Abstract
We determined how social stimuli that vary in behavioral relevance differentially activate functional networks in the frog hypothalamus. As measured by egr-1 mRNA levels, activity in three hypothalamic nuclei varied with acoustic stimulus, and these responses were correlated with egr-1 responses in different auditory regions regardless of stimulus. The correlations among hypothalamic nuclei, however, varied as a function of the behavioral relevance of the stimuli. Thus relevant social cues shift the functional connectivity within the hypothalamus, consistent with principles that underlie the simultaneous processing of sensory information in cognitive tasks.
Keywords: amphibian, immediate early gene, mate choice, neural network, túngara frog
The complex anatomical connections characteristic of networks in the brain pose a challenge for understanding neural circuits and their dual capacities to motivate and respond to behavior. A traditional approach in studies of neural systems underlying social behavior has been to investigate properties of individual nuclei or centers. Alternatively, cognitive neuroscience studies have more recently focused on variation in the functional relationships among neocortical areas, that is, on network properties indicated by correlated patterns of activity among brain regions, or functional connectivity (1-3). Functional connectivity has been assessed by using metabolic measures such as positron emission tomography (1) or fluorodeoxy-glucose incorporation (4) to infer neural activity patterns within brain networks.
The limbic and hypothalamic areas are critical candidates for network analyses, because those nuclei are heavily and reciprocally interconnected anatomically and participate in complex ways in multiple aspects of behavior and physiology (5). The brain directs appropriate behavioral and physiological responses to diverse sensory inputs by partitioning interconnected brain regions into independent subnetworks that subserve different tasks (5). Newman (5) described the flexibility of functional connections involved in a variety of aggressive, sexual, and maternal behaviors in the context of a social behavior network of six limbic regions. A similar but not entirely congruent network of limbic regions has been described in geckos (6) in which developmental influences on functional connectivity have been linked to aggressive behaviors (7). Newman (5) predicted that sensory inputs modulate the transient subnetworks that mediate continuously varying behavioral responses to social cues. In this paper, we extend Newman's prediction to consider how socially relevant auditory inputs demarcate subnetworks in the hypothalamus, a brain division that has a critical role in mediating reproductive responses to social cues.
Anurans are tractable models for such an investigation. Behavioral responses to conspecific vocal signals are well documented, and we have a rich understanding of how the auditory system differentially responds to behaviorally relevant acoustic stimuli (8, 9). Neuroanatomical connections between auditory and hypothalamic regions have been identified (10-15), and the physiological outputs of the hypothalamus may be elicited by stimulation with mating calls (14, 16-21). At present, however, functional localization within the anuran hypothalamus is poorly understood, although it is known that several hypothalamic regions participate in endocrine control and reproductive behavior of both sexes (22-25). Here we document neural activation in different acoustic conditions to determine whether hypothalamic regions show selectivity among mating calls, how hypothalamic activity relates to auditory system inputs, and how acoustic stimulation modifies functional connectivity within the hypothalamus.
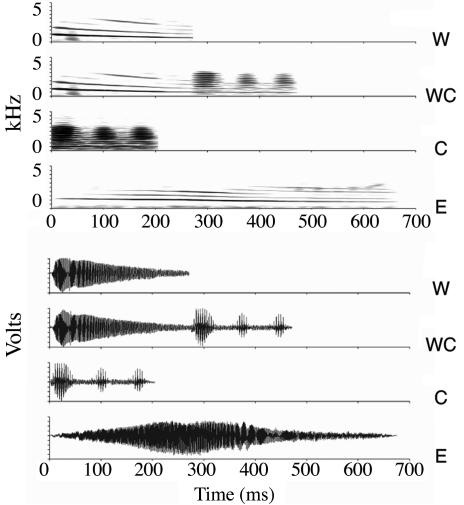
We examined the hypothalamic responses to acoustic social cues in the túngara frog, Physalaemus pustulosus. As for most frogs, critical reproductive activities are motivated by species-specific mating calls (26, 27). Females use these calls to identify males of the correct species and to further choose among potential conspecific mates. Males of this species produce simple “whine” and complex “whine-chuck” calls (Fig. 1), both of which elicit female reproductive behavior, whereas the latter is more attractive to females (26). The chuck portion of the complex call by itself does not elicit responses from females, nor is it ever produced in nature without the accompanying whine (28, 29). We exposed females to one of five acoustic treatments: no stimulus, one of two irrelevant calls (conspecific chuck-only or heterospecific Physalaemus enesefae whine) or one of two behaviorally relevant calls (conspecific whine or whine-chuck). To determine the hypothalamic responses to these relevant and irrelevant calls, we examined mRNA levels of the activity marker egr-1 (also called krox-24, zif268, NGFI-A, TIS8, or ZENK) after acoustic stimulation (30). We measured egr-1 in seven hypothalamic nuclei (Fig. 2) and seven auditory midbrain and thalamic nuclei.
Fig. 1.
Recordings of natural mating calls used as acoustic stimuli. Sonograms (Upper) and waveforms (Lower) of acoustic stimuli broadcast to frogs. Top to bottom for each: P. pustulosus whine, P. pustulosus whine-chuck, chuck-only, and heterospecific P. enesefae whine.
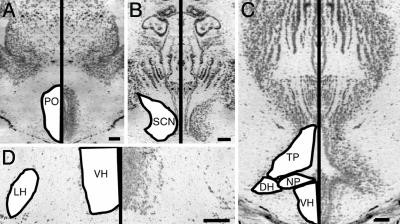
Fig. 2.
Photomicrographs of cresyl-violet-stained transverse sections through frog brain. Left half of photographs are mirror images of right half for clarity. Hypothalamic nuclei are labeled on figures based on standard nomenclature (31) with the following abbreviations: DH, dorsal hypothalamus; NP, nucleus of the periventricular organ; POA, anterior POA; VH, ventral hypothalamus. (Bar, 100 μm.) (A) The second-most rostral section we used for POA analysis. (B) Section containing the SCN comparable to figure 3A in ref. 31. (C) Third-most rostral section containing the infundibular hypothalamus, equivalent to figure 5A in ref. 31. (D) Higher-magnification image showing sparse cells in the LH at a level corresponding to figure 4A of ref. 31.
Materials and Methods
Exposure of Frogs to Acoustic Stimuli. We collected P. pustulosus females in amplexus between 1930 and 2330 h between 23 July and 2 August 2002 from natural breeding aggregations near the Smithsonian Tropical Research Institute in Gamboa, Panama. To confirm mate receptivity, each female was tested once by using standard phonotaxis tests with whine and whine-chuck stimuli broadcast at 82 dB SPL (sound pressure level, relative to 20 μP) from speakers at opposite sides of an acoustic chamber, then responsive females were placed inside sound-dampening boxes. After 2 h without stimulation, we exposed females to one of five acoustic treatments as described (30): silence, natural heterospecific P. enesefae call, natural P. pustulosus whine, natural P. pustulosus whine-chuck, or P. pustulosus chuck trimmed from the whine-chuck stimulus (Fig. 1).
Analysis of egr-1 Expression. We performed egr-1 radioactive in situ hybridization as described (30) and used custom automated-counting procedures to measure egr-1 expression in each brain region in digital photomicrographs with unbiased sampling detailed below. Our measure of egr-1 levels was silver grain density, calculated from the area covered by cell bodies and from grain number within a standard-sized sampling frame as described (30).
We determined borders of hypothalamic subdivisions based on cytoarchitecture following the description of the bullfrog diencephalon (31). We selected sections to be analyzed for each region by using the following criteria. Four sections spaced at least 32 μm apart within the anterior preoptic area (POA) were selected based on cytoarchitecture. The rostral-most appearance of the POA, with its circular preoptic recess, marked the first section. The second section was identified by the dorsoventral elongation of the preoptic recess (Fig. 2 A). More caudally, laminations appeared in the POA, indicating the third section. The fourth POA section resembled figure 1B in ref. 31. We located the suprachiasmatic nucleus (SCN) by using the optic chiasm as the rostral landmark, then selected three sections corresponding to figures 2B and 3 A and B in ref. 31 for egr-1 quantification (Fig. 2B). We chose six sections for analysis of the infundibular hypothalamus by matching cytoarchitecture pictured in figures 4 A and B, 5 A and B, and 6 A and B of ref. 31 (see Fig. 2 C and D for examples). Sections that were torn, missing, or angled were excluded from the analysis, resulting in exclusion of some frogs in which no sections were appropriate to measure one or more brain regions. The resultant number of animals per group is listed in Table 2, which is published as supporting information on the PNAS web site.
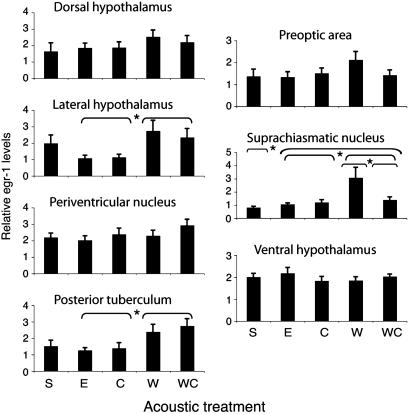
Fig. 3.
SCN, PT, and LH show significant egr-1 variation with stimulus. Mean egr-1 levels (grains/pixel×104) for frogs in each stimulus condition in seven hypothalamic nuclei. Group differences in egr-1 levels were assessed by ANOVA with three contrasts (Sound: S vs. E, C, W, WC; Relevance: E, C vs. W, WC; Preference, W vs. WC). Asterisks indicate which contrasts were significant (P < 0.05). Error bars indicate standard error. S, silence; E, P. enesefae whine; C, chuck-only; W, P. pustulosus whine; WC, P. pustulosus whine-chuck.
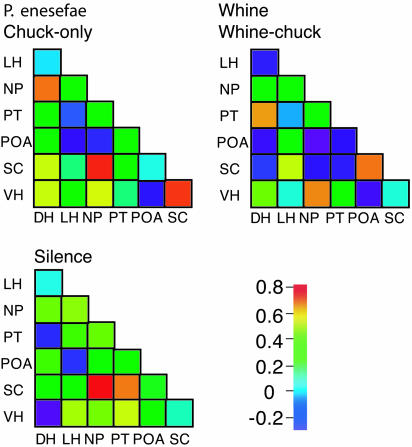
Fig. 4.
Pair-wise correlations between hypothalamic nuclei vary with acoustic stimulus. Pair-wise Pearson correlation coefficients between nuclei calculated for unstimulated animals, for animals hearing irrelevant calls (chuck-only and P. enesefae whine), and for frogs exposed to behaviorally relevant calls (conspecific whine and whine-chuck). Coefficient coded by color scale (Lower Right) with red colors representing high and blue indicating low coefficients.
Fig. 5.
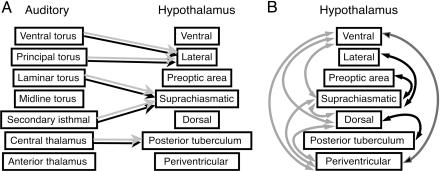
Functional connectivity of the hypothalamus. Gray arrows show significant relationships (P < 0.05) in frogs that heard irrelevant acoustic stimuli (P. enesefae whine and chuck-only), and black arrows indicate relationships in frogs exposed to behaviorally relevant stimuli (conspecific whine and whine-chuck). (A) egr-1 levels in midbrain and thalamic nuclei implicated in auditory processing are significant predictors of hypothalamic expression patterns. Relationships between auditory and hypothalamic regions do not vary with relevance of stimulus. (B) egr-1 correlations between hypothalamic regions differ based on behavioral relevance of acoustic stimulus.
We measured the egr-1 expression in each brain region in digital photomicrographs (Optronics camera, Olympus BX60 microscope with ×100 objective) spaced within the larger hypothalamic nuclei as described below. The POA was spanned by three photomicrographs beginning at a random position within 100 μm of the ventral POA border, then spaced by 60 μm (rostral-most section), 75 μm (second-most rostral section), or 150 μm (caudal two sections). The last image in the series fell outside the borders of the POA in some sections and was excluded. We selected the first image of the SCN and then collected a second image in each section spaced 100 μm away across the midline. The two photomicrographs in the dorsal hypothalamus (DH) and posterior tuberculum (PT) were spaced by 100 and 150 μm, respectively, along the long axis of the nucleus within one hemisphere (mediolateral for DH, varying for PT depending on rostrocaudal position). We selected a position within the ventral-most 100 μm of the ventral hypothalamus at random for the first image and captured the second image in the same hemisphere 150 μm away in the dorsal or dorsolateral directions. Because of their small size, the lateral hypothalamus (LH) and nucleus of the periventricular organ each yielded only one photomicrograph per section.
We also measured egr-1 levels in several auditory regions in the thalamus and midbrain for correlation with hypothalamic egr-1 levels. Photomicrographs in four toral subdivisions were collected as described (30). One photomicrograph centered in the small anterior thalamic nucleus was taken in one to three sections at least 32 μm apart. We analyzed two photomicrographs in the central thalamic nucleus, spaced by 150 μm dorsoventrally, in one to three sections at least 32 μm apart. Two to six sections spaced 32 μm apart each yielded one photomicrograph encompassing the central portion of the secondary isthmal nucleus.
Statistics. We used spss 11 (SPSS, Chicago) and mplus 3.11 (Muthén & Muthén, Los Angeles) for statistical analyses.
To determine how the acoustic stimuli influenced mean egr-1 expression in the hypothalamus, we used ANOVA with acoustic stimulus as the between-subjects factor for each subdivision separately and further tested the specific effects of acoustic stimulus by using three orthogonal contrasts. To test for the effect of sound, we compared females that heard any mating call with those that heard no sound (Contrast: Sound). Comparing females that heard the whine or whine-chuck with females that heard the chuck-only or P. enesefae whine tested for the effect of call relevance (Contrast: Relevance). Neither the heterospecific call nor the chuck-only stimulus elicits phonotaxis from females, and neither is heard naturally in Panama (26, 27), whereas both natural whine and whine-chuck cause robust phonotactic responses (28). Grouping of the two irrelevant stimuli was supported by the lack of significant differences in egr-1 levels between animals presented with chuck-only and P. enesefae whine in all 14 auditory or hypothalamic regions (t test, P > 0.1; data not shown). Because female P. pustulosus prefer conspecific whine-chuck stimuli to whines (28), we tested for differences between conspecific calls, comparing females that heard the whine with those that heard the whine-chuck (Contrast: Preference). Several variables were not normally distributed because of positive skew and kurtosis. Log-transformation of these egr-1 levels produced normal distributions. We ran all ANOVA analyses by using both log-transformed and untransformed data and found identical patterns of statistical significance with one exception: the overall ANOVA F ratio for the LH was significant at the α = 0.05 level by using log-transformed but not untransformed data. Because of the similarity in results after transformation, this paper reports results by using untransformed data.
We compared hypothalamic egr-1 levels with those in auditory regions of the thalamus and midbrain by using multiple regression analyses. Seven separate regressions were analyzed by using mean egr-1 levels in the seven hypothalamic regions as dependent variables. Mean egr-1 levels in seven auditory thalamic and midbrain regions (four regions of the torus: laminar, midline, principal, and ventral; secondary isthmal nucleus; anterior thalamic nucleus; and central thalamic nucleus) (11, 32) were independent variables entered simultaneously. Adjusted R2 is reported because of small sample size. Significance of the regression model was tested by ANOVA, and contribution of each independent variable was calculated as regression coefficients and tested by t test. Differences in auditory-hypothalamic relationships based on stimulus relevance were assessed by using hierarchical linear regressions comparing basic regressions as above with those adding predictor variables representing call relevance and interaction terms. Relevance was encoded for each individual based on acoustic treatment as follows: one predictor variable indicating irrelevant stimuli (silence, 0; chuck-only or P. enesefae, 1; whine, or whine-chuck, 0) and one for relevant calls (silence, 0; chuck-only or P. enesefae, 0; whine or whine-chuck, 1). Interaction terms were the product of the irrelevance or relevance variable (value 0 or 1) and egr-1 levels in one of the auditory regions. We limited the number of total predictor variables by including only those interaction terms based on the one or two auditory regions that contributed significantly to the basic regression by t test. Improvements in regressions with relevance and interaction variables were determined by using F tests comparing the two nested models.
We examined functional connectivity among hypothalamic nuclei by using correlation and covariance analyses. We calculated pair-wise Pearson correlation coefficients to assess linear relationships between egr-1 levels in all 21 possible pairs of the seven hypothalamic nuclei, calculating correlations separately in three groups: silence (n = 7), frogs that heard irrelevant calls (chuck-only and P. enesefae; n = 13), and frogs that heard behaviorally relevant calls (whine and whine-chuck; n = 12). We then compared covariance matrices by using mplus 3.11 to test cross-sample models based on two of the three groups defined above: frogs that heard irrelevant calls and frogs that heard behaviorally relevant calls. All 21 pair-wise covariances between hypothalamic nuclei were included in the model, and corresponding covariances were constrained to be equal across groups. Parameters were estimated by using mean-adjusted maximum-likelihood procedures and compared with the independence model in which cross-group constraints were lifted. In addition to statistical tests, we examined Bentler's Comparative Fit Index (CFI) and Standardized Root-Mean-Square Residual (SRMR) to assess model fit (criteria for good fit: CFI >0.96, SRMR <0.10) (33).
Results
Acoustic Stimulation Alters egr-1 Expression. Three hypothalamic regions showed modulation of egr-1 levels by acoustic stimulus, and response biases differed among regions (Fig. 3, and Table 2, and Table 3, which is published as supporting information on the PNAS web site). We characterized mating call selectivity with univariate ANOVAs followed by three orthogonal contrasts: (i) unstimulated animals compared with animals that heard any stimulus, (ii) frogs that heard behaviorally relevant mating calls (conspecific whine or whine-chuck) with those that heard irrelevant mating calls (chuck-only or heterospecific whine), and (iii) animals presented with whine compared with those exposed to whine-chuck. Both the LH (t29 = 3.058, P = 0.005) and PT (t29 = 3.243, P = 0.003) exhibited significant egr-1 induction in response to either relevant mating call. The SCN showed a distinct selectivity, with significant differences in egr-1 expression for relevant vs. irrelevant calls (t28 = 2.591, P = 0.015), as well as for unstimulated vs. stimulated animals (t28 = 3.530, P = 0.001) and for whine vs. whine-chuck comparisons (t28 = 3.747, P = 0.001). Four other hypothalamic nuclei did not show significant egr-1 variation with stimulus (all P > 0.1).
Auditory-Hypothalamic Relationships. egr-1 levels in each acoustically responsive hypothalamic nucleus were linearly related to gene expression in midbrain and thalamic auditory nuclei, and these relationships depended on different auditory regions. Expression in the LH was significantly related to egr-1 levels in the principal and ventral regions of the torus semicircularis, the anuran homolog of mammalian inferior colliculus (Table 1, multiple regression: R2 = 0.558, F7,15 = 4.969, P = 0.004; Table 4, which is published as supporting information on the PNAS web site, significant predictors: principal β = 0.678, t15 = 3.316, P = 0.005 and ventral β = -0.506, t15 = 2.79, P = 0.014). egr-1 levels in the PT were significantly predicted by central thalamic nucleus expression (Table 1, multiple regression: R2 = 0.809, F7,14 = 13.728, P < 0.001; Table 5, which is published as supporting information on the PNAS web site, significant predictor: central β = 0.952, t14 = 5.31, P < 0.001), and SCN egr-1 expression was significantly associated with egr-1 levels of the midbrain secondary isthmal nucleus as well as the laminar nucleus of the torus semicircularis (Table 1, multiple regression: R2 = 0.539, F7,15 = 4.675, P = 0.006; Table 6, which is published as supporting information on the PNAS web site, significant predictors: secondary isthmal β = 0.798, t15 = 7.055, P < 0.001 and laminar β = 0.363, t15 = 2.847, P = 0.013). No other hypothalamic regions had egr-1 measures that were linearly related to auditory region levels (Table 1). None of these multiple regressions depended significantly on whether frogs heard behaviorally relevant (conspecific whine or whine-chuck) or irrelevant (heterospecific or chuck-only) stimuli (Table 1). Thus different auditory streams predict gene expression in distinct hypothalamic regions, and the relationships between these auditory and hypothalamic regions are stable despite changing behavioral relevance of acoustic stimuli.
Table 1. Linear associations between hypothalamic and auditory regions do not depend on behavioral relevance of stimulus.
| Auditory-hypothalamic regression
|
Behavioral relevance included
|
|||||
|---|---|---|---|---|---|---|
| Hypothalamic region | Adjusted R2 | F | P | R2 change | F change | P |
| Dorsal hypothalamus | 0.295 | 2.313 | 0.082 | 0.015 | 0.214 | 0.810 |
| Lateral hypothalamus | 0.558 | 4.969 | 0.004 | 0.128 | 1.105 | 0.428 |
| Periventricular nucleus | 0.386 | 1.348 | 0.296 | 0.032 | 0.353 | 0.709 |
| Preoptic area | 0.088 | 1.289 | 0.324 | 0.055 | 0.598 | 0.565 |
| Posterior tuberculum | 0.539 | 4.765 | 0.006 | 0.099 | 1.266 | 0.341 |
| Suprachiasmatic nucleus | 0.809 | 13.728 | 0.000 | 0.043 | 0.920 | 0.510 |
| Ventral hypothalamus | −0.176 | 0.530 | 0.799 | 0.136 | 1.329 | 0.298 |
The auditory-hypothalamic regression column summarizes relationships between egr-1 levels in all seven auditory regions (independent predictor variables) and each hypothalamic region (dependent variable). The behavioral relevance column shows little improvement in auditory-hypothalamic multiple regressions when variables encoding behavioral relevance of stimulus and interaction terms are added. (see Statistics in Materials and Methods).
Relationships Among Hypothalamic Nuclei. Unlike the auditory-hypothalamic associations, the functional connectivity among hypothalamic nuclei varied with biological relevance of the stimuli (Fig. 4). We examined pair-wise correlations in egr-1 levels between hypothalamic nuclei in frogs exposed to no stimulus, behaviorally relevant, or irrelevant stimuli. Unstimulated frogs had only one significant correlation in egr-1 levels within the hypothalamus (SC and nucleus of the periventricular organ). Frogs that heard either behaviorally relevant (whine, whine-chuck) or irrelevant stimuli (P. enesefae, chuck-only) had several significant pair-wise correlations in regional hypothalamic activity, but the pattern of correlations, that is, the functional connectivity, differed depending on stimulus relevance (Figs. 4 and 5). Results from a structural equation model (34) rejected the null hypothesis that the intrahypothalamic covariance matrices did not differ between frogs hearing relevant and irrelevant mating calls; the intercorrelation pattern was significantly different between treatment groups (Sartorra-Bentler 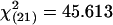 , P = 0.001, Comparative Fit Index = 0.646, Standardized Root-Mean-Square Residual = 0.345). The inter- and intraregional network properties of the hypothalamus, therefore, show fundamentally different responses to social stimuli (Fig. 5): auditory inputs have stable regressions with hypothalamic nuclei regardless of stimulus; however, the intercorrelated functional networks within the hypothalamus differ depending on stimulus relevance.
, P = 0.001, Comparative Fit Index = 0.646, Standardized Root-Mean-Square Residual = 0.345). The inter- and intraregional network properties of the hypothalamus, therefore, show fundamentally different responses to social stimuli (Fig. 5): auditory inputs have stable regressions with hypothalamic nuclei regardless of stimulus; however, the intercorrelated functional networks within the hypothalamus differ depending on stimulus relevance.
Discussion
Functional neuroanatomical analyses may be focused either on the assessment of individual brain regions or on the activity of neural networks that participate in sensory and motor tasks (3). We show here that both types of analyses contribute to understanding signal processing and highlight the potential for simultaneous consideration of multiple brain regions when estimating neural activity by using egr-1 expression, a marker previously used to map functional responses to sensory stimuli in individual brain nuclei (e.g., refs. 35-38). Our analysis centered on the processing of acoustic communication signals in the túngara frog. The behavioral responses of these female frogs to acoustic signals vary depending on their recognition of the signals as normal conspecific calls or as behaviorally irrelevant acoustic stimuli and further depending on the degree of attractiveness of those conspecific calls. For the signals we used here, túngara frogs exhibit phonotaxis to whines and whine-chucks and strongly prefer the whine-chuck when both are presented (26). Conversely, heterospecific P. enesefae calls and chucks presented alone elicit little or no response from P. pustulosus females (27-29). We find two types of significant differences in hypothalamic egr-1 expression coinciding with the different behavioral relevance of these signals: quantitative differences in gene activation and qualitative differences in network properties. The first type of response was an increase in mean egr-1 expression in select nuclei, nominating them as particularly important in generating behavioral or physiological responses to social signals. The second type of response was a differential configuration of functional networks within the hypothalamus for behaviorally relevant vs. irrelevant calls. How these two neural responses relate to behavioral and physiological responses to calls or to the cognitive processes underlying decisions, and how these responses differ in species with divergent behavioral selectivity, are important questions for future study.
egr-1 expression patterns in three hypothalamic regions varied with acoustic stimulus. The SCN, LH, and PT all receive auditory inputs (10-13, 15), but no studies have reported auditory responsiveness or call selectivity. We found that all three regions showed egr-1 elevation in response to conspecific calls, with the PT and LH increasing egr-1 levels following both conspecific stimuli, whereas the SCN had greater egr-1 responsiveness to the whine than the whine-chuck. Inferring electrophysiological activity from egr-1 responses is complicated, because the egr-1 and electrical responses to presynaptic neurotransmitter release may be uncoupled by differential dependence on the protein contingent in the postsynaptic cell (39, 40); nonetheless egr-1 induction suggests that these three nuclei are electrically active during reception of conspecific mating calls and therefore may contribute to female responses. The LH has not been previously implicated in mating behavior or physiology, nor has the SCN, which in the green treefrog receives retinal input in one portion and may influence circadian rhythms as found in other vertebrates (41). The PT has been implicated in reproductive behavior in female frogs: lesioning dopamine-containing neurons decreases locomotive response to mating calls, and remaining phonotactic behavior is correlated with the number of surviving dopaminergic cells in the PT (25). Two nuclei previously identified as acoustically responsive by using electrophysiology, ventral hypothalmus and POA (20), did not show egr-1 induction in response to mating calls, perhaps because of the hormonal status of amplexed females, the absence of protein components necessary for egr-1 induction, or the mixture of both cells that decrease and cells that increase activity in response to sound. How hypothalamic auditory specificity relates to the separate roles of each brain region in mating responses will require additional functional studies combining behavioral and physiological measures with analyses of auditory activation within the hypothalamus.
The auditory egr-1 responses of the hypothalamus differ not only in their selectivity but also in their associations with egr-1 levels in midbrain and thalamic auditory regions. Correlated egr-1 responses depend on anatomical connections, but functional and anatomical connectivity may be dissociated by distinct thresholds for egr-1 induction in different brain regions and by the context-dependent preponderance of a subset of anatomical inputs in downstream neural activation. Therefore, significant functional associations may be based either on active anatomical connections between the specific auditory and hypothalamic regions or on common inputs to the correlated regions. For example, the laminar nucleus of the torus does send at least minor projections to the SCN (13), and the PT receives central thalamic inputs (12). In each case, the significant regression coefficients may be caused by that anatomical link: the auditory neurons increase neurotransmitter release in response to acoustic stimulation, and that neurotransmitter release initiates egr-1 induction. In this way, strong relationships between a hypothalamic region and a midbrain or thalamic region may indicate direct functional links between those regions. The anatomical and functional connections between these hypothalamic and auditory regions would therefore warrant more complete investigation. Even in cases in which correlations may not rely on direct anatomical connections, the distinct auditory-hypothalamic relationships suggest that hypothalamic nuclei receive separate auditory inputs and thus may focus on different aspects of mating call stimuli. That hypothalamic nuclei may be driven by different parts of the auditory system may reflect different functional subsystems whose components and roles in mating behavior and physiology remain to be described.
In addition to auditory-hypothalamic relationships, we also found correlated activation among hypothalamic nuclei. Here we show that the shifts in functional connectivity characterizing the social behavior network hypothesis (5) can be extended to the frog hypothalamus in which network activity patterns vary dynamically according to behavioral significance of sensory inputs. These fast and presumably transient changes in egr-1 levels cannot be caused by differences in anatomical connections between groups, thus differences in functional connectivity within the hypothalamus most likely rely on variation in synaptic activity in the auditory-hypothalamic projections. This type of network analysis has not been applied previously to immediate early gene expression data. Future analyses of emergent networks in response to varied social cues may enable us to causally link sensory inputs with consequent emergent networks within the hypothalamus and the behaviors produced by such transient networks.
These experiments highlight principles by which neural networks may generate appropriate responses to sensory inputs. Specific responses of individual hypothalamic regions are derivative of the responses of the auditory nuclei to which they are connected, whereas the functional connectedness within the hypothalamus is an emergent property modulated by the relevance of social context. The principles of parallel processing and distributed functional networks that we describe here in the frog hypothalamus are remarkably similar to those processes posited in cognitive neuroscience including perception, memory, and decision-making (42, 43). In both the frog hypothalamus and human cortex, sensory inputs may be processed in separate streams, and these streams may provide independent inputs to integrative regions. Differently configured, transient functional networks of interacting integrative regions may subserve different cognitive tasks in the human cortex (44, 45) and different reproductive roles in the frog hypothalamus, as suggested here. Hypothalamic and cognitive networks differ in that processes such as attention adjust responses to sensory inputs in cortical circuits (2, 43, 46), whereas the frog hypothalamic nuclei show consistent influence from auditory inputs regardless of stimulus relevance, at least in reproductively active females. Although cognitive networks may invoke more levels of modulation, the patterns of correlations we have described may represent fundamental properties common to all complex interconnected networks in the brain.
Supplementary Material
Acknowledgments
We thank X. Bernal, S. Burmeister, K. Lynch, K. Lampbert, and A. S. Rand for contributions to experiments. The Smithsonian Tropical Research Institute and the National Science Foundation (IBN 9816564) provided generous funding for this research.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: POA, preoptic area; SCN, suprachiasmatic nucleus; PT, posterior tuberculum; LH, lateral hypothalamus.
References
- 1.Friston, K. J., Frith, C. D., Liddle, P. F. & Frackowiak, R. S. (1993) J. Cereb. Blood Flow Metab. 13, 5-14. [DOI] [PubMed] [Google Scholar]
- 2.Bressler, S. L. (1995) Brain Res. Rev. 20, 288-304. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz, B., Tagamets, M. A. & McIntosh, A. R. (1999) Trends Cognit. Sci. 3, 91-98. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh, A. R. & Gonzalez-Lima, F. (1991) Brain Res. 547, 295-302. [DOI] [PubMed] [Google Scholar]
- 5.Newman, S. W. (1999) Ann. N.Y. Acad. Sci. 877, 242-257. [DOI] [PubMed] [Google Scholar]
- 6.Crews, D. (2003) Dev. Psychobiol. 43, 1-10. [DOI] [PubMed] [Google Scholar]
- 7.Sakata, J. T., Coomber, P., Gonzalez-Lima, F. & Crews, D. (2000) Brain Behav. Evol. 55, 139-151. [DOI] [PubMed] [Google Scholar]
- 8.Gerhardt, H. C. & Huber, F. (2002) Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions (Univ. of Chicago Press, Chicago).
- 9.Ryan, M. J., ed. (2001) Anuran Communication (Smithsonian Institution Press, Washington, DC).
- 10.Wilczynski, W. & Northcutt, R. G. (1983) J. Comp. Neurol. 214, 321-332. [DOI] [PubMed] [Google Scholar]
- 11.Neary, T. J. & Wilczynski, W. (1986) Neurosci. Lett. 71, 142-146. [DOI] [PubMed] [Google Scholar]
- 12.Hall, J. C. & Feng, A. S. (1987) J. Comp. Neurol. 258, 407-419. [DOI] [PubMed] [Google Scholar]
- 13.Endepols, H. & Walkowiak, W. (2001) J. Comp. Physiol. A 186, 1119-1133. [DOI] [PubMed] [Google Scholar]
- 14.Allison, J. D. & Wilczynski, W. (1991) Brain Behav. Evol. 38, 322-331. [DOI] [PubMed] [Google Scholar]
- 15.Endepols, H., Roden, K., Luksch, H., Dicke, U. & Walkowiak, W. (2004) J. Comp. Neurol. 468, 299-310. [DOI] [PubMed] [Google Scholar]
- 16.Brzoska, J. & Obert, H. (1980) J. Comp. Physiol. A 140, 25-29. [Google Scholar]
- 17.Burmeister, S. S. & Wilczynski, W. (2000) Horm. Behav. 38, 201-209. [DOI] [PubMed] [Google Scholar]
- 18.Burmeister, S. S. & Wilczynski, W. (2005) Brain Behav. Evol. 65, 26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lea, J., Dyson, M. & Halliday, T. (2001) Anim. Behav. 61, 373-377. [DOI] [PubMed] [Google Scholar]
- 20.Allison, J. D. (1992) J. Comp. Physiol. A 171, 387-395. [DOI] [PubMed] [Google Scholar]
- 21.Wilczynski, W. & Allison, J. D. (1989) Brain Behav. Evol. 33, 317-324. [DOI] [PubMed] [Google Scholar]
- 22.Ball, J. N. (1981) Gen. Comp. Endocrinol. 44, 135-170. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt, R. S. (1968) Behaviour 30, 239-257. [Google Scholar]
- 24.Schmidt, R. S. (1989) Horm. Behav. 23, 1-9. [DOI] [PubMed] [Google Scholar]
- 25.Endepols, H., Schul, J., Gerhardt, H. C. & Walkowiak, W. (2004) J. Neurobiol. 60, 395-410. [DOI] [PubMed] [Google Scholar]
- 26.Ryan, M. J. (1980) Science 209, 523-525. [DOI] [PubMed] [Google Scholar]
- 27.Ryan, M. J. & Rand, A. S. (1995) Science 269, 390-392. [DOI] [PubMed] [Google Scholar]
- 28.Rand, A. S. & Ryan, M. J. (1981) Z. Tierpsychol. 57, 209-214. [Google Scholar]
- 29.Farris, H. E., Rand, A. S. & Ryan, M. J. (2002) Brain Behav. Evol. 60, 181-188. [DOI] [PubMed] [Google Scholar]
- 30.Hoke, K. L., Burmeister, S. S., Fernald, R. D., Rand, A. S., Ryan, M. J. & Wilczynski, W. (2004) J. Neurosci. 24, 11264-11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neary, T. J. & Northcutt, R. G. (1983) J. Comp. Neurol. 213, 262-278. [DOI] [PubMed] [Google Scholar]
- 32.Wilczynski, W. & Capranica, R. R. (1984) Prog. Neurobiol. 22, 1-38. [DOI] [PubMed] [Google Scholar]
- 33.Hu, L. & Bentler, P. M. (1999) Struct. Eq. Model. Multidiscip. J. 6, 1-55. [Google Scholar]
- 34.Bentler, P. M. (1995) EQS Structural Equations Program Manual (Multivariate Software, Encino, CA).
- 35.Mello, C. V., Vicario, D. S. & Clayton, D. F. (1992) Proc. Natl. Acad. Sci. USA 89, 6818-6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarvis, E. D., Schwabl, H., Ribeiro, S. & Mello, C. V. (1997) NeuroReport 8, 2073-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarvis, E. D. & Mello, C. V. (2000) J. Comp. Neurol. 419, 1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarvis, E. D., Ribeiro, S., da Silva, M. L., Ventura, D., Vielliard, J. & Mello, C. V. (2000) Nature 406, 628-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clayton, D. F. (2000) Neurobiol. Learn. Mem. 74, 185-216. [DOI] [PubMed] [Google Scholar]
- 40.Jarvis, E. D. (2004) in Nature's Music: The Science of Birdsong, eds. Marler, P. & Slabberkoorn, H. (Elsevier-Academic, New York), pp. 239-275.
- 41.Allison, J. D. & Wilczynski, W. (1994) Brain Behav. Evol. 43, 129-139. [DOI] [PubMed] [Google Scholar]
- 42.Bullier, J. & Nowak, L. G. (1995) Curr. Opin. Neurobiol. 5, 497-503. [DOI] [PubMed] [Google Scholar]
- 43.Buchel, C. & Friston, K. J. (1997) Cereb. Cortex 7, 768-778. [DOI] [PubMed] [Google Scholar]
- 44.McIntosh, A. R. (1998) Ann. N.Y. Acad. Sci. 855, 556-571. [DOI] [PubMed] [Google Scholar]
- 45.Hirsch, J., Moreno, D. R. & Kim, K. H. (2001) J. Cognit. Neurosci. 13, 389-405. [DOI] [PubMed] [Google Scholar]
- 46.Mesulam, M. M. (1998) Brain 121, 1013-1052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.