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mishra A, Maiti R, Mishra BR, Jena M. Comparative efficacy and safety of pharmacological interventions for smoking cessation in healthy adults: A network meta-analysis. Pharmacol Res. 2021;166:105478. doi: 10.1016/j.phrs.2021.105478. [DOI] [PubMed] [Google Scholar]
- 180.Laniado-Laborín R. Smoking cessation intervention: An evidence-based approach. Postgrad Med. 2010;122:74–82. doi: 10.3810/pgm.2010.03.2124. [DOI] [PubMed] [Google Scholar]
- 181.Shang X, Fenfen E, Guo K, Yang C, Zhou L, Wu Y, et al. Effectiveness and safety of varenicline for smoking cessation: An overview and meta-analysis. J Addict Med. 2023;17:536–43. doi: 10.1097/ADM.0000000000001171. [DOI] [PubMed] [Google Scholar]
- 182.Lindson N, Theodoulou A, Ordóñez-Mena JM, Fanshawe TR, Sutton AJ, Livingstone-Banks J, et al. Pharmacological and electronic cigarette interventions for smoking cessation in adults: Component network meta-analyses. Cochrane Database Syst Rev. 2023;9:CD015226. doi: 10.1002/14651858.CD015226.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Thomas KH, Dalili MN, López-López JA, Keeney E, Phillippo D, Munafò MR, et al. Smoking cessation medicines and e-cigarettes: A systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess. 2021;25:1–224. doi: 10.3310/hta25590. [DOI] [PubMed] [Google Scholar]
- 184.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The lung health study. JAMA. 1994;272:1497–505. [PubMed] [Google Scholar]
- 185.Wells JM, Estepar RS, McDonald MN, Bhatt SP, Diaz AA, Bailey WC, et al. Clinical, physiologic, and radiographic factors contributing to development of hypoxemia in moderate to severe COPD: A cohort study. BMC Pulm Med. 2016;16:169. doi: 10.1186/s12890-016-0331-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ergan B, Nava S. Long-term oxygen therapy in COPD patients who do not meet the actual recommendations. COPD. 2017;14:351–66. doi: 10.1080/15412555.2017.1319918. [DOI] [PubMed] [Google Scholar]
- 187.Pierson DJ. Pathophysiology and clinical effects of chronic hypoxia. Respir Care. 2000;45:39–51. [PubMed] [Google Scholar]
- 188.Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93:391–8. doi: 10.7326/0003-4819-93-3-391. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 189.Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1:681–6. [PubMed] [Google Scholar]
- 190.Long-Term Oxygen Treatment Trial Research Group. Albert RK, Au DH, Blackford AL, Casaburi R, Cooper JA, Jr., et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375:1617–27. doi: 10.1056/NEJMoa1604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;7:CD004104. doi: 10.1002/14651858.CD004104.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995;151:1799–806. doi: 10.1164/ajrccm.151.6.7767523. [DOI] [PubMed] [Google Scholar]
- 193.Carrera M, Marín JM, Antón A, Chiner E, Alonso ML, Masa JF, et al. A controlled trial of noninvasive ventilation for chronic obstructive pulmonary disease exacerbations. J Crit Care. 2009;24:473. doi: 10.1016/j.jcrc.2008.08.007. e7-14. [DOI] [PubMed] [Google Scholar]
- 194.Celikel T, Sungur M, Ceyhan B, Karakurt S. Comparison of noninvasive positive pressure ventilation with standard medical therapy in hypercapnic acute respiratory failure. Chest. 1998;114:1636–42. doi: 10.1378/chest.114.6.1636. [DOI] [PubMed] [Google Scholar]
- 195.Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–22. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 196.Barbé F, Togores B, Rubí M, Pons S, Maimó A, Agustí AG. Noninvasive ventilatory support does not facilitate recovery from acute respiratory failure in chronic obstructive pulmonary disease. Eur Respir J. 1996;9:1240–5. doi: 10.1183/09031936.96.09061240. [DOI] [PubMed] [Google Scholar]
- 197.Macrea M, Oczkowski S, Rochwerg B, Branson RD, Celli B, Coleman JM, 3rd, et al. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202:e74–87. doi: 10.1164/rccm.202006-2382ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ergan B, Oczkowski S, Rochwerg B, Carlucci A, Chatwin M, Clini E, et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur Respir J. 2019;54:1901003. doi: 10.1183/13993003.01003-2019. [DOI] [PubMed] [Google Scholar]
- 199.Pitre T, Abbasi S, Su J, Mah J, Zeraatkar D. Home high flow nasal cannula for chronic hypercapnic respiratory failure in COPD: A systematic review and meta-analysis. Respir Med. 2023;219:107420. doi: 10.1016/j.rmed.2023.107420. [DOI] [PubMed] [Google Scholar]
- 200.Tan D, Walline JH, Ling B, Xu Y, Sun J, Wang B, et al. High-flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease patients after extubation: A multicenter, randomized controlled trial. Crit Care. 2020;24:489. doi: 10.1186/s13054-020-03214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Oczkowski S, Ergan B, Bos L, Chatwin M, Ferrer M, Gregoretti C, et al. ERS clinical practice guidelines: High-flow nasal cannula in acute respiratory failure. Eur Respir J. 2022;59:2101574. doi: 10.1183/13993003.01574-2021. [DOI] [PubMed] [Google Scholar]
- 202.Al Nufaiei ZF, Al Zhranei RM. High-flow nasal cannula oxygen therapy versus non-invasive ventilation in patients at very high risk for extubating failure: A systematic review of randomized controlled trials. PLoS One. 2024;19:e0299693. doi: 10.1371/journal.pone.0299693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Simon S, Joean O, Welte T, Rademacher J. The role of vaccination in COPD: Influenza, SARS-CoV-2, pneumococcus, pertussis, RSV and varicella zoster virus. Eur Respir Rev. 2023;32:230034. doi: 10.1183/16000617.0034-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Alshehri A, Ahmed M, Bagazi D, Alghamdi A. Healthcare providers’ adherence to recommended pneumococcal and influenza vaccination in patients discharged with respiratory diseases from general medical wards. Vaccines (Basel) 2023;11:431. doi: 10.3390/vaccines11020431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hak E, van Essen GA, Buskens E, Stalman W, de Melker RA. Is immunising all patients with chronic lung disease in the community against influenza cost effective? Evidence from a general practice based clinical prospective cohort study in Utrecht, The Netherlands. J Epidemiol Community Health. 1998;52:120–5. doi: 10.1136/jech.52.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Poole PJ, Chacko E, Wood-Baker RW, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006:CD002733. doi: 10.1002/14651858.CD002733.pub2. [DOI] [PubMed] [Google Scholar]
- 207.Alfageme I, Vazquez R, Reyes N, Muñoz J, Fernández A, Hernandez M, et al. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax. 2006;61:189–95. doi: 10.1136/thx.2005.043323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–25. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 209.Alqahtani JS, Oyelade T, Aldhahir AM, Alghamdi SM, Almehmadi M, Alqahtani AS, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: A rapid systematic review and meta-analysis. PLoS One. 2020;15:e0233147. doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kwok WC, Leung SH, Tam TC, Ho JC, Lam DC, Ip MS, et al. Efficacy of mRNA and inactivated whole virus vaccines against COVID-19 in patients with chronic respiratory diseases. Int J Chron Obstruct Pulmon Dis. 2023;18:47–56. doi: 10.2147/COPD.S394101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Redondo E, Rivero-Calle I, Mascarós E, Ocaña D, Jimeno I, Gil Á, et al. Respiratory syncytial virus vaccination recommendations for adults aged 60 years and older: The neumoexperts prevention group position paper. Arch Bronconeumol. 2024;60:161–70. doi: 10.1016/j.arbres.2024.01.004. [DOI] [PubMed] [Google Scholar]
- 212.Cong B, Dighero I, Zhang T, Chung A, Nair H, Li Y. Understanding the age spectrum of respiratory syncytial virus associated hospitalisation and mortality burden based on statistical modelling methods: A systematic analysis. BMC Med. 2023;21:224. doi: 10.1186/s12916-023-02932-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Melgar M, Britton A, Roper LE, Talbot HK, Long SS, Kotton CN, et al. Use of respiratory syncytial virus vaccines in older adults: Recommendations of the advisory committee on immunization practices – United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:793–801. doi: 10.15585/mmwr.mm7229a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Safonova E, Yawn BP, Welte T, Wang C. Risk factors for herpes zoster: Should people with asthma or COPD be vaccinated? Respir Res. 2023;24:35. doi: 10.1186/s12931-022-02305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kawai K, Yawn BP. Risk factors for herpes zoster: A systematic review and meta-analysis. Mayo Clin Proc. 2017;92:1806–21. doi: 10.1016/j.mayocp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 216.Rochester CL, Vogiatzis I, Holland AE, Lareau SC, Marciniuk DD, Puhan MA, et al. An official American Thoracic Society/European Respiratory Society policy statement: Enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. 2015;192:1373–86. doi: 10.1164/rccm.201510-1966ST. [DOI] [PubMed] [Google Scholar]
- 217.Rochester CL, Alison JA, Carlin B, Jenkins AR, Cox NS, Bauldoff G, et al. Pulmonary rehabilitation for adults with chronic respiratory disease: An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2023;208:e7–26. doi: 10.1164/rccm.202306-1066ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Vogiatzis I, Rochester CL, Spruit MA, Troosters T, Clini EM American Thoracic Society/European Respiratory Society Task Force on Policy in Pulmonary Rehabilitation. Increasing implementation and delivery of pulmonary rehabilitation: Key messages from the new ATS/ERS policy statement. Eur Respir J. 2016;47:1336–41. doi: 10.1183/13993003.02151-2015. [DOI] [PubMed] [Google Scholar]
- 219.Garvey C, Bayles MP, Hamm LF, Hill K, Holland A, Limberg TM, et al. Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: Review of selected guidelines: An official statement from the American Association of Cardiovascular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev. 2016;36:75–83. doi: 10.1097/HCR.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 220.Ryrsø CK, Godtfredsen NS, Kofod LM, Lavesen M, Mogensen L, Tobberup R, et al. Lower mortality after early supervised pulmonary rehabilitation following COPD-exacerbations: A systematic review and meta-analysis. BMC Pulm Med. 2018;18:154. doi: 10.1186/s12890-018-0718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Alison JA, McKeough ZJ, Johnston K, McNamara RJ, Spencer LM, Jenkins SC, et al. Australian and New Zealand pulmonary rehabilitation guidelines. Respirology. 2017;22:800–19. doi: 10.1111/resp.13025. [DOI] [PubMed] [Google Scholar]
- 222.Zeng Y, Jiang F, Chen Y, Chen P, Cai S. Exercise assessments and trainings of pulmonary rehabilitation in COPD: A literature review. Int J Chron Obstruct Pulmon Dis. 2018;13:2013–23. doi: 10.2147/COPD.S167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lu HY, Chen CF, Lee DL, Tsai YJ, Lin PC. Effects of early pulmonary rehabilitation on hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2023;18:881–93. doi: 10.2147/COPD.S397361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Du Y, Lin J, Wang X, Zhang Y, Ge H, Wang Y, et al. Early pulmonary rehabilitation in acute exacerbation of chronic obstructive pulmonary disease: A meta-analysis of randomized controlled trials. COPD. 2022;19:69–80. doi: 10.1080/15412555.2022.2029834. [DOI] [PubMed] [Google Scholar]
- 225.Rochester CL. Barriers to pulmonary rehabilitation. Respir Care. 2024;69:713–23. doi: 10.4187/respcare.11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Spitzer KA, Stefan MS, Priya A, Pack QR, Pekow PS, Lagu T, et al. Participation in pulmonary rehabilitation after hospitalization for chronic obstructive pulmonary disease among Medicare beneficiaries. Ann Am Thorac Soc. 2019;16:99–106. doi: 10.1513/AnnalsATS.201805-332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Criner GJ, Cordova F, Sternberg AL, Martinez FJ. The National Emphysema Treatment Trial (NETT) part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med. 2011;184:881–93. doi: 10.1164/rccm.201103-0455CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Koster TD, Dijk MV, Slebos DJ. Bronchoscopic lung volume reduction for emphysema: Review and update. Semin Respir Crit Care Med. 2022;43:541–51. doi: 10.1055/s-0042-1747938. [DOI] [PubMed] [Google Scholar]
- 229.Buttery SC, Banya W, Bilancia R, Boyd E, Buckley J, Greening NJ, et al. Lung volume reduction surgery versus endobronchial valves: A randomised controlled trial. Eur Respir J. 2023;61:2202063. doi: 10.1183/13993003.02063-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Leard LE, Holm AM, Valapour M, Glanville AR, Attawar S, Aversa M, et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2021;40:1349–79. doi: 10.1016/j.healun.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chambers DC, Perch M, Zuckermann A, Cherikh WS, Harhay MO, Hayes D, Jr., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report – 2021; Focus on recipient characteristics. J Heart Lung Transplant. 2021;40:1060–72. doi: 10.1016/j.healun.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Maltais F, Bjermer L, Kerwin EM, Jones PW, Watkins ML, Tombs L, et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: The EMAX randomised trial. Respir Res. 2019;20:238. doi: 10.1186/s12931-019-1193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 234.de Jong YP, Uil SM, Grotjohan HP, Postma DS, Kerstjens HA, van den Berg JW. Oral or IV prednisolone in the treatment of COPD exacerbations: A randomized, controlled, double-blind study. Chest. 2007;132:1741–7. doi: 10.1378/chest.07-0208. [DOI] [PubMed] [Google Scholar]
- 235.Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: A prospective randomised controlled trial. Lancet. 1999;354:456–60. doi: 10.1016/s0140-6736(98)11326-0. [DOI] [PubMed] [Google Scholar]
- 236.Alía I, de la Cal MA, Esteban A, Abella A, Ferrer R, Molina FJ, et al. Efficacy of corticosteroid therapy in patients with an acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. Arch Intern Med. 2011;171:1939–46. doi: 10.1001/archinternmed.2011.530. [DOI] [PubMed] [Google Scholar]
- 237.Singh JM, Palda VA, Stanbrook MB, Chapman KR. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease: A systematic review. Arch Intern Med. 2002;162:2527–36. doi: 10.1001/archinte.162.22.2527. [DOI] [PubMed] [Google Scholar]
- 238.Niewoehner DE. The role of systemic corticosteroids in acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Med. 2002;1:243–8. doi: 10.1007/BF03256615. [DOI] [PubMed] [Google Scholar]
- 239.Walters JA, Tan DJ, White CJ, Wood-Baker R. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;3:CD006897. doi: 10.1002/14651858.CD006897.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhao Z, Lou O, Wang Y, Yin R, Gong C, Deng F, et al. Long- versus short-duration systemic corticosteroid regimens for acute exacerbations of COPD: A systematic review and meta-analysis of randomized trials and cohort studies. PLoS One. 2023;18:e0296470. doi: 10.1371/journal.pone.0296470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Leuppi JD, Schuetz P, Bingisser R, Bodmer M, Briel M, Drescher T, et al. Short-term versus conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: The REDUCE randomized clinical trial. JAMA. 2013;309:2223–31. doi: 10.1001/jama.2013.5023. [DOI] [PubMed] [Google Scholar]
- 242.Maselli DJ, Peters JI. 5 days of prednisone was noninferior to 14 days in patients with acute COPD exacerbation. Ann Intern Med. 2013;159:JC5. doi: 10.7326/0003-4819-159-6-201309170-02005. [DOI] [PubMed] [Google Scholar]
- 243.Tse G, Emmanuel B, Ariti C, Bafadhel M, Papi A, Carter V, et al. A long-term study of adverse outcomes associated with oral corticosteroid use in COPD. Int J Chron Obstruct Pulmon Dis. 2023;18:2565–80. doi: 10.2147/COPD.S433326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Vanoverschelde A, Van Hoey C, Buyle F, Den Blauwen N, Depuydt P, Van Braeckel E, et al. In-hospital antibiotic use for severe chronic obstructive pulmonary disease exacerbations: A retrospective observational study. BMC Pulm Med. 2023;23:138. doi: 10.1186/s12890-023-02426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Llor C, Moragas A, Miravitlles M, Mesquita P, Cordoba G. Are short courses of antibiotic therapy as effective as standard courses for COPD exacerbations? A systematic review and meta-analysis. Pulm Pharmacol Ther. 2022;72:102111. doi: 10.1016/j.pupt.2022.102111. [DOI] [PubMed] [Google Scholar]
- 246.Adams SG, Melo J, Luther M, Anzueto A. Antibiotics are associated with lower relapse rates in outpatients with acute exacerbations of COPD. Chest. 2000;117:1345–52. doi: 10.1378/chest.117.5.1345. [DOI] [PubMed] [Google Scholar]
- 247.Zhou L, Deng Y, Liu K, Liu H, Liu W. The use of antibiotics in the early stage of acute exacerbation of chronic obstructive pulmonary disease in patients without obvious signs of infection: A multicenter, randomized, parallel-controlled study. Front Pharmacol. 2024;15:1380939. doi: 10.3389/fphar.2024.1380939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Vollenweider DJ, Jarrett H, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD010257. doi: 10.1002/14651858.CD010257. [DOI] [PubMed] [Google Scholar]
- 249.Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: A systematic review and metaanalysis. Chest. 2008;133:756–66. doi: 10.1378/chest.07-1207. [DOI] [PubMed] [Google Scholar]
- 250.Wang JX, Zhang SM, Li XH, Zhang Y, Xu ZY, Cao B. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: A prospective randomized controlled trial. Int J Infect Dis. 2016;48:40–5. doi: 10.1016/j.ijid.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 251.Jørgensen AF, Coolidge J, Pedersen PA, Petersen KP, Waldorff S, Widding E. Amoxicillin in treatment of acute uncomplicated exacerbations of chronic bronchitis. A double-blind, placebo-controlled multicentre study in general practice. Scand J Prim Health Care. 1992;10:7–11. doi: 10.3109/02813439209014027. [DOI] [PubMed] [Google Scholar]
- 252.Llor C, Moragas A, Hernández S, Bayona C, Miravitlles M. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:716–23. doi: 10.1164/rccm.201206-0996OC. [DOI] [PubMed] [Google Scholar]
- 253.Sachs AP, Koëter GH, Groenier KH, van der Waaij D, Schiphuis J, Meyboom-de Jong B. Changes in symptoms, peak expiratory flow, and sputum flora during treatment with antibiotics of exacerbations in patients with chronic obstructive pulmonary disease in general practice. Thorax. 1995;50:758–63. doi: 10.1136/thx.50.7.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wilson R, Anzueto A, Miravitlles M, Arvis P, Alder J, Haverstock D, et al. Moxifloxacin versus amoxicillin/clavulanic acid in outpatient acute exacerbations of COPD: MAESTRAL results. Eur Respir J. 2012;40:17–27. doi: 10.1183/09031936.00090311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.van Velzen P, Ter Riet G, Bresser P, Baars JJ, van den Berg BT, van den Berg JW, et al. Doxycycline for outpatient-treated acute exacerbations of COPD: A randomised double-blind placebo-controlled trial. Lancet Respir Med. 2017;5:492–9. doi: 10.1016/S2213-2600(17)30165-0. [DOI] [PubMed] [Google Scholar]
- 256.Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: A randomised placebo-controlled trial. Lancet. 2001;358:2020–5. doi: 10.1016/S0140-6736(01)07097-0. [DOI] [PubMed] [Google Scholar]
- 257.Khilnani GC, Saikia N, Banga A, Sharma SK. Non-invasive ventilation for acute exacerbation of COPD with very high PaCO (2): A randomized controlled trial. Lung India. 2010;27:125–30. doi: 10.4103/0970-2113.68308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Nava S, Grassi M, Fanfulla F, Domenighetti G, Carlucci A, Perren A, et al. Non-invasive ventilation in elderly patients with acute hypercapnic respiratory failure: A randomised controlled trial. Age Ageing. 2011;40:444–50. doi: 10.1093/ageing/afr003. [DOI] [PubMed] [Google Scholar]
- 259.Thys F, Roeseler J, Reynaert M, Liistro G, Rodenstein DO. Noninvasive ventilation for acute respiratory failure: A prospective randomised placebo-controlled trial. Eur Respir J. 2002;20:545–55. doi: 10.1183/09031936.02.00287402. [DOI] [PubMed] [Google Scholar]
- 260.Echevarria C, Brewin K, Horobin H, Bryant A, Corbett S, Steer J, et al. Early supported discharge/hospital at home for acute exacerbation of chronic obstructive pulmonary disease: A review and meta-analysis. COPD. 2016;13:523–33. doi: 10.3109/15412555.2015.1067885. [DOI] [PubMed] [Google Scholar]
- 261.Nissen I, Jensen MS. Nurse-supported discharge of patients with exacerbation of chronic obstructive pulmonary disease. Ugeskr Laeger. 2007;169:2220–3. [PubMed] [Google Scholar]
- 262.Utens CM, Goossens LM, Smeenk FW, Rutten-van Mölken MP, van Vliet M, Braken MW, et al. Early assisted discharge with generic community nursing for chronic obstructive pulmonary disease exacerbations: Results of a randomised controlled trial. BMJ Open. 2012;2:e001684. doi: 10.1136/bmjopen-2012-001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ojoo JC, Moon T, McGlone S, Martin K, Gardiner ED, Greenstone MA, et al. Patients’ and carers’ preferences in two models of care for acute exacerbations of COPD: Results of a randomised controlled trial. Thorax. 2002;57:167–9. doi: 10.1136/thorax.57.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Aimonino Ricauda N, Tibaldi V, Leff B, Scarafiotti C, Marinello R, Zanocchi M, et al. Substitutive “hospital at home” versus inpatient care for elderly patients with exacerbations of chronic obstructive pulmonary disease: A prospective randomized, controlled trial. J Am Geriatr Soc. 2008;56:493–500. doi: 10.1111/j.1532-5415.2007.01562.x. [DOI] [PubMed] [Google Scholar]
- 265.Goossens LM, Utens CM, Smeenk FW, van Schayck OC, van Vliet M, van Litsenburg W, et al. Cost-effectiveness of early assisted discharge for COPD exacerbations in The Netherlands. Value Health. 2013;16:517–28. doi: 10.1016/j.jval.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 266.MacNee W. Systemic inflammatory biomarkers and co-morbidities of chronic obstructive pulmonary disease. Ann Med. 2013;45:291–300. doi: 10.3109/07853890.2012.732703. [DOI] [PubMed] [Google Scholar]
- 267.Thomsen M, Ingebrigtsen TS, Marott JL, Dahl M, Lange P, Vestbo J, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309:2353–61. doi: 10.1001/jama.2013.5732. [DOI] [PubMed] [Google Scholar]
- 268.Perez T, Mal H, Aguilaniu B, Brillet PY, Chaouat A, Louis R, et al. COPD and inflammation: Statement from a French expert group. Phenotypes related to inflammation. Rev Mal Respir. 2011;28:192–215. doi: 10.1016/j.rmr.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 269.Santos NC, Miravitlles M, Camelier AA, Almeida VD, Maciel RR, Camelier FW. Prevalence and impact of comorbidities in individuals with chronic obstructive pulmonary disease: A systematic review. Tuberc Respir Dis (Seoul) 2022;85:205–20. doi: 10.4046/trd.2021.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Greulich T, Weist BJ, Koczulla AR, Janciauskiene S, Klemmer A, Lux W, et al. Prevalence of comorbidities in COPD patients by disease severity in a German population. Respir Med. 2017;132:132–8. doi: 10.1016/j.rmed.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 271.Collop N. Sleep and sleep disorders in chronic obstructive pulmonary disease. Respiration. 2010;80:78–86. doi: 10.1159/000258676. [DOI] [PubMed] [Google Scholar]
- 272.McNicholas WT. Chronic obstructive pulmonary disease and obstructive sleep apnea: Overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. Am J Respir Crit Care Med. 2009;180:692–700. doi: 10.1164/rccm.200903-0347PP. [DOI] [PubMed] [Google Scholar]
- 273.Maharjan S, Dua R, Saini LK, Kumar N, Gupta R. Prevalence and predictors of restless legs syndrome among patients having stable chronic obstructive pulmonary disease. Sleep Med. 2024;118:32–8. doi: 10.1016/j.sleep.2024.03.041. [DOI] [PubMed] [Google Scholar]
- 274.Du D, Zhang G, Xu D, Liu L, Hu X, Chen L, et al. Prevalence and clinical characteristics of sleep disorders in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Sleep Med. 2023;112:282–90. doi: 10.1016/j.sleep.2023.10.034. [DOI] [PubMed] [Google Scholar]
- 275.van Zeller M, Basoglu OK, Verbraecken J, Lombardi C, McNicholas WT, Pepin JL, et al. Sleep and cardiometabolic comorbidities in the obstructive sleep apnoea-COPD overlap syndrome: Data from the European Sleep Apnoea Database. ERJ Open Res. 2023;9:00676–2022. doi: 10.1183/23120541.00676-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fanaridis M, Bouloukaki I, Stathakis G, Steiropoulos P, Tzanakis N, Moniaki V, et al. Prevalence and characteristics of patients with obstructive sleep apnea and chronic obstructive pulmonary disease: Overlap syndrome. Life (Basel) 2024;14:547. doi: 10.3390/life14050547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Marin-Oto M, Marin JM. Obstructive sleep apnea effects on chronic airway disease exacerbations-missed opportunities for improving outcomes in chronic obstructive pulmonary disease and asthma. Sleep Med Clin. 2024;19:275–82. doi: 10.1016/j.jsmc.2024.02.007. [DOI] [PubMed] [Google Scholar]
- 278.Jaoude P, El-Solh AA. Predictive factors for COPD exacerbations and mortality in patients with overlap syndrome. Clin Respir J. 2019;13:643–51. doi: 10.1111/crj.13079. [DOI] [PubMed] [Google Scholar]
- 279.Tockman MS, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med. 1987;106:512–8. doi: 10.7326/0003-4819-106-4-512. [DOI] [PubMed] [Google Scholar]
- 280.Wasswa-Kintu S, Gan WQ, Man SF, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: A systematic review and meta-analysis. Thorax. 2005;60:570–5. doi: 10.1136/thx.2004.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Su Z, Jiang Y, Li C, Zhong R, Wang R, Wen Y, et al. Relationship between lung function and lung cancer risk: A pooled analysis of cohorts plus Mendelian randomization study. J Cancer Res Clin Oncol. 2021;147:2837–49. doi: 10.1007/s00432-021-03619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mu H, Yang X, Li Y, Zhou B, Liu L, Zhang M, et al. Three-year follow-up study reveals improved survival rate in NSCLC patients underwent guideline-concordant diagnosis and treatment. Front Oncol. 2024;14:1382197. doi: 10.3389/fonc.2024.1382197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bonney A, Malouf R, Marchal C, Manners D, Fong KM, Marshall HM, et al. Impact of low-dose computed tomography (LDCT) screening on lung cancer-related mortality. Cochrane Database Syst Rev. 2022;8:CD013829. doi: 10.1002/14651858.CD013829.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Edwards DM, Pirzadeh M, Van T, Jiang R, Tate A, Schaefer G, et al. Impact of lung cancer screening on stage migration and mortality among the national Veterans Health Administration population with lung cancer. Cancer. 2024;130:2910–7. doi: 10.1002/cncr.35340. [doi: 10.1002/cncr.35340] [DOI] [PubMed] [Google Scholar]
- 285.Bethesda: GOLD; 2024. Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for Prevention, Diagnosis and Management of COPD: 2024 Report. [Google Scholar]
- 286.US Preventive Services Task Force, Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:962–70. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 287.Kauczor HU, Baird AM, Blum TG, Bonomo L, Bostantzoglou C, Burghuber O, et al. ESR/ERS statement paper on lung cancer screening. Eur Radiol. 2020;30:3277–94. doi: 10.1007/s00330-020-06727-7. [DOI] [PubMed] [Google Scholar]
- 288.Pereira LF, Santos RS, Bonomi DO, Franceschini J, Santoro IL, Miotto A, et al. Lung cancer screening in Brazil: Recommendations from the Brazilian Society of Thoracic Surgery, Brazilian Thoracic Association, and Brazilian College of Radiology and Diagnostic Imaging. J Bras Pneumol. 2024;50:e20230233. doi: 10.36416/1806-3756/e20230233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Silva M, Picozzi G, Sverzellati N, Anglesio S, Bartolucci M, Cavigli E, et al. Low-dose CT for lung cancer screening: Position paper from the Italian college of thoracic radiology. Radiol Med. 2022;127:543–59. doi: 10.1007/s11547-022-01471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wolf AM, Oeffinger KC, Shih TY, Walter LC, Church TR, Fontham ET, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. 2024;74:50–81. doi: 10.3322/caac.21811. [DOI] [PubMed] [Google Scholar]
- 291.Amicizia D, Piazza MF, Marchini F, Astengo M, Grammatico F, Battaglini A, et al. Systematic review of lung cancer screening: Advancements and strategies for implementation. Healthcare (Basel) 2023;11:2085. doi: 10.3390/healthcare11142085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.de Miguel Díez J, Chancafe Morgan J, Jiménez García R. The association between COPD and heart failure risk: A review. Int J Chron Obstruct Pulmon Dis. 2013;8:305–12. doi: 10.2147/COPD.S31236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Lainscak M, Hodoscek LM, Düngen HD, Rauchhaus M, Doehner W, Anker SD, et al. The burden of chronic obstructive pulmonary disease in patients hospitalized with heart failure. Wien Klin Wochenschr. 2009;121:309–13. doi: 10.1007/s00508-009-1185-8. [DOI] [PubMed] [Google Scholar]
- 294.Ni H, Nauman DJ, Hershberger RE. Managed care and outcomes of hospitalization among elderly patients with congestive heart failure. Arch Intern Med. 1998;158:1231–6. doi: 10.1001/archinte.158.11.1231. [DOI] [PubMed] [Google Scholar]
- 295.Cuthbert JJ, Pellicori P, Clark AL. Optimal management of heart failure and chronic obstructive pulmonary disease: Clinical challenges. Int J Gen Med. 2022;15:7961–75. doi: 10.2147/IJGM.S295467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Leong P, Osadnik CR, King PT, MacDonald MI, Ko BS, Lau KK, et al. Right ventricular end-diastolic volume and outcomes in exacerbations of COPD. Respirology. 2022;27:56–65. doi: 10.1111/resp.14170. [DOI] [PubMed] [Google Scholar]
- 297.Osundolire S, Goldberg RJ, Lapane KL. Descriptive epidemiology of chronic obstructive pulmonary disease in US nursing home residents with heart failure. Curr Probl Cardiol. 2023;48:101484. doi: 10.1016/j.cpcardiol.2022.101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Rutten FH, Cramer MJ, Zuithoff NP, Lammers JW, Verweij W, Grobbee DE, et al. Comparison of B-type natriuretic peptide assays for identifying heart failure in stable elderly patients with a clinical diagnosis of chronic obstructive pulmonary disease. Eur J Heart Fail. 2007;9:651–9. doi: 10.1016/j.ejheart.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 299.Etminan M, Jafari S, Carleton B, FitzGerald JM. Beta-blocker use and COPD mortality: A systematic review and meta-analysis. BMC Pulm Med. 2012;12:48. doi: 10.1186/1471-2466-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Feng Z, Zhang L, Wang Y, Guo H, Liu J. Efficacy and safety of bisoprolol in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2023;18:3067–83. doi: 10.2147/COPD.S438930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Goedemans L, Bax JJ, Delgado V. COPD and acute myocardial infarction. Eur Respir Rev. 2020;29:190139. doi: 10.1183/16000617.0139-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Polman R, Hurst JR, Uysal OF, Mandal S, Linz D, Simons S. Cardiovascular disease and risk in COPD: A state of the art review. Expert Rev Cardiovasc Ther. 2024;22:177–91. doi: 10.1080/14779072.2024.2333786. [DOI] [PubMed] [Google Scholar]
- 303.Yang HM, Ryu MH, Carey VJ, Kinney GL, Hokanson JE, Dransfield MT, et al. Chronic obstructive pulmonary disease exacerbations increase the risk of subsequent cardiovascular events: A longitudinal analysis of the COPD gene study. J Am Heart Assoc. 2024;13:e033882. doi: 10.1161/JAHA.123.033882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Gologanu D, Stanescu C, Ursica T, Balea MI, Ionita D, Bogdan MA. Prevalence and characteristics of pulmonary hypertension associated with COPD – A pilot study in patients referred to a pulmonary rehabilitation program clinic. Maedica (Bucur) 2013;8:243–8. [PMC free article] [PubMed] [Google Scholar]
- 305.Chaouat A, Bugnet AS, Kadaoui N, Schott R, Enache I, Ducoloné A, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:189–94. doi: 10.1164/rccm.200401-006OC. [DOI] [PubMed] [Google Scholar]
- 306.Hurdman J, Condliffe R, Elliot CA, Swift A, Rajaram S, Davies C, et al. Pulmonary hypertension in COPD: Results from the ASPIRE registry. Eur Respir J. 2013;41:1292–301. doi: 10.1183/09031936.00079512. [DOI] [PubMed] [Google Scholar]
- 307.Dauriat G, Reynaud-Gaubert M, Cottin V, Lamia B, Montani D, Canuet M, et al. Severe pulmonary hypertension associated with chronic obstructive pulmonary disease: A prospective French multicenter cohort. J Heart Lung Transplant. 2021;40:1009–18. doi: 10.1016/j.healun.2021.04.021. [DOI] [PubMed] [Google Scholar]
- 308.Isa N, Mudhafar D, Ju C, Man KK, Lau WC, Cheng LY, et al. Effects of phosphodiesterase-5 inhibitors in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. COPD. 2022;19:300–8. doi: 10.1080/15412555.2022.2067525. [DOI] [PubMed] [Google Scholar]
- 309.Arif R, Pandey A, Zhao Y, Arsenault-Mehta K, Khoujah D, Mehta S. Treatment of pulmonary hypertension associated with COPD: A systematic review. ERJ Open Res. 2022;8:00348–2021. doi: 10.1183/23120541.00348-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Liu X, Chen Z, Li S, Xu S. Association of chronic obstructive pulmonary disease with arrhythmia risks: A systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:732349. doi: 10.3389/fcvm.2021.732349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Wilchesky M, Ernst P, Brophy JM, Platt RW, Suissa S. Bronchodilator use and the risk of arrhythmia in COPD: Part 2: Reassessment in the larger Quebec cohort. Chest. 2012;142:305–11. doi: 10.1378/chest.11-1597. [DOI] [PubMed] [Google Scholar]
- 312.Keogh E, Mark Williams E. Managing malnutrition in COPD: A review. Respir Med. 2021;176:106248. doi: 10.1016/j.rmed.2020.106248. [DOI] [PubMed] [Google Scholar]
- 313.Hallin R, Koivisto-Hursti UK, Lindberg E, Janson C. Nutritional status, dietary energy intake and the risk of exacerbations in patients with chronic obstructive pulmonary disease (COPD) Respir Med. 2006;100:561–7. doi: 10.1016/j.rmed.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 314.Yuan FZ, Xing YL, Xie LJ, Yang DL, Shui W, Niu YY, et al. The relationship between prognostic nutritional indexes and the clinical outcomes of patients with acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2023;18:1155–67. doi: 10.2147/COPD.S402717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Putcha N, Anzueto AR, Calverley PM, Celli BR, Tashkin DP, Metzdorf N, et al. Mortality and exacerbation risk by body mass index in patients with COPD in TIOSPIR and UPLIFT. Ann Am Thorac Soc. 2022;19:204–13. doi: 10.1513/AnnalsATS.202006-722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 317.Ferreira IM, Brooks D, White J, Goldstein R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD000998. doi: 10.1002/14651858.CD000998.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Lattanzi G, Lelli D, Antonelli Incalzi R, Pedone C. Effect of macronutrients or micronutrients supplementation on nutritional status, physical functional capacity and quality of life in patients with COPD: A systematic review and meta-analysis. J Am Nutr Assoc. 2024;43:473–87. doi: 10.1080/27697061.2024.2312852. [DOI] [PubMed] [Google Scholar]
- 319.Scoditti E, Massaro M, Garbarino S, Toraldo DM. Role of diet in chronic obstructive pulmonary disease prevention and treatment. Nutrients. 2019;11:1357. doi: 10.3390/nu11061357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Alkhunizan M, Almasoud N, Munia Abdulmowla M, Khalid Z. The prevalence of osteoporosis and osteopenia among older adults in a community-based setting in Riyadh, Saudi Arabia. Cureus. 2022;14:e32765. doi: 10.7759/cureus.32765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Ardawi MS, Sibiany AM, Bakhsh TM, Qari MH, Maimani AA. High prevalence of Vitamin D deficiency among healthy Saudi Arabian men: Relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporos Int. 2012;23:675–86. doi: 10.1007/s00198-011-1606-1. [DOI] [PubMed] [Google Scholar]
- 322.Sadat-Ali M, Al-Habdan IM, Al-Turki HA, Azam MQ. An epidemiological analysis of the incidence of osteoporosis and osteoporosis-related fractures among the Saudi Arabian population. Ann Saudi Med. 2012;32:637–41. doi: 10.5144/0256-4947.2012.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Al Nozha OM, El Tarhouny S, Taha I, Sultan I, Abdu Allah AM, Hammoda MA, et al. Association between Vitamin D level and z-score changes of bone density in college-age Saudi girls: A cross-sectional study. Int J Gen Med. 2023;16:865–74. doi: 10.2147/IJGM.S396536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ghosh AJ, Moll M, Hayden LP, Bon J, Regan E, Hersh CP, et al. Vitamin D deficiency is associated with respiratory symptoms and airway wall thickening in smokers with and without COPD: A prospective cohort study. BMC Pulm Med. 2020;20:123. doi: 10.1186/s12890-020-1148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–8. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 326.Franco CB, Paz-Filho G, Gomes PE, Nascimento VB, Kulak CA, Boguszewski CL, et al. Chronic obstructive pulmonary disease is associated with osteoporosis and low levels of Vitamin D. Osteoporos Int. 2009;20:1881–7. doi: 10.1007/s00198-009-0890-5. [DOI] [PubMed] [Google Scholar]
- 327.Kaenmuang P, Keeratichananont W, Geater SL, Chantamanee N, Srikaew P. Prevalence, predictors, dynamic bone change, and treatment efficacy of osteoporosis among chronic obstructive pulmonary disease patients: A prospective cohort study. Front Med (Lausanne) 2023;10:1214277. doi: 10.3389/fmed.2023.1214277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Watanabe R, Tai N, Hirano J, Ban Y, Inoue D, Okazaki R. Independent association of bone mineral density and trabecular bone score to vertebral fracture in male subjects with chronic obstructive pulmonary disease. Osteoporos Int. 2018;29:615–23. doi: 10.1007/s00198-017-4314-7. [DOI] [PubMed] [Google Scholar]
- 329.Inoue D, Watanabe R, Okazaki R. COPD and osteoporosis: Links, risks, and treatment challenges. Int J Chron Obstruct Pulmon Dis. 2016;11:637–48. doi: 10.2147/COPD.S79638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Graat-Verboom L, Wouters EF, Smeenk FW, van den Borne BE, Lunde R, Spruit MA. Current status of research on osteoporosis in COPD: A systematic review. Eur Respir J. 2009;34:209–18. doi: 10.1183/09031936.50130408. [DOI] [PubMed] [Google Scholar]
- 331.Jolliffe DA, Greenberg L, Hooper RL, Mathyssen C, Rafiq R, de Jongh RT, et al. Vitamin D to prevent exacerbations of COPD: Systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax. 2019;74:337–45. doi: 10.1136/thoraxjnl-2018-212092. [DOI] [PubMed] [Google Scholar]
- 332.Lehouck A, Mathieu C, Carremans C, Baeke F, Verhaegen J, Van Eldere J, et al. High doses of Vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: A randomized trial. Ann Intern Med. 2012;156:105–14. doi: 10.7326/0003-4819-156-2-201201170-00004. [DOI] [PubMed] [Google Scholar]
- 333.Rafiq R, Aleva FE, Schrumpf JA, Daniels JM, Bet PM, Boersma WG, et al. Vitamin D supplementation in chronic obstructive pulmonary disease patients with low serum Vitamin D: A randomized controlled trial. Am J Clin Nutr. 2022;116:491–9. doi: 10.1093/ajcn/nqac083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Demay MB, Pittas AG, Bikle DD, Diab DL, Kiely ME, Lazaretti-Castro M, et al. Vitamin D for the prevention of disease: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2024;109:1907–47. doi: 10.1210/clinem/dgae290. [DOI] [PubMed] [Google Scholar]
- 335.Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23:345–9. doi: 10.1183/09059180.00007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Putman-Casdorph H, McCrone S. Chronic obstructive pulmonary disease, anxiety, and depression: State of the science. Heart Lung. 2009;38:34–47. doi: 10.1016/j.hrtlng.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 337.Zareifopoulos N, Bellou A, Spiropoulou A, Spiropoulos K. Prevalence, contribution to disease burden and management of comorbid depression and anxiety in chronic obstructive pulmonary disease: A narrative review. COPD. 2019;16:406–17. doi: 10.1080/15412555.2019.1679102. [DOI] [PubMed] [Google Scholar]
- 338.Mou Y, Shan L, Liu Y, Wang Y, He Z, Li X, et al. Risk factors for anxiety and its impacts on acute exacerbation in older patients with chronic obstructive pulmonary disease. Front Med (Lausanne) 2024;11:1340182. doi: 10.3389/fmed.2024.1340182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Hong YJ, Kim Y, Moon JY, Park S, Lee JK, Jung KS, et al. Associations between depression and anxiety index and frequency of acute exacerbation in chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2023;17:17534666231216591. doi: 10.1177/17534666231216591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: A systematic review and meta-analysis. Chest. 2013;144:766–77. doi: 10.1378/chest.12-1911. [DOI] [PubMed] [Google Scholar]
- 341.Zhao X, Liu G, Liu D, Zou L, Huang Q, Chen M, et al. Clinical and economic burden of anxiety/depression among older adult COPD patients: Evidence from the COPD-AD China registry study. Front Psychiatry. 2023;14:1221767. doi: 10.3389/fpsyt.2023.1221767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Kunik ME, Roundy K, Veazey C, Souchek J, Richardson P, Wray NP, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127:1205–11. doi: 10.1378/chest.127.4.1205. [DOI] [PubMed] [Google Scholar]
- 343.Maurer J, Rebbapragada V, Borson S, Goldstein R, Kunik ME, Yohannes AM, et al. Anxiety and depression in COPD: Current understanding, unanswered questions, and research needs. Chest. 2008;134:43S–56S. doi: 10.1378/chest.08-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Licker MJ, Widikker I, Robert J, Frey JG, Spiliopoulos A, Ellenberger C, et al. Operative mortality and respiratory complications after lung resection for cancer: Impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg. 2006;81:1830–7. doi: 10.1016/j.athoracsur.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 345.Fields AC, Divino CM. Surgical outcomes in patients with chronic obstructive pulmonary disease undergoing abdominal operations: An analysis of 331,425 patients. Surgery. 2016;159:1210–6. doi: 10.1016/j.surg.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 346.Li Y, Zheng H, Yan W, Cao N, Yan T, Zhu H, et al. The impact of chronic obstructive pulmonary disease on the prognosis outcomes of patients with percutaneous coronary intervention or coronary artery bypass grafting: A meta-analysis. Heart Lung. 2023;60:8–14. doi: 10.1016/j.hrtlng.2023.02.017. [DOI] [PubMed] [Google Scholar]
- 347.Pompili C, Tariq J, Dalmia S, Harle A, Gilbert A, Valuckiene L, et al. Cohort study investigating evolution and factors associated with dyspnoea after anatomic lung resection. J Thorac Dis. 2024;16:113–22. doi: 10.21037/jtd-23-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur Respir J. 2009;34:17–41. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- 349.Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT American College of Chest Physicians. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines. Chest. (2nd) 2007;132:161S–77S. doi: 10.1378/chest.07-1359. [DOI] [PubMed] [Google Scholar]
- 350.Marlow LL, Lee AH, Hedley E, Grocott MP, Steiner MC, Young JD, et al. Findings of a feasibility study of pre-operative pulmonary rehabilitation to reduce post-operative pulmonary complications in people with chronic obstructive pulmonary disease scheduled for major abdominal surgery. F1000Res. 2020;9:172. doi: 10.12688/f1000research.22040.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Smetana GW, Lawrence VA, Cornell JE American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: Systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581–95. doi: 10.7326/0003-4819-144-8-200604180-00009. [DOI] [PubMed] [Google Scholar]
- 352.Ergan B, Akgun M, Pacilli AM, Nava S. Should I stay or should I go? COPD and air travel. Eur Respir Rev. 2018;27:180030. doi: 10.1183/16000617.0030-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Ahmedzai S, Balfour-Lynn IM, Bewick T, Buchdahl R, Coker RK, Cummin AR, et al. Managing passengers with stable respiratory disease planning air travel: British Thoracic Society recommendations. Thorax. 2011;66(Suppl 1):i1–30. doi: 10.1136/thoraxjnl-2011-200295. [DOI] [PubMed] [Google Scholar]
- 354.Berg BW, Dillard TA, Rajagopal KR, Mehm WJ. Oxygen supplementation during air travel in patients with chronic obstructive lung disease. Chest. 1992;101:638–41. doi: 10.1378/chest.101.3.638. [DOI] [PubMed] [Google Scholar]
- 355.Edvardsen A, Akerø A, Christensen CC, Ryg M, Skjønsberg OH. Air travel and chronic obstructive pulmonary disease: A new algorithm for pre-flight evaluation. Thorax. 2012;67:964–9. doi: 10.1136/thoraxjnl-2012-201855. [DOI] [PubMed] [Google Scholar]
- 356.Coker RK, Armstrong A, Church AC, Holmes S, Naylor J, Pike K, et al. BTS clinical statement on air travel for passengers with respiratory disease. Thorax. 2022;77:329–50. doi: 10.1136/thoraxjnl-2021-218110. [DOI] [PMC free article] [PubMed] [Google Scholar]


