Abstract
The Tat protein of human immunodeficiency virus (HIV) type 1 is a transactivator of viral gene expression that is required for virus replication and spread. Moreover, Tat is released by acutely HIV-infected cells via a leaderless secretory pathway and in a biologically active form that exerts effects on both HIV-infected and uninfected cells from different organs and systems. This review focuses on the activities of extracellular Tat protein on endothelial cells, on angiogenesis, and on the pathogenesis of AIDS-associated angioproliferative diseases such as Kaposi's sarcoma. In particular, we discuss results from different groups indicating that Tat mimics the proangiogenic activities of extracellular matrix molecules and that it enhances the effects of angiogenic factors.
INTRODUCTION
New blood vessel formation (angiogenesis) requires coordinated and sequential steps. Specifically, after the degradation of the basement membrane of capillaries or veins by specific proteases, endothelial cells sprout from the preexisting vessel, migrate directionally toward the angiogenic stimuli into the perivascular space, and then proliferate, elongating the new vessel (64). These events are tightly regulated by stimulatory and inhibitory factors, and any failure in these control mechanisms may lead to abnormal blood vessel growth (64).
A disease characterized by pathological blood vessel growth is Kaposi's sarcoma (KS), an angioproliferative disease particularly frequent in human immunodeficiency virus type 1 (HIV-1)-infected patients. KS generally arises on the skin of the extremities as multiple patches, plaques, or nodular lesions, but it can also involve mucosas and visceral organs (158). A milder form of KS occurs sporadically in elderly men of the Mediterranean Eastern-European areas (classical KS) (73) or in organ transplant recipients upon immunosuppressive therapy (posttransplant KS) (136). However, KS has gained much interest since it represents the most frequent and aggressive tumor of HIV-infected individuals (AIDS-KS), particularly homo- and bisexual men (68, 93, 153). Another aggressive form of KS is African KS, which is endemic in subequatorial Africa (169).
In spite of their different clinical courses, the lesions of all forms of KS share a common histopathology. Specifically, early lesions (patch or plaque) appear as a granulation-type reaction infiltrated by immune cells and characterized by intense angiogenesis and proliferating spindle-shaped cells of endothelial and macrophage cell origin, which are considered to be the tumor cells of KS (KS cells [KSC]) (145, 153, 158). In late (nodular) lesions, KSC become the predominant cell type and lesions acquire a fibrosarcoma-like aspect, although neoangiogenesis remains always evident (145, 153, 158).
KSC obtained from early-stage KS lesions do not show a transformed phenotype and, when injected in nude mice, do not promote the development of true tumors, but rather they induce angioproliferative lesions of mouse cell origin which closely resemble early KS lesions. Mouse, KS-like lesions as KS can sporadically regress in humans (51, 154). In contrast, it has been reported that KSC obtained from late-stage KS show in some cases a transformed phenotype, as indicated by the acquisition of monoclonality (141), microsatellite instability (20), altered expression of proto-oncogenes (22, 25, 88, 102, 161), and the capability of promoting tumor development in mice (84, 117). These findings suggest that KS develops as a reactive process which, in time, may evolve into a true sarcoma. However, advanced KS can also be polyclonal, suggesting that transformation to a real monoclonal tumor is a sporadic event (76). In particular, the finding that transformed KSC lines obtained from late-stage KS lesions result in tumors in SCID but not in nude mice (84, 117) suggests that KSC transformation is likely to occur only in severely immunocompromised hosts. This is consistent with the fact that microsatellite instability has been observed in KSC from AIDS-KS lesions but not from classic KS lesions (20).
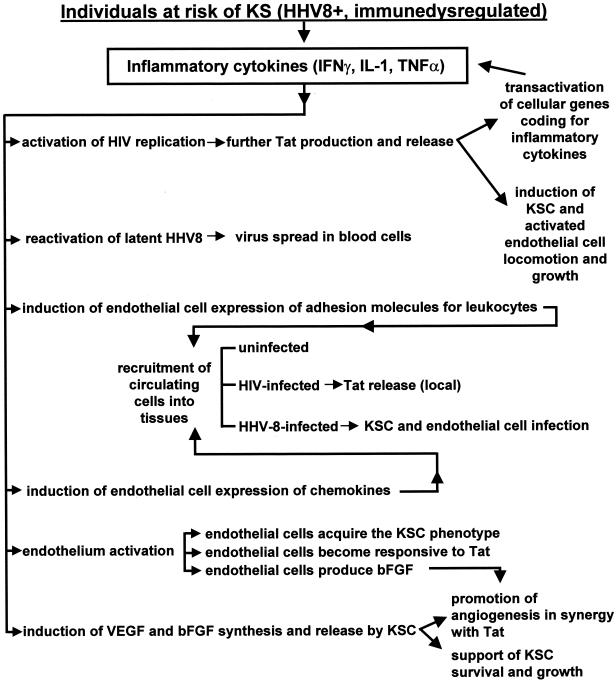
Several studies indicated that a network of soluble inflammatory and angiogenic cytokines promotes KS lesion development and progression by acting both in an autocrine and paracrine fashion (Fig. 1). In particular, all patients with KS or at risk for KS show signs of immune activation such as increased levels in blood of intercellular adhesion molecule 1, neopterin, soluble CD8, and inflammatory cytokines, including interleukin 1 (IL-1), IL-6, tumor necrosis factor (TNF), and gamma interferon (IFN-γ) (19, 37, 47, 57, 69, 85, 86, 111, 134, 148, 149, 166, 188, 196). In addition, high levels of inflammatory cytokines are detected in early KS lesions, which are infiltrated by numerous CD8+ T cells and monocytes that precede KSC appearance (37, 57, 60, 69, 85, 111, 133, 145, 148, 166, 188, 196).
FIG. 1.
Interplay among HHV-8, inflammatory cytokines, angiogenic factors and HIV-1 Tat protein in AIDS-KS pathogenesis.
Of interest, upon their activation by inflammatory cytokines, endothelial cells acquire the phenotypic and functional features of KSC (Fig. 1). These include the spindle cell morphology; the expression of activation markers such as intercellular adhesion molecule 1, vascular cell adhesion molecule type 1, and e-selectin; the down-regulation of factor VIII-related antigen expression; and the production and release of angiogenic factors which, in turn, mediate the development of angioproliferative lesions in vivo (18, 51, 54, 55, 59, 60, 156, 157).
Among the angiogenic factors expressed by KSC in vitro and in vivo, basic fibroblast growth factor (bFGF) is a key mediator of KS lesion formation since it promotes both KSC and endothelial cell locomotion and proliferation in an autocrine and paracrine manner, respectively (51, 54, 55, 197). Injection of bFGF in mice results in the formation of KS-like lesions (54), while bFGF antagonists that include specific antisense oligodeoxynucleotides or neutralizing antibodies block the development of angioproliferative lesions induced by primary KSC upon injection in nude mice (54, 55).
Another angiogenic molecule expressed in vitro and in vivo by KSC is the vascular endothelial cell growth factor type A (VEGF-A) (11, 43, 157, 190), a homodimer belonging to the cysteine knot family of growth factors (181). In KS lesions, VEGF-A is responsible for edema formation and synergizes with bFGF in inducing endothelial cell growth and angiogenesis (43, 157). However, differently from bFGF, VEGF-A has no autocrine growth effect on KSC, although, like endothelial cells, they express both VEGF-A tyrosine kinase receptors: the VEGF receptor 1, encoded by the Flt-1 gene, and the VEGF receptor 2, encoded by the Flk-1 gene (also known as the KDR gene) (43, 70, 157, 190). In contrast, VEGF-C, another member of the VEGF family, stimulates the growth of some KSC isolates which, similarly to lymphatic endothelial cells, express the receptor for this angiogenic factor (95, 118, 192).
Altogether, these data suggest that inflammatory cytokines may behave as KS “initiating factors” since they trigger the production of angiogenic factors which, in turn, promote KS development and progression (Fig. 1). Clinical evidence confirms that immune activation rather than immunodeficiency may play a role in lesion development. In particular, both patients with classic KS and those with African KS show evidence of CD8+ T-cell activation with production of Th-1-type cytokines (19, 98). In addition, AIDS-KS not only can arise in the absence of immune deficiency (14, 29, 112), but it also develops in individuals showing signs of immune activation and Th-1-type cytokine production (47, 159). Furthermore, treatment of AIDS patients with recombinant inflammatory cytokines has resulted in KS onset or progression (1, 6). Finally, immune activation is likely to occur also in posttransplant KS in which, in spite of immune suppressive therapy, allogeneic stimulation may induce local foci of activated immune cells.
Together with increased levels of inflammatory and angiogenic cytokines, another feature common to all forms of KS is infection with human herpesvirus 8 (HHV-8) (126, 133, 175-177). Increased HHV-8 seroprevalence occurs in populations with a high incidence of KS, and elevated anti-HHV-8 antibody titers are predictive of KS development in at-risk individuals (48, 50, 71, 94, 96, 110, 119, 146, 147, 164, 194). However, in those countries where KS is endemic, the prevalence of HHV-8 infection is much higher than KS incidence (73, 194), suggesting that HHV-8 acts as a cofactor in KS development. In particular, HHV-8 could be responsible for immune cell activation and inflammatory cytokine production, as observed for other herpesviruses such as Epstein-Barr virus (36). However, high levels of inflammatory cytokines are also found in uninvolved skin of KS patients or in patients at risk for KS and prior to detection of HHV-8 in blood or tissues examined by PCR (60).
It is noteworthy that inflammatory cytokines reactivate latent HHV-8, increasing the virus load and inducing virus spread to all blood cells (Fig. 1) (7, 81, 124; M. C. Sirianni, L. Vincenzi, S. Topino, E. Scala, A. Angeloni, R. Gonnella, S. Uccini, and A. Faggioni, Letter, J. Infect. Dis. 176:541, 1997). Following HHV-8 spreading, an immune response against the virus is established (96, 110, 147, 164). However, due to the decline in CD4+ T cells (182, 183), decreased NK cytotoxic responses (M. C. Sirianni, L. Vincenzi, S. Topino, A. Giovannetti, F. Mazzetta, C. Alario, and B. Ensoli, Abstr. 2nd Int. Workshop KSHV/HHV-8 Related Agents, abstr., 1999), or up-regulation of inhibitory receptors in killer cells (M. C. Sirianni, C. Alario, F. Libi, D. Scaramuzzi, S. Topino, F. Ensoli, and P. Monini, Abstr. 3rd Int. Workshop Kaposi's Sarcoma-Associated Herpesvirus Related Agents, abstr., 2000), the immune control of HHV-8 infection appears to be impaired in individuals with late-stage AIDS-KS. Subtle immune defects can also occur in patients with late-stage classic and African KS (169; Sirianni et al. abstr., 1999, and abstr., 2000).
Thus, immune activation and inflammatory cytokine production initiate KS, whereas immune defects and HHV-8 escape mechanisms may be key for KS progression.
Paradoxically, the immune response against HHV-8 may even exacerbate KS progression via production of inflammatory cytokines (60, 124). In fact, the increased inflammatory cytokines in individuals with (or at risk for) KS induce both endothelial cells and leukocytes to express adhesion molecules which mediate the adhesion of HIV-infected, HHV-8-infected, or uninfected leukocytes to the endothelium and their migration into tissues (Fig. 1) (60, 203). The recruitment of leukocytes in the lesion is enhanced by several chemokines (e.g., MIP1α and -β, RANTES, monocyte chemotactic protein-1, IL-8, Mig, and IFN-γ-inducible protein 1) that are expressed by KSC, activated leukocytes, or endothelial cells (160).
When HHV-8-infected leukocytes migrate to tissues, they can release virus, establishing a persistent, latent infection of KSC and endothelial cells (Fig. 1). These cells express viral latent gene products (23, 26, 46, 175-177). Among them, the viral homologues of the FLICE inhibitory proteins and cyclins D may provide KSC and endothelial cells with growth and/or antiapoptotic signals (46, 115, 172, 175-177). The long-lasting expression of these HHV-8 latency genes together with the deregulated expression of cellular oncogenes and/or oncosuppressor genes, including int-2, c-myc, bcl-2, and p53 (22, 25, 88, 102, 161), are likely to participate in KS progression toward a real tumor. These findings suggest that the role of HHV-8 in KS pathogenesis may be more important after KS initiation. This hypothesis is supported by the fact that in early KS lesions only a small fraction of KSC is infected by HHV-8, whereas in late-stage lesions most KSC are latently infected (50).
As mentioned above, high levels of inflammatory and angiogenic cytokines and infection by HHV-8 are present in lesions from all forms of KS (56, 60, 126, 149, 166, 175-177). However, KS is much more frequent and aggressive in the setting of HIV-1 infection (68, 153). This suggests that HIV itself or molecules produced during HIV infection could play a role in KS development and progression. The low incidence and the regression of AIDS-KS in individuals treated with highly active antiretroviral therapy, which lowers HIV-1 load in blood and tissues (109), support this concept.
Although HIV can infect endothelial cells in specific anatomical regions (8, 12, 104, 105, 128, 129, 137, 184), this does not lead to endothelial cell growth or angiogenesis; rather, it leads to endothelial cell damage and death (9, 41, 87, 104, 105, 128, 163). Thus, a role for HIV in KS pathogenesis should be indirect. In particular, the development of dermal lesions resembling KS in transgenic mice carrying the HIV-1 tat gene (42, 187) suggested earlier that the Tat protein of HIV-1 could be the pathogenetic link between HIV-1 infection and the highly aggressive form of KS occurring in infected individuals.
Tat PROTEIN OF HIV-1: RELEASE, UPTAKE AND EXTRACELLULAR FUNCTIONS
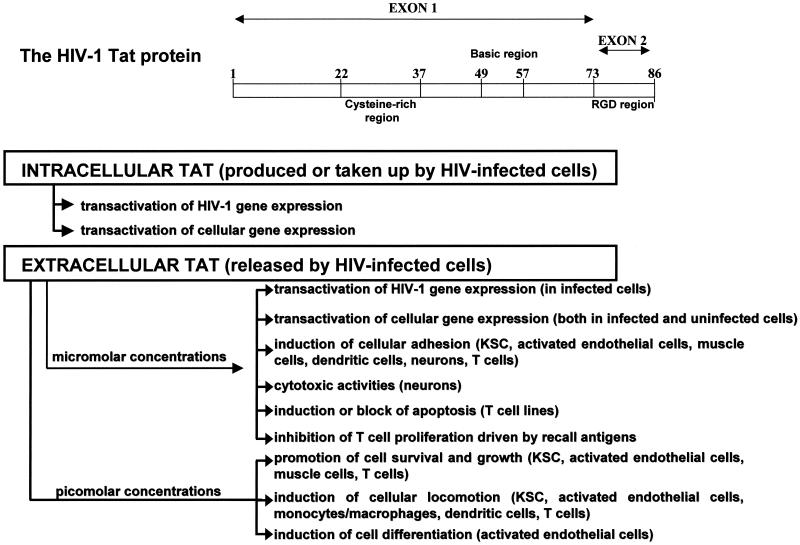
The HIV-1 genome encodes several regulatory proteins that coordinate the expression, transport and assembly of mature viral particles that lead to virus production and transmission (10, 45, 62, 170). Among the HIV-1 regulatory proteins, Tat, a small polypeptide of 86 to 102 amino acids (depending on the viral strain), is essential for virus gene expression and replication (10, 170). In particular, Tat transactivates HIV-1 long terminal repeat-directed gene expression, increasing the expression of all HIV-1 transcripts (Fig. 2) (10, 62). The transcriptional effect of Tat is mediated through its interaction with the Tat activation response element present in all the viral RNAs (72, 170) and occurs through increased rates of transcription, initiation, and elongation (24, 108).
FIG. 2.
Structure and biological properties of HIV-1 Tat protein.
Tat-mediated transactivation requires both the cysteine-rich region of Tat (with seven cysteines in positions 22 to 37) and the highly basic region of Tat (two lysines and six arginines in positions 48 to 57) (Fig. 2). The former mediates the formation of metal-linked dimers, which appear to be necessary for protein activity, and the latter is important for nuclear localization of Tat and nucleic acid binding (66).
The 14 amino acids present in the C-terminal region of Tat are not necessary for the transactivation of HIV-1 gene expression (66). However, among these residues is the arginine-glycine-aspartic acid (RGD) sequence (Fig. 2) that enables Tat to bind and signal through the same integrin receptors that recognize the RGD region of extracellular matrix molecules (16-18, 28, 54, 70, 121).
During acute infection of T cells by HIV-1, Tat is released in the extracellular compartment (Fig. 2) in the absence of cell death or cell permeability changes, even if it lacks a secretory signal (38, 52, 53). This occurs via a specific leaderless secretory pathway that is independent from the endoplasmic reticulum and the Golgi apparatus, as found for IL-1, bFGF, and acidic FGF (38, 77, 92, 150). Tat is also released in vivo both in the tissues and in the blood of HIV-infected individuals (34, 193, 195). Release of Tat occurs very early after infection, at the moment of maximal tat gene expression, and is undetectable when tat expression is low, such as in HIV-1 chronically infected cells or tat permanently expressing cell lines (38, 52, 53). In addition, Tat release depends upon the presence of cytoplasmic localized protein. In fact, mutations in the Tat basic region which reduce the nuclear localization of the protein increase its cytoplasmic localization and release (53).
After release, a fraction of extracellular Tat binds, through its basic residues, to the heparan sulfate proteoglycans present in the cell membrane and extracellular matrix (38). Binding to heparan sulfate proteoglycans protects extracellular Tat from proteolytic degradation, as previously found for several growth factors (reviewed in reference 142). Consistent with its heparin-binding properties, Tat can be purified to homogeneity by heparin-affinity chromatography (38). Of note, the addition of heparin blocks Tat-promoted transactivation of HIV-1 gene expression as well as the other functions of extracellular Tat since it traps the protein and blocks receptor binding and/or the uptake of the protein by the cells (38).
Extracellular Tat, in fact, can act both from the outside of cells and inside cells, exerting effects that depend upon its concentrations and on the target cell type (Fig. 2). Experimental evidence indicates that the biological effects exerted by low (picomolar) concentrations of extracellular Tat are mediated through the interaction with cell surface receptors and by the engagement of specific signal transduction pathways (Fig. 2). In particular, extracellular Tat has been shown to bind the α5β1 and αvβ3 integrin receptors and VEGF receptor 2 (3, 16-18, 28, 101, 123, 171, 186, 189, 201). By these interactions, picomolar concentrations of extracellular Tat have been shown to phosphorylate tyrosine kinases localized at cellular focal adhesion plaques. These include related adhesion focal tyrosine kinase, p130cas, c-Jun amino-terminal kinase, Src kinase, and paxillin (70, 121, 123). In particular, phosphorylation of the focal adhesion kinase 125 by extracellular Tat results in the generation of downstream intracellular signals such as the induction of phosphoinositide 3-kinase activity (120, 121, 123). In contrast, the biological effects of high (nanomolar to micromolar) concentrations of extracellular Tat result mostly from the internalization of the protein followed by its nuclear localization and transcriptional effects on viral and/or cellular gene expression (Fig. 2). Specifically, extracellular Tat can be taken up by a variety of cells in a concentration- and time-dependent fashion and rapidly localizes into the nucleus (53, 58, 65). When taken up by HIV-infected cells, extracellular Tat transactivates long terminal repeat-directed HIV-1 gene expression, increasing HIV-1 production or rescuing Tat-defective HIVs (53, 65). Consistent with these findings, anti-Tat antibodies can inhibit HIV-1 replication in infected cells (35, 173).
Tat can also transactivate, in both infected and uninfected cells, the expression of cellular genes coding for cytokines, chemokines or their receptors, extracellular matrix molecules, and integrins (33, 106, 139, 144, 179, 191; M. Lotz, J. Kekow, M. T. Cronin, J. A. McCutchan; I. Clark-Lewis, D. A. Carson, and W. Wachsman, abstract from the American Association of Immunologists Joint Meeting 1990, FASEB J. 4:2014, 1990). The abnormal expression of these genes may contribute to virus spreading, AIDS development, and/or worsening of the AIDS clinical course (97, 196).
Results from other studies indicated that Tat can also play a role in the tissue destruction often seen in AIDS patients—either directly, by exerting cytotoxic activities (101, 152), or indirectly, through phagocyte recruitment (107).
Micromolar concentrations of extracellular Tat inhibit T-cell proliferation driven by recall antigens (39, 178, 185) and induce apoptosis of T-cell lines (113, 140). In contrast, picomolar concentrations of Tat promote the survival and the growth of the same cell types (143, 198-200). The pleiotropic activities that Tat displays on cell survival and growth are likely mediated by Tat capability of modulating the expression and the activity of genes relevant for cell survival, including p53 (114), bcl-2 (200), and the gene coding for CD95 ligand (193).
One of the most striking properties of extracellular Tat is its capability of mimicking the effect of extracellular matrix proteins on the locomotion, adhesion, and growth of KSC and endothelial cells, thus enhancing angiogenesis and KS progression in HIV-infected patients (Fig. 2).
EFFECTS OF TAT ON PRIMARY KSC CULTURES
Conditioned media from both tat-transfected and HIV-1 acutely infected cells contain extracellular, biologically active Tat. Consistent with the role of Tat in AIDS-KS pathogenesis, these conditioned media stimulate KSC growth in a manner that correlates directly with their Tat content (52, 53). KSC growth induced by the Tat-containing media is specifically inhibited by anti-Tat antibodies (52, 53). In addition, recombinant HIV-1 Tat protein stimulate KSC proliferation at levels comparable to those induced by KSC mitogens such as bFGF (52, 53).
Tat promotes KSC growth at concentrations ranging from 0.05 to 50 ng/ml, whereas no growth stimulation is observed with Tat concentrations higher than 100 ng/ml (52, 53). On the contrary, micromolar concentrations of extracellular Tat are required to transactivate HIV-1 or cellular gene expression (53, 65). This indicates that Tat induces transactivation events and KSC growth through different pathways.
Picomolar concentrations of extracellular Tat also promote KSC directional migration (chemotaxis) and induce KSC to degrade and traverse the basement membrane (invasion) (2). The latter effect of Tat is due to its ability to promote the expression and the activation of matrix metalloproteinase 2 (MMP-2) (17, 54), which plays a major role in both tumor invasion and angiogenesis (32, 162).
The fact that extracellular Tat promotes KSC growth and locomotion may explain, at least in part, the high prevalence and aggressiveness of KS in HIV-1-infected individuals. This is sustained by in vivo findings indicating that Tat increases KS-like lesion formation induced by bFGF injection into mice (18, 54) and that the KSC growth rate increases in the presence of circulating Tat protein (138).
However, other results indicate that Tat alone may not be sufficient for AIDS-KS induction. In fact, although tat-transgenic mice develop KS (42, 187), injection of Tat protein alone promotes neither angiogenesis nor KS-like lesion formation in mice (18, 54). This is consistent with the in vitro finding that Tat cannot induce the growth and locomotion of primary endothelial cells unless they are previously activated with inflammatory cytokines (2, 15, 52, 61).
ACTIVATION OF ENDOTHELIAL CELLS BY INFLAMMATORY CYTOKINES IS REQUIRED FOR Tat BIOLOGICAL EFFECTS
As mentioned above, inflammatory cytokines that are increased in patients with KS or at risk for KS induce endothelial cells to acquire the KSC phenotypic and functional features. Among these features is the responsiveness to the motogenic and mitogenic effects of extracellular Tat. Specifically, after activation by inflammatory cytokines, endothelial cells express MMP-2, invade the extracellular matrix, migrate, and proliferate in response to Tat (2, 15, 17, 18, 59, 61). In this regard we speculated that the high levels of intracellular Tat expressed in tissues of tat-transgenic mice (42) could transactivate inflammatory cytokine gene expression, as previously observed in other experimental systems (Fig. 1) (33, 144; Lotz et al., FASEB J. 4:2014, 1990). This will increase inflammatory cytokine levels in tat-transgenic mice, thus rendering murine endothelial cells responsive to the angiogenic effects of extracellular Tat protein.
The main inflammatory cytokine that is required for endothelial cell response to Tat is IFN-γ, although TNF alpha (TNF-α) and IL-1β contribute to these effects in a synergistic fashion (61). This is consistent with IFN-γ's capability of upregulating TNF receptor expression (135) and with the fact that TNF and IL-1 augment IFN-γ receptor levels (103).
Extracellular Tat also induces activated endothelial cells to differentiate and to organize into interconnecting tubular structures, the new capillaries (2). As it promotes endothelial cell locomotion, growth, and differentiation, extracellular Tat can be considered to be an angiogenic factor. Similarities between Tat and angiogenic factors include the following: (i) heparin-binding properties and presence as both soluble and extracellular bound forms (4, 38, 151), (ii) phosphorylation of focal adhesion kinases and generation of phosphoinositide 3-kinase activity (3, 70, 120, 121, 123), (iii) promotion of vascular cell growth (3, 15, 17, 18, 52-54, 59, 61, 123, 171), (iv) promotion of vascular cell migration (2-4, 17, 18, 59, 61, 123), (v) promotion of vascular cell invasion by inducing release of active metalloproteinases (2, 17, 18, 54, 61, 123), (vi) promotion of endothelial cell differentiation on tridimensional matrices (2), and (vii) promotion of angiogenesis in vivo (chicken chorioallantoic membrane assay, nude mouse model) (3, 4, 18, 27, 54, 123). However, differently from a true angiogenic molecule such as bFGF, Tat acts only on endothelial cells that are activated by inflammatory cytokines (2, 15-18, 54, 61) (Table 1).
TABLE 1.
Comparative analysis of Tat and bFGF angiogenic effectsa
| Biological effect | Effect exhibited in response to:
|
|
|---|---|---|
| Tat | bFGF | |
| EC invasion | No | Yes |
| IC-EC invasion | Yes | Yesb |
| KSC invasion | Yes | Yesc |
| EC migration | No | Yes |
| IC-EC migration | Yes | Yesb |
| KSC migration | Yes | Yesc |
| EC growth | No | Yes |
| IC-EC growth | Yes | Yesd |
| KSC growth | Yes | Yesc |
Human umbilical vein endothelial cells were cultured for 5 days in the absence (endothelial cells [EC]) or in the presence of human recombinant IL- 1β, TNF-α, and IFN-γ (endothelial cells activated by inflammatory cytokines [IC-EC]) combined together at 5, 0.5, and 0.1 ng/ml, respectively. KSC were obtained from AIDS-KS lesions, cultured, and characterized as previously reported (130, 154). Data from references 2, 15, 17, 52-54, and 59-61.
IC-EC show enhanced invasion and migration in response to bFGF compared to EC. This is probably due to a synergy between bFGF and inflammatory cytokines in the induction of metalloproteases (80).
The effect of bFGF on KSC invasion, migration, and growth is less evident than that exerted on EC. This is probably due to autocrine bFGF production by KSC (51, 54, 55).
Exogenous bFGF promotes IC-EC growth to a lesser extent than EC. This is in agreement with IL-1's capability of down-regulating the expression of high-affinity bFGF receptors in the endothelium (44).
By inducing endothelial cell responsiveness to Tat, inflammatory cytokines are likely to play a major role in AIDS-KS induction and progression. In fact, the same inflammatory cytokines that induce endothelial cell responsiveness to Tat also activate HIV-1 replication, thus leading to further production of Tat, which then acts as a KS progression factor (15). In addition, when Tat and inflammatory cytokines are present at the same time in the microenvironment, KSC growth is further enhanced (Fig. 1) (15).
Thus, in order to exert its in vitro angiogenic effects, Tat requires the cooperation of IFN-γ, IL-1β, and TNF-α, whose levels are increased in blood and lesions of AIDS-KS patients or in individuals at risk for KS. These results suggest that the simultaneous presence of Tat and inflammatory cytokines may be responsible for the high frequency and aggressiveness of KS in HIV-1-infected individuals.
Concerning the forms of KS that are not associated with HIV infection, such as classic and posttransplant KS, inflammatory cytokines are sufficient to initiate KS, by inducing the synthesis of angiogenic growth factors, including bFGF and VEGF (18, 59, 60, 155-157). After KS initiation, HHV-8 is likely to play a major role in KSC transformation and/or KS progression (46, 115, 172, 175-177). Nevertheless, the absence of Tat could explain why classic and posttransplant KS have a milder clinical course than AIDS-KS.
Tat BINDS VASCULAR CELLS THROUGH MULTIPLE DOMAINS
The finding that Tat acts only on cytokine-activated endothelial cells suggests that its angiogenic effects are mediated by an inducible receptor. This hypothesis is supported by the finding that Tat promotes the adhesion of endothelial cells only when cells are activated by the same inflammatory cytokines that induce the responsiveness to the other effects of Tat (16).
By promoting cellular adhesion, Tat mimics the effect of extracellular matrix molecules such as fibronectin or vitronectin. Similarities between Tat and extracellular matrix molecules include the following: (i) presence of an RGD sequence (16, 28), (ii) presence of a heparin-binding domain (4, 17, 38, 151), (iii) binding to integrin receptors (αvβ3, αvβ5, and α5β1) (16-18, 54, 61, 123, 171, 186, 201), (iv) phosphorylation of the focal adhesion kinases (70, 120, 121, 123), (v) promotion of cellular adhesion and modulation of cellular response to mitogens (16-18, 28, 54, 101, 123, 171), (vi) promotion of cellular migration (2-5, 17, 18, 21, 61, 106, 107, 123), (vii) induction of the synthesis of matrix metalloproteases (17, 54), and (viii) modulation of the balance between the soluble and sequestered fraction of angiogenic molecules (17). Consistent with these findings, the same inflammatory cytokines that induce endothelial cells to attach onto immobilized Tat also augment endothelial cell adhesion to fibronectin or vitronectin (16).
Results from studies performed to identify the domain(s) mediating the cell adhesion effect of Tat indicate that, as for fibronectin or vitronectin, vascular cell attachment to Tat is mainly mediated by the RGD region (amino acids 84 to 86) of the protein (16, 28) (Table 2 and Fig. 1). The RGD region of extracellular matrix molecules, including fibronectin and vitronectin, binds cell surface receptors belonging to the integrin family (90). Tat can compete with fibronectin or vitronectin for binding to these same integrins. Specifically, Tat inhibits KSC and endothelial cell attachment to fibronectin or vitronectin, and RGD peptides spanning the cell attachment domain of fibronectin block KSC and endothelial cell adhesion to Tat (16). Consistent with these findings, antibodies directed against the α5β1 integrin (the main fibronectin receptor) and the αvβ3 integrin (the main vitronectin receptor) strongly inhibit KSC and endothelial cell adhesion to immobilized Tat (16). This indicates that Tat is specifically recognized by these RGD-binding integrins. This finding is likely to have relevance in AIDS-KS pathogenesis since in primary AIDS-KS lesions extracellular Tat costains with αvβ3 and α5β1, which are highly expressed by both KSC and activated endothelial cells lining the blood vessels (54, 59-61).
TABLE 2.
Interaction of Tat domains with vascular cell surface molecules mediating Tat angiogenic effects
| Effect on endothelial cells | Tat domain involved | Cell surface molecule(s) involved |
|---|---|---|
| Focal adhesion kinase phosphorylationa | RGD | α5β1 and αvβ3 |
| Basic | VEGF receptor 2 | |
| Cysteine rich (participates) | VEGF receptor 2 | |
| Adhesionb | RGD (main mediator) | α5β1 and αvβ3 |
| Basic (participates) | Heparan sulfate proteoglycans | |
| Growthc | Basic (main mediator) | VEGF receptor 2, heparan sulfate proteoglycans |
| Cysteine rich (participates) | VEGF receptor 2? | |
| RGD (participates) | α5β1 and αvβ3 | |
| Migration and/or invasiond | RGD (main mediator) | α5β1 and αvβ3 |
| Basic (participates) | Heparan sulfate proteoglycans, VEGF receptor 2? |
See references 70, 123, and 171.
See references 16, 17, 54, and 123.
See references 4, 16-18, 52-54, 59-61, 123, 127, 138, and 171.
See references 2-4, 17, 18, 54, 61, 123, and 171.
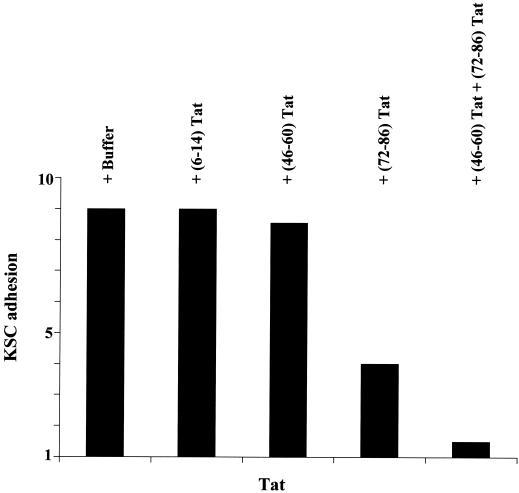
However, anti-α5β1 and -αvβ3 antibodies cannot completely block vascular cell adhesion to Tat. This occurs only when cells are preincubated with peptides spanning both the RGD and the basic region of Tat (16) (Table 2 and Fig. 3). In agreement with these results, Tat mutants in the basic or RGD sequence have a reduced capability of promoting endothelial cell adhesion (123). Thus, both the basic and RGD sequences of Tat are involved in the adhesion effect of Tat. This is consistent with the finding that both Tat basic and RGD peptides mimic Tat's capability of phosphorylating the focal adhesion tyrosine kinases (70, 120, 121, 123) (Table 2).
FIG. 3.
KSC adhesion to Tat is mediated by the RGD region of the protein, whereas Tat basic sequence facilitates this interaction. KSC were suspended by trypsinization and preincubated for 90 min at 4°C with rotation and with one of the following competitor peptides (at 10 μg/ml): (6-14)Tat, a peptide spanning the Tat N-terminal region; (46-60)Tat, a peptide spanning the Tat basic region; or (72-86)Tat, a peptide spanning the Tat C-terminal region and containing the RGD sequence (all from American Biotechnologies Inc.). After incubation, KSC were seeded on plastic plates (Flow Laboratories) coated with recombinant Tat protein (30 μg/cm2) and incubated for 1 h at 37°C in a CO2 atmosphere. Nonadherent cells were removed by washing the plates with phosphate-buffered saline solution, whereas adherent cells were fixed, stained, and quantitated as previously described (16, 28). The number of adherent cells is expressed as the fold increase in KSC attachment compared to the adhesion seen with of bovine serum albumin (30 μg/cm2). This did not induce KSC attachment and was given a unitary value.
Data obtained with nonactivated endothelial cells indicated that the Tat RGD region binds cells with a low affinity, while the Tat cysteine-rich and basic regions bind cells with high affinity (123). Therefore, the binding affinity of the different Tat domains may depend upon the endothelial cell activation state (2, 15, 59, 61).
To identify the domains mediating the effects of Tat on KSC and endothelial cells, overlapping peptides spanning the Tat amino acid sequence have been tested for the ability to promoting the invasion, migration, and growth of cytokine-activated endothelial cells or KSC. Results indicated that multiple domains mediate these in vitro angiogenic effects of Tat. In particular, a pivotal role is played by both the basic and the RGD sequences of Tat, consistent with the finding that both these domains also mediate the effects of Tat on neurons, monocytes, and dendritic cells (21, 101, 152). In addition, the Tat cysteine-rich region has also been found to possess angiogenic activities (27).
Tat RGD SEQUENCE MEDIATES THE LOCOMOTION OF VASCULAR CELLS AND PROVIDES THE CELLS WITH THE ADHESION SIGNAL THEY REQUIRE IN ORDER TO PROLIFERATE
The αvβ3 and α5β1 integrins play a major role in new blood vessel formation. In particular, α5β1 modulates endothelial cell response to angiogenic growth factors (91) and endothelial cell migration toward fragments of extracellular matrix molecules (89, 202). The αvβ3 integrin is required for in vivo angiogenesis (30, 31), representing a survival system for neoforming vessels. In particular, the αvβ3-mediated endothelial cell adhesion to extracellular matrix suppresses the activity of the p53 gene and of the p53-inducible cell cycle inhibitor p21waf-1/cip-1 and increases bcl-2 levels, thus inhibiting cell apoptosis (174). Triggering of αvβ3 also promotes endothelial cell migration (202). In addition, αvβ3 binds MMP-2, facilitating the oriented cellular invasion (32). Finally, αvβ3 participates in bFGF-promoted angiogenesis (30, 31) and is required for the full activation of VEGF receptor 2 triggered by VEGF-A (171). The fact that the RGD region of Tat can bind αvβ3 and α5β1 suggests that these integrins mediate, at least in part, Tat-induced angiogenesis.
Our recent work, in fact, indicates that the binding of the Tat RGD region to α5β1 and αvβ3 specifically triggers KSC and endothelial cell locomotion (Table 2). In particular, among various peptides spanning the Tat amino acid sequence, only the Tat RGD peptide induces KSC and endothelial cell chemotaxis (17). This is consistent with the chemotactic activity of the RGD region of fibronectin and vitronectin (references 89 and 202 and data presented above). In fact, as found for fibronectin and vitronectin, anti-α5β1 and/or anti-αvβ3 monoclonal antibodies specifically inhibit KSC or endothelial cell migration promoted by Tat or the Tat RGD peptide (17).
Peptides spanning Tat basic residues do not induce a significant endothelial cell migration (17). This is different from what was observed in monocytes, whose migration is promoted by both the RGD and the basic Tat peptide (21). This discrepancy may be explained by the finding that Tat-promoted monocyte migration is mediated by the binding of Tat basic residues to the VEGF receptor 1 (122), a receptor mediating VEGF-induced cell migration (131). In contrast, Tat basic residues are not capable of binding and activating VEGF receptor 1 in endothelial cells (3). Nevertheless, Tat mutants in the basic region show a reduced capability of promoting endothelial cell migration, thus suggesting that also the basic sequence participates in the chemotactic effect of Tat (123). This may be due to the heparin-binding properties of the Tat basic region (38), which facilitates Tat binding to the integrins, as found for the Tat adhesion effect (16) (Fig. 3 and Table 2) and for cellular migration promoted by extracellular matrix molecules (reference 202 and data presented above). However, it is also possible that the mutated Tat protein utilized by Mitola et al. (123) could present an altered conformation and spatial configuration of the Tat RGD domain.
Anti-α5β1 and anti-αvβ3 antibodies also inhibit Tat-induced vascular cell invasion (17). This is consistent with the Tat RGD region's capability of activating MMP-2 expression at levels comparable to those observed with full-length Tat (17, 54). In fact, the triggering of α5β1 or αvβ3 by the RGD sequence of fibronectin or vitronectin activates MMP-2 gene expression (32, 162). These results are also supported by the finding that the Tat RGD region induces the expression of focal adhesion kinase 125, which is activated by integrin triggering and has a major role in cellular locomotion (107, 121).
It is well established that a specific growth factor can act only on cells expressing specific integrins (75). The interactions between growth factors and integrins play a key role in the control of cellular functions. In fact, integrin-induced signaling pathways are also activated by growth factor binding to their receptors (75). Consistent with these findings, the binding of the Tat RGD domain to α5β1 and αvβ3 is also involved in the vascular cell growth induced by this viral protein. In fact, mutations of the Tat RGD region impair Tat's capability of promoting endothelial cell proliferation (123). In particular, endothelial cells show an increased proliferative response to bFGF when they are seeded onto immobilized Tat protein (17, 54). This phenomenon, which was previously observed for fibronectin or vitronectin (30, 91), suggests that the binding of the Tat RGD sequence to the integrins provides endothelial cells with the adhesion signal they require in order to grow. This is also supported by Tat's capability of promoting KSC expression of the same mitogen-activated protein kinases induced by integrin triggering (70).
Consistent with these data, we have recently shown that α5β1 and αvβ3 antagonists, such as monoclonal antibodies directed against these receptors or cyclic RGD peptides, specifically inhibit FGF-induced proliferation of endothelial cells seeded onto immobilized Tat (17).
Altogether these results demonstrate that Tat enhances angiogenesis and KS development by a molecular mimicry of extracellular matrix molecules. This is supported by the recent finding that transgenic mice expressing an RGD deletion mutant of Tat develop a milder form of KS than transgenic mice carrying wild-type Tat (138).
However, cell adhesion is not sufficient to induce endothelial cell proliferation that also requires soluble angiogenic factors (30, 31, 78, 91).
Tat BASIC REGION RETRIEVES SEQUESTERED, EXTRACELLULAR-BOUND bFGF INTO A SOLUBLE FORM AND INDUCES VEGF RECEPTOR 2 PHOSPHORYLATION
Peptides encompassing the Tat basic sequence induce the growth of both KSC and cytokine-activated endothelial cells (17). In agreement with this finding, Tat proteins with mutations in the basic sequence show a reduced capability of promoting endothelial cell growth (123).
The highly basic charge of Tat residues 48 to 57 has previously suggested that binding of Tat to the cell surface may be the result of nonspecific ionic interactions (28). In fact, Tat basic residues can bind several different molecules expressed on the membrane of several cell types (3, 4, 17, 21, 38, 122, 123, 125, 127, 151, 152, 171, 186, 189, 193). For endothelial cells, these are αvβ5, an integrin that recognizes the vitronectin basic sequence (186); cell membrane- or extracellular matrix-associated heparan sulfate proteoglycans (4, 17, 38, 151); VEGF receptor 2 (3, 70, 123, 125, 127, 171); and VEGF receptor 1 (122).
The interaction between the Tat basic region and αvβ5 is unlikely to play a significant role in AIDS-KS pathogenesis, since anti-αvβ5 antibodies do not inhibit Tat angiogenic effects (17, 186).
In contrast, the binding of Tat to heparin and heparan sulfate proteoglycans has profound effects on Tat-promoted angiogenesis. In particular, the interaction between Tat and proteoglycans on one hand facilitates the binding of the Tat RGD region to the integrins (Fig. 3 and Table 2; see also data presented under “Tat Binds Vascular Cells through Multiple Domains”) and on the other hand enables Tat to compete with bFGF for the same heparin-binding sites and to release bound bFGF in a soluble form. In fact, similarly to Tat, bFGF binds to heparan sulfate proteoglycans of the cell surface and extracellular matrix, remaining soluble only in fraction (13, 63, 155). Both soluble and extracellularly bound bFGFs are biologically active (13, 63). However, the bound bFGF fraction represents a localized storage of the growth factor, protected from proteolytic degradation and solubilized only under emergency conditions such as wound repair (13, 63). Sequestered, extracellularly bound bFGF can be retrieved into a soluble form by heparin or Tat or the Tat basic peptide (13, 17, 38, 63, 155). This may explain why the combination of Tat and heparin exerts angiogenic effects in vivo (4).
By displacing preformed extracellularly bound bFGF through a competitive effect for heparin-binding sites of the cell surface and extracellular matrix (17), Tat basic peptide increases soluble bFGF to amounts that promote KSC and endothelial cell growth (17). Neutralizing anti-bFGF antibodies or specific antisense oligomers that inhibit bFGF synthesis and release (55) block Tat-promoted KSC or endothelial cell growth (17). Thus, bFGF triggers the mitogenic effect of Tat on vascular cell types.
Tat basic sequence can also bind VEGF receptor 2, as already observed for the binding of the basic sequence of VEGF-A to VEGF receptor 2 (99). Although Tat has the RKK (66) and not the RKH sequence that is required by VEGF-A in order to bind VEGF receptor 2 (99), Tat can stimulate VEGF receptor 2 phosphorylation in both endothelial cells (3, 123, 171) and KSC (70). In addition, Tat binding to VEGF receptor 2 leads to the activation of the same signal transduction pathways which are triggered by VEGF-A binding to VEGF receptor 2 (70, 123). Tat basic peptides can mimic VEGF receptor 2 activation exerted by the full-length Tat protein (123). However, Tat proteins with mutations in the basic region are still capable of activating signal transduction pathways triggered by VEGF receptor 2 phosphorylation and capable of promoting endothelial cell proliferation (123). This may be due to the Tat RGD region, which provides the adhesion signal required for cellular growth (see “Tat RGD Sequence Mediates the Locomotion of Vascular Cells…” above). In fact, most of the signaling pathways activated by Tat or VEGF-A binding to VEGF receptor 2 are the same as those induced by vitronectin, fibronectin, or Tat binding to αvβ3 or α5β1 (40, 70, 82, 120, 121).
Concerning Tat interaction with VEGF receptor 1, Tat binds this receptor on monocytes (122) but not on endothelial cells (3). Although these data are somewhat odd since VEGF receptor 1 is the same in the two cell types, they indicate that Tat behaves differently from VEGF-A, which binds both VEGF receptor 1 and VEGF receptor 2 in endothelial cells (131). While the triggering of VEGF receptor 2 by VEGF-A promotes both endothelial cell proliferation and migration, VEGF-A binding to VEGF receptor 1 induces only endothelial cell migration (131). This is because VEGF receptor 1 does not activate mitogen-activated protein kinases (131). Thus, the VEGF receptor 2 tyrosine kinase receptor is the main mediator of VEGF-A angiogenic activity. Nevertheless, a VEGF dimer can bind and link together either two VEGF receptors 2 or VEGF receptor 1 and VEGF receptor 2. Like the VEGF receptor 2 homodimers, the VEGF receptor 1-VEGF receptor 2 heterodimers also undergo autophosphorylation following VEGF-A binding, and this can mediate VEGF-A angiogenic effects (131). The finding that Tat does not bind VEGF receptor 1 on endothelial cells may contribute to explaining why Tat and VEGF-A have different effects on endothelial cells. A first important difference between Tat and VEGF is that VEGF-A induces the growth and locomotion of endothelial cells, which constitutively express VEGF receptor 1 and VEGF receptor 2 (131), whereas Tat cannot, unless cells are preactivated by inflammatory cytokines (2, 15, 59, 61). In this regard, it should be noted that bFGF but not VEGF is synthesized and released by endothelial cells activated by inflammatory cytokines that induce their responsiveness to Tat (155-157). These in vitro data are also consistent with the in vivo finding that, differently from VEGF-A, injection of purified Tat protein alone does not trigger angiogenesis in mice (18, 54), despite the expression of VEGF receptor 1 and VEGF receptor 2 in mouse tissues (49, 180). Another difference is that Tat promotes KSC growth, while exogenous VEGF-A has no growth effects on these cells (157). Moreover, Tat does not synergize with VEGF in promoting endothelial cell proliferation (18). In contrast, Tat enhances the mitogenic effect of bFGF on endothelial cells, consistent with its capability of maintaining exogenously added bFGF into a soluble form (17, 18, 54).
An intriguing possible explanation for the lack of synergy between Tat and VEGF-A is that Tat could bridge its cysteine-rich region with that of one VEGF monomer, forming VEGF-Tat heterodimers. This phenomenon, which was previously described for the placenta growth factor, may reduce the availability of active VEGF molecules, since VEGF heterodimers cannot signal as well as VEGF homodimers (132). Alternatively, Tat may behave as platelet factor 4, which, because of its heparin-binding property, compromises the interactions between VEGF and heparan sulfate proteoglycans, thus inhibiting VEGF binding to VEGF receptor 2 (74). Another possible explanation for the lack of synergy of Tat and VEGF is the competition of Tat for vitronectin binding to αvβ3 (16), which is required for a full VEGF receptor 2 activation (171).
Indeed, other results suggest that Tat binding to VEGF receptor 2 may even inhibit some VEGF-A biological effects. In particular, VEGF blocks Tat-induced endothelial cell migration (3), consistent with the finding that VEGF can inhibit Tat binding to both endothelial cells and monocytes (3, 122). However, in KS lesions VEGF amounts are much greater than those of Tat (54, 157) making it difficult for VEGF receptor 2 competition by Tat and VEGF to occur in vivo.
THE CYSTEINE-RICH REGION OF Tat IS ANGIOGENIC IN VIVO
Boykins and coworkers have recently shown that peptides spanning the cysteine-rich domain of Tat promote angiogenesis in the chicken chorioallantoic membrane assay (27). This is the first report indicating that a cysteine-rich peptide can exert direct angiogenic effects.
The cysteine residues mediate the formation of Tat dimers, which are required for the transactivating property of this protein (66). Sequences rich in cysteines similar to that of Tat are also present in angiogenic molecules such as VEGF, placenta growth factor, and platelet-derived growth factor. These sequences mediate growth factor dimerization, which is required for receptor binding (181). At present little is known about the mechanisms explaining the angiogenic effect of the Tat cysteine-rich peptide. Although it has been shown that the cysteine-rich domain of Tat can bind endothelial cell membrane with high affinity (see “Tat Binds Vascular Cells through Multiple Domains” above), mutations in the cysteine-rich region do not affect Tat's capability of binding endothelial cells and of promoting their adhesion (123). Recent studies indicate that mutations in the cysteine-rich region reduce Tat's capability of inducing endothelial cell migration (123), although peptides spanning the Tat cysteine-rich region promote monocyte but not endothelial cell migration (5). Furthermore, Tat proteins with mutations in the cysteine-rich region show a decreased capability of promoting endothelial cell growth in vitro and angiogenesis in vivo (123), and they fail to transduce in endothelial cells the intracellular signals normally triggered by wild-type Tat, such as the induction of phosphoinositide 3-kinase activity (123). These results suggest that Tat dimerization caused by the bridging of cysteine residues from two Tat monomers may be essential for Tat binding to a receptor mediating the mitogenic and motogenic effects of Tat. However, the findings that Tat basic peptides directly promote endothelial cell growth and that Tat RGD peptides directly induce endothelial cell migration argue against this hypothesis.
Tat REQUIRES ADDITIONAL FACTORS TO EXERT ANGIOGENIC EFFECTS IN VIVO
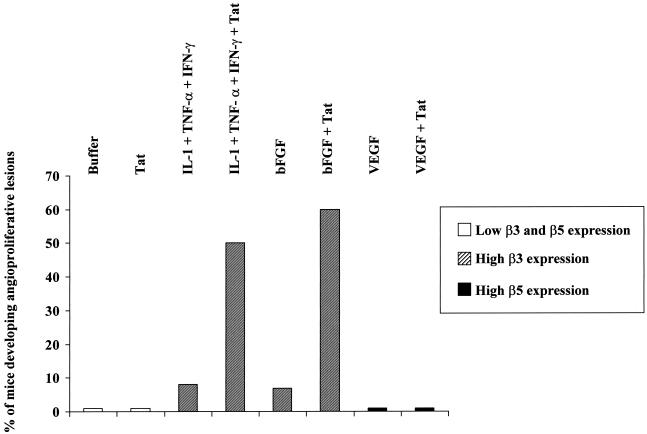
As described above under “Activation of Endothelial Cells by Inflammatory Cytokines Is Required for Tat Biological Effects,” primary endothelial cells need to be activated by inflammatory cytokines in order to respond to Tat motogenic and mitogenic effects. The requirement of other factors for Tat angiogenic effects is observed also in vivo. In fact, although tat-transgenic mice develop dermal lesions closely resembling human KS (42, 187), injection of recombinant purified and biologically active Tat protein in nude mice has no effects (18, 54) (Fig. 4). In contrast, when Tat is injected in combination with suboptimal (non-lesion-forming) amounts of bFGF it promotes the development of angioproliferative, KS-like lesions in nude mice (18, 54) (Fig. 4). The angiogenic effect of Tat and bFGF is reproduced by the injection of Tat and combined IL-1β, TNF-α, and IFN-γ, which are the same cytokines that induce endothelial cell responsiveness to Tat in vitro (18) (Fig. 4).
FIG. 4.
Combined IL-1β, TNF-α, and IFN-γ or bFGF, but not VEGF, synergizes with Tat in inducing the development of angioproliferative, KS-like lesions in nude mice. Nude mice received subcutaneous injections with recombinant IL-1β, TNF-α, and IFN-γ (0.5 μg of each), bFGF (0.1 μg), VEGF (1 μg), or Tat (10 μg), alone or combined. Results are expressed as the percentage of mice developing macroscopic angioproliferative lesions 6 days after protein inoculation (18, 54). Eight to twelve mice were inoculated per experimental condition. Results from staining experiments performed with rabbit polyclonal antibodies by the peroxidase-antiperoxidase method (18, 54) on frozen tissues from nude mice injected with the above-mentioned cytokines are expressed as β3 or β5 expression level increases over the expression of these integrins in tissues from mice injected with protein resuspension buffer (phosphate-buffered saline solution-0.1% bovine serum albumin) (18).
Concentrations of IL-1β, TNF-α, and IFN-γ exerting angiogenic synergy with Tat in vivo induce both bFGF and VEGF expression in mouse tissues (18). These two angiogenic molecules are highly expressed in primary KS lesions, where they synergize in promoting angiogenesis and vascular permeability (43, 51, 54, 55, 155-157, 197). These findings suggested that both bFGF and VEGF represent the triggering signal of Tat-induced angiogenesis. However, as discussed above, differently from bFGF, VEGF does not synergize with Tat in promoting in vivo angiogenesis (18) (Fig. 4). Consistent with these in vivo results, and as found for inflammatory cytokines (see “Tat Binds Vascular Cells through Multiple Domains” above), exposure to bFGF induces endothelial cells to adhere to Tat, while VEGF has no effects (18).
Tat ANGIOGENIC EFFECTS CORRELATE WITH β3 INTEGRIN EXPRESSION INDUCED BY INFLAMMATORY CYTOKINES OR bFGF, BUT NOT WITH VEGF-PROMOTED β5 EXPRESSION
Although triggered by soluble angiogenic factors, angiogenesis is modulated by integrin receptors mediating the adhesive interactions between endothelial cells and extracellular matrix molecules (64). In particular, previous studies indicated that bFGF promotes angiogenesis by inducing the α5β1 and αvβ3 integrins (30, 67, 100), which bind the RGD region of fibronectin, vitronectin (90), and Tat (16-18). In contrast, VEGF-induced angiogenesis requires the engagement of αvβ5 (67), an integrin that binds the basic residues of both vitronectin and Tat (186).
As mentioned above under “Tat RGD Sequence Mediates the Locomotion of Vascular Cells…” and “Tat Basic Region Retrieves Sequestered, Extracellularly Bound bFGF,” the interaction between the Tat RGD region and α5β1 or αvβ3 results in events that lead to new blood vessel formation, whereas Tat binding to αvβ5 is not involved in Tat angiogenic effects (Table 2). In agreement with these findings, immunohistochemical stainings of tissues from mice injected with inflammatory cytokines, bFGF, or VEGF indicate that the in vivo angiogenic effect of Tat correlates with expressions of β3 and, to a lesser extent, β1, which are induced by inflammatory cytokines or bFGF, but not with β5 expression, which is promoted by VEGF (18) (Fig. 4). In addition, Tat's capability of increasing the soluble fraction of bFGF further upregulates the expression of the αvβ3 and α5β1 integrins, which in turn mediate Tat-promoted endothelial cell invasion, migration, and adhesion (18). Thus, inflammatory cytokines, through bFGF induction, or exogenous bFGFs synergize with Tat in promoting neoangiogenesis, because they upregulate the expression of α5β1 and αvβ3 integrins, which function as the receptors for Tat (16). These results explain why Tat requires bFGF, or those cytokines that induce bFGF production and release, to exert its activities. On the contrary, VEGF-A and Tat do not synergize in vivo (18) (Fig. 4), either because VEGF promotes the expression of αvβ5, which does not mediate the angiogenic effects of Tat (17, 188), or because VEGF-A and Tat share (and compete for) the same receptor (3, 123). Consistent with these in vivo data, exposure to bFGF induces endothelial cells to migrate and proliferate in response to Tat, while VEGF has no effect (18). Thus, Tat interaction with αvβ3 and, to a lesser extent, α5β1 has a major role in the angiogenic, KS-promoting effect of this viral protein.
The inhibition of the interaction between the Tat RGD region and the integrins has been shown to inhibit Tat binding to the cell surface and Tat angiogenic effects (16-18, 21, 28, 54, 70, 120, 121, 123). Similarly, the angiogenic effects of VEGF-A and bFGF are blocked by αvβ3 antagonists, although these molecules do not inhibit VEGF-A or bFGF binding to endothelial cells (17, 30, 31, 123, 171). In particular, αvβ3 has been recently shown to participate in the full activation of VEGF receptor 2 triggered by VEGF-A. In fact, αvβ3-mediated adhesion to vitronectin enhances in endothelial cells the phosphorylation of VEGF receptor 2 and the proliferative response to VEGF (171). This is because VEGF receptor 2 triggering by VEGF-A induces the formation of a VEGF receptor 2/β3 complex (171).
Thus, antagonists of RGD-binding integrins, previously shown to inhibit angiogenesis in several in vitro and in vivo models (30, 31, 79), could be useful in blocking Tat, bFGF, and VEGF angiogenic effects that lead to KS development and progression. In agreement with this hypothesis, integrin antagonists such as cyclic RGD peptides (79, 83) specifically block the development of angioproliferative, KS-like lesions induced in nude mice by the injection of combined Tat and bFGF (18).
CONCLUSIONS
The angiogenic properties of extracellular Tat have linked this HIV-1 protein to AIDS-KS pathogenesis. However, in order to exert its angiogenic effects, Tat requires the cooperation of inflammatory cytokines, including IL-1β, TNF-α, and IFN-γ, whose levels are increased in the blood and tissues of individuals at risk for KS or in AIDS-KS patients (2, 15-18, 59-61, 166). These cytokines augment (in KSC) or induce (in endothelial cells) the synthesis and release of bFGF, an angiogenic molecule highly expressed in primary KS lesions (54, 55, 155, 157, 197). Indeed, Tat enhances bFGF's capability of promoting angiogenesis both in vitro and in vivo (17, 18, 54). This is because, by competing for heparin-binding sites, the highly basic residues of Tat retrieve extracellularly bound bFGF into a soluble form which promotes endothelial cell growth and upregulates the expression of α5β1 and αvβ3 (17, 18). These integrins, in turn, bind the Tat RGD region and, by this interaction, mediate Tat-promoted locomotion of KSC and activated endothelial cells and provide these vascular cell types with the adhesion signal they require in order to grow in response to bFGF (16, 17, 54). These effects of the Tat RGD region are consistent with the role of the RGD region of extracellular matrix molecules in angiogenesis (30, 31, 79, 89).
The same inflammatory cytokines that cooperate with Tat in promoting angiogenesis and KS progression also increase KSC production of VEGF-A, another angiogenic factor expressed in KS lesions (18, 43, 157). It is noteworthy that Tat binds and phosphorylates VEGF receptor 2, the receptor that mediates most of VEGF-A's angiogenic effects (3, 70, 123, 127). The fact that Tat and VEGF-A share the same receptor may explain why, as opposed to what occurs with Tat and bFGF, Tat does not enhance VEGF angiogenic effects either in vitro or in vivo (18). However, in AIDS-KS lesions VEGF concentrations are much higher than those of Tat (43, 54, 155, 157, 190), making unlikely an inhibitory or activating effect of Tat on VEGF receptor 2 in vivo.
In conclusion, although some discrepancies due to differences in the experimental systems employed exist, results from several groups point out HIV-1 Tat as a key progression factor of KS since it enhances all the biological steps of angiogenesis and KS progression.
Elevated levels of bFGF, VEGF, and inflammatory cytokines are detectable in AIDS-KS lesions, where extracellular Tat costains with α5β1 and αvβ3 in both KSC and endothelial cells (54). This suggests that the mechanisms of Tat action described here operate in vivo and that Tat acts as a progression factor for AIDS-KS, which is the most aggressive form of the disease. In fact, different from what is seen with HHV-8, inflammatory cytokines, and angiogenic factors that are present in all forms of KS, Tat is present only in AIDS-KS.
Thus, KS is a multifactorial disease, and the combined action of HHV-8 latency genes, deregulated cellular proto-oncogenes, and Tat may favor the transformation of KS into a true tumor, particularly in immunocompromised individuals.
The data described herein suggest new intervention strategies for AIDS-KS based on the use of Tat and integrin competitors, combined with anti-inflammatory and antiangiogenic drugs, but also suggest novel immunotherapeutic strategies, such as the development of a Tat-based vaccine. This vaccine has recently been shown to contain virus replication and to block the onset of AIDS in vaccinated monkeys upon challenge with a pathogenic virus (34, 35). Since no anti-Tat antibody responses are found in AIDS-KS patients, unlike what has been found in our observations of HIV-infected individuals without KS (our unpublished data), it is likely that a vaccine based on Tat may be a key to reducing KS incidence and progression in HIV-1-infected individuals. To this end, studies comparing the Tat immune responses (both humoral and cellular) in AIDS-KS patients with those in other HIV-infected individuals are in progress.
Acknowledgments
The results described herein were obtained through research supported by grants from the AIDS Program of the Italian Ministry of Health and the Italian Association for Cancer Research (AIRC).
We thank Cecilia Sgadari, Elena Toschi, Stefano Buttò, and Paolo Monini (Laboratory of Virology, Istituto Superiore di Sanità, Rome, Italy) and Federico Bussolino (University of Turin, Turin, Italy) for helpful discussion and A. Lippa and A. Carinci for editorial assistance.
REFERENCES
- 1.Aboulafia, D., S. A. Miles, S. R. Saks, and R. T. Mitsuyasu. 1989. Intravenous recombinant tumor necrosis factor in the treatment of AIDS-related Kaposi's sarcoma. J. Acquir. Immune Defic. Syndr. 2:54-58. [PubMed] [Google Scholar]
- 2.Albini, A., G. Barillari, R. Benelli, R. C. Gallo, and B. Ensoli. 1995. Angiogenic properties of human immunodeficiency virus type 1 Tat protein. Proc. Natl. Acad. Sci. USA 92:4838-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albini, A., R. Soldi, D. Giunciuglio, E. Giraudo, R. Benelli, L. Primo, D. Noonan, M. Salio, G. Camussi, W. Rockl, and F. Bussolino. 1996. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat. Med. 2:1371-1375. [DOI] [PubMed] [Google Scholar]
- 4.Albini, A., R. Benelli, M. Presta, M. Rusnati, M. Ziche, A. Rubartelli, G. Paglialunga, F. Bussolino, and D. Noonan. 1996. HIV-1 tat protein is a heparin binding angiogenic growth factor. Oncogene 12:289-297. [PubMed] [Google Scholar]
- 5.Albini, A., R. Benelli, D. Giunciuglio, T. Cai, G. Mariani, S. Ferrini, and D. Noonan. 1998. Identification of a novel domain of HIV tat involved in monocyte chemotaxis. J. Biol. Chem. 273:15895-15900. [DOI] [PubMed] [Google Scholar]
- 6.Albrecht, H., H. J. Stellbrink, G. Gross, B. Berg, U. Helmchen, and H. Mensing. 1994. Treatment of atypical leishmaniasis with interferon-gamma resulting in progression of Kaposi's sarcoma in an AIDS patient. Clin. Investig. 72:1041-1047. [DOI] [PubMed] [Google Scholar]
- 7.Ambroziak, J. A., D. J. Blackbourn, B. G. Herndier, R. G. Glogau, J. H. Gullett, A. R. McDonald, E. T. Lennette, and J. A. Levy. 1995. Herpes-like sequences in HIV-infected and uninfected Kaposi's sarcoma patients. Science 268:582-583. [DOI] [PubMed] [Google Scholar]
- 8.An, S. F., M. Groves, B. Giometto, A. A. Beckett, and F. Scaravilli. 1999. Detection and localisation of HIV-1 DNA and RNA in fixed adult AIDS brain by polymerase chain reaction/in situ hybridisation technique. Acta Neuropathol. (Berlin) 98:481-487. [DOI] [PubMed] [Google Scholar]
- 9.Annunziata, P., C. Cioni, S. Toneatto, and E. Paccagnini. 1998. HIV-1 gp120 increases the permeability of rat brain endothelium cultures by a mechanism involving substance P. AIDS 12:2377-2385. [DOI] [PubMed] [Google Scholar]
- 10.Arya, S. K., C. Guo, S. F. Josephs, and F. Wong-Staal. 1985. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science 229:69-73. [DOI] [PubMed] [Google Scholar]
- 11.Ascherl, G., C. Hohenadl, O. Schatz, E. Shumay, J. Bogner, L. Eckart, E. Tschacler, P. Monini, B. Ensoli, and M. Stürzl. 1999. Infection with human immunodeficiency virus-1 increases expression of vascular endothelial growth factor in T cells: implications for acquired immunodeficiency syndrome-associated vasculopathy. Blood 93:4232-4241. [PubMed] [Google Scholar]
- 12.Bagasra, O., E. Lavi, L. Bobroski, K. Khalili, J. P. Pestaner, R. Tawadros, and R. J. Pomerantz. 1996. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS 10:573-585. [DOI] [PubMed] [Google Scholar]
- 13.Baird, A., D. Schubert, N. Ling, N., and R. Guillemin. 1988. Receptor- and heparin-binding domains of basic fibroblast growth factor. Proc. Natl. Acad. Sci. USA 85:2324-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballard, H. S. 1985. Disseminated Kaposi's sarcoma without lymphocyte abnormalities. Arch. Intern. Med. 145:547.. [PubMed] [Google Scholar]
- 15.Barillari, G., L. Buonaguro, V. Fiorelli, J. Hoffman, F. Michaels, R. C. Gallo, and B. Ensoli. 1992. Effects of cytokines from activated immune cells on vascular cell growth and HIV-1 gene expression. Implications for AIDS Kaposi's sarcoma pathogenesis. J. Immunol. 149:3727-3734. [PubMed] [Google Scholar]
- 16.Barillari, G., R. Gendelman, R. C. Gallo, and B. Ensoli. 1993. The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc. Natl. Acad. Sci. USA 90:7941-7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barillari, G., C. Sgadari, V. Fiorelli, F. Samaniego, S. Colombini, V. Manzari, A. Modesti, B. C. Nair, A. Cafaro, M. Stürzl, and B. Ensoli. 1999. The Tat protein of human immunodeficiency virus type 1 promotes vascular cell growth and locomotion by engaging the α5β1 and αvβ3 integrins and by mobilizing sequestered basic fibroblast growth factor. Blood 94:663-672. [PubMed] [Google Scholar]
- 18.Barillari, G., C. Sgadari, C. Palladino, R. Gendelman, A. Caputo, C. B. Morris, B. C. Nair, P. Markham, A. Nel, M. Stürzl, and B. Ensoli. 1999. Inflammatory cytokines synergize with the HIV-1 Tat protein to promote angiogenesis and Kaposi's sarcoma via induction of basic fibroblast growth factor and the αvβ3 integrin. J. Immunol. 163:1929-1935. [PubMed] [Google Scholar]
- 19.Beckstead, J. H. 1992. Oral presentation of Kaposi's sarcoma in a patient without severe immunodeficiency. Arch. Pathol. Lab. Med. 116:543-545. [PubMed] [Google Scholar]
- 20.Bedi, G. C., W. H. Westra, H. Farzadegan, P. M. Pitha, and D. Sidransky. 1995. Microsatellite instability in primary neoplasms from HIV+ patients. Nat. Med. 1:65-68. [DOI] [PubMed] [Google Scholar]
- 21.Benelli, R., R. Mortarini, A. Anichini, D. Giunciuglio, D. M. Noonan, S. Montalti, C. Tacchetti, and A. Albini. 1998. Monocyte-derived dendritic cells and monocytes migrate to HIV-Tat RGD and basic peptides. AIDS 12:261-268. [DOI] [PubMed] [Google Scholar]
- 22.Bergman, R., M. Ramon, S. Kilim, C. Lichtig, and R. Friedman-Birnbaum. 1996. An immunohistochemical study of p53 protein expression in classical Kaposi's sarcoma. Am. J. Dermatol. 18:367-370. [DOI] [PubMed] [Google Scholar]
- 23.Blasig, C., C. Zietz, B. Haar, F. Neipel, S. Esser, N. H. Brockmeyer, E. Tschachler, S. Colombini, B. Ensoli, and M. Stürzl. 1997. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. J. Virol. 71:7963-7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohan Morris, C. A., F. Kashanchi, B. Ensoli, L. Buonaguro, K. A. Boris-Lawrie, and J. Brady. 1992. Analysis of Tat transactivation of human immunodeficiency virus transcription in vitro. Gene Expr. 2:391-407. [PMC free article] [PubMed] [Google Scholar]
- 25.Bohan Morris, C. A., R. Gendelman, A. J. Marrogi, M. Lu, J. M. Lockyer, W. Alperin-Lea, and B. Ensoli. 1996. Immunohistochemical detection of Bcl-2 in AIDS-associated and classical Kaposi's sarcoma. Am. J. Pathol. 148:1055-1063. [PMC free article] [PubMed] [Google Scholar]
- 26.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 27.Boykins, R. A., R. Mahieux, U. T. Shankavaram, Y. S. Gho, S. F. Lee, I. K. Hewlett, L. M. Wahl, H. K. Kleinman, J. N. Brady, K. M. Yamada, and S. Dhawan. 1999. Cutting edge: a short polypeptide domain of HIV-1 Tat protein mediates pathogenesis. J. Immunol. 163:15-20. [PubMed] [Google Scholar]
- 28.Brake, D. A., C. Debouck, and G. Biesecker. 1990. Identification of an Arg-Gly-Asp (RGD) cell adhesion site in human immunodeficiency virus type I transactivation protein tat. J. Cell Biol. 111:1275-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks, J. J. 1986. Kaposi's sarcoma: a reversible hyperplasia. Lancet ii:1309-1311. [DOI] [PubMed] [Google Scholar]
- 30.Brooks, P. C., R. A. Clark, and D. A. Cheresh. 1994. Requirement of vascular integrin αvβ3 for angiogenesis. Science 264:569-571. [DOI] [PubMed] [Google Scholar]
- 31.Brooks, P. C., A. M. Montgomery, M. Rosenfeld, R. A. Reisfeld, T. Hu, G. Klier, and D. A. Cheresh. 1994. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79:1157-1164. [DOI] [PubMed] [Google Scholar]
- 32.Brooks, P. C., S. Stromblad, L. C. Sanders, T. L. von Schalscha, R. T. Aimes, W. G. Stetler-Stevenson, J. P. Quigley, and D. A. Cheresh. 1996. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell 85:683-693. [DOI] [PubMed] [Google Scholar]
- 33.Buonaguro, L., G. Barillari, H. K. Chang, C. A. Bohan, V. Kao, R. Morgan, R. C. Gallo, and B. Ensoli. 1992. Effects of the human immunodeficiency virus type I Tat protein on the expression of inflammatory cytokines. J. Virol. 66:7159-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cafaro, A., A. Caputo, C. Fracasso, M. T. Maggiorella, D. Goletti, S. Baroncelli, M. Pace, L. Sernicola, M. L. Koanga-Mogtomo, M. Betti, A. Borsetti, R. Belli, L. Åkerblom, F. Corrias, S. Buttò, J. Heeney, P. Verani, F. Titti, and B. Ensoli. 1999. Control of SHIV89.6P infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat. Med. 5:643-650. [DOI] [PubMed] [Google Scholar]
- 35.Cafaro, A., F. Titti, C. Fracasso, M. T. Maggiorella, S. Baroncelli, A. Caputo, D. Goletti, A. Borsetti, M. Pace, E. Fanales-Belasio, B. Ridolfi. D. R. M. Negri, L. Sernicola, R. Belli, F. Corrias, I. Macchia, P. Leone, Z. Michelini, P. ten Haaft, S. Buttò, P. Verani, and B. Ensoli. 2001. Vaccination with DNA containing tat coding sequences and unmethylated CpG motifs protects cynomolgus monkeys upon infection with simian/human immunodeficiency virus (SHIV89.6P). Vaccine 19:2862-2877. [DOI] [PubMed] [Google Scholar]
- 36.Callan, M. F., N. Steven, P. Krausa, J. D. Wilson, P. A. Moss, G. M. Gillespie, J. I. Bell, A. B. Rickinson, and A. J. McMichael. 1996. Large clonal expansion of CD8+ T cells in acute infectious mononucleosis. Nat. Med. 2:906-911. [DOI] [PubMed] [Google Scholar]
- 37.Caruso, A., R. Gonzales, R. Stellini, A. Scalzini, L. Peroni, and A. Turano. 1990. Interferon-gamma marks activated T lymphocytes in AIDS patients. AIDS Res. Hum. Retrovir. 6:899-904. [DOI] [PubMed] [Google Scholar]
- 38.Chang, H. C., F. Samaniego, B. C. Nair, L. Buonaguro, and B. Ensoli. 1997. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix associated heparan sulphate proteoglycans through its basic region. AIDS 11:1421-1431. [DOI] [PubMed] [Google Scholar]
- 39.Chirmule, N., S. Than, S. A. Khan, and S. Pahwa. 1995. Human immunodeficiency virus Tat induces functional responsiveness in T cells. J. Virol. 69:492-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark, E. A., and J. S. Brugge. 1995. Integrin and signal transduction pathways: the road taken. Science 268:233-239. [DOI] [PubMed] [Google Scholar]
- 41.Conaldi, P. G., C. Serra, A. Dolei, F. Basolo, V. Falcone, G. Mariani, P. Speziale, and A. Toniolo. 1995. Productive HIV-1 infection of human vascular endothelial cells requires cell proliferation and is stimulated by combined treatment with interleukin-1 beta plus tumor necrosis factor-alpha. J. Med. Virol. 47:355-363. [DOI] [PubMed] [Google Scholar]
- 42.Corallini, A., G. Altavilla, L. Pozzi, F. Bignozzi, M. Negrini, P. Rimessi, F. Gualandi, and G. Barbanti-Brodano. 1993. Systemic expression of HIV-1 tat gene in transgenic mice induces endothelial proliferation and tumors of different histotypes. Cancer Res. 53:5569-5575. [PubMed] [Google Scholar]
- 43.Cornali, E., C. Zietz, R. Benelli, W. Weninger, L. Masiello, G. Breier, E. Tschachler, A. Albini, and M. Stürzl. 1996. Vascular endothelial growth factor regulates angiogenesis and vascular permeability in Kaposi's sarcoma. Am. J. Pathol. 149:1851-1869. [PMC free article] [PubMed] [Google Scholar]
- 44.Cozzolino, F., M. Torcia, D. Aldinucci, M. Ziche, F. Almerigogna, D. Bani, and D. M. Stern. 1990. Interleukin-1 is an autocrine regulator of endothelial cell growth. Proc. Natl. Acad. Sci. USA 87:6487-6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cullen, B. R., and W. C. Greene. 1989. Regulatory pathways governing HIV-1 replication. Cell 58:423-426. [DOI] [PubMed] [Google Scholar]
- 46.Davis, M. A., M. A. Stürzl, C. Blasig, A. Schreier, H. G. Guo, M. Reitz, S. R. Opalenik, and P. J. Browning. 1997. Expression of human herpesvirus 8-encoded cyclin D in Kaposi's sarcoma spindle cells. J. Natl. Cancer Inst. 89:1868-1874. [DOI] [PubMed] [Google Scholar]
- 47.DePaoli, P., C. Caffau, M. D'Andrea, M. Tavio, U. Tirelli, and G. Santini. 1994. Serum levels of intercellular adhesion molecule 1 in patients with HIV-1 related Kaposi's sarcoma. J. Acquir. Immune Defic. Syndr. 7:695-699. [PubMed] [Google Scholar]
- 48.de-The, G., G. Bestetti, M. van Beveren, and A. Gessain. 1999. Prevalence of human herpesvirus 8 infection before the acquired immunodeficiency disease syndrome-related epidemic of Kaposi's sarcoma in East Africa. J. Natl. Cancer Inst. 91:1888-1889. [DOI] [PubMed] [Google Scholar]
- 49.de-Vries, C., J. A. Escobedo, H. Ueno, K. Houck, N. Ferrara, and L. T. Wuilliams. 1992. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 255:989-991. [DOI] [PubMed] [Google Scholar]
- 50.Dupin, N., C. Fisher, P. Kellam, S. Ariad, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ensoli, B., S. Nakamura, S. Z. Salahuddin, P. Biberfeld, L. Larsson, B. Beaver, F. Wong-Staal, and R. C. Gallo. 1989. AIDS-Kaposi's sarcoma derived cells express cytokines with autocrine and paracrine growth effects. Science 243:223-226. [DOI] [PubMed] [Google Scholar]
- 52.Ensoli, B., G. Barillari, S. Z. Salahuddin, R. C. Gallo, and F. Wong-Staal. 1990. Tat protein of HIV-1 stimulates the growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature 345:84-86. [DOI] [PubMed] [Google Scholar]
- 53.Ensoli, B., L. Buonaguro, G. Barillari, V. Fiorelli, R. Gendelman, R. A. Morgan, P. Wingfeld, and R. C. Gallo. 1993. Release, uptake and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67:277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ensoli, B., R. Gendelman, P. Markham, V. Fiorelli, S. Colombini, M. Raffeld, A. Cafaro, H. K. Chang, J. N. Brady, and R. C. Gallo. 1994. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi's sarcoma. Nature 371:674-680. [DOI] [PubMed] [Google Scholar]
- 55.Ensoli, B., P. Markham, V. Kao, G. Barillari, V. Fiorelli, R. Gendelman, M. Raffeld, G. Zon, and R. C. Gallo. 1994. Block of AIDS-Kaposi's sarcoma (KS) cell growth, angiogenesis, and lesion formation in nude mice by antisense oligonucleotide targeting basic fibroblast growth factor. A novel strategy for the therapy of KS. J. Clin. Investig. 94:1736-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fagiolo, U., A. Cossarizza, E. Scala, E. Fanales-Belasio, C. Ortolani, E. Cozzi, D. Monti, C. Franceschi, and R. Paganelli. 1993. Increased cytokine production in mononuclear cells of healthy elderly people. Eur. J. Immunol. 23:2375-2378. [DOI] [PubMed] [Google Scholar]
- 57.Fan, J., Z. H. Bass, and J. L. Fahey. 1993. Elevated IFN-γ and decreased IL-2 gene expression are associated with HIV infection. J. Immunol. 151:5031-5040. [PubMed] [Google Scholar]
- 58.Fawell, S., J. Seery, Y. Daikh, C. Moore, L. L. Chen, B. Pepinsky, and J. Barsoum. 1994. Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. USA 91:664-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fiorelli, V., R. Gendelman, F. Samaniego, P. Markham, and B. Ensoli. 1995. Cytokines from activated T cells induce normal endothelial cells to acquire the phenotypic and functional features of AIDS-Kaposi's sarcoma spindle cells. J. Clin. Investig. 95:1723-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fiorelli, V., R. Gendelman, M. C. Sirianni, H. K. Chang, S. Colombini, P. D. Markham, P. Monini, J. Sonnabend, A. Pintus, R. C. Gallo, and B. Ensoli. 1998. Gamma-interferon produced by CD8+ T cells infiltrating Kaposi's sarcoma induces spindle cells with angiogenic phenotype and synergy with human immunodeficiency virus type I Tat protein: an immune response to human herpesvirus-8 infection? Blood 91:956-967. [PubMed] [Google Scholar]
- 61.Fiorelli, V., G. Barillari, E. Toschi, C. Sgadari, P. Monini, M. Sturzl, and B. Ensoli. 1999. IFN-gamma induces endothelial cells to proliferate and to invade the extracellular matrix in response to HIV-1 Tat protein: implications for AIDS-Kaposi's sarcoma pathogenesis. J. Immunol. 162:1165-1171. [PubMed] [Google Scholar]
- 62.Fisher, A. G., M. B. Feinberg, S. F. Josephs, M. E. Harper, L. M. Marselle, G. Reyes, M. A. Gonda, A. Aldovini, C. Debouck, R. C. Gallo, et al. 1986. The transactivator gene of HTLV-III is essential for virus replication. Nature 320:367-371. [DOI] [PubMed] [Google Scholar]
- 63.Folkman, J., M. Klagsbrun, J. Sasse, M. Wadzinski, D. Ingberg, and I. Vlodavsky. 1988. A heparin-binding angiogenic protein--basic fibroblast growth factor--is stored within basement membrane. Am. J. Pathol. 130:393-400. [PMC free article] [PubMed] [Google Scholar]
- 64.Folkman, J., and Y. Shing. 1992. Angiogenesis. J. Biol. Chem. 267:10931-10934. [PubMed] [Google Scholar]
- 65.Frankel, A. D., and C. O. Pabo. 1988. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55:1189-1193. [DOI] [PubMed] [Google Scholar]
- 66.Frankel, A. D., S. Biancalana, and D. Hudson. 1989. Activity of synthetic peptides from the Tat protein of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 86:7397-7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedlander, M., P. C. Brooks, R. W. Shaffer, C. M. Kincaid, J. A. Varner, and D. A. Cheresh. 1995. Definition of two angiogenic pathways by distinct αv integrins. Science 270:1500-1502. [DOI] [PubMed] [Google Scholar]
- 68.Friedman-Kien, A. E. 1981. Disseminated Kaposi's sarcoma syndrome in young homosexual men. J. Am. Acad. Dermatol. 5:468-471. [DOI] [PubMed] [Google Scholar]
- 69.Fuchs, D., A. Hausen, G. Reibnegger, E. R. Werner, G. Werner-Felmayer, M. P. Dierich, and H. Watcher. 1989. Interferon-gamma concentrations are increased in sera from individuals infected with human immunodeficiency virus-1. J. Acquir. Immune Defic. Syndr. 2:158-162. [PubMed] [Google Scholar]
- 70.Ganju, R. K., N. Munshi, B. C. Nair, Z. Y. Liu, P. Gill, and J. E. Groopman. 1998. Human immunodeficiency virus Tat modulates the Flk-1/KDR receptor, mitogen-activated protein kinase and components of focal adhesion in Kaposi's sarcoma cells. J. Virol. 72:6131-6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao, S. J., L. Kingsley, M. Li, W. Zheng, C. Parravicini, J. Ziegler, R. Newton, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, Y. Chang, and P. S. Moore. 1996. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat. Med. 2:925-928. [DOI] [PubMed] [Google Scholar]
- 72.Garcia, J. A., D. Harrich, L. Pearson, R. Mitsuyasu, and R. B. Gaynor. 1988. Functional domains required for tat-induced transcriptional activation of the HIV-1 long terminal repeat. EMBO J. 7:3143-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geddes, M., S. Franceschi, A. Barchielli, F. Falcini, S. Carli, G. Cocconi, E. Conti, P. Crosignani, L. Gafa, L. Giarelli, et al. 1994. Kaposi's sarcoma in Italy before and after the AIDS epidemic. Br. J. Cancer 69:333-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gengrinovitch, S., S. M. Greenerg, T. Cohen, H. Gitay-Goren, P. Rockwell, T. E. Maione, B. Z. Levi, and G. Neufeld. 1995. Platelet factor-4 inhibits the mitogenic activity of VEGF121 and VEGF165 using several concurrent mechanisms. J. Biol. Chem. 270:15059-15065. [DOI] [PubMed] [Google Scholar]
- 75.Giancotti, F. G. 1997. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr. Opin. Cell Biol. 9:691-700. [DOI] [PubMed] [Google Scholar]
- 76.Gill, P., Y. Tsai, A. P. Rao, and P. Jones. 1997. Clonality in Kaposi's sarcoma. N. Engl. J. Med. 337:570-571. [DOI] [PubMed] [Google Scholar]
- 77.Globus, R. K., J. Plouet, and D. Gospodarowitcz. 1989. Cultured bovine bone cells synthesize basic fibroblast growth factor and store it in their extracellular matrix. Endocrinology 124:1539-1547. [DOI] [PubMed] [Google Scholar]
- 78.Gospodarowitcz, D., G. Greenburg, and C. R. Birdwell. 1978. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 38:4155-4171. [PubMed] [Google Scholar]
- 79.Hammes, H. P., M. Brownlee, A. Jonczyk, A. Sutter, and K. T. Preissner. 1996. Subcutaneous injection of a cyclic peptide antagonist of vitronectin receptor-type integrins inhibits retinal neovascularization. Nat. Med. 2:529-533. [DOI] [PubMed] [Google Scholar]
- 80.Hanemaaijer, R., P. Koolwijk, L. Le Clerk, W. J. de Vree, and V. W. van Hinsberg. 1993. Regulation of matrix metalloprotease expression in human vein and microvascular endothelial cells. Effects of TNFα, IL-1 and phorbol ester. Biochem. J. 296:803-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harrington, W. J., O. Bagasra, C. E. Sosa, L. E. Bobroski, M. Baum, X. L. Wen, L. Cabral, G. E. Byrne, R. J. Pomerantz, and C. Wood. 1996. Human herpesvirus type 8 DNA sequences in cell-free plasma and mononuclear cells of Kaposi's sarcoma patients. J. Infect. Dis. 174:1101-1105. [DOI] [PubMed] [Google Scholar]
- 82.Hatai, M., H. Hashi, A. Mogi, H. Soga, J. Yokota, and Y. Yaoi. 1994. Stimulation of tyrosine- and serine-phosphorylation of focal adhesion kinase in mouse 3T3 cells by fibronectin and fibroblast growth factor. FEBS Lett. 350:113-116. [DOI] [PubMed] [Google Scholar]
- 83.Hayman, E. G., M. D. Piershbacher, and E. Ruoslahti. 1985. Detachment of cells from culture substrate by soluble fibronectin peptides. J. Cell Biol. 100:1948-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herndier, B. G., A. Werner, P. Arnstein, N. W. Abbey, F. Demartis, R. L. Cohen, M. A. Shuman, and J. A. Levy. 1994. Characterization of a human Kaposi's sarcoma cell line that induces angiogenic tumors in animals. AIDS 8:575-581. [DOI] [PubMed] [Google Scholar]
- 85.Hober, D., A. Haque, P. Wattre, G. Beaucaire, Y. Mouton, and A. Capron. 1989. Production of tumor necrosis factor alpha (TNF-alpha) and interleukin-1 (IL-1) in patients with AIDS. Clin. Exp. Immunol. 78:329-333. [PMC free article] [PubMed] [Google Scholar]
- 86.Honda, M., K. Kitamura, Y. Mizutani, M. Oishi, M. Arai, T. Okura, K. Igarahi, K. Yasukawa, T. Hirano, and Y. Kishimoto. 1990. Quantitative analysis of serum IL-6 and its correlation with increased levels of serum IL-2R in HIV-induced diseases. J. Immunol. 145:4059-4064. [PubMed] [Google Scholar]
- 87.Huang, M. B., M. Hunter, and V. C. Bond. 1999. Effect of extracellular human immunodeficiency virus type 1 glycoprotein 120 on primary human vascular endothelial cell cultures. AIDS Res. Hum. Retrovir. 15:1265-1277. [DOI] [PubMed] [Google Scholar]
- 88.Huang, Y. Q., J. J. Li, D. Moscatelli, C. Basilico, A. Nicolaides, W. G. Zhang, B. J. Poiesz, and A. E. Friedman-Kien. 1993. Expression of int-2 oncogene in Kaposi's sarcoma lesions. J. Clin. Investig. 91:1191-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Humpries, M. J., M. Obara, K. Olden, and K. M. Yamada. 1989. Role of fibronectin in adhesion, migration and metastasis. Cancer Investig. 7:373-393. [DOI] [PubMed] [Google Scholar]
- 90.Hynes, R. O. 1992. Integrins: versatility, modulation and signaling in cell adhesion. Cell 69:11-24. [DOI] [PubMed] [Google Scholar]
- 91.Ingber, D. E., D. Prusty, J. V. Frangioni, E. J. Cragoe, Jr., C. Lechene, and M. A. Schwartz. 1990. Control of intracellular pH and growth by fibronectin in capillary endothelial cells. J. Cell Biol. 110:1803-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jackson, A., F. Tarantini, S. Gamble, S. Friedman, and T. Maciag. 1995. The release of fibroblast growth factor-1 from NIH-3T3 cells in response to temperature involves the function of cysteine residues. J. Biol. Chem. 270:33-36. [DOI] [PubMed] [Google Scholar]
- 93.Jacobson, L. P., A. Munoz, R. Fox, J. P. Phair, J. Dudley, G. I. Obrams, L. A. Kingsley, B. F. Polk, and The Multicenter AIDS Cohort Study Group. 1990. Incidence of Kaposi's sarcoma in a cohort of homosexual men infectected with HIV-1. J. Acquir. Immune Defic. Syndr. 3:S24-S31. [PubMed] [Google Scholar]
- 94.Jacobson, L. P., F. J. Jenkins, G. Springer, A. Munoz, K. V. Shah, J. Phair, Z. Zhang, and H. Armenian. 2000. Interaction of human immunodeficiency virus type 1 and human herpesvirus type 8 infections on the incidence of Kaposi's sarcoma. J. Infect. Dis. 181:1940-1949. [DOI] [PubMed] [Google Scholar]
- 95.Jussila, L., R. Valtola, T. A. Partanen, P. Salven, P. Heikkila, M. T. Matikainen, R. Renkonen, A. Kaipainen, M. Detmar, E. Tschachler, R. Alitalo, and K. Alitalo. 1998. Lymphatic endothelium and Kaposi's sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res. 58:1599-1604. [PubMed] [Google Scholar]
- 96.Kedes, D. H., E. Operskalski, M. Busch, R. Kohn, J. Flood, and D. Ganem. 1996. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat. Med. 2:918-924. [DOI] [PubMed] [Google Scholar]
- 97.Kekow, J., W. Wachsman, J. A. McCutchan, W. L. Gross, M. Zachariah, D. A. Carson, and M. Lotz. 1991. Transforming growth factor-beta and suppression of humoral immune responses in HIV infection. J. Clin. Investig. 87:1010-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kestens, L., M. Melbye, R. J. Biggar, W. J. Stevens, P. Piot, A. De Muynck, H. Taelman, M. De Feyter, L. Paluku, and P. L. Gigase. 1985. Endemic African KS is not associated with immunodeficiency. Int. J. Cancer 36:49-54. [DOI] [PubMed] [Google Scholar]
- 99.Keyt, B. A., L. T. Berleau, H. U. Nguyen, H. Chen, H. Heinsohn, R. Vandlen, and N. Ferrara. 1996. The carboxyl-terminal domain (111-165) of vascular endothelial growth factor is critical for its mitogenic potency. J. Biol. Chem. 271:7788-7795. [DOI] [PubMed] [Google Scholar]
- 100.Klein, S., F. G. Giancotti, M. Presta, S. M. Albeda, C. A. Buck, and D. B. Rifkin. 1993. Basic fibroblast growth factor modulates integrin expression in microvascular endothelial cells. Mol. Cell. Biol. 4:973-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kolson, D. L., J. Buchalter, R. Collman, B. Hellmig, C. F. Farrel, C. Debouck, and F. Gonzalez-Scarano. 1993. HIV-1 Tat alters normal organization of neurons and astrocytes in primary rodent brain cell cultures: RGD sequence dependence. AIDS Res. Hum. Retrovir. 9:677-685. [DOI] [PubMed] [Google Scholar]
- 102.Koster, R., L. M. Blatt, M. Streubert, C. Zietz, H. Hermeking, W. Brysch, and M. Stürzl. 1996. Consensus-interferon and platelet-derived growth factor adversely regulate proliferation and migration of Kaposi's sarcoma cells by control of c-myc expression. Am. J. Pathol. 149:1871-1885. [PMC free article] [PubMed] [Google Scholar]
- 103.Krakauer, T., and J. J. Oppenheim. 1993. IL-1 and tumor necrosis factor-α each upregulate both the expression of IFNγ and enhance IFNγ-induced HLA-DR expression on human monocytes and on a human monocytic cell line. J. Immunol. 150:1205-1211. [PubMed] [Google Scholar]
- 104.Lafon, M. E., J. L. Gendrault, C. Royer, D. Jaeck, A. Kirn, and A. M. Steffan. 1993. Human endothelial cells isolated from the hepatic sinusoids and the umbilical vein display a different permissiveness for HIV-1. Res Virol. 144:99-104. [DOI] [PubMed] [Google Scholar]
- 105.Lafon, M. E., A. M. Steffan, C. Royer, D. Jaeck, A. Beretz, A. Kirn, and J. L. Gendrault. 1994. HIV-1 infection induces functional alterations in human liver endothelial cells in primary culture. AIDS 8:747-752. [DOI] [PubMed] [Google Scholar]
- 106.Lafrenie, R. M., L. M. Wahal, J. S. Epstein, I. K. Hewlett, K. M. Yamada, and S. Dhawan. 1996. HIV-1 Tat modulates the function of monocytes and alters their interactions with microvessel endothelial cells. J. Immunol. 156:1638-1645. [PubMed] [Google Scholar]
- 107.Lafrenie, R. M., L. M. Wahal, J. S. Epstein, I. K. Hewlett, K. M. Yamada, and S. Dhawan. 1996. HIV-1 Tat protein promotes chemotaxis and invasive behavior by monocytes. J. Immunol. 157:974-977. [PubMed] [Google Scholar]
- 108.Laspia, M. F., A. P. Rice, and M. B. Matthews. 1989. HIV-1 Tat protein increases transcriptional initiation and stabilizes elongation. Cell 59:283-292. [DOI] [PubMed] [Google Scholar]
- 109.Lebbè, C., L. Blum, C. Pellet, G. Blanchard, O. Verola, P. Morel, O. Danne, and F. Calvo. 1998. Clinical and biological impact of antiretroviral therapy with protease inhibitors on HIV-related Kaposi's sarcoma. AIDS 12:F45-F49. [DOI] [PubMed] [Google Scholar]
- 110.Lennette, E. T., D. J. Blackbourn, and J. A. Levy. 1996. Antibodies to human herpesvirus type 8 in the general population and in Kaposi's sarcoma patients. Lancet 348:858-861. [DOI] [PubMed] [Google Scholar]
- 111.Lepe-Zuniga, J. L., P. W. A. Mansell, and E. M. Hersh. 1987. Idiopathic production of interleukin-1 in acquired immune deficiency syndrome. J. Clin. Microbiol. 25:1695-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Levy, J. A., and J. L. Ziegler. 1983. Acquired immunodeficiency syndrome is an opportunistic infection and Kaposi's sarcoma results from secondary immune stimulation. Lancet ii:78-81. [DOI] [PubMed] [Google Scholar]
- 113.Li, C. J., D. J. Friedman, C. Wang, V. Metelev, and A. B. Pardee. 1995. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268:429-431. [DOI] [PubMed] [Google Scholar]
- 114.Li, C. J., C. Wang, D. J. Friedman, and A. B. Pardee. 1995. Reciprocal modulations between p53 and Tat of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 92:5461-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li, M., H. Lee, D. W. Yoon, J. C. Albrecht, B. Fleckenstein, F. Neipel, and J. U. Jung. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional cyclin. J. Virol. 71:1984-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reference deleted.
- 117.Lunardi-Iskandar, Y., P. Gill, V. H. Lam, R. A. Zeman, F. Michaels, D. L. Mann, M. S. Reitz, Jr., M. Kaplan, Z. N. Berneman, D. Carter, J. L. Bryant, and R. C. Gallo. 1995. Isolation and characterization of an immortal neoplastic cell line (KS Y-1) from AIDS-associated Kaposi's sarcoma. J. Natl. Cancer Inst. 87:974-981. [DOI] [PubMed] [Google Scholar]
- 118.Marchiò, S., L. Primo, M. Pagano, G. Palestro, A. Albini, T. Veikkola, I. Cascone, K. Alitalo, and F. Bussolino. 1999. Vascular endothelial growth factor-C stimulates the migration and proliferation of Kaposi's sarcoma cells. J. Biol. Chem. 274:27617-27622. [DOI] [PubMed] [Google Scholar]
- 119.Martin, J. N., D. E. Ganem, D. H. Osmond, K. A. Page-Shafer, D. Macrae, and D. H. Kedes. 1998. Sexual transmission and the natural history of human herpesvirus 8 infection. N. Engl. J. Med. 338:948-954. [DOI] [PubMed] [Google Scholar]
- 120.Milani, D., M. Mazzoni, P. Borgatti, G. Zauli, L. Cantley, and S. Capitani. 1996. Extracellular human immunodeficiency virus type-1 Tat protein activates phosphatidylinositol 3-kinase in PC12 neuronal cells. J. Biol. Chem. 271:22961-22964. [DOI] [PubMed] [Google Scholar]
- 121.Milani, D., M. Mazzoni, G. Zauli, C. Mischiati, D. Gibellini, M. Giacca, and S. Capitani. 1998. HIV-1 Tat induces phosphorylation of p125fak and its association with phosphoinositide 3-kinase in PC12 cells. AIDS 12:1275-1284. [DOI] [PubMed] [Google Scholar]
- 122.Mitola, S., S. Sozzani, W. Luini, L. Primo, A. Borsatti, H. Weich, and F. Bussolino. 1997. Tat-human immunodeficiency virus-1 induces human monocyte chemotaxis by activation of vascular endothelial growth factor receptor-1. Blood 90:1365-1372. [PubMed] [Google Scholar]
- 123.Mitola, S., R. Soldi, I. Zanon, L. Barra, M. I. Gutierrez, B. Berkhout, M. Giacca, and F. Bussolino. 2000. Identification of specific molecular structures of human immunodeficiency virus type-1 Tat relevant for its biological effects on vascular endothelial cells. J. Virol. 74:344-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Monini, P., S. Colombini, M. Stürzl, D. Goletti, A. Cafaro, C. Sgadari, S. Buttò, M. Franco, P. Leone, S. Fais, P. Leone, G. Melucci-Vigo, C. Chiozzini, F. Carlini, G. Ascherl, E. Cornali, C. Ziezt, E. Ramazzotti, F. Ensoli, M. Andreoni, P. Pezzotti, G. Rezza, R. Yarchoan, R. C. Gallo, and B. Ensoli. 1999. Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi's sarcoma. Blood 93:4044-4058. [PubMed] [Google Scholar]
- 125.Montaldo, F., A. Maffe, M. Morini, D. Noonan, S. Giordano, A. Albini, and M. Prat. 2000. Expression of functional tyrosine kinases on immortalized Kaposi's sarcoma cells. J. Cell. Physiol. 184:246-254. [DOI] [PubMed] [Google Scholar]
- 126.Moore, P. S., and Y. Chang. 1995. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma patients with and without HIV infection. N. Engl. J. Med. 332:1181-1185. [DOI] [PubMed] [Google Scholar]
- 127.Morini, M., R. Benelli, D. Giunciuglio, S. Carlone, G. Arena, D. M. Noonan, and A. Albini. 2000. Kaposi's sarcoma cells of different etiologic origins respond to HIV-Tat through the Flk-1/KDR (VEGF receptor-2): relevance in AIDS-KS pathology. Biochem. Biophys. Res. Commun. 273:267-271. [DOI] [PubMed] [Google Scholar]
- 128.Moses, A. V., and J. A. Nelson. 1994. HIV infection of human brain capillary endothelial cells: implications for AIDS dementia. Adv. Neuroimmunol. 4:239-247. [DOI] [PubMed] [Google Scholar]
- 129.Moses, A. V., S. G. Stenglein, J. G. Strussenberg, K. Wehrly, B. Chesebro, and J. A. Nelson. 1996. Sequences regulating tropism of human immunodeficiency virus type 1 for brain capillary endothelial cells map to a unique region on the viral genome. J. Virol. 70:3401-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakamura, S., S. Z. Salahuddin, P. Biberfeld, B. Ensoli, P. D. Markham, F. Wong-Staal, and R. C. Gallo. 1988. Kaposi's sarcoma cells: long-term culture with growth factor from retrovirus-infected CD4+ T cells. Science 242:426-430. [DOI] [PubMed] [Google Scholar]
- 131.Neufeld, G., T. Cohen, S. Gengrinovitch, and Z. Poltorak. 1999. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 13:9-22. [PubMed] [Google Scholar]
- 132.Nicosia, R. F. 1998. What is the role of vascular endothelial growth factor-related molecules in tumor angiogenesis? Am. J. Pathol. 153:11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Orenstein, J. M., S. Alkan, A. Blauvelt, K. T. Jeang, M. D. Weinstein, D. Ganem, and B. Herndier. 1997. Visualization of human herpesvirus type 8 in Kaposi's sarcoma by light and transmission electron microscopy. AIDS 11:F35-F45. [DOI] [PubMed] [Google Scholar]
- 134.Oxholm, A., P. Oxholm, H. Permin, and K. Bendtzen. 1989. Epidermal tumor necrosis factor alpha and interleukin 6-like activities in AIDS-related Kaposi's sarcoma. APMIS 97:533-538. [DOI] [PubMed] [Google Scholar]
- 135.Pandita, R., E. Pocsik, and B. B. Aggarwal. 1992. Interferon γ induces cell surface expression of both types of tumor necrosis factor receptors. FEBS Lett. 312:87-90. [DOI] [PubMed] [Google Scholar]
- 136.Penn, I. 1979. Kaposi's sarcoma in organ transplant recipiens: report of 20 cases. Transplantation 27:8-11. [DOI] [PubMed] [Google Scholar]
- 137.Poland, S. D., G. P. Rice, and G. A. Dekaban. 1995. HIV-1 infection of human brain-derived microvascular endothelial cells in vitro. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8:437-445. [DOI] [PubMed] [Google Scholar]
- 138.Prakash, O., Z. Y. Tang, Y. E. He, M. S. Ali, R. Coleman, J. Gill, G. Farr, and F. Samaniego. 2000. Human Kaposi's sarcoma cell-mediated tumorigenesis in human immunodeficiency type 1 tat-expressing transgenic mice. J. Natl. Cancer Inst. 92:721-728. [DOI] [PubMed] [Google Scholar]
- 139.Puri, R. K., and B. B. Aggarwal. 1992. Human immunodeficiency virus type-1 tat gene up-regulates interleukin 4 receptors on a human B-lymphoblastoid cell line. Cancer Res. 52:3787-3790. [PubMed] [Google Scholar]
- 140.Purvis, S. F., J. W. Jacobberger, R. M. Sramkoski, A. H. Patki, and M. M. Lederman. 1995. HIV type 1 Tat protein induces apoptosis and death in Jurkat cells. AIDS Res. Hum. Retrovir. 11:443-450. [DOI] [PubMed] [Google Scholar]
- 141.Rabkin, C. S., S. Janz, A. Lash, A. E. Coleman, E. Musaba, L. Liotta, R. J. Biggar, and Z. Zhuang. 1997. Monoclonal origin of multicentric Kaposi's sarcoma lesions. N. Engl. J. Med. 336:988-993. [DOI] [PubMed] [Google Scholar]
- 142.Raines, E. W., and R. Ross. 1992. Compartmentalization of PDGF on extracellular binding sites dependent on exon 6-encoded sequences. J. Cell Biol. 116:533-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ranki, A., A. Lagerstedt, V. Ovod, E. Aavik, and K. J. E. Krohn. 1994. Expression kinetics and subcellular localization of HIV-1 regulatory proteins Nef, Tat and Rev in acutely and chronically infected lymphoid cell lines. Arch. Virol. 139:365-378. [DOI] [PubMed] [Google Scholar]
- 144.Rautonen, J., N. Rautonen, N. L. Martin, and D. W. Wara. 1994. HIV type 1 Tat protein induces immunoglobulin and interleukin 6 synthesis by uninfected peripheral blood mononuclear cells. AIDS Res. Hum. Retrovir. 10:781-785. [DOI] [PubMed] [Google Scholar]
- 145.Regezi, J., L. A. McPhail, T. E. Daniels, Y. G. DeSouza, J. S. Greenspan, and D. Greenspan. 1993. Human immunodeficiency virus-associated oral Kaposi's sarcoma. A heterogeneous population dominated by spindle-shaped endothelial cells. Am. J. Pathol. 43:240-249. [PMC free article] [PubMed] [Google Scholar]
- 146.Rezza, G., E. T. Lennette, M. Giuliani, P. Pezzotti, F. Caprilli, P. Monini, S. Buttò, G. Lodi, A. Di Carlo, J. A. Levy, and B. Ensoli. 1998. Prevalence and determinants of anti-lytic and anti-latent antibodies to human herpesvirus-8 among Italian individuals at risk of sexually and parenterally transmitted infections. Int. J. Cancer 77:361-365. [DOI] [PubMed] [Google Scholar]
- 147.Rezza, G., M. Andreoni, M. Dorrucci, P. Pezzotti, P. Monini, R. Zerboni, B. Salassa, V. Colangeli, L. Sarmati, E. Nicastri, M. Barbanera, R. Pristera, F. Aiuti, L. Ortona, and B. Ensoli. 1999. Human herpesvirus 8 seropositivity and risk of Kaposi's sarcoma and other acquired immunodeficiency syndrome-related diseases. J. Natl. Cancer Inst. 91:1468-1474. [DOI] [PubMed] [Google Scholar]
- 148.Rinaldo, C., P. Piazza, Y. Z. Wang, J. Armstrong, P. Gupta, M. Ho, S. Petteway, D. Reed, D. Lyter, and L. Kingsley. 1988. HIV-1 specific production of IFN-γ and modulation by recombinant IL-2 during early HIV-1 infection. J. Immunol. 140:3389-3393. [PubMed] [Google Scholar]
- 149.Rizzardini, G., S. Piconi, S. Ruzzante, M. L. Fusi, M. Lukwiya, S. Declich, M. Tamburini, M. L. Villa, M. Fabiani, F. Milazzo, and M. Clerici. 1996. Immunological activation markers in the serum of African and European HIV-seropositive and seronegative individuals. AIDS 10:1535-1542. [DOI] [PubMed] [Google Scholar]
- 150.Rubartelli, A., F. Cozzolino, M. Talio, and R. Sitia. 1990. A novel secretory pathway for interleukin-1 β, a protein lacking a signal sequence. EMBO J. 9:1503-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rusnati, M., G. Tulipano, C. Urbinati, E. Tanghetti, R. Giuliani, M. Giacca, M. Ciomei, A. Corallini, and M. Presta. 1998. The basic domain in HIV-1 Tat protein as a target for polysulfonated heparin-mimicking extracellular Tat antagonists. J. Biol. Chem. 273:16027-16037. [DOI] [PubMed] [Google Scholar]
- 152.Sabatier, J. M., E. Vives, K. Mabrouk, A. Benjouad, H. Rochat, A. Duval, B. Hue, and E. Bahraoui. 1991. Evidence for neurotoxic activity of Tat from human immunodeficiency virus type 1. J. Virol. 65:961-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Safai, B., K. G. Johnson, S. Myskowski, B. Koziner, S. Y. Yang, S. Cunningham-Rundles, J. H. Godbold, and B. Dupont. 1985. The natural history of Kaposi's sarcoma in the acquired immunodeficiency syndrome. Ann. Intern. Med. 103:744-750. [DOI] [PubMed] [Google Scholar]
- 154.Salahuddin, S. Z., S. Nakamura, P. Biberfeld, M. H. Kaplan, P. D. Markham, L. Larsson, and R. C. Gallo. 1988. Angiogenic properties of Kaposi's sarcoma-derived cells after long-term culture in vitro. Science 242:430-433. [DOI] [PubMed] [Google Scholar]
- 155.Samaniego, F., P. D. Markham, R. C. Gallo, and B. Ensoli. 1995. Inflammatory cytokines induce AIDS-Kaposi's sarcoma-derived spindle cells to produce and release basic fibroblast growth factor and enhance Kaposi's sarcoma-like lesion formation in nude mice. J. Immunol. 154:3582-3592. [PubMed] [Google Scholar]
- 156.Samaniego, F., P. Markham, R. Gendelman, R. C. Gallo, and B. Ensoli. 1997. Inflammatory cytokines induce endothelial cells to produce and release basic fibroblast growth factor and to promote Kaposi's sarcoma-like lesions in nude mice. J. Immunol. 158:1887-1894. [PubMed] [Google Scholar]
- 157.Samaniego, F., P. D. Markham, R. Gendelman, Y. Watanabe, V. Kao, K. Kowalski, N. Ferrara, J. A. Sonnabend, A. Pintus, G. Zon, R. C. Gallo, and B. Ensoli. 1998. Vascular endothelial growth factor and basic fibroblast growth factor present in Kaposi's sarcoma (KS) are induced by inflammatory cytokines and synergize to promote vascular permeability and KS lesion development. Am. J. Pathol. 152:433-443. [PMC free article] [PubMed] [Google Scholar]
- 158.Santucci, M., N. Pimpinelli, S. Moretti, and B. Giannotti. 1988. Classic and immunodeficiency-associated Kaposi's sarcoma. Arch. Pathol. Lab. Med. 112:1214-1220. [PubMed] [Google Scholar]
- 159.Schlesinger, M., F. N. Chu, M. Badamchian, G. D. Jiang, J. P. Roboz, A. L. Goldstein, and J. G. Bekesi. 1994. A distinctive form of soluble CD8 is secreted by stimulated CD8+ cells in HIV-1 infected and high-risk individuals. Clin. Immunol. Immunopathol. 73:252-260. [DOI] [PubMed] [Google Scholar]
- 160.Sciacca, F. L., M. Sturzl, F. Bussolino, M. Sironi, H. Brandstetter, C. Zietz, D. Zhou, C. Matteucci, G. Peri, S. Sozzani, R. Benelli, M. Arese, A. Albini, F. Colotta, and A. Mantovani. 1994. Expression of adhesion molecules, platelet activating factor and chemokines by Kaposi's sarcoma cells. J. Immunol. 153:4816-4825. [PubMed] [Google Scholar]
- 161.Scinicariello, F., M. J. Dolan, I. Nedelcu, S. K. Tyring, and J. K. Hilliard. 1994. Occurrence of human papillomavirus and p53 gene mutations in Kaposi's sarcoma. Virology 203:153-157. [DOI] [PubMed] [Google Scholar]
- 162.Seftor, R. E., E. A. Seftor, W. G. Stetler-Stevensen, and M. J. Hendrix. 1993. The 72 kDa type IV collagenase is modulated via differential expression of αvβ3 and α5β1 integrins during human melanoma cell invasion. Cancer Res. 53:3411-3415. [PubMed] [Google Scholar]
- 163.Shi, B., U. De Girolami, J. He, S. Wang, A. Lorenzo, J. Busciglio, and D. Gabuzda. 1996. Apoptosis induced by HIV-1 infection of the central nervous system. J. Clin. Investig. 98:1979-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Simpson, G. R., T. F. Schulz, D. Whitby, P. M. Cook, C. Boshoff, L. Rainbow, M. R. Howard, J. J. Gao, R. A. Bohenzky, P. Simmonds, C. Lee, A. de Ruiter, A. Hatzakis, R. S. Tedder, I. V. Weller, R. A. Weiss, and P. S. Moore. 1996. Prevalence of Kaposi's sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet 348:1133-1138. [DOI] [PubMed] [Google Scholar]
- 165.Reference deleted.
- 166.Sirianni, M. C., L. Vincenzi, V. Fiorelli, S. Topino, E. Scala, S. Uccini, A. Angeloni, A. Faggioni, D. Cerimele, F. Cottoni, F. Aiuti, and B. Ensoli. 1998. Gamma-interferon production in peripheral blood mononuclear cells and tumor infiltrating lymphocytes from Kaposi's sarcoma patients: correlation with the presence of human herpesvirus-8 in peripheral blood mononuclear cells and lesional macrophages. Blood 91:968-976. [PubMed] [Google Scholar]
- 167.Reference deleted.
- 168.Reference deleted.
- 169.Slavin, G., H. M. Cameron, and H. Singh. 1969. Kaposi's sarcoma in mainland Tanzania: a report of 117 cases. Br. J. Cancer 23:349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sodroski, J., R. Patarca, C. Rosen, F. Wong-Staal, and W. Haseltine. 1985. Location of the trans-activating region of the genome of human T-cell lymphotropic virus type III. Science 229:74-77. [DOI] [PubMed] [Google Scholar]
- 171.Soldi, R., S. Mitola, M. Strasly, P. Defilippi, G. Tarone, and F. Bussolino. 1999. Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 18:882-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Steinaa, L., A. M. Sorensen, J. O. Nielsen, and J. E. Hansen. 1994. Antibody to HIV-1 Tat protein inhibits the replication of virus in culture. Arch. Virol. 139:263-271. [DOI] [PubMed] [Google Scholar]
- 174.Stromblad, S., J. C. Becker, M. Yebra, P. C. Brooks, and D. A. Cheresh. 1996. Suppression of p53 activity and p21waf/cp1 expression by vascular cell integrin αvβ3 during angiogenesis. J. Clin. Investig. 98:426-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stürzl, M., C. Blasig, A. Schreier, F. Neipel, C. Hohenadl, E. Cornali, G. Ascherl, S. Esser, N. H. Brockmeyer, M. Ekman, E. E. Kaaya, E. Tschachler, and P. Biberfeld. 1997. Expression of HHV-8 latency-associated T0.7 RNA in spindle cells and endothelial cells of AIDS-associated, classical and African Kaposi's sarcoma. Int. J. Cancer 72:68-71. [DOI] [PubMed] [Google Scholar]
- 176.Stürzl, M., C. Hohenadl, C. Zietz, E. Castanos-Velez, A. Wunderlich, G. Ascherl, P. Biberfeld, P. Monini, P. J. Browning, and B. Ensoli. 1999. Expression of K13/v-FLIP gene of human herpesvirus 8 and apoptosis in Kaposi's sarcoma spindle cells. J. Natl. Cancer Inst. 91:1725-1733. [DOI] [PubMed] [Google Scholar]
- 177.Stürzl, M., A. Wunderlich, G. Ascherl, C. Hohenadl, P. Monini, C. Zietz, P. J. Browning, F. Neipel, P. Biberfeld, and B. Ensoli. 1999. Human herpesvirus-8 (HHV-8) gene expression in Kaposi's sarcoma (KS) primary lesions: an in situ hybridization study. Leukemia 13:S110-S112. [DOI] [PubMed] [Google Scholar]
- 178.Subramanyam, M., W. G. Gutheil, W. W. Bachovchin, and B. T. Huber. 1993. Mechanism of HIV-1 Tat induced inhibition of antigen-specific T cell responsiveness. J. Immunol. 150:2544-2553. [PubMed] [Google Scholar]
- 179.Taylor, J. P., C. Cupp, A. Diaz, M. Chowdhury, K. Khalili, S. A. Jimenez, and S. Amini. 1992. Activation of expression of genes coding for extracellular matrix proteins in Tat-producing glioblastoma cells. Proc. Natl. Acad. Sci. USA 89:9617-9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Terman, B. I., M. Dougher-Vermazen, M. E. Carrion, D. Dimitrov, D. C. Armellino, D. Gospodarowicz, and P. Bohlen. 1992. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem. Biophys. Res. Commun. 187:1579-1586. [DOI] [PubMed] [Google Scholar]
- 181.Thomas, K. A. 1996. Vascular endothelial growth factor, a potent and selective angiogenic agent. J. Biol. Chem. 271:603-606. [DOI] [PubMed] [Google Scholar]
- 182.Touloumi, G., A. Hatzakis, I. Potouridou, I. Milona, J. Strarigos, A. Katsambas, G. Giraldo, E. Beth-Giraldo, R. J. Biggar, N. Mueller, and D. Trichopoulos. 1999. The role of immunosuppression and immune-activation in classic Kaposi's sarcoma. Int. J. Cancer 82:817-821. [DOI] [PubMed] [Google Scholar]
- 183.Urassa, W. K., E. E. Kaaya, J. N. Kitinya, L. L. Lema, H. Amir, J. Luande, G. Biberfeld, F. S. Mhalu, and P. Biberfeld. 1998. Immunological profile of endemic and epidemic Kaposi's sarcoma patients in Dar-es-Salaam, Tanzania. Int. J. Mol. Med. 1:979-982. [DOI] [PubMed] [Google Scholar]
- 184.Valle, L. D., S. Croul, S. Morgello, S. Amini, J. Rappaport, and K. Khalili. 2000. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J. Neurovirol. 6:221-228. [DOI] [PubMed] [Google Scholar]
- 185.Viscidi, R. P., K. Mayur, H. M. Ledreman, and A. D. Frankel. 1989. Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science 246:1606-1608. [DOI] [PubMed] [Google Scholar]
- 186.Vogel, B. E., S. J. Lee, A. Hieldebrand, W. Craig, M. D. Pierschbacher, F. Wong-Staal, and E. Ruoslahti. 1993. A novel integrin specificity exemplified by binding of the αvβ5 integrin to the basic domain of the HIV Tat protein and vitronectin. J. Cell Biol. 121:461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vogel, J., S. H. Hinrichs, R. K. Reynolds, P. A. Luciw, and G. Jay. 1988. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature 335:606-611. [DOI] [PubMed] [Google Scholar]
- 188.Vyakarnam, A., P. Matear, A. Meager, G. Kelly, B. Stanley, I. Weller, and P. Beverly. 1991. Altered production of tumor necrosis factor α and β and interferon γ by HIV-infected individuals. Clin. Exp. Immunol. 84:109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Weeks, B. S., K. Desai, P. M. Lowenstein, M. E. Klotman, P. E. Klotman, M. Green, and H. K. Kleinman. 1993. Identification of a novel cell attachment domain in the HIV-1 Tat protein and its 90-kDa cell surface binding protein. J. Biol. Chem. 268:5279-5284. [PubMed] [Google Scholar]
- 190.Weindel, K., D. Marme, and H. A. Weich. 1992. AIDS-associated Kaposi's sarcoma cells in culture express vascular endothelial growth factor. Biochem. Biophys. Res. Commun. 183:1167-1174. [DOI] [PubMed] [Google Scholar]
- 191.Weiss, J. M., A. Nath, E. O. Major, and J. W. Berman. 1999. HIV-1 Tat induces monocyte chemoattractant protein-1 mediated monocyte transmigration across a model of the human blood-brain barriers and up-regulates CCR5 expression on human monocytes. J. Immunol. 163:2953-2959. [PubMed] [Google Scholar]
- 192.Weninger, W., T. A. Partanen, S. Breiteneder-Geleff, C. Mayer, H. Kowalski, M. Mildner, J. Pammer, M. Stürzl, D. Kerjaschki, K. Alitalo, and E. Tschachler. 1998. Expression of vascular endothelial growth factor receptor-3 and podoplanin suggets a lymphatic endothelial cell origin of Kaposi's sarcoma tumor cells. Lab. Investig. 79:243-251. [PubMed] [Google Scholar]
- 193.Westendorp, M. O., R. Frank, C. Ochsenbauer, K. Stricker, J. Dhein, H. Walczak, K. M. Debatin, and P. H. Krammer. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497-500. [DOI] [PubMed] [Google Scholar]
- 194.Whitby, D., M. Luppi, P. Barozzi, C. Boshoff, R. A. Weiss, and G. Torelli. 1998. Human herpesvirus 8 seroprevalence in blood donors and lymphoma patients from different regions of Italy. J. Natl. Cancer Inst. 90:395-397. [DOI] [PubMed] [Google Scholar]
- 195.Wieland, U., J. E. Kuhn, C. Jassoy, H. Rubsamen-Waigmann, V. Wolber, and R. W. Ruben. 1990. Antibodies to recombinant HIV-1 Vif, Tat and Nef proteins in human sera. Med. Microbiol. Immunol. 179:1-11. [DOI] [PubMed] [Google Scholar]
- 196.Wright, S. C., A. Jewett, R. Mitsuyasu, and B. Bonavida. 1988. Spontaneous cytotoxicity and tumor necrosis factor production by peripheral blood monocytes from AIDS patients. J. Immunol. 141:99-104. [PubMed] [Google Scholar]
- 197.Xerri, L., J. Hassoun, J. Planche, V. Guigou, J. J. Grob, P. Parc, D. Birnbaum, and O. De Lapeyriere. 1991. Fibroblast growth factor gene expression in AIDS-Kaposi's sarcoma detected by in situ hybridization. Am. J. Pathol. 138:9-15. [PMC free article] [PubMed] [Google Scholar]
- 198.Zauli, G., D. Gibellini, D. Milani, M. Mazzoni, P. Borgatti, M. La Placa, and S. Capitani. 1993. Human immunodeficiency virus type-1 Tat protein protects lymphoid, epithelial and neuronal cell lines from death by apoptosis. Cancer Res. 53:4481-4485. [PubMed] [Google Scholar]
- 199.Zauli, G., M. La Placa, M. Vignoli, M. C. Re, D. Gibellini, G. Furlini, D. Milani, M. Marchisio, M. Mazzoni, and S. Capitani. 1995. An autocrine loop of HIV type-1 Tat protein is responsible for the improved survival/proliferation capacity of permanently tat-transfected cells and required for optimal human immunodeficiency virus type 1 long terminal repeat transactivating activity. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10:306-316. [PubMed] [Google Scholar]
- 200.Zauli, G., D. Gibellini, A. Caputo, A. Bassini, M. Negrini, M. Monne, M. Mazzoni, and S. Capitani. 1995. The human immunodeficiency virus type-1 Tat protein upregulates Bcl-2 gene expression in Jurkat T cell lines and primary peripheral blood mononuclear cells. Blood 86:3823-3834. [PubMed] [Google Scholar]
- 201.Zauli, G., D. Gibellini, C. Celeghini, C. Mischiati, A. Bassini, M. La Placa, and S. Capitani. 1996. Pleiotropic effects of immobilized versus soluble recombinant HIV-1 Tat protein on CD3-mediated activation, induction of apoptosis, and HIV-1 long terminal repeat transactivation in purified CD4+ T lymphocytes. J. Immunol. 157:2216-2224. [PubMed] [Google Scholar]
- 202.Zetter, B. R., and S. E. Brightman. 1990. Cell motility and the extracellular matrix. Curr. Opin. Cell Biol. 2:850-856. [DOI] [PubMed] [Google Scholar]
- 203.Zietz, C., B. Hotz, M. Stürzl, E. Rauch, R. Penning, and U. Lohrs. 1996. Aortic endothelium in HIV-1 infection: chronic injury, activation, and increased leukocyte adherence. Am. J. Pathol. 149:1887-1898. [PMC free article] [PubMed] [Google Scholar]