Abstract
The thyroid-stimulating hormone receptor (TSHR) is a G protein–linked, 7–transmembrane domain (7-TMD) receptor that undergoes complex posttranslational processing unique to this glycoprotein receptor family. Due to its complex structure, TSHR appears to have unstable molecular integrity and a propensity toward over- or underactivity on the basis of point genetic mutations or antibody-induced structural changes. Hence, both germline and somatic mutations, commonly located in the transmembrane regions, may induce constitutive activation of the receptor, resulting in congenital hyperthyroidism or the development of actively secreting thyroid nodules. Similarly, mutations leading to structural alterations may induce constitutive inactivation and congenital hypothyroidism. The TSHR is also a primary antigen in autoimmune thyroid disease, and some TSHR antibodies may activate the receptor, while others inhibit its activation or have no influence on signal transduction at all, depending on how they influence the integrity of the structure. Clinical assays for such antibodies have improved significantly and are a useful addition to the investigative armamentarium. Furthermore, the relative instability of the receptor can result in shedding of the TSHR ectodomain, providing a source of antigen and activating the autoimmune response. However, it may also provide decoys for TSHR antibodies, thus influencing their biological action and clinical effects. This review discusses the role of the TSHR in the physiological and pathological stimulation of the thyroid.
The master switch in the regulation of the thyroid gland, including its growth and differentiation, is the thyroid-stimulating hormone (TSH) receptor (TSHR). The TSHR is a 7–transmembrane domain (7-TDM) G protein–coupled receptor anchored to the surface of the plasma membrane of thyrocytes and a variety of other cell types (1). In addition, the TSHR has been implicated in a range of thyroid diseases (Table 1). For example, certain TSHR mutations cause constitutive overactivity of thyroid cells, leading to active nodule formation or rare cases of congenital hyperthyroidism. In contrast, other TSHR mutations have resulted in receptor inactivation or rare cases of congenital hypothyroidism (2). The TSHR is also a major autoantigen in autoimmune thyroid disease (AITD). In particular, the TSHR is the target of the immune response in patients with Graves disease, who exhibit unique TSHR-stimulating antibodies (1, 3). This review, therefore, encompasses those diseases involving TSHR structural variants and in which TSHR is a major antigenic target.
Table 1.
Diseases of the TSHR
An overview of the TSHR
TSHR structure.
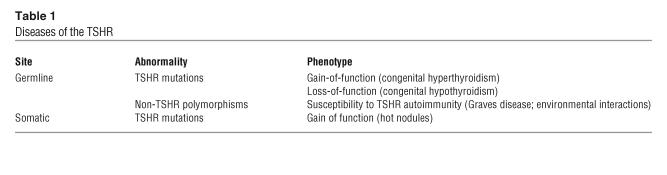
Prior to successful cloning, the subunit structure of the TSHR had been deduced by affinity labeling of thyrocyte membranes using radiolabeled and photoactivated TSH (4). The cloning of the canine TSHR in 1989 resulted from cross-hybridization procedures with a luteinizing hormone (LH; also known as lutropin) receptor probe (5) and was soon followed by the cloning of the human gene (6–8). The deduced protein structure established its membership in the family of G protein–coupled receptors having sequence similarity with the adrenergic-rhodopsin receptors (Figure 1). The TSHR gene on chromosome 14q3 (9) codes for a 764-aa protein, which comprises a signal peptide of 21 aa; a large, glycosylated ectodomain of 394 residues encoded by 9 exons; and 349 residues encoded by the tenth and largest exon, which constitute the 7 TMDs and cytoplasmic tail. The sequence also revealed 2 nonhomologous segments within the TSHR ectodomain (residues 38–45 and 316–366) not found in otherwise closely related glycoprotein hormone receptors such as those for LH and follicle-stimulating hormone (FSH; also known as follitropin) (3). The initial TSH cross-linking studies described above indicated that the mature TSHR contained 2 subunits (4), and the subsequent molecular cloning of the TSHR indicated that both subunits were encoded by a single gene, which indicated that intramolecular cleavage must have occurred (4, 10, 11), something not observed with the LH and FSH receptors. One TSHR subunit consists of a large extracellular domain (or ectodomain; mostly the α, or A, subunit), and the second contains the short membrane-anchored and intracellular portion of the receptor (the β, or B, subunit) (Figure 1).
Figure 1.
TSHR structure. This computer model of the TSHR shows the 7 TMDs (spirals) embedded within the plasma membrane and a short cytoplasmic tail, which together make up the β/B subunit. The unique 50-aa–long cleaved region (about 316–366 aa) is shown in gray. Forming a long array, the 9 LRRs, each consisting of 20–24 aa, are depicted as spirals (α helices and β pleated sheets) on the ectodomain of the receptor and make up the major portion of the α/A subunit. The LRRs have a characteristic horseshoe shape with a concave inner surface. C, C-terminus; N, N-terminus. Figure adapted with permission from Thyroid (28).
The TSH-binding pocket on the TSHR.
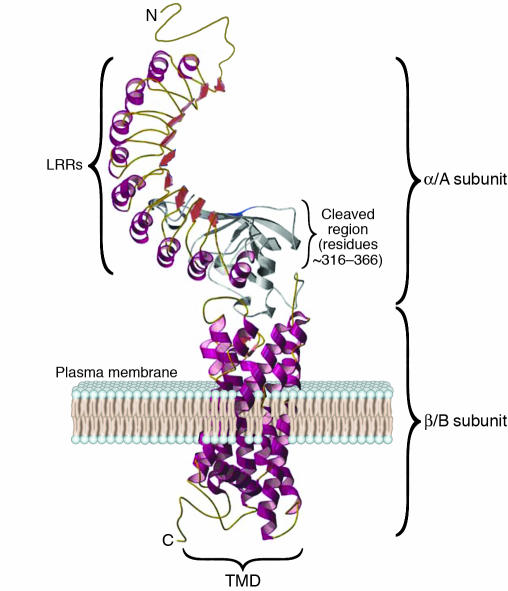
Expression on the plasma membrane of the TSHR ectodomain with a short lipid tail is sufficient for high-affinity binding of TSH (12–14). Hence, the TSHR ectodomain, consisting mainly of 9 leucine-rich repeats (LRRs) and an N-terminal tail, encoded by exons 2–8, forms the binding domain for TSH. The 7 TMDs are joined intracellularly by connecting loops that interact with G proteins when the receptor is activated (15), whereas the exoplasmic loops, outside the cell, have ancillary roles in receptor structure and activation (16). Investigation of the 2 nonhomologous segments within the ectodomain showed that deletion of residues 38–45 abrogated TSH binding (17), whereas deletion of residues 316–366 did not (17, 18), and the TSHR-transfected cells were still capable of TSH-mediated signaling. A detailed mutational analysis of residues 38–45 showed that Cys41 was the critical residue required for TSHR expression (19, 20), and data also indicated that Cys41 interaction with other neighboring Cys residues in the TSHR ectodomain, via disulfide bonds, was essential for high-affinity TSH binding (20). Hence, cysteine bonding helped restrain the structure critical for TSH ligand binding (17). In order to better define the TSH-binding sites on the TSHR, mutagenesis of functional TSHRs was undertaken together with the use of synthetic peptides. Such studies showed that there were multiple TSH-binding sites in the region of the LRRs of the TSHR, which is consistent with the existence of a conformational binding site (21, 22). Another approach employed a panel of epitope-mapped TSHR antibodies to block labeled TSH binding to the native TSHR. This approach defined TSH binding based on TSHR sequences recognized by the antibodies and suggested 3 distinct TSH binding regions in the TSHR: aa 246–260, 277–296, and 381–385 (23, 24). These regions may fold together to form a complex TSH-binding pocket (Figure 2).
Figure 2.
The TSH-binding pocket and TSHR antibody epitopes. (A) Schematic representation of the TSHR ectodomain showing the major regions (black dots) of TSH binding. Figure adapted with permission from Thyroid (24). (B) Model of the TSH-binding pocket, with TSH ligand making contact with the epitopes within and outside of the LRRs. Figure adapted with permission from Thyroid (28).
TSHR cleavage and shedding.
A unique posttranslational proteolytic event clips the TSHR into 2 subunits (3, 4) as indicated in Figure 1. Intramolecular cleavage results in removal of the unique, intervening, approximately 50-aa polypeptide segment in the ectodomain (aa 316–366) (10, 25). This cleavage step may involve an uncharacterized MMP-like enzyme acting at the cell surface (26, 27). Following cleavage, the α and β subunits are disulfide-bonded by cysteine residues flanking the now-absent cleaved 50-aa region, a structure compatible with molecular modeling of the TSHR (28). Subsequently these α-β disulfide links are broken, by protein disulfide isomerase (29) and possibly by progression of β subunit degradation toward the membrane (30), which leads to shedding of the α subunit from the membrane-bound receptor. This explains the high ratio of TSHR β to TSHR α subunits (up to 3:1) found in normal thyroid membrane preparations (10, 11). However, TSHR signal transduction is not dependent on ectodomain cleavage, as has been demonstrated with a noncleavable construct (18).
TSHR multimerization.
Recent studies have documented the propensity of G protein–coupled receptors to form homo- and heterodimeric forms, and these forms may have functional roles in protein trafficking (31), internalization (32), receptor stability (33), and signaling (34). We have found that the TSHR also forms oligomers in both TSHR-transfected cells and native thyrocytes (35). While unstimulated TSHRs were found in multimeric forms (36), this multimeric state was reversed by TSH (37). We still do not understand the physiologic consequences of TSHR multimerization, intramolecular cleavage, and subunit shedding. These fascinating processes most likely have a major effect on thyroid cell function and the interface between the TSHR and the immune repertoire, and they may play a role in the susceptibility of the thyroid to immune attack.
The open and closed TSHR hypothesis.
Unlike other glycoprotein receptors, the TSHR is constitutively active and susceptible to enhanced constitutive activation by mutation, deletions, and even mild trypsin digestion (38, 39). Studies using mutational analyses have suggested that the putative electrostatic interactions between the ectodomain and the extracellular loops of the TMDs in the TSHR may be critical for the maintenance of a relatively inactive “closed” state (16). When these constraints are absent or removed, for example by a mutation or ligand binding, an “open” conformation ensues. This 2-state model predicts that the open format of the receptor, when stabilized, would lead to full activation. Further support for this was provided by the finding that constitutive activation developed when the TSHR ectodomain was truncated, which suggested that the presence of the ectodomain dampened a constitutively active β subunit (40, 41). Additionally, a recent TSHR computer modeling and docking study (28) has shown that several mutations in the TMD that are associated with increased TSHR basal activity are caused by the formation of new interactions that stabilize the open, activated form of the receptor. Therefore, the TSHR must maintain an equilibrium between the inactive (closed) and active (opened) formats at any given time, and any factor (such as a mutation, high-affinity ligand binding, or high-affinity antibody binding) may shift this equilibrium from a quiescent structure (closed) to an active (opened) format.
TSHR and lipid rafts.
Lipid rafts are sphingolipid- and cholesterol-rich membrane microdomains on the plasma membrane. The association of sphingolipids with cholesterol condenses the packing of the sphingolipids, leading to an enhanced mobility within the membrane (42, 43). Lipid rafts have been associated with signal transduction within cells because they sequester signaling proteins. Our observation of the dissociation of TSHR multimers following TSH binding, as discussed above, suggested that these dissociated TSHRs may move more easily into lipid rafts to facilitate signaling (1). Using labeled cholera toxin, which binds to lipid rafts enriched in GM1 gangliosides, we showed that the TSHRs that were localized to lipid rafts in fact moved out with TSH activation (44). Other researchers, using classical differential lipid extraction methodology, were unable to detect either stimulated TSHRs or their main Gsα (called gsp; G–stimulatory protein) signaling partner, within caveolin-restricted lipid rafts (45). These data suggest that the size of monomeric and multimeric receptors may determine their movement in and out of such domains. This, in turn, may influence signal transduction both by TSH and TSHR-stimulating antibodies.
Role of the TSHR in thyroid development.
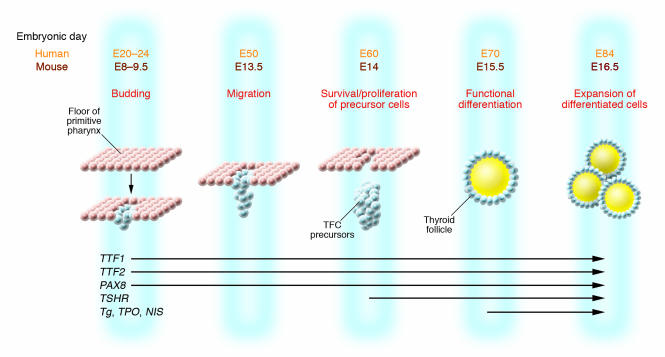
The mouse thyroid gland begins to develop at E8.5 as an endodermal thickening in the floor of the primitive pharynx (46). Thyroid precursor cells express a combination of transcription factors (Figure 3), and by E14, TSHR mRNA appears and the molecular and morphological differentiation of the thyroid gland occurs (47). At E17, the formation of thyroglobulin (Tg; a colloidal protein stored in the lumen of thyroid follicles and the substrate for synthesis of thyroid hormones by iodination) then begins (48). Since the activation of the TSH/TSHR signaling pathway is concurrent with the expression of genes needed for thyroid hormone synthesis and secretion, the TSHR must play an important role in the growth of the thyroid gland (49, 50). This has been confirmed by studies in TSHR-defective mice. In several mutant mouse lines derived to possess a nonfunctional TSHR (e.g., TshrhytTshrhyt) (51), the thyroid gland developed to a much smaller size than normal at 2 months of age in the absence of a functional TSHR. Furthermore, the expression levels of thyroid peroxidase (TPO; the enzyme responsible for Tg iodination) and the sodium iodine symporter (NIS; which transports iodine into the thyroid cell) were greatly reduced. Conversely, no significant changes were detected in the amounts of Tg and transcription factors such as paired box gene 8 (PAX8) and thyroid transcription factors 1 and 2 (TTF1 and TTF2). Separately, in a TSHR-KO mouse model, developed by homologous recombination (52), we found that the thyroid glands were smaller than those of control littermates. Histology of these thyroid glands showed that the TSHR-KO thyroid had fewer follicles and more non–follicle-associated interstitial cells within the gland compared with wild-type thyroids. In humans, fetal thyroid gland formation occurs during weeks 7–9 of gestation, and thyroid embryogenesis is largely complete by 12 weeks of gestation (Figure 3). At 12 weeks, the fetal thyroid is capable of concentrating iodide and synthesizing thyroid hormones, which lessens the dependence on maternal thyroid hormone in the second trimester. Fetal TSH is first detectable at 10 weeks of gestation by bioassay and radioimmunoassay (53). The level of serum TSH at this time is low until a structurally matured thyroid gland is developed at approximately week 18 (53).
Figure 3.
Schematic representation of the stages of development of the thyroid follicular cells and the expression of relevant genes. At mouse E8 (E20 in human), the median thyroid bud appears as a thickening in the floor of the pharynx and expresses a combination of transcription factors such as PAX8, the transcription factor essential for the thyrocyte promoter activation of TPO; Tg and NIS; and TTF1 and TTF2, responsible for morphogenesis of the thyroid gland and maintenance of the thyrocyte cell type. At about E13.5 in mouse and E50 in human, the thyroid diverticulum starts its migration from the pharyngeal floor and reaches its definitive pretracheal position. By E14 (E60 in human), the thyroid follicular cell (TFC) precursors express the TSHR. By E15.5, the thyroid follicular organization appears with the expression of a series of proteins that are essential for thyroid hormone biosynthesis, including TPO, Tg, and NIS. Figure modified with permission from Clinical Genetics (47).
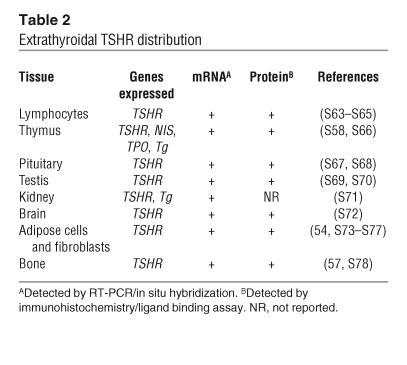
Extrathyroidal TSHRs.
TSHR expression is not unique to thyroid tissue, as originally thought. TSHR mRNA and protein have been detected in a variety of extrathyroidal cell types (1) (Table 2). For example, recent studies using TSHR antibodies have demonstrated the presence of TSHR-specific immunoreactivity in fibroblasts and adipose tissue from the retro-orbital and pretibial space of patients with Graves ophthalmopathy and pretibial myxedema (54, 55). TSHR signal transduction and its consequences in nonthyroidal cells have been poorly investigated to date but appear to modulate a variety of cellular processes. For example, TSH ligand has been implicated in the induction of lipolysis (56) and in the modulation of osteoclast activity (57), and clearly such widespread expression of the TSHR must impact self tolerance to such an important antigen and the immune repertoire.
Table 2.
Extrathyroidal TSHR distribution
Genetic alterations in the TSHR
Germline mutations.
The apparent instability of the TSHR means that point mutations could be expected to result in changes in TSHR function either due to a change in its constitutive signaling ability or, as with any such glycoprotein, secondary to a change in receptor expression. With inactivating mutations, there may be only a partial reduction in TSH function causing partial TSH resistance, and inappropriately increased serum TSH levels, or a more marked loss of TSHR function, resulting in congenital hypothyroidism. The syndromes of TSH resistance, therefore, are heterogeneous in phenotype, genotype, and mode of inheritance.
Compensated partial TSH resistance.
Resistance to TSH is assessed by measuring serum TSH levels (58). Partial TSH resistance is defined as the maintenance of adequate thyroid function resulting in mild (6–15 mU/l) to moderate (20–50 mU/l) elevations in serum TSH associated with normal free thyroid hormone concentrations. In patients suffering from this type of resistance, thyroid volume may be normal, or there may be a modest degree of hypoplasia. While inactivating mutations of the TSHR may be inherited from one or both parents, partial TSH resistance is most commonly inherited from one parent. In cases where there are defects inherited from both parents, the TSH resistance more often becomes more severe. For example, a compound heterozygote (with a P162A mutation on the maternal allele and a C600R mutation on the paternal allele) exhibited a high TSH level, while in cases where only 1 allele was mutated, the TSH levels were lower (59). Figure 4 shows some of the reported mutations resulting in compensated partial TSH resistance. Such mutant TSHR sequences have also been studied using transient expression systems. Their constitutive TSH-independent cAMP activities were markedly reduced compared with those of wild-type TSHRs, and inadequate increases in cAMP generation were seen following TSH stimulation (60). In addition, flow cytometry experiments on the transfected cells showed reduced expression of some mutant TSHRs at the cell membrane. Hence, there is a variety of loss-of-function mutations of the TSHR gene resulting in inappropriately elevated TSH levels in patients. Such patients often have normal thyroid hormone levels, so their condition thus meets the definition of mild thyroid failure (58). However, serum thyroid autoantibodies are usually not detected in these patients.
Figure 4.
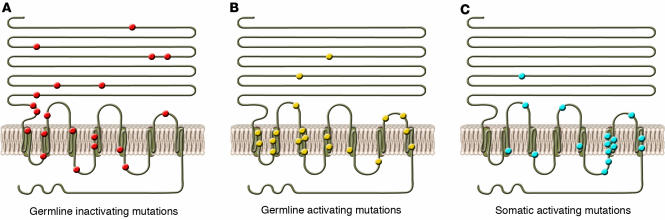
Examples of mutation in human and mouse TSHR. The locations of constitutively germline inactivating (A), germline activating (B), and somatic activating (C) mutations are represented. Most activating mutations (shown in B and C) have been localized to exon 10, which codes for the transmembrane and cytoplasmic regions of the receptor. Figure adapted with permission from The New England Journal of Medicine (S62).
Congenital hypothyroidism.
Mutations in a variety of genes, particularly those involved in thyroid ontogeny, cause thyroid dysgenesis and congenital hypothyroidism at a rate of 1 in 4,000 births. Thyroid dysgenesis can take the form of complete, ectopic, or hypoplastic thyroid development, or the hypothyroidism may be secondary to dyshormonogenesis. Genes known to be involved in this disorder in humans, in addition to TSHR, include TTF1 and TTF2, PAX8, NIS, TPO, and Tg (Figure 3). The sonic hedgehog gene has also been implicated in congenital hypothyroidism in mice (46, 61, 62). Large elevations in TSH levels, along with decreased thyroid hormone levels, are generally found in babies with thyroid hypoplasia or agenesis along with low or absent serum Tg. Such children may have either homozygous or compound heterozygous mutations in their TSHR, which cause uncompensated TSH resistance (63, 64) (Figure 4A). For example, a substitution of threonine in place of a highly conserved alanine at position 553 in the fourth TMD was found to be homozygous in 2 congenitally hypothyroid siblings and heterozygous in both parents and 2 unaffected siblings, resulting in compensated partial TSH resistance in these individuals (60, 65). Functional analysis of cells expressing transfected receptors with this mutation showed extremely low levels of TSHR expression at the surface of these cells compared with cells expressing wild-type receptors. However, not all TSH resistance is secondary to mutations in the TSHR itself (66).
Congenital hyperthyroidism.
Congenital hyperthyroidism is a rare disorder that until recently was considered almost exclusively to be a consequence of maternal Graves disease and transplacental passage of thyroid-stimulating TSHR antibodies (67). However, sporadic cases and a few familial cases of nonautoimmune congenital hyperthyroidism have been reported in which the affected individuals possess activating mutations of the TSHR — often, but certainly not exclusively, in exon 10, which encodes the transmembrane region and intracellular tail (2, 68–70) (Figure 4B). The treatment of congenital hyperthyroidism in the absence of an autoimmune cause involves controlling thyroid function with antithyroid drugs and performing a total thyroidectomy when the child is older than 5 years of age.
Models of congenital hypothyroidism in the mouse.
Two models of complete uncompensated TSH resistance with high TSH levels and low thyroid hormone levels have been developed in the mouse: the TshrhytTshrhyt mouse and the TSHR-KO mouse. Defective thyroid ontogenesis and inherited primary hypothyroidism were first described in the TshrhytTshrhyt mouse in 1981 (71). These mice have thyroids with small and sparse follicles poorly endowed with cytoplasm and typical of marked thyroid hypoplasia. The characteristic defect of the TshrhytTshrhyt mouse is a mutation in the TSHR at aa residue 556, in which a proline is converted to a leucine in TMD IV (72), which results in loss of TSH binding and loss of signal transduction (73). This model also provided proof of the importance of the interaction between extracellular and transmembrane regions that appears to be necessary to facilitate TSH binding and signal transduction and that underlies the molecules’ innate instability. Similarly, the TSHR-KO mouse also showed congenital hypothyroidism and thyroid hypoplasia (52). However, relatively normal thyroid follicles may still be found in both of these animal models, which would indicate that the TSHR is not essential for follicle formation. In the TSHR-KO mouse, the endogenous TSHR promoter results in the expression of GFP (52). Therefore, we were able to use the expression of this reporter gene to confirm the widespread expression of the TSHR in extrathyroidal tissues (1).
Somatic mutations of TSHR that lead to thyroid nodule formation.
There appears to be an increased rate of mutagenesis in the stimulated thyroid gland as seen in rats that develop thyroid nodules following prolonged stimulation with TSH (74). The mechanism of this effect is unknown. Claims that the TSHR may be an oncogene have not been substantiated (75). Common thyroid adenomas are usually well encapsulated benign neoplasms (called nodules or adenomata), which sometimes (approximately 5–10% of the time) may appear to take up excess amounts of radioiodine (“hot” nodules) but more commonly exhibit normal or decreased uptake and retention of radioiodine compared with normal tissue (called “cold” nodules or nontoxic adenomata) (76).
Activating TSHR somatic mutations.
Only somatic mutations with a positive effect on both growth and cell function would be expected to induce clonal expansion and thyroid nodule formation. Autonomous benign and malignant thyroid nodules have been shown to have a variety of point mutations leading to constitutive activation of the TSHR — often referred to as gain-of-function mutations and once again most commonly occurring in the TMDs (as summarized in Figure 4C) (77, 78). Additional tumors may have defects in Gsα rather than the TSHR itself (76). However, hyperthyroidism resulting from single or multiple adenomas (toxic multinodular goiters) is less common than hyperthyroidism resulting from Graves disease. Indeed, the degree of disparity in the prevalence of these entities may be dependent upon the iodine intake of the population at risk (76). Hence, nodules with activating TSHR mutations are more likely to cause hyperthyroidism in the presence of normal or high iodine intake and may be clinically uncommon in geographic areas of iodine deficiency.
Inactivating TSHR somatic mutations.
In contrast to toxic adenomas, inactivating mutations of the TSHR would reduce the uptake of radioiodine into the thyroid follicular cells and would be unlikely to cause nodule formation, since the mutation would fail to induce thyroid cell proliferation. Hence, inactivating somatic TSHR mutations are unusual in these tumors (76). Thyroid cancers frequently exhibit reduced TSHR expression, which is presumably secondary to ongoing dedifferentiation, and this reduces expression of all thyroid-specific genes (76).
Multinodular goiter.
Examination of toxic multinodular goiters (Plummer disease) has revealed separate and distinct activating mutations in different hot nodules within the same thyroid gland. Hence, the autonomous nodules are polyclonal in origin. Activating mutations of the TSHR are present before the formation of an actual nodule (78, 79) and can be seen throughout the gland in toxic multinodular goiters, indicative of their role in true nodule formation.
G protein mutations.
Although outside the scope of this review, the classic example of activating defects of the thyroid arising as a result of mutant Gsα in sporadic cases of thyroid adenomas (76). In these adenomas, somatic point mutations in, for example, Arg201 or Gln227, inhibited GTP hydrolysis and led to the development of autonomous hyperfunctioning nodules (75).
“Specificity crossover” at the TSHR
Promiscuity of the TSHR.
Ligand specificity between TSH, FSH, LH, and human chorionic gonadotropin (hCG) and their cognate receptors is determined by the conformation of the horseshoe structure in the LRRs of their receptor ectodomains, as discussed above (22, 28). The N-terminal portion of the β subunit is additionally important for ligand binding, as indicated, for example, by loss of TSH binding after mutation of Y385 (80) or disruption of sulfation of Tyr385 and Tyr387 (81). However, it has been known for many years that both LH and hCG can interact with the TSHR — a phenomenon termed specificity crossover (82, 83) — and can initiate second signal (84). TSH, in contrast, has poor crossover with the gonadotropin receptors, once again suggestive of the innate instability of the TSHR itself and the ease with which it can be stimulated or blocked. Furthermore, we also showed that normal hCG was able to induce thyroid cell growth (85) as well as activate a recombinant TSHR expressed in mammalian cells (86).
Gestational thyrotoxicosis.
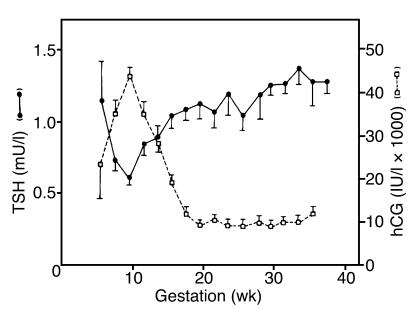
Some degree of increased thyroid stimulation is normal during early pregnancy and is associated with increasing concentrations of serum placental hCG, which peaks at 10–12 weeks (10–40 IU/ml) (Figure 5) (87). In multiple pregnancies, serum hCG levels tend to be even higher, with greater potential impact on thyroid function. Thyroid enlargement, low TSH levels — a sensitive reflection of enhanced thyroid hormone levels — and sometimes a detectable increase in free thyroid hormone levels may all be seen transiently in apparently normal pregnancy (87). This first-trimester phenomenon is often referred to as gestational thyrotoxicosis and is secondary to specificity crossover by hCG at the TSHR. As hCG levels fall, results of thyroid function tests return to normal. However, this degree of transient, mild thyroid overactivity does not require treatment.
Figure 5.
Gestational thyrotoxicosis. Shown here is the inverse relationship between serum hCG and TSH levels in early pregnancy. The level of TSH falls as thyroid function increases. Simultaneously, hCG levels increase. Adapted with permission from The Journal of Clinical Endocrinology and Metabolism (87).
Hyperemesis gravidarum.
Excessive vomiting in early pregnancy is usually self-limited, but 30–50% of women with this disorder have increased thyroid function and may develop mild clinical thyrotoxicosis (82, 83). Such cases are usually associated with high hCG levels, and hCG may show increased thyroid-stimulating activity due to posttranslational changes including reduced sialic acid content. However, not all women with severe hyperemesis have such high hCG levels. A missense mutation in the germline TSHR ectodomain has been reported to cause the TSHR to become supersensitive to hCG and accounts for the development of thyrotoxicosis (88). This mutation resulted in a lysine being replaced with an arginine at position 183 in the fifth LRR. In fact, many mutations at position 183 increase the affinity of hCG for the TSHR (89), and these may occur more commonly in hyperemesis than previously thought (90).
Hydatidiform mole, trophoblastic tumors, and choriocarcinoma.
Enormous quantities of hCG may be secreted by hydatidiform moles, trophoblastic tumors, and choriocarcinoma tissue. When the level exceeds approximately 200 IU/ml, patients develop hyperthyroidism (82, 83). Effective chemotherapy of the tumor returns the patient to euthyroidism. Molecular variants of hCG with increased thyrotropic potency have been described in patients with choriocarcinoma (91, 92). In such patients, the hCG with increased thyroid-stimulating potency may have reduced sialic acid content or a truncated C-terminal tail, among other possibilities (93).
TSHR autoimmunity
AITD.
AITD traditionally includes autoimmune thyroiditis (Hashimoto disease) and autoimmune hyperthyroidism (Graves disease). Graves disease, in particular, is the result of a complex autoimmune response to the TSHR that results in the production of TSHR-specific T cells and TSHR autoantibodies, thought to be secondary to an interaction between susceptibility genes and environmental triggers.
Genetic susceptibility.
The paradigm of a genetic contribution to TSHR autoimmunity is supported by abundant epidemiological and experimental data (for a review, see ref. 94). Graves disease has been known for many years to be familial (95–97), and in a recent study, we have shown that the sibling risk ratio (referred to as 8s) in Graves disease was high, at 11.6 (98). Furthermore, twin studies have shown a concordance rate for Graves disease in monozygotic twins of 20–35% compared with 2–7% in dizygotic twins (99, 100). Several genes and genetic regions have been identified as being linked or associated with AITD (see Potential factors causing susceptibility to TSHR autoimmunity). However, evidence suggesting the association of polymorphisms in the most obvious candidate, the TSHR gene, with Graves disease has been surprisingly weak (101). In contrast, HLA-DR3 was the first Graves disease susceptibility gene to be identified, with an odds ratio of 2–3 (102). More recently, we have shown that the presence of arginine at position 74 of the HLA-DR β1 chain confers most of this susceptibility (102). CTLA-4 is an important costimulatory molecule that participates in the interaction between T cells and APCs (103) and serves mainly to downregulate T cell activation by APCs. The CTLA4 gene has been shown to be weakly and functionally associated with several autoimmune diseases including Graves disease (104–109). Another general autoimmunity gene that was recently identified is protein tyrosine phosphatase non-receptor type 22 (PTPN22). The PTPN22 gene was originally found to be associated with rheumatoid arthritis (110) and was later found to be associated with systemic lupus erythematosus, type 1 diabetes, and Graves disease, with an odds ratio of 1.9 (111–113). Another costimulatory molecule that was recently found to be associated with Graves disease is CD40 (114, 115); however, some investigators have been unable to show this association (116). In addition to these immune response–related genes, we (117) and others (118) have recently shown that the gene encoding Tg is a major gene involved in TSHR-related autoimmunity. Amino acid substitutions in this gene predisposed individuals to Graves disease and autoimmune thyroiditis (117). Most likely, these susceptibility genes interact and influence disease phenotype and severity. For example, we have reported a marked synergistic increase in the risk ratio for individuals carrying a specific HLA-DR3/Tg haplotype (117).
Environmental susceptibility.
While the significantly higher concordance rate for Graves disease in monozygotic twins points to a strong genetic susceptibility, the fact that the concordance rate in monozygotic twins was in fact only approximately 30% rather than 90% may point to significant environmental influences (99, 119). The most important nongenetic potential risk factors contributing to the etiology of AITD, including TSHR autoimmunity, are thought to be dietary iodine intake (95, 120), smoking (121, 122), stress (123, 124), pregnancy, exposure to radiation, and infection (119, 124, 125). All of these suggest environmental triggers; however, their association with AITD requires further investigation and validation.
The TSHR as an autoantigen
Graves disease, T cells, and the discovery of TSHR autoimmunity.
Although a restricted set of T cells reacts to TSHR antigen in patients with TSHR autoimmunity (S1, S2), the fact that a stimulating antibody to the TSHR is the primary cause of the hyperthyroidism has tended to dominate research on this disease. The presence of long-acting thyroid stimulator in the sera of patients with hyperthyroid Graves disease was discovered almost 50 years ago (S3). Patient sera stimulated radioiodine release from prelabeled guinea pig thyroid for a much longer time period than did a pituitary TSH preparation. This prolonged stimulating activity was then found to reside in the IgG fraction of serum from many patients with Graves disease (S4). With the availability of biologically active radiolabeled TSH, it became possible to probe thyroid membranes for the TSHR; the Graves IgG was shown to compete with TSH for receptor occupancy (S5) and contained TSHR antibodies acting as TSH agonists. Autoantibodies that bind to the TSHR and initiate a second signal are TSHR-stimulating, while those that only bind may be TSHR-blocking or TSHR-neutral (Figure 6). The original, and brave, self-infusion of plasma from patients with Graves disease by Adams and colleagues and the resulting thyroid stimulation (S6) was the absolute confirmation of the role of TSHR antibody in the induction of human hyperthyroidism and was one of the first demonstrations of antibody transfer causing autoimmune disease.
Figure 6.
A hypothetical model simplified to explain the effect of structural changes in the TSH-binding site by diverse TSHR antibodies. The TSH-binding pocket, represented by the LRRs, is shown by spirals representing the α helix and the β pleated sheets represented by the wide red arrows. The gray region represents the unique cleaved region (316–366 aa) of the receptor. (A) Epitope A1 represents the site where thyroid-stimulating antibodies bind in part to the LRRs, bringing about a structural change in the receptor that leads to signal transduction; Epitope A2 represents a similar competing site, where TSH-blocking antibodies bind (both illustrated as a best fit). (B) Epitope B is the least common site, where TSHR-blocking antibodies may bind but do not compete with antibodies binding to Epitope A. They bind in part to the LRR region but do not bring about the required structural change for signal transduction yet are still able to hinder TSH binding to this site (illustrated as a good fit). (C) Epitope C is where neutral antibodies bind to the cleaved region and/or the N terminus of the TSHR ectodomain, bringing no appropriate structural alteration to the TSHR and thus leaving the LRR region free for TSH, and other TSHR antibodies, to bind. Thus, neutral antibodies result in no signal transduction and do not block TSH binding (illustrated as no fit).
Animal models of TSHR autoimmunity.
Initial attempts to generate a Graves disease animal model used recombinant human TSHR ectodomain protein from prokaryotic and eukaryotic cell expression systems (S7, S8). The resulting TSHR antibodies blocked the action of TSH on the TSHR (S9, S10). The Shimojo mouse model of Graves disease (S11, S12) was the first system for generating hyperthyroid mice. Fibroblasts expressing both the TSHR and MHC class II antigens were injected intraperitoneally into syngeneic mice, which resulted in thyroid hypertrophy and the presence of TSHR-stimulating antibodies in the serum. The first 260 aa of the TSHR ectodomain, chimeric with the C-terminal ectodomain of the LH receptor, were essential to induce the production of TSHR antibodies (S13), which indicated that TSHR antibody–binding sites existed in the LRR-containing domain (22). This approach also revealed the necessity of immunizing with the intact conformation of the TSHR ectodomain in order to induce TSHR-stimulating antibodies and was later confirmed utilizing TSHR-expressing plasmid and viral vectors (S14– S16). Similarly, the first approximately 289 aa residues of the TSHR α subunit, expressed by an adenovirus vector, induced Graves hyperthyroidism more frequently than did a noncleaving receptor (S17).
TSHR-stimulating antibodies.
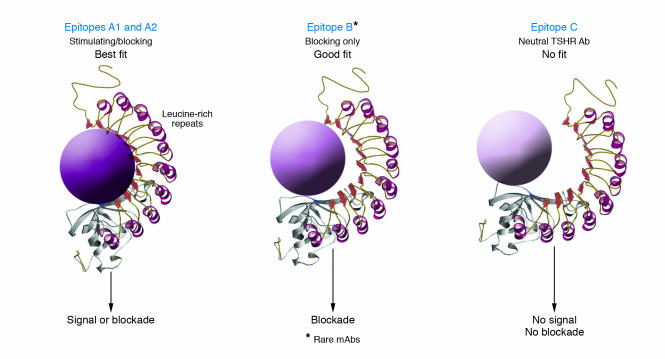
TSHR antibodies are usually of the IgG1 subclass, which has been suggested as evidence of their oligoclonality (S18). At least in animal models of immunization-induced Graves disease, the role of the Th1 immune response may be primary in directing the formation of TSHR antibodies (S19). The conformational binding site of TSHR-stimulating monoclonal antibodies generated from human (S20), hamster (S21), and mouse (S22) is shared and on the TSHR α subunit (approximately 316 aa) (designated as Epitope A on the α/A subunit) (Figure 6A) (S23). Recently, Costagliola et al. have localized the conformational binding site of 1 mouse monoclonal stimulating antibody specific to the TSHR as the N-terminal region of the LRRs in the ectodomain (S24), and this is likely to be the major stimulatory epitope (Figures 2B and 6A). Of additional importance is an observation that shed α subunits preferentially recognize TSHR antibodies compared with intact membrane-bound receptor (S25), which indicates easier access to the epitope. Hence, shed TSHR α subunits have the potential to act as a systemic antigenic stimulus and may contribute to the breakdown of TSHR tolerance in susceptible individuals (S17, S26). However, such fragments may also have the potential to act as “decoys” to divert stimulating antibodies away from the intact TSHR.
TSHR-blocking antibodies.
This class of antibody is able to induce hypothyroidism by blocking the thyrotropic action of TSH. Both TSHR-blocking and -stimulating antibodies are seen in patients with Graves disease and in animal models. In addition, TSHR-blocking antibodies can also be seen in individuals with atrophic Hashimoto disease (S27). In Graves disease, blocking antibodies may play a role in the fluctuation of thyroid function and may contribute to the poor correlation between thyroid function and serum titers of TSHR autoantibodies (S28–S30). During the course of treatment of patients with Graves disease, TSHR-blocking antibodies may become the more prevalent antibody, thus contributing to the development of hypothyroidism (S31). Cases of transient congenital hypothyroidism have been well documented as secondary to placental transfer of maternal TSHR-blocking antibodies (67, S32). It has also been shown that TSHR antibodies from Graves disease patients and atrophic Hashimoto thyroiditis patients can compete for binding to the N terminus of the TSHR β subunit (S33). Recently, a human TSHR-stimulating mAb has been isolated and also found to compete with TSHR autoantibodies in both Graves disease and atrophic Hashimoto thyroiditis (S20). These studies indicate that the binding site for TSHR-stimulating mAbs (designated as Epitope A on the TSHR) is also similar to that of some TSHR blocking antibodies (Figure 6) (S34) and that routine binding competition assays cannot, therefore, distinguish between stimulating and blocking varieties of TSHR antibodies. However, the majority of TSHR-blocking antibodies do not compete with TSHR-stimulating antibodies and bind to an area we have designated as Epitope B (Figure 6).
TSHR-neutral autoantibodies.
The neutral TSHR antibodies, by definition, do not affect TSH binding to the TSHR and bind to a site designated as Epitope C (Figure 6). Their presence was first clearly reported in a patient with Graves disease and monoclonal gammopathy (S35). Results of studies using affinity-purified TSHR have suggested that normal control sera may also contain IgG that binds the TSHR and lacks stimulating or blocking activity and may represent a natural TSHR antibody (S36). In the animal models of Graves disease (S11, S14–S16), the major linear epitopes (small binding regions of aa not influenced by the conformation of the receptor), which were recognized by neutral TSHR antibodies, were mainly the approximately 20 N-terminal aa and/or the TSHR cleaved region (S37) (Figures 1 and 2). Neither of these epitopes appear to be critical for TSH binding to the TSHR (i.e., they are outside the TSH binding pocket; see ref. 24). The clinical or pathophysiological relevance of neutral TSHR antibodies remains unclear. Our recent data, however, indicate that they are capable of inhibiting TSHR cleavage and as a consequence prolong the half-life of the TSHR (S38).
Detecting TSHR antibodies.
TSHR antibodies in the serum of patients with AITD are most commonly measured by receptor protein–binding assays in which labeled TSH is competed with monoclonal TSHR antibodies for binding to target TSHRs. TSH competition assays (112, S39) compete TSHR antibodies with radioiodine-labeled bovine TSH binding to solubilized porcine TSHRs and are available in most commercial clinical laboratories. Hence, the levels of TSHR antibodies can be routinely determined in patients with Graves disease as a measure of disease activity. However, the biological activity of stimulating antibodies must still be measured in a thyroid cell or another mammalian cell with transfected TSHRs in order to detect cAMP generated in response to TSHR antibody stimulation via the TSHR. Levels of TSHR-blocking antibodies can be measured in a similar way by detecting a reduction in TSH-mediated cAMP-generated response. When the patient is hyperthyroid, there is no need for a bioassay to determine the stimulating or blocking activity of the TSHR antibodies present, since the patient serves as the “bioassay” (S40). As discussed earlier, there are also autoantibodies specific to the TSHR that do not compete for TSH binding and, therefore, are not detectable in the current clinical assays (the neutral antibodies).
The new generations of clinical assays for TSHR antibodies.
The original 1974 protein-binding assay for TSHR antibodies used a crude thyroid membrane preparation with a crude Ig fraction of the patients serum but was considerably nonspecific (S41). The first generation of commercial assays, introduced in 1982, used detergent-solubilized porcine TSHRs, with greatly improved specificity compared with that seen in the earlier experimental systems (S42). These were liquid-phase assays in which polyethylene glycol (PEG) was used to separate bound and free, labeled TSH. Similar results were obtained with recombinant human TSHRs and porcine TSHRs, in keeping with the similar sequences of the human and porcine TSHR regions thought to be important for TSHR antibody binding (S43). The second generation of assays was developed in the late 1990s. With the availability of mAbs to the TSHR, it was possible to use solid-phase assays with the TSHR immobilized on plastic tubes (S44) or plates (S45). These serum systems were significantly more sensitive than the PEG-based assays and, when standardized appropriately, yielded results consistent with those of many different commercial assays. Although TSHR antibody assays based on the use of labeled IgG from Graves disease patients were described many years ago (S46), a third generation of apparently more sensitive assays for TSHR antibody detection has been developed only recently (S39). These assays followed the isolation of thyroid-stimulating mAbs to the TSHR. Such solid-phase systems use competition for labeled monoclonal TSHR-stimulating mAb binding to immobilized TSHRs and appear to be more stable than assays employing labeled TSH. Using these newer techniques, many investigators have found that more than 95% of untreated patients with Graves disease have detectable TSHR antibodies at the time of diagnosis.
The clinical utility of TSHR antibody measurement
TSHR antibody measurements serve as a marker for TSHR autoimmunity, particularly in Graves disease. The putative clinical indications for the measurement of TSHR antibodies are summarized in Possible clinical indications for TSHR antibody assessment.
Changes in TSHR antibody levels with administration of antithyroid drugs.
TSHR antibody levels fall after treatment of patients with antithyroid drugs. However, the majority of patients with hyperthyroid Graves disease relapse after stopping a 12-month course of antithyroid drugs, with the actual percentage varying among populations and with their levels of iodine intake (40–70%) (S47). The fall in TSHR antibody levels with antithyroid drug treatment was most clearly demonstrated in a study on patients with Hashimoto thyroiditis (S5). This effect is secondary to a combination of their mild immunosuppressive action and their induction of a decrease in thyroid antigen expression (S48–S50). The measurement of TSHR antibodies in patients with Graves disease has proven to be a useful predictor of relapse and remission after antithyroid drug treatment only if the levels of TSHR antibody remain high (S40, S47, S50, S51). Unfortunately, many studies of the efficacy of using TSHR antibody levels to predict relapse and remission of Graves disease have used TSHR antibody assays of dubious sensitivity, precision, and specificity (S52). Recent improvements in TSHR antibody assays with the introduction of solid-phase systems described above (S39, S44, S45, S53) should improve the accuracy of relapse prediction and treatment evaluation in patients with Graves disease.
Potential factors interfering with the action of TSHR antibodies.
There are a number of potential reasons why measurement of TSHR antibodies may be unreliable in the prediction of thyrotoxic recurrence after antithyroid drug administration in addition to problems with the assay (S40). These include: (a) low antibody quantity (S54); (b) variable antibody affinity; and (c) reduced thyroid function, for example, secondary to concurrent thyroiditis or iodine deficiency. Hence, a negative TSHR antibody assay result is of little help to the clinician. The loss of previously detectable TSHR antibodies indicates that the immune system may have become more tolerant, but such patients may still have a high recurrence rate after discontinuing antithyroid drugs because the TSHR antibodies were undetectable.
Changes in TSHR antibody levels after surgery and radioiodine treatment.
TSHR antibody levels also fall after removal or ablation of the thyroid gland. Since the TSHR is expressed in a variety of extrathyroidal tissues, this effect cannot be secondary to removal of the antigen. More likely, the release of a large quantity of antigen at the time of surgery may induce widespread apoptosis of thyroid-specific T cells and B cells, while radioiodine may be cytotoxic to intrathyroidal immune cells as well as thyroid cells. However, after radioiodine ablation of the thyroid, there is first a marked, but transient, increase in TSHR antibody levels, possibly due to the radiosensitivity of regulatory T cells, before the long-term decrease in levels is seen (S47, S55). This transient phenomenon has been implicated in the deterioration of Graves ophthalmic eye disease after radioiodine administration (S56, S57).
The role of the TSHR in the extrathyroidal manifestations of Graves disease.
As discussed earlier (Table 2), the TSHR is expressed in a wide variety of tissues (1) including the thymus, where it normally allows deletion of TSHR-specific T cells (S58). However, the TSHR is also considered a major antigen leading to the manifestation of extrathyroidal diseases including Graves ophthalmopathy and pretibial myxedema (S59, S60). There is highly suggestive evidence that links the TSHR as a shared thyroidal, retrobulbar, and dermal antigen in patients with Graves disease. For example, TSHR expression in retro-orbital fibroblasts is exacerbated in patients with ophthalmopathy (S59, S61).
Conclusions
Diseases of the thyroid are common, and the TSHR is associated with a great many of these disorders. The TSHR undergoes complex posttranslational processing, which results in a unique structure of 2 covalently linked subunits in its ectodomain. This structure appears to provide inherent instability, since point mutations, mostly in the transmembrane regions, confer constitutive overactivity or underactivity indicative of the conformational changes that ensue. Similarly, the TSHR is highly susceptible to specificity crossover by other glycoprotein hormones such as hCG, which suggests that the receptor can be easily switched from a closed to an open state. The TSHR-stimulating antibodies of Graves disease are similarly dependent on the correct conformation of the TSH and have no interaction with nonglycosylated or reduced receptor, while shed TSHR ectodomains may act as immune stimulants or antibody decoys. Hence, unique posttranslational processing appears to explain the propensity of the TSHRs to be involved in human disease.
Supplementary Material
Acknowledgments
We thank the NIH, the Marvin Sinkoff Endowment, the David Owen Segal Endowment, and the Joseph and Arita Steckler Fund for continuing support.
Note: References S1–S78 are available online with this article; doi:10.1172/JCI26031DS1.
Footnotes
Nonstandard abbreviations used: AITD, autoimmune thyroid disease; FSH, follicle-stimulating hormone; hCG, human chorionic gonadotropin; LH, luteinizing hormone; LRR, leucine-rich repeat; NIS, sodium iodine symporter; PAX8, paired box gene 8; Tg, thyroglobulin; TMD, transmembrane domain; TPO, thyroid peroxidase; TSH, thyroid-stimulating hormone; TSHR, TSH receptor; TTF, thyroid transcription factor.
Conflict of interest: Terry F. Davies is a consultant to Kronus Inc. and RSR Ltd., which manufacture and distribute tests for the measurement of thyroid antibodies including TSHR antibodies, and to the Abbott Corp., which manufactures a thyroid hormone preparation.
References
- 1.Davies TF, Marians R, Latif R. The TSH receptor reveals itself. J. Clin. Invest. 2002;110:161–164. doi:10.1172/JCI200216234. doi: 10.1172/JCI16234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duprez L, et al. Pathology of the TSH receptor. J. Pediatr. Endocrinol. Metab. 1999;12(Suppl. 1):295–302. [PubMed] [Google Scholar]
- 3.Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies [review] Endocr. Rev. 1998;19:673–716. doi: 10.1210/edrv.19.6.0352. [DOI] [PubMed] [Google Scholar]
- 4.Kajita Y, Rickards CR, Buckland PR, Howell RD, Rees Smith B. Analysis of thyrotropin receptors by photoaffinity labeling. Orientation of receptor subunits in the cell membrane. Biochem. J. 1985;227:413–420. doi: 10.1042/bj2270413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parmentier M, et al. Molecular cloning of the thyrotropin receptor. Science. 1989;246:1620–1622. doi: 10.1126/science.2556796. [DOI] [PubMed] [Google Scholar]
- 6.Libert F, et al. Cloning, sequencing and expression of the human thyrotropin (TSH) receptor: evidence for binding of autoantibodies. Biochem. Biophys. Res. Commun. 1989;165:1250–1255. doi: 10.1016/0006-291x(89)92736-8. [DOI] [PubMed] [Google Scholar]
- 7.Nagayama Y, Kaufman KD, Seto P, Rapoport B. Molecular cloning, sequence and functional expression of the cDNA for the human thyrotropin receptor. Biochem. Biophys. Res. Commun. 1989;165:1250–1255. doi: 10.1016/0006-291x(89)92727-7. [DOI] [PubMed] [Google Scholar]
- 8.Misrahi M, et al. Cloning, sequencing and expression of human TSH receptor. Biochem. Biophys. Res. Commun. 1990;166:394–403. doi: 10.1016/0006-291x(90)91958-u. [DOI] [PubMed] [Google Scholar]
- 9.Libert F, Passage E, Lefort A, Vassart G, Mattei MG. Localization of human thyrotropin receptor gene to chromosome region 14q3 by in situ hybridization. Cytogenet. Cell Genet. 1990;54:82–83. doi: 10.1159/000132964. [DOI] [PubMed] [Google Scholar]
- 10.Loosfelt H, et al. Two-subunit structure of the human thyrotropin receptor. Proc. Natl. Acad. Sci. U. S. A. 1992;89:3765–3769. doi: 10.1073/pnas.89.9.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Misrahi M, et al. Processing of the precursors of the human thyroid-stimulating hormone receptor in various eukaryotic cells (human thyrocytes, transfected L cells and baculovirus-infected insect cells) Eur. J. Biochem. 1994;222:711–719. doi: 10.1111/j.1432-1033.1994.tb18916.x. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, Zou M, Parhar RS, Farid NR. High-affinity binding of thyrotropin to the extracellular domain of its receptor transfected in Chinese hamster ovary cells. Thyroid. 1993;3:129–133. doi: 10.1089/thy.1993.3.129. [DOI] [PubMed] [Google Scholar]
- 13.Da Costa CR, Johnstone AP. Production of the thyrotrophin receptor extracellular domain as a glycosylphosphatidylinositol-anchored membrane protein and its interaction with thyrotrophin and autoantibodies. J. Biol. Chem. 1998;273:11874–11880. doi: 10.1074/jbc.273.19.11874. [DOI] [PubMed] [Google Scholar]
- 14.Costagliola S, Khoo D, Vassart G. Production of bioactive amino-terminus domain of the thyrotropin receptor via insertion in the plasma membrane by a glycosylphosphatidylinositol anchor. FEBS Lett. 1998;436:427–433. doi: 10.1016/s0014-5793(98)01177-6. [DOI] [PubMed] [Google Scholar]
- 15.Chazenbalk GD, Nagayama Y, Russo D, Wadsworth HL, Rapoport B. Functional analysis of the cytoplasmic domains of the human thyrotropin receptor by site-directed mutagenesis. J. Biol. Chem. 1990;265:20970–20975. [PubMed] [Google Scholar]
- 16.Vlaeminck-Guillem V, Ho SC, Rodien P, Vassart G, Costagliola S. Activation of the cAMP pathway by the TSH receptor involves switching of the ectodomain from a tethered inverse agonist to an agonist. Mol. Endocrinol. 2002;16:736–746. doi: 10.1210/mend.16.4.0816. [DOI] [PubMed] [Google Scholar]
- 17.Wadsworth HL, Chazenbalk GD, Nagayama Y, Russo D, Rapoport B. An insertion in the human thyrotropin receptor critical for high affinity hormone binding. Science. 1990;249:1423–1425. doi: 10.1126/science.2169649. [DOI] [PubMed] [Google Scholar]
- 18.Chazenbalk GD, Tanaka K, McLachlan SM, Rapoport B. On the functional importance of thyrotropin receptor intramolecular cleavage. Endocrinology. 1999;140:4516–4520. doi: 10.1210/endo.140.10.7031. [DOI] [PubMed] [Google Scholar]
- 19.Wadsworth HL, Russo D, Nagayama Y, Chazenbalk GD, Rapoport B. Studies on the role of amino acids 38-45 in the expression of a functional thyrotropin receptor. Mol. Endocrinol. 1992;6:394–398. doi: 10.1210/mend.6.3.1584215. [DOI] [PubMed] [Google Scholar]
- 20.Chen CR, Tanaka K, Chazenbalk GD, McLachlan SM, Rapoport B. A full biological response to autoantibodies in Graves’ disease requires a disulfide-bonded loop in the thyrotropin receptor N terminus homologous to a laminin epidermal growth factor-like domain. J. Biol. Chem. 2001;276:14767–14772. doi: 10.1074/jbc.M008001200. [DOI] [PubMed] [Google Scholar]
- 21.Nagayama Y, Russo D, Wadsworth HL, Chazenbalk GD, Rapoport B. Eleven amino acids (Lys-201 to Lys-211) and 9 amino acids (Gly-222 to Leu-230) in the human thyrotropin receptor are involved in ligand binding. J. Biol. Chem. 1991;266:14926–14930. [PubMed] [Google Scholar]
- 22.Smits G, et al. Glycoprotein hormone receptors: determinants in leucine-rich repeats responsible for ligand specificity. EMBO J. 2003;22:2692–2703. doi: 10.1093/emboj/cdg260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagayama Y, Wadsworth HL, Russo D, Chazenbalk GD, Rapoport B. Binding domains of stimulatory and inhibitory thyrotropin (TSH) receptor autoantibodies determined with chimeric TSH-lutropin/chorionic gonadotropin receptors. J. Clin. Invest. 1991;88:336–340. doi: 10.1172/JCI115297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffreys J, et al. Characterization of the thyrotropin binding pocket. Thyroid. 2002;12:1051–1061. doi: 10.1089/105072502321085144. [DOI] [PubMed] [Google Scholar]
- 25.Chazenbalk GD, et al. Evidence that the thyrotropin receptor ectodomain contains not one, but two, cleavage sites. Endocrinology. 1997;138:2893–2899. doi: 10.1210/endo.138.7.5259. [DOI] [PubMed] [Google Scholar]
- 26.Couet J, et al. Shedding of human thyrotropin receptor ectodomain. Involvement of a matrix metalloprotease. J. Biol. Chem. 1996;271:4545–4552. doi: 10.1074/jbc.271.8.4545. [DOI] [PubMed] [Google Scholar]
- 27.de Bernard S, et al. Sequential cleavage and excision of a segment of the thyrotropin receptor ectodomain. J. Biol. Chem. 1999;274:101–107. doi: 10.1074/jbc.274.1.101. [DOI] [PubMed] [Google Scholar]
- 28.Nunez Miguel RN, et al. Analysis of the TSH receptor-TSH interaction by comparative modeling. Thyroid. 2004;14:991–1011. doi: 10.1089/thy.2004.14.991. [DOI] [PubMed] [Google Scholar]
- 29.Couet J, et al. Cell surface protein disulfide-isomerase is involved in the shedding of human thyrotropin receptor ectodomain. Biochemistry. 1996;35:14800–14805. doi: 10.1021/bi961359w. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka K, Chazenbalk GD, McLachlan SM, Rapoport B. Subunit structure of thyrotropin receptors expressed on the cell surface. J. Biol. Chem. 1999;274:33979–33984. doi: 10.1074/jbc.274.48.33979. [DOI] [PubMed] [Google Scholar]
- 31.Jordan BA, Trapaidze N, Gomes I, Nivarthi R, Devi LA. Oligomerization of opioid receptors with beta 2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation. Proc. Natl. Acad. Sci. U. S. A. 2001;98:343–348. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devi LA, Brady LS. Dimerization of G-protein coupled receptors [review] Neuropsychopharmacology. 2000;23(4 Suppl.):S3–S4. doi: 10.1016/S0893-133X(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 33.Trapaidze N, Gomes I, Cvejic S, Bansinath M, Devi LA. Opioid receptor endocytosis and activation of MAP kinase pathway. Brain Res. Mol. Brain Res. 2000;76:220–228. doi: 10.1016/s0169-328x(00)00002-4. [DOI] [PubMed] [Google Scholar]
- 34.Overton MC, Blumer KJ. G-protein-coupled receptors function as oligomers in vivo. Curr. Biol. 2000;10:341–344. doi: 10.1016/s0960-9822(00)00386-9. [DOI] [PubMed] [Google Scholar]
- 35.Graves PN, Vlase H, Bobovnikova Y, Davies TF. Multimeric complex formation by the thyrotropin receptor in solubilized thyroid membranes. Endocrinology. 1996;137:3915–3920. doi: 10.1210/endo.137.9.8756566. [DOI] [PubMed] [Google Scholar]
- 36.Latif R, Graves P, Davies TF. Oligomerization of the human thyrotropin receptor: fluorescent protein-tagged hTSHR reveals post-translational complexes. J. Biol. Chem. 2001;276:45217–45224. doi: 10.1074/jbc.M103727200. [DOI] [PubMed] [Google Scholar]
- 37.Latif R, Graves P, Davies TF. Ligand-dependent inhibition of oligomerization at the human thyrotropin receptor. J. Biol. Chem. 2002;277:45059–45067. doi: 10.1074/jbc.M206693200. [DOI] [PubMed] [Google Scholar]
- 38.Van Sande J, et al. Specific activation of the thyrotropin receptor by trypsin. Mol. Cell. Endocrinol. 1996;119:161–168. doi: 10.1016/0303-7207(96)03804-x. [DOI] [PubMed] [Google Scholar]
- 39.Vassart G, Pardo L, Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem. Sci. 2004;29:119–126. doi: 10.1016/j.tibs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Szkudlinski MW, Fremont V, Ronin C, Weintraub BD. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol. Rev. 2002;82:473–502. doi: 10.1152/physrev.00031.2001. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M, et al. The extracellular domain suppresses constitutive activity of the transmembrane domain of the human TSH receptor: implications for hormone-receptor interaction and antagonist design. Endocrinology. 2000;141:3514–3517. doi: 10.1210/endo.141.9.7790. [DOI] [PubMed] [Google Scholar]
- 42.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 43.Simons K, Toomre D. Lipid rafts and signal transduction [review] Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 44.Latif R, Ando T, Daniel S, Davies TF. Localization and regulation of thyrotropin receptors within lipid rafts. Endocrinology. 2003;144:4725–4728. doi: 10.1210/en.2003-0932. [DOI] [PubMed] [Google Scholar]
- 45.Costa MJ, et al. Sphingolipid-cholesterol domains (lipid rafts) in normal human and dog thyroid follicular cells are not involved in thyrotropin receptor signaling. Endocrinology. 2004;145:1464–1472. doi: 10.1210/en.2003-1432. [DOI] [PubMed] [Google Scholar]
- 46.Di Lauro, R., and De Felice, M. 2001. Thyroid gland: anatomy and development in endocrinology. 4th edition. L.J. DeGroot and J.L. Jameson, editors. WB Saunders Co. Philadelphia, Pennsylvania, USA. 1268–1277.
- 47.Van Vliet G. Development of the thyroid gland: lessons from congenitally hypothyroid mice and men [review] Clin. Genet. 2003;63:445–455. doi: 10.1034/j.1399-0004.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- 48.De Felice M, et al. A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat. Genet. 1998;19:395–398. doi: 10.1038/1289. [DOI] [PubMed] [Google Scholar]
- 49.Brown RS. Minireview: developmental regulation of thyrotropin receptor gene expression in the fetal and newborn thyroid. Endocrinology. 2004;145:4058–4061. doi: 10.1210/en.2004-0458. [DOI] [PubMed] [Google Scholar]
- 50.De Felice M, Postiglione MP, Di Lauro R. Minireview: thyrotropin receptor signaling in development and differentiation of the thyroid gland: insights from mouse models and human diseases. Endocrinology. 2004;145:4062–4067. doi: 10.1210/en.2004-0501. [DOI] [PubMed] [Google Scholar]
- 51.Postiglione MP, et al. Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15462–15467. doi: 10.1073/pnas.242328999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marians RC, et al. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15776–15781. doi: 10.1073/pnas.242322099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N. Engl. J. Med. 1994;331:1072–1078. doi: 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- 54.Bahn RS, et al. Thyrotropin receptor expression in Graves’ orbital adipose/connective tissues: potential autoantigen in Graves’ ophthalmopathy. J. Clin. Endocrinol. Metab. 1998;83:998–1002. doi: 10.1210/jcem.83.3.4676. [DOI] [PubMed] [Google Scholar]
- 55.Daumerie C, Ludgate M, Costagliola S, Many MC. Evidence for thyrotropin receptor immunoreactivity in pretibial connective tissue from patients with thyroid-associated dermopathy. Eur. J. Endocrinol. 2002;146:35–38. doi: 10.1530/eje.0.1460035. [DOI] [PubMed] [Google Scholar]
- 56.Vizek K, Razova M, Melichar V. Lipolytic effect of TSH, glucagon and hydrocortisone on the adipose tissue of newborns and adults in vitro. Physiol. Bohemoslov. 1979;28:325–331. [PubMed] [Google Scholar]
- 57.Abe E, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 58.Refetoff S. The syndrome of resistance to thyroid stimulating hormone. J. Chin. Med. Assoc. 2003;66:441–452. [PubMed] [Google Scholar]
- 59.Alberti L, et al. Germline mutations of TSH receptor gene as cause of nonautoimmune subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2002;87:2549–2555. doi: 10.1210/jcem.87.6.8536. [DOI] [PubMed] [Google Scholar]
- 60.Park SM, Clifton-Bligh RJ, Betts P, Chatterjee VK. Congenital hypothyroidism and apparent athyreosis with compound heterozygosity or compensated hypothyroidism with probable hemizygosity for inactivating mutations of the TSH receptor. Clin. Endocrinol. (Oxf.). 2004;60:220–227. doi: 10.1111/j.1365-2265.2004.01967.x. [DOI] [PubMed] [Google Scholar]
- 61.Fagman H, Grande M, Gritli-Linde A, Nilsson M. Genetic deletion of sonic hedgehog causes hemiagenesis and ectopic development of the thyroid in mouse. Am. J. Pathol. 2004;164:1865–1872. doi: 10.1016/S0002-9440(10)63745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Damante G, Tell G, Di Lauro R. A unique combination of transcription factors controls differentiation of thyroid cells. Prog. Nucleic Acid Res. Mol. Biol. 2001;66:307–356. doi: 10.1016/s0079-6603(00)66033-6. [DOI] [PubMed] [Google Scholar]
- 63.Sunthornthepvarakui T, Gottschalk ME, Hayashi Y, Refetoff S. Brief report: resistance to thyrotropin caused by mutations in the thyrotropin-receptor gene. N. Engl. J. Med. 1995;332:155–160. doi: 10.1056/NEJM199501193320305. [DOI] [PubMed] [Google Scholar]
- 64.Refetoff S. Resistance to thyrotropin. J. Endocrinol. Invest. 2003;26:770–779. doi: 10.1007/BF03347364. [DOI] [PubMed] [Google Scholar]
- 65.Abramowicz MJ, Duprez L, Parma J, Vassart G, Heinrichs C. Familial congenital hypothyroidism due to inactivating mutation of the thyrotropin receptor causing profound hypoplasia of the thyroid gland. J. Clin. Invest. 1997;99:3018–3024. doi: 10.1172/JCI119497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie J, et al. Resistance to thyrotropin (TSH) in three families is not associated with mutations in the TSH receptor or TSH. J. Clin. Endocrinol. Metab. 1997;82:3933–3940. doi: 10.1210/jcem.82.12.4418. [DOI] [PubMed] [Google Scholar]
- 67.McKenzie JM, Zakarija M. Fetal and neonatal hyperthyroidism and hypothyroidism due to maternal TSH receptor antibodies. Thyroid. 1992;2:155–159. doi: 10.1089/thy.1992.2.155. [DOI] [PubMed] [Google Scholar]
- 68.Duprez L, et al. Germline mutations in the thyrotropin receptor gene cause non-autoimmune autosomal dominant hyperthyroidism. Nat. Genet. 1994;7:396–401. doi: 10.1038/ng0794-396. [DOI] [PubMed] [Google Scholar]
- 69.Kopp P, et al. Brief report: congenital hyperthyroidism caused by a mutation in the thyrotropin-receptor gene. N. Engl. J. Med. 1995;332:150–154. doi: 10.1056/NEJM199501193320304. [DOI] [PubMed] [Google Scholar]
- 70.Tonacchera M, et al. Functional characteristics of three new germline mutations of the thyrotropin receptor gene causing autosomal dominant toxic thyroid hyperplasia. J. Clin. Endocrinol. Metab. 1996;81:547–554. doi: 10.1210/jcem.81.2.8636266. [DOI] [PubMed] [Google Scholar]
- 71.Beamer WJ, Eicher EM, Maltais LJ, Southard JL. Inherited primary hypothyroidism in mice. Science. 1981;212:61–63. doi: 10.1126/science.7209519. [DOI] [PubMed] [Google Scholar]
- 72.Stein SA, et al. Identification of a point mutation in the thyrotropin receptor of the hyt/hyt hypothyroid mouse. Mol. Endocrinol. 1994;8:129–138. doi: 10.1210/mend.8.2.8170469. [DOI] [PubMed] [Google Scholar]
- 73.Stein SA, et al. The site of the molecular defect in the thyroid gland of the hyt/hyt mouse: abnormalities in the TSH receptor-G protein complex. Thyroid. 1991;1:257–266. doi: 10.1089/thy.1991.1.257. [DOI] [PubMed] [Google Scholar]
- 74.Fernandez Rodriguez A, et al. Induction of thyroid proliferative changes in rats treated with antithyroid compound. Anat. Histol. Embryol. 1991;20:289–298. doi: 10.1111/j.1439-0264.1991.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 75.Matsuo K, Friedman E, Gejman PV, Fagin JA. The thyrotropin receptor (TSH-R) is not an oncogene for thyroid tumors: structural studies of the TSH-R and the alpha-subunit of Gs in human thyroid neoplasms. J. Clin. Endocrinol. Metab. 1993;76:1446–1451. doi: 10.1210/jcem.76.6.8501149. [DOI] [PubMed] [Google Scholar]
- 76.Krohn K, Paschke R. Somatic mutations in thyroid nodular disease. Mol. Genet. Metab. 2002;75:202–208. doi: 10.1006/mgme.2001.3290. [DOI] [PubMed] [Google Scholar]
- 77.Rodien P, Ho SC, Vlaeminck V, Vassart G, Costagliola S. Activating mutations of TSH receptor. Ann. Endocrinol. (Paris). 2003;64:12–16. [PubMed] [Google Scholar]
- 78.Krohn K, Wohlgemuth S, Gerber H, Paschke R. Hot microscopic areas of iodine-deficient euthyroid goitres contain constitutively activating TSH receptor mutations. J. Pathol. 2000;192:37–42. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH650>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 79.Tonacchera M, et al. Activating thyrotropin receptor mutations are present in nonadenomatous hyperfunctioning nodules of toxic or autonomous multinodular goiter. J. Clin. Endocrinol. Metab. 2000;85:2270–2274. doi: 10.1210/jcem.85.6.6634. [DOI] [PubMed] [Google Scholar]
- 80.Kosugi S, Ban T, Akamizu T, Kohn LD. Site-directed mutagenesis of a portion of the extracellular domain of the rat thyrotropin receptor important in autoimmune thyroid disease and nonhomologous with gonadotropin receptors. Relationship of functional and immunogenic domains. J. Biol. Chem. 1991;266:19413–19418. [PubMed] [Google Scholar]
- 81.Costagliola S, et al. Tyrosine sulfation is required for agonist recognition by glycoprotein hormone receptors. EMBO J. 2002;21:504–513. doi: 10.1093/emboj/21.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hershman JM. Human chorionic gonadotropin and the thyroid: hyperemesis gravidarum and trophoblastic tumors [review] Thyroid. 1999;9:653–657. doi: 10.1089/thy.1999.9.653. [DOI] [PubMed] [Google Scholar]
- 83.Hershman JM. Physiological and pathological aspects of the effect of human chorionic gonadotropin on the thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2004;18:249–265. doi: 10.1016/j.beem.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 84.Davies T, Taliadouros G, Catt K, Nisula B. Assessment of urinary thyrotropin-competing activity in choriocarcinoma and thyroid disease: further evidence for human chorionic gonadotropin interacting at the thyroid cell membrane. J. Clin. Endocrinol. Metab. 1979;49:353–357. doi: 10.1210/jcem-49-3-353. [DOI] [PubMed] [Google Scholar]
- 85.Davies TF, Platzer M. hCG-induced TSH receptor activation and growth acceleration in FRTL-5 thyroid cells. Endocrinology. 1986;118:2149–2151. doi: 10.1210/endo-118-5-2149. [DOI] [PubMed] [Google Scholar]
- 86.Tomer Y, Huber GK, Davies TF. Human chorionic gonadotropin (hCG) interacts directly with recombinant human TSH receptors. J. Clin. Endocrinol. Metab. 1992;74:1477–1479. doi: 10.1210/jcem.74.6.1317388. [DOI] [PubMed] [Google Scholar]
- 87.Glinoer D, et al. Regulation of maternal thyroid during pregnancy. J. Clin. Endocrinol. Metab. 1990;71:276–287. doi: 10.1210/jcem-71-2-276. [DOI] [PubMed] [Google Scholar]
- 88.Rodien P, et al. Familial gestational hyperthyroidism caused by a mutant thyrotropin receptor hypersensitive to human chorionic gonadotropin. N. Engl. J. Med. 1998;339:1823–1826. doi: 10.1056/NEJM199812173392505. [DOI] [PubMed] [Google Scholar]
- 89.Smits G, et al. Lysine 183 and glutamic acid 157 of the TSH receptor: two interacting residues with a key role in determining specificity toward TSH and human CG. Mol. Endocrinol. 2002;16:722–735. doi: 10.1210/mend.16.4.0815. [DOI] [PubMed] [Google Scholar]
- 90.Rodien P, et al. Abnormal stimulation of the thyrotrophin receptor during gestation. Hum. Reprod. Update. 2004;10:95–105. doi: 10.1093/humupd/dmh008. [DOI] [PubMed] [Google Scholar]
- 91.Tsuruta E, et al. Pathogenic role of asialo human chorionic gonadotropin in gestational thyrotoxicosis. J. Clin. Endocrinol. Metab. 1995;80:350–355. doi: 10.1210/jcem.80.2.7852489. [DOI] [PubMed] [Google Scholar]
- 92.Pekary AE, et al. Increased in vitro thyrotropic activity of partially sialated human chorionic gonadotropin extracted from hydatidiform moles of patients with hyperthyroidism. J. Clin. Endocrinol. Metab. 1993;76:70–74. doi: 10.1210/jcem.76.1.8421106. [DOI] [PubMed] [Google Scholar]
- 93.Kraiem Z, Lahat N, Sadeh O, Blithe DL, Nisula BC. Desialylated and deglycosylated human chorionic gonadotropin are superagonists of native human chorionic gonadotropin in human thyroid follicles. Thyroid. 1997;7:783–788. doi: 10.1089/thy.1997.7.783. [DOI] [PubMed] [Google Scholar]
- 94.Tomer Y, Davies TF. Searching for the autoimmune thyroid disease susceptibility genes: from gene mapping to gene function. Endocr. Rev. 2003;24:694–717. doi: 10.1210/er.2002-0030. [DOI] [PubMed] [Google Scholar]
- 95.Bartels, E.D. 1941. Heredity in Graves’ disease. Munksgaard. Copenhagen, Denmark. 384 pp.
- 96.Martin L. The heredity and familial aspects of exophthalmic goitre and nodular goitre. Q. J. Med. 1945;14:207–219. [PubMed] [Google Scholar]
- 97.Hall R, Stanbury JB. Familial studies of autoimmune thyroiditis. Exp. Clin. Endocrinol. 1967;2:719–725. [PMC free article] [PubMed] [Google Scholar]
- 98.Villanueva R, Greenberg DA, Davies TF, Tomer Y. Sibling recurrence risk in autoimmune thyroid disease. Thyroid. 2003;13:761–764. doi: 10.1089/105072503768499653. [DOI] [PubMed] [Google Scholar]
- 99.Brix TH, Kyvik KO, Christensen K, Hegedus L. Evidence for a major role of heredity in Graves’ disease: a population-based study of two Danish twin cohorts. J. Clin. Endocrinol. Metab. 2001;86:930–934. doi: 10.1210/jcem.86.2.7242. [DOI] [PubMed] [Google Scholar]
- 100.Ringold DA, et al. Further evidence for a strong genetic influence on the development of autoimmune thyroid disease: the California twin study. Thyroid. 2002;12:647–653. doi: 10.1089/105072502760258613. [DOI] [PubMed] [Google Scholar]
- 101.Ban Y, Greenberg DA, Concepcion ES, Tomer Y. A germline single nucleotide polymorphism at the intracellular domain of the human thyrotropin receptor does not have a major effect on the development of Graves’ disease. Thyroid. 2002;12:1079–1083. doi: 10.1089/105072502321085171. [DOI] [PubMed] [Google Scholar]
- 102.Ban Y, et al. Arginine at position 74 of the HLA-DR beta1 chain is associated with Graves’ disease. Genes Immun. 2004;5:203–208. doi: 10.1038/sj.gene.6364059. [DOI] [PubMed] [Google Scholar]
- 103.Chambers CA, Allison JP. Co-stimulation in T cell responses. Curr. Opin. Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 104.Yanagawa T, Hidaka Y, Guimaraes V, Soliman M, DeGroot LJ. CTLA-4 gene polymorphism associated with Graves’ disease in a Caucasian population. J. Clin. Endocrinol. Metab. 1995;80:41–45. doi: 10.1210/jcem.80.1.7829637. [DOI] [PubMed] [Google Scholar]
- 105.Kouki T, et al. CTLA-4 gene polymorphism at position 49 in exon 1 reduces the inhibitory function of CTLA-4 and contributes to the pathogenesis of Graves’ disease. J. Immunol. 2000;165:6606–6611. doi: 10.4049/jimmunol.165.11.6606. [DOI] [PubMed] [Google Scholar]
- 106.Tomer Y, Greenberg DA, Barbesino G, Concepcion E, Davies TF. CTLA-4 and not CD28 is a susceptibility gene for thyroid autoantibody production. J. Clin. Endocrinol. Metab. 2001;86:1687–1693. doi: 10.1210/jcem.86.4.7372. [DOI] [PubMed] [Google Scholar]
- 107.Ban Y, Tomer Y. The contribution of immune regulatory and thyroid specific genes to the etiology of Graves’ and Hashimoto’s diseases. Autoimmunity. 2003;36:367–379. doi: 10.1080/08916930310001603037. [DOI] [PubMed] [Google Scholar]
- 108.Ueda H, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 109.Ban Y, Tozaki T, Taniyama M, Tomita M. Association of a thyroglobulin gene polymorphism with Hashimoto’s thyroiditis in the Japanese population. Clin. Endocrinol. (Oxf.). 2004;61:263–268. doi: 10.1111/j.1365-2265.2004.02096.x. [DOI] [PubMed] [Google Scholar]
- 110.Begovich AB, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 2004;75:330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bottini N, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat. Genet. 2004;36:337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 112.Smyth D, et al. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53:3020–3023. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- 113.Velaga MR, et al. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves’ disease. J. Clin. Endocrinol. Metab. 2004;89:5862–5865. doi: 10.1210/jc.2004-1108. [DOI] [PubMed] [Google Scholar]
- 114.Tomer Y, Concepcion E, Greenberg DA. A C/T single-nucleotide polymorphism in the region of the CD40 gene is associated with Graves’ disease. Thyroid. 2002;12:1129–1135. doi: 10.1089/105072502321085234. [DOI] [PubMed] [Google Scholar]
- 115.Kim TY, et al. A C/T polymorphism in the 5′-untranslated region of the CD40 gene is associated with Graves’ disease in Koreans. Thyroid. 2003;13:919–925. doi: 10.1089/105072503322511319. [DOI] [PubMed] [Google Scholar]
- 116.Houston FA, et al. Role of the CD40 locus in Graves’ disease. Thyroid. 2004;14:506–509. doi: 10.1089/1050725041517039. [DOI] [PubMed] [Google Scholar]
- 117.Ban Y, et al. Amino acid substitutions in the thyroglobulin gene are associated with susceptibility to human and murine autoimmune thyroid disease. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15119–15124. doi: 10.1073/pnas.2434175100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Collins JE, et al. Association of a rare thyroglobulin gene microsatellite variant with autoimmune thyroid disease. J. Clin. Endocrinol. Metab. 2003;88:5039–5042. doi: 10.1210/jc.2003-030093. [DOI] [PubMed] [Google Scholar]
- 119.Tomer Y, Davies TF. Infection, thyroid disease, and autoimmunity. Endocr. Rev. 1993;14:107–120. doi: 10.1210/edrv-14-1-107. [DOI] [PubMed] [Google Scholar]
- 120.Bulow Pedersen I, et al. Large differences in incidences of overt hyper- and hypothyroidism associated with a small difference in iodine intake: a prospective comparative register-based population survey. J. Clin. Endocrinol. Metab. 2002;87:4462–4469. doi: 10.1210/jc.2002-020750. [DOI] [PubMed] [Google Scholar]
- 121.Prummel MF, Wiersinga WM. Smoking and risk of Graves’ disease. JAMA. 1993;269:479–482. [PubMed] [Google Scholar]
- 122.Vestergaard P, et al. Smoking as a risk factor for Graves’ disease, toxic nodular goiter, and autoimmune hypothyroidism. Thyroid. 2002;12:69–75. doi: 10.1089/105072502753451995. [DOI] [PubMed] [Google Scholar]
- 123.Santos AM, et al. Grave’s disease and stress [abstract] Acta. Med. Port. 2002;15:423–427. [PubMed] [Google Scholar]
- 124.Winsa B, et al. Stressful life events and Graves’ disease. Lancet. 1991;338:1475–1479. doi: 10.1016/0140-6736(91)92298-g. [DOI] [PubMed] [Google Scholar]
- 125.Sonino N, et al. Life events in the pathogenesis of Graves’ disease. A controlled study. Acta. Endocrinol. (Copenh.). 1993;128:293–296. doi: 10.1530/acta.0.1280293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.