Abstract
Antagonists of growth hormone-releasing hormone (GHRH) were shown to inhibit the growth of various cancers. We investigated the antitumor activity and the mechanism of action of GHRH antagonists in human non-Hodgkin's lymphomas (NHL). Nude mice bearing xenografts of RL and HT human NHL were treated with GHRH antagonists MZ-5-156 and MZ-J-7-138 at a dose of 40 μg twice daily. The concentrations of serum IGF-1 and GHRH, bFGF, and VEGF in tumor tissue were measured by radioimmunoassays. Expression of GHRH and splice variant 1 of the GHRH receptor in both cell lines was examined by RT-PCR. The effects of MZ-5-156, MZ-J-7-138 and GHRH on cell proliferation were evaluated in vitro. Treatment with MZ-5-156 and MZ-J-7-138 significantly (P < 0.05) inhibited the growth of RL and HT tumors by 59.9-73.9%. High-affinity binding sites for GHRH and mRNA for GHRH and splice variant-1 of the GHRH receptors were found on RL and HT tumors. RL and HT cells contained GHRH peptide, and their growth in vitro was significantly inhibited by both antagonists. IGF-I levels in serum of mice were significantly decreased by antagonist MZ-5-156. Therapy with GHRH antagonists also significantly reduced tumoral bFGF, whereas VEGF levels were not suppressed. Our findings suggest that GHRH antagonists inhibit the growth of RL and HT lymphomas by direct effects mediated by tumoral receptors for GHRH. GHRH antagonists could offer a new therapeutic modality for the management of advanced NHL.
Keywords: growth factor inhibitors, GHRH-antagonist, receptor splice variant 1, fibroblast growth factor
Non-Hodgkin's lymphoma (NHL) is the most frequently diagnosed hematological malignancy and in the past five decades its incidence has steadily increased (1, 2). In the United States, ≈56,000 new cases are expected to be diagnosed in 2005, with an estimated 21,000 deaths (1, 2). Depending on the NHL subtype, the treatment of choice can include surgical intervention, conventional chemotherapy, radiotherapy, or a combination of the three therapeutic modalities (2, 3). However, the prognosis is poor for patients with advanced stages of NHL, with a 5-year survival rate of 73% for patients at low risk, but only 26% for those at high risk (3). Thus, new, more effective therapies for NHL are needed.
The discovery of various growth factors in neoplastic tissues prompted the development of a unique class of target-specific anticancer agents that inhibit mainly receptors for growth factors (4-9). In an endeavor to develop a class of anticancer agents, antagonistic analogues of growth hormone-releasing hormone (GHRH) were synthesized in our institute (10-13). These GHRH antagonists were tested in vitro and in vivo in models of various human tumors and found to inhibit growth of breast (14), prostatic (10, 15), colorectal (16), pancreatic (17), renal (10), bone (18, 19), and lung cancers (10, 20, 21) as well as gliomas (10). Antitumor activity of the GHRH antagonists appeared to be mediated indirectly through suppression of growth hormone (GH)-IGF-I axis or exerted by direct action on the neoplastic cells. Thus, GHRH antagonists suppress the release of GH from the pituitary, which results in a decrease in hepatic production of IGF-I (13, 14). High doses of the GHRH-antagonists MZ-4-71 and MZ-5-156 lowered IGF-I levels in the liver and serum and inhibited the growth of experimental osteosarcomas, Ewing sarcomas, and prostatic, renal, and lung carcinomas (10). Because IGF-I is a well known mitogen for various malignancies (22), a reduction of the serum IGF-I level may contribute to the antitumor activity of GHRH antagonists. However, when lower doses of GHRH antagonists or more recently developed analogues JV-1-36, JV-1-38 and MZ-J-7-118 with different structural features were administered in further in vivo experiments, the growth of human pancreatic, colorectal, prostatic, breast, ovarian, and lung cancers was inhibited in the absence of any significant effects on serum IGF-I (16, 17, 20, 21, 23-26). Therefore, it may be assumed that the main mechanism responsible for tumor inhibition is a direct effect of the antagonists on neoplastic cells (10, 13). In addition, GHRH-antagonists decreased the proliferation of diverse human cancer cell lines by direct action in vitro, under conditions in which the contribution of the hypothalamic GHRH/pituitary GH/hepatic IGF-I axis is clearly excluded (10, 14, 16, 17, 19-21, 23-31). These findings may be explained by recent discoveries on the role of tumoral GHRH and GHRH receptors in the proliferation of cancers. Although it has been known for >20 years that some cancers produce GHRH (32), it was only recently proposed that GHRH might function as an autocrine growth factor in neoplastic cells. Thus, various cancer lines were found to proliferate in response to exogenous GHRH and its agonistic analogues (21, 23, 24, 29, 33). mRNA for GHRH or its peptide product were also found in surgical specimens of human endometrial, ovarian, breast and prostate cancers (34-36). mRNAs encoding four splice variants (SVs) of GHRH receptors as well as specific high-affinity binding sites for GHRH and its antagonistic analogs have been identified in diverse cancer lines and specimens of human tumors (20, 23-25, 30, 31, 33, 34, 37-40). Thus, the direct antiproliferative action of GHRH-antagonists could be exerted through the disruption of an autocrine/paracrine stimulatory loop formed by tumoral GHRH and its tumoral receptors (10, 21, 30, 31, 33, 39-42).
GHRH peptide was localized in cells of the immune system such as monocytes and B and T cells (43, 44). It was found that transgenic mice, which overexpress human GHRH, have increased numbers of haematopoetic stem cells in the spleen (45). Interestingly, in cell lines of immune cell-derived tumors, higher levels of GHRH mRNA were detected as compared to the nonmalignant cells, suggesting that a dysregulation of GHRH gene transcription may contribute to the pathogenesis of some of these malignancies (44). These findings prompted us to examine two human NHL cell lines of B cell origin, RL and HT, for GHRH expression and to evaluate the effects of two antagonistic analogues of GHRH in vitro and in vivo. One of these antagonists, MZ-5-156, has a high affinity to the pituitary GHRH receptors and strongly inhibits the endocrine axis for pituitary GH and hepatic IGF-I (46, 47). The other antagonist, MZ-J-7-138, was recently developed in our laboratory and is characterized by weaker endocrine effects, but a higher antitumor activity.
Materials and Methods
Peptides and Reagents. GHRH antagonists MZ-5-156, MZ-J-7-138 and JV-1-42, as well as human GHRH(1-29)NH2 were synthesized in our laboratory by solid phase methods (12, 13, 46). The chemical structure of MZ-5-156 is [PhAc-Tyr-1,d-Arg-2,Phe(4-C1)6,Abu15,Nle-27]hGHRH(1-28)Agm, that of MZ-J-7-138 is (CH3-(CH2)6-CO-Ty r-1,d-Arg-2,Phe(4-C1)6,Ala-8,His-9,Tyr(Et)10,His-11,Orn-12,Abu15, His-20,Orn-21,Nle-27,d-Arg-28,Har29) hGHRH(1-29)NH2, and that of JV-1-42 is (PhAc-His-1,d-Arg-2,Phe(4-C1)6,Arg-9,Abu15,Nle-27,d-Arg-28,Har29)hGHRH(1-29)NH2, where PhAc is phenylacetyl, Phe(4-C1) is 4-chlorophenylalanine, Tyr(Et) is O-ethyltyrosine, Orn is ornithine, Abu is α-aminobutyric acid, Nle is norleucine, Har is homoarginine, and Agm is agmatine. For daily injection, MZ-5-156 and MZ-J-7-138 were dissolved in 0.1% DMSO in 10% aqueous propylene glycol solution (vehicle solution).
Cell Lines and Cell Culture. RL and HT human NHL cell lines were obtained from American Type Culture Collection. RL is a mature, EBV-negative, CD20+ B cell line derived from a diffuse large-cell lymphoma and carries a chromosomal marker t(14, 18)(q32;q21). HT is also an EBV-negative, CD20+ B cell line, derived from a diffuse mixed small- and large-cell lymphoma. RL and HT cells were maintained in RPMI medium 1640 supplemented with 10% FBS (Atlanta Biologicals) and 1 mM sodium pyruvate (Sigma), 50 μg/ml penicillin G, 50 μg/ml gentamicin, and 100 μg/ml streptomycin (GIBCO).
Animals. Five- to six-week-old female athymic nude mice (Ncr nu/nu) were obtained from the National Cancer Institute (Bethesda). The animals were housed in sterile cages under laminar flow hoods in a temperature-controlled room with a 12-h light/12-h dark schedule. They were fed autoclaved chow and water ad libitum.
In Vivo Experiments. RL and HT human NHL cells growing exponentially were implanted into five female nude mice by s.c. injection of 107 cells into both flanks. Tumors resulting after 4-6 weeks in donor animals were aseptically dissected and mechanically minced. In both experiments, 3-mm3 pieces of tumor tissue were transplanted s.c. into the experimental animals by a trocar needle. Tumor volume (length × width × height × 0.5236) and body weight were measured weekly. At the end of each experiment, mice were killed under anesthesia and necropsy was performed. Tumors and organs were removed and weighed. Tumor and liver specimens were snap frozen and stored at -70°C. All experiments were in accordance with the institutional guidelines for the welfare of animals in experiments.
In experiment 1, when RL tumors had reached a volume of 70-80 mm3, the mice were divided into three groups. The animals received the following treatment as s.c. injections twice per day: group 1, control, no treatment (11 mice); group 2, MZ-5-156 at a dose of 40 μg (10 mice); group 3, MZ-J-7-138 at a dose of 40 μg (10 mice). Because of the large tumor volume in the control group, the experiment was terminated on day 22.
In experiment 2, animals bearing HT tumors of ≈60 mm3 were assigned to three groups of 11 animals each, which received the same treatment as in experiment 1. Experiment 2 was terminated on day 29.
In Vitro Proliferation Studies. RL and HT human NHL cells were seeded into 96-well microplates (Falcon, Lincoln Park, NJ). After a recovery period of 24 h, the culture medium was removed and replaced with RPMI medium 1640 with 5% FBS and 1 mM sodium pyruvate (Invitrogen). The test compounds were GHRH(1-29)NH2, MZ-5-156, and MZ-J-7-138 at concentrations of 0.1 and 1 μM. Each treatment was performed in octuplicate wells, and the experiments were repeated three times. After 96 h, the crystal violet assay was performed to determine the optical density (OD) of the wells as described (26). Results were calculated as %T/C, where T = OD600 of treated cultures and C = OD600 of control cultures (medium plus vehicle).
RT-PCR Analysis of mRNAs for GHRH, GHRH Receptor SV1, and the IGF-I Receptor (IGF-IR). The presence of various mRNAs in the RL and HT tumors was determined by RT-PCR. Total RNAs were extracted following the TRI-Reagent protocol (Sigma) from two control tumor xenografts of each NHL (HT and RL). Two micrograms of total RNA or mRNA were reverse transcribed into cDNA by using the iScript cDNA synthesis kit (Bio-Rad).
The primer pairs (sense and antisense) for the amplification of the cDNA of GHRH were 5′-ATGCAGATGCCATCTTCACCAA-3′ and 5′-TGCTGTCTACCTGACGACCAA-3′, yielding a product of 150 bp; for GHRH receptor SV1, 5′-TGGGGAGAGGGAAGGAGTTGT-3′ and 5′-GCGAGAACCAGCCACCAGAA-3′, yielding a product of 523 bp; for β-actin, 5′-CTGGAACGGTGAAGGTGACA-3′ and 5′-AAGGGACTTCCTGTAACAATG-3′, yielding a product of 140 bp; and for IGF-IR, 5′-AAACCACGAGGCTGAGAAGCT-3′ and 5′-CAGCATAATCACCAACCCTC-3′, yielding a product of 446 bp. Two microliters of cDNA were amplified following the recommendations of the protocol for the AmpliTaq Gold Polymerase (Applied Biosystems) on an Applied Biosystems PCR system 2700. An initial step at 95°C for 10′ was followed by 40 cycles at 94°C for 30 s and 60°C for 60 s for GHRH amplification, 40 cycles at 94°C for 30 s and 62°C for 60 s followed for SV1 amplification, 35 cycles at 94°C for 30 s and 60°C for 60 s for β-actin, and 35 cycles at 94°C for 30 s and 65°C for 60 s for IGF-IR amplification, respectively. The PCRs were terminated in a final extension at 65°C for 10 min. For negative controls, no cDNA was added to the PCR. The PCR products were analyzed by electrophoresis on a 2% agarose gel stained with ethidium bromide.
Radioligand Binding Studies. Radioiodinated derivative of GHRH antagonist JV-1-42 was prepared by the chloramine-T method as described (34). Preparation of the membrane fractions from RL and HT human NHLs grown in nude mice was carried out as reported (38). Receptor binding of GHRH was performed by using in vitro ligand competition assays based on binding of the radiolabeled GHRH antagonist JV-1-42 to tumor membrane fractions described in detail (34, 38). The ligand-pc computerized curve-fitting software of Munson and Rodbard (48) was used to determine the type of receptor binding, dissociation constant (Kd) and maximal binding capacity of the receptors (Bmax).
Real-Time PCR Analysis of IGF-I mRNA Levels in Mouse Liver. Total RNA was isolated by using the Aurum Total RNA Mini kit (Bio-Rad). One microgram of total RNA was reverse transcribed into cDNA with 1× iScript Reaction mix (Bio-Rad) and 1 μl of iScript Reverse Transcriptase (Bio-Rad) in a total volume of 20 μl.
The iCycler iQ Real-Time PCR detection system (Bio-Rad) was used for sample cDNA quantification. The PCR contained 2 μl of the reverse transcription mixture, 300 nM of the gene specific primers, and 1× iQ SYBRO Green Supermix (Bio-Rad) in a total volume of 25 μl. The specific primer sequences were: CTGTGCCCCACTGAAGCCTA for mouse IGF-1 sense, GGACTTCTGAGTCTTGGGCATG for mouse IGF-1 antisense, AGATCAAGATCATTGCTCCTCCT for β-actin sense, and GGGTGTAAA ACGCAGCTCAG for β-actin antisense. The thermal cycling conditions comprised an initial denaturation step at 95°C for 3 min, then 40 cycles of two-step PCR including 95°C for 15 s and 60°C for 1 min. Data were collected during the 60°C annealing step and were further analyzed with the iCycler iQ Optical System's software. Each PCR included the five points of the calibration curve using serially diluted mouse liver cDNA. PCR efficiencies were 95.6% and 101.9% for β-actin and IGF-I, respectively. All samples were analyzed in triplicates. Negative controls included PCR amplification without cDNA and reverse transcriptase, respectively. Relative expression ratio was calculated by using the mathematical model described by Pfaffl (49), with IGF-1 as target gene and β-actin as reference gene.
RIA for VEGF, bFGF, IGF-I, and GHRH. Human bFGF and VEGF levels were determined by RIA in homogenates from tumor tissue of both experiments. Mouse IGF-I was measured in the serum from mice of experiment 1. GHRH was determined in cells of both cell lines.
Tumors were homogenized in an ice-cold extraction buffer (50 mM Tris·HCl/5 mM EDTA/5 mM MgCl2, pH 7.6 containing protease inhibitor mixture). The supernatant was obtained by centrifugation (12,000 × g for 20 min) and frozen at -70°C until use. VEGF-A was extracted from tumor tissue, and mouse IGF-I was extracted from serum by using a modified acid-ethanol cryo-precipitation method (50). The total protein content was determined by using the Bio-Rad protein assay kit.
The standard for VEGF was recombinant human VEGF, 38.2 kDa, consisting of 165 amino acids. Anti-human VEGF was an affinity-purified polyclonal antibody. Both VEGF and anti-human VEGF were purchased from Pepro Tech (Rocky Hill, NJ). The standard was used in a range of 0.006-12.8 ng per tube. The antibody was used at a final dilution of 1:200,000. VEGF was iodinated by the lactoperoxidase method and purified by high-performance liquid chromatography using a reverse-phase Vydac C4 column. The assay buffer for VEGF consisted of 0.01 M sodium phosphate (pH 7.6), 0.025 M EDTA, 0.14 M NaCl, and 1% BSA. The antibody and tracer were added simultaneously and incubated overnight at 4°C. Bound and free fractions were separated by the polyethylene-glycol double antibody method.
The standard for bFGF was a recombinant human growth factor, 17.2 kDa, consisting of 154 amino acid residues (Pepro Tech). The standard curve ranged from 0.125 to 256 ng per tube. The affinity-purified polyclonal antibody generated against bFGF in rabbits was purchased from Pepro Tech and used at the final dilution of 1:2,500. [125I] Bolton Hunter-labeled human recombinant bFGF was purchased from NEN. The specific activity of [125I]bFGF was >1,200 Ci·mmol-1 (>70 mCi·mg-1, 1 Ci = 37 GBq), and it was purified by affinity chromatography. The assay buffer consisted of 0.1% (wt/vol) gelatin, 0.01 M phosphate buffer (pH 7.6), 0.025 M EDTA, 0.14 M NaCl, 0.05% (vol/vol) Tween-20, and 0.002 M DTT. The bound and free fractions were separated by the polyethylene-glycol double antibody method.
For the RIA of IGF-I, recombinant murine IGF-I (Pepro Tech) was used as standard in the range of 2-2,000 pg per tube, and also for iodination with the chloramine-T method. Goat anti-mouse IGF-I (Pepro Tech) was used at a final dilution of 1:40,000.
For the measurement of GHRH content of RL and HT NHL cells in vitro, 5-6 × 106 cells were seeded in 75-cm2 flasks and allowed to attach for 24 h, when the medium was replaced by McCoy 5A medium (Gibco). After 48 h, the human GHRH content of RL and HT cells was determined as described (20, 31).
The RIA results from all samples were evaluated in a computer-controlled gamma Counter (Packard Cobra System). The intra- and interassay coefficients of were <10% and 15%, respectively.
Statistical Analysis. Data are expressed as means ± SE. Differences between mean values were evaluated by two-tailed Student's t test. P < 0.05 was considered significant.
Results
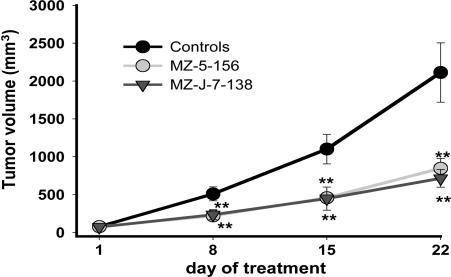
Effects of MZ-5-156 and MZ-J-7-138 on the Growth of Human RL and HT NHL Xenografted into Nude Mice. In experiment 1, both GHRH antagonists administered for 21 days at a dose of 40 μg twice daily significantly (P < 0.01) inhibited the growth of RL human NHL. MZ-5-156 significantly decreased tumor volume by 59.9% and tumor weight by 62.1%, and MZ-J-7-138 reduced tumor volume by 66.1% and tumor weight by 66.9%. Both antagonists significantly (P < 0.05) prolonged tumor volume doubling time (Fig. 1 and Table 1).
Fig. 1.
Mean tumor volumes in nude mice bearing RL human NHL xenografts during treatment with GHRH-antagonists MZ-5-156 and MZ-J-7-138 at a daily dose of 2 × 40 μg. Vertical bars indicate SE (**, P < 0.01).
Table 1. Effects of therapy with GHRH antagonists MZ-5-156 and MZ-J-7-138 at doses of 40 μg twice daily on the growth of RL and HT human NHL xenografted into nude mice.
| Final tumor volume, mm3 (% inhibition) | Tumor weight, mg (% inhibition) | Tumor doubling time, days | |
|---|---|---|---|
| Experiment 1 (RL)* | |||
| Control | 2,112.7 ± 391.2 | 3,052.8 ± 507.7 | 5.1 ± 0.3 |
| MZ-5-156 | 846.7 ± 130.2† (59.9) | 1,156.0 ± 161.2† (62.1) | 7.4 ± 1.0‡ |
| MZ-J-7-138 | 715.2 ± 116.2† (66.1) | 1,011.3 ± 140.6† (66.9) | 7.2 ± 0.8‡ |
| Experiment 2 (HT)* | |||
| Control | 1,087.2 ± 184.7 | 1,742.9 ± 251.0 | 7.3 ± 0.6 |
| MZ-5-156 | 371.4 ± 69.0‡ (65.8) | 609.1 ± 120.0† (65.1) | 14.3 ± 2.7‡ |
| MZ-J-7-138 | 283.9 ± 57.1† (73.9) | 412.5 ± 76.6† (76.3) | 22.5 ± 6.1‡ |
All doses were twice daily as 40 μg. †, P < 0.01 vs. controls. ‡, P < 0.05 vs. controls.
The initial tumor volume was 57-78 mm3.
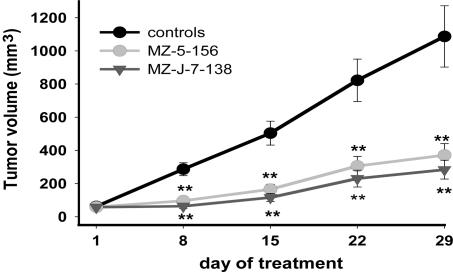
In experiment 2, MZ-5-156 and MZ-J-7-138 injected at a dose of 2 × 40 μg twice daily for 4 weeks significantly (P < 0.01) decreased tumor volume of HT NHL by 65.8% and 73.9%, respectively. Tumor weight was significantly (P < 0.01) reduced by 65.1% in mice treated with MZ-5-156 and by 76.3% in animals treated with MZ-J-7-138 (Fig. 2 and Table 1). Tumor volume doubling time was significantly (P < 0.05) prolonged from 7.3 to 14.3 days in the MZ-5-156 group and to 22.5 days in the MZ-J-7-138 group (Table 1).
Fig. 2.
Mean tumor volumes in nude mice bearing HT human NHL xenografts during treatment with antagonists MZ-5-156 and MZ-J-7-138 at a daily dose of 2 × 40 μg. Vertical bars indicate SE (**, P < 0.01).
At the end of both experiments, no significant differences in body weights or weights of various organs were observed between treated and control animals, indicating that MZ-5-156 and MZ-J-138 at a dose of 40 μg twice daily had no toxic side effects (data not shown).
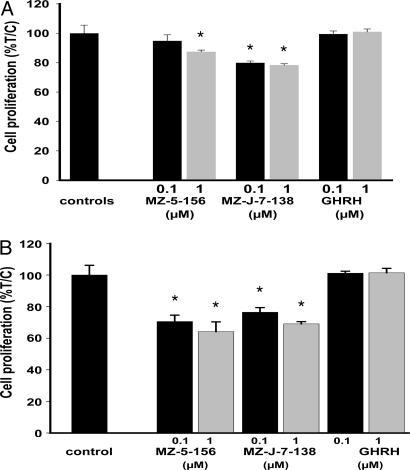
Effect of GHRH(1-29)NH2 and GHRH Antagonists MZ-5-156 and MZ-J-7-138 on the Proliferation of RL and HT Cells in Vitro. In three independent experiments, RL and HT cells cultured in vitro were exposed to two concentrations of GHRH(1-29)NH2, MZ-5-156, and MZ-J-7-138, and the effect on the proliferation was followed by crystal violet assay. GHRH(1-29)NH2 at 0.1 and 1 μM did not influence the growth of RL or HT cells. GHRH antagonist MZ-5-156 did not inhibit the proliferation of RL cells at a dose of 0.1 μM, but a significant (P < 0.05) inhibition of 8.7 to 12.3% was observed using a 1 μM concentration.
GHRH antagonist MZ-J-7-138 at doses of 0.1 μM and 1 μM significantly (P < 0.05) decreased the proliferation of RL cells by 14.2-20.1% and 13.6-21.8%, respectively. The proliferation of HT cells was significantly (P < 0.05) decreased by 19.3-29.4% and 22.1-35.8% by GHRH antagonist MZ-5-156 at doses of 0.1 and 1 μM.
MZ-J-7-138 significantly (P < 0.05) inhibited growth of HT cells by 13.8-23.6% at a dose of 0.1 μM and by 21.0-30.9% at a dose of 1 μM. The results of a representative experiment are shown in Fig. 3.
Fig. 3.
Effect of GHRH antagonists MZ-5-156 and MZ-J-7-138 on the proliferation of RL (A) and HT (B) human NHL cell lines. These experiments are representative of three independent experiments per cell line, each performed in octuplicate wells. The relative cell number in treated and control wells was determined by crystal violet staining and expressed as percent T/C values, where T is the absorbance of treated cultures and C is absorbance of control cultures. (*, P < 0.05.)
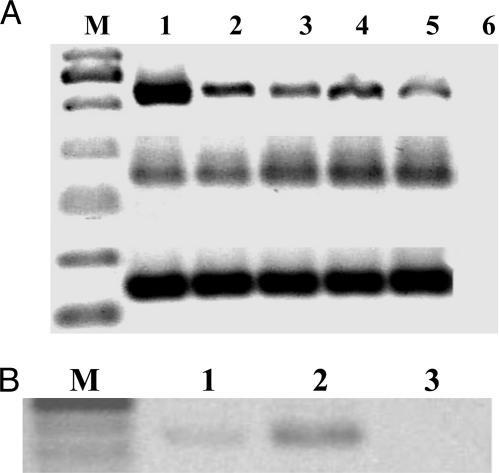
Presence of mRNA for GHRH, for the GHRH Receptor SV1 and for IGF-IR in Human RL and HT NHL Samples. Human RL and HT NHL samples expressed mRNA for GHRH, SV1, and β actin as reflected by PCR products of the expected sizes (Fig. 4A), mRNA expression for IGF-IR was also found in both cell lines (Fig. 4B). No PCR products were detected in the negative controls for all tested genes.
Fig. 4.
RT-PCR analysis of mRNA expression for GHRH receptor SV1 (A Top), GHRH (A Middle), β-actin (A Bottom), and IGF-IR (B). (A) RT-PCR was performed in two samples of each human NHL tumor tissues HT (lanes 2 and 3) and RL (lanes 4 and 5). As positive controls, amplifications of SV1 mRNA from human prostate cancer cell line LNCaP (Top, lane 1) and amplifications of GHRH and beta-actin mRNA from human pituitary tissue (Middle and Bottom, lane 1) were used. DNA molecular weight markers are shown under M. All PCR products resulted in the expected sizes of 523 bp for GHRH receptor SV1, 150 bp for GHRH, and 140 bp for β-actin. No amplification was detected in the negative control (lane 6). (B) Expected PCR-products of 446 bp for IGF-IR were detected in the RL (lane 1) and HT (lane 2) NHL tumors. No amplification was detected in the negative control (lane 3).
GHRH Receptor-Binding Studies. In membranes of untreated human RL and HT NHL, radiolabeled JV-1-42 was bound to a single class of high-affinity binding sites. The mean dissociation constant (Kd) was 4.9 ± 0.37 nM for RL and 6.9 ± 0.20 nM for HT. The mean maximal binding capacities (Bmax) for RL and HT human NHL were 365.6 ± 43.9 fmol per mg of protein and 422.8 ± 35.0 fmol per mg of protein, respectively.
GHRH Peptide in RL and HT NHL Cell Lines Cultured in Vitro. The GHRH peptide content of RL and HT cells was measured by RIA. A concentration of 3,702 pg per mg of protein was found in ≈3.0 × 107 cultured RL cells and 5,559 pg per mg of protein in the same number of HT cells.
Effects of MZ-5-156 and MZ-J-7-138 on Serum IGF-1 in Nude Mice. The effects of administration of the two GHRH antagonists MZ-5-156 and MZ-J-7-138 at doses of 40 μg twice daily on serum IGF-I levels in nude mice were examined by RIA. In experiment 1, MZ-5-156 significantly (P < 0,05) decreased serum IGF-I by 27.1%, whereas MZ-J-7-138 had no significant effect (Table 2). The serum samples of the control group of experiment 2 were accidentally lost and no values are available, but the IGF-1 level of mice treated with MZ-5-156 was significantly lower (P < 0.01) than that of mice given MZ-J-7-138 (Table 2).
Table 2. Effects of GHRH antagonists MZ-5-156 and MZ-J-7-138 on tumoral levels of bFGF and VEGF-A and the serum levels of IGF-I as measured by RIA.
| bFGF, pg per mg of protein | VEGF-A, pg per mg of protein | Serum IGF-1, ng/ml | |
|---|---|---|---|
| Experiment 1 (RL) | |||
| Control | 278.0 ± 46.6 | 631.9 ± 37.3 | 182.2 ± 13.5 |
| MZ-5-156 | ND* | 652.5 ± 42.1 | 132.8 ± 11.6† |
| MZ-J-7-138 | ND* | 643.3 ± 48.6 | 163.5 ± 18.42 |
| Experiment 2 (HT) | |||
| Control | 580.3 ± 116.2 | 442.2 ± 21.4 | NM |
| MZ-5-156 | 235.2 ± 104.8‡ | 475.8 ± 26.4 | 132.7 ± 11.8‡ |
| MZ-J-7-138 | 5.11 ± 2.3* | 462.4 ± 24.8 | 177.0 ± 12.2 |
Doses were 40 μg given twice daily. ND, not detectable; NM, not measured because the sample was lost.
* P < 0.01 vs. control. †, P < 0.05 vs. control. ‡, P < 0.01 vs. MZ-J-7-138.
Effects of MZ-5-156 and MZ-J-7-138 on the mRNA Levels of IGF-I in Livers of Nude Mice. Total RNA from livers of mice treated with MZ-5-156 and MZ-J-7-138 and untreated controls (experiment 1) was isolated and subjected to real-time RT-PCR analysis. The relative expression ratios were 0.36 and 1.07 for the groups treated with MZ-5-156 and MZ-J-7-138 (46), indicating a down-regulation of IGF-I mRNA by 64% in the MZ-5-156 group, but no changes in the MZ-J-7-138 group. No significant amount of PCR products were detected from the negative controls.
Effects of MZ-5-156 and MZ-J-7-138 on the Concentration of bFGF and VEGF in RL an HT Tumors. The average concentrations of bFGF and VEGF in untreated RL tumors were 278.0 pg per mg of protein and 631.9 pg per mg of protein, respectively. After therapy with the GHRH antagonists MZ-5-156 and MZ-J-7-138 for 21 days, bFGF was strongly suppressed and protein concentration was below detectable levels for RIA in all tumors examined. The concentration of VEGF did not change in either treatment group (Table 2).
In untreated HT tumors, the concentration of bFGF was 580.3 pg per mg of protein. Treatment with MZ-5-156 and MZ-J-7-138 decreased bFGF by 59.5% and 99.1%, respectively. VEGF was not influenced by the two GHRH antagonists as in experiment 2 (Table 2).
Discussion
GHRH antagonists appear to inhibit both the endocrine and autocrine/paracrine pathways, which stimulate the growth of neoplastic cells (10, 11). The antitumor activity of GHRH antagonists has been demonstrated in a wide range of human experimental cancers, but their possible effects on NHL have not been studied. In the present study, GHRH antagonists MZ-5-156 and MZ-J-7-138 significantly inhibited the growth of RL and HT NHL tumors xenografted in nude mice. Tumor volume doubling time was also significantly prolonged by both antagonists. Toxic side effects were not observed in any of the experiments. Our findings suggest that GHRH antagonists could be considered for a new approach to the therapy of NHL.
GHRH antagonists were initially developed to suppress the pituitary GH secretion and consequently the hepatic IGF-I in serum, which functions as a mitogen in many malignancies (18). However, more recent studies suggest that locally produced GHRH and its tumoral binding sites are involved in the pathogenesis of various cancers. Thus, the main effects of GHRH antagonists may be mediated directly through the receptors for GHRH on malignant cells (10, 20, 21, 24, 25, 30, 31, 33, 34, 37-42). Although the pituitary type of GHRH receptors has not been reported in cancer cells so far (14, 24, 29, 37, 38, 51), we recently identified splice variants of GHRH receptors on tumors that might mediate direct effects of GHRH and its antagonists (37, 38). These tumoral receptors for GHRH differ from the pituitary-type GHRH receptors or receptors for other peptides of the VIP/secretin/glucagon family. All of the SVs of the GHRH receptor contain a retained intronic sequence at the 5′ end but lack the first three exons (37, 38). The lack of the first three exons in SV1 may result in a tumoral receptor protein, in which most of the large NH2-terminal extracellular domain, characteristic of the pituitary receptor, is truncated (37, 38). We assume that the mRNA for SV1 encodes a functional receptor protein that binds GHRH and its analogues and is different from the pituitary GHRH receptor (38). The transfection of SV1 in NIH 3T3 mouse fibroblast cells supports the participation of SV1 in cell proliferation signaling (42). 3T3 cells transfected with SV1 display specific high-affinity binding sites for radiolabeled GHRH antagonist JV-1-42 and show augmented sensitivity to GHRH analogs. The expression of SV1 increases the mitogenic responses to GHRH or GHRH agonist JI-38 and the antimitogenic response to GHRH antagonist JV-1-38 compared to SV1-negative cells transfected with the empty vector.
In the current study, we were able to demonstrate that human RL and HT NHLs express specific, high-affinity binding sites for GHRH antagonists as well as mRNA for SV1. The receptor protein encoded by mRNA for SV1 was also recently detected by Western Blot analysis in these cell lines (52). In addition, we found GHRH protein in appreciable quantities in cultured RL and HT cells and the corresponding mRNA for GHRH was also detected in both cell lines. Whereas the proliferation of RL and HT cells in vitro was significantly inhibited by the two GHRH antagonists MZ-5-156 and MZ-J-7-138, an equivalent concentration of GHRH could not stimulate the growth of both cell lines; this may be due to the high local secretion of endogenous GHRH of the NHL cells, which causes a maximal stimulation of cell growth. Thus, no additional growth promoting effect could be achieved by exogenous GHRH administration.
To determine whether the endocrine effects of MZ-5-156 and MZ-J-7-138 contributed to the antitumor activity in vivo, we measured the levels of IGF-I in serum and liver of mice bearing RL tumors. MZ-5-156 significantly decreased serum IGF-I by 27.1% and the concentration of IGF-I mRNA in hepatic tissue by 64%, whereas the recently developed antagonist MZ-J-7-138 did not alter the hepatic IGF-I or serum IGF-I level; this might be due to structural differences between these antagonists. Thus, earlier GHRH antagonists such as MZ-5-156 preferentially block the pituitary receptors for GHRH and inhibit serum IGF-1, whereas the antagonist MZ-J-7-138 exhibits a stronger direct antitumor effect based on its higher binding affinity to the tumoral receptors for GHRH, which differ from the pituitary receptors. Other recent in vivo studies in our laboratory indicate that MZ-J-7-138 has only a weak suppressive effect on GH levels in rats, whereas MZ-5-156 has high GH inhibitory activity (>70% suppression of GH in this test). As MZ-J-7-138 inhibited tumor growth more effectively than MZ-5-156, endocrine suppression of serum IGF-I may be of minor importance for the antitumor activity of this class of GHRH antagonists. However, GHRH was expressed by RL and HT cells and receptors for GHRH were detected in the tumors of both cell lines. This suggests that GHRH functions as an autocrine growth factor in both cell lines. Thus, the direct effects of the GHRH antagonists on the tumoral GHRH receptors appear to be the dominating mechanism of action in RL and HT human NHL.
Angiogenetic factors such as bFGF and VEGF play an important role in the pathogenesis of haematological malignancies, and high levels of bFGF and VEGF are associated with poor prognosis in patients with NHL (53-56). As both growth factors were decreased by GHRH antagonists in some previous studies (15, 57, 58), we compared the protein expression of bFGF and VEGF in treated and control tumor samples of both experiments. bFGF was strongly decreased after treatment with MZ-5-156 and MZ-J-7-138 both in RL and HT tumors. Therefore, it can be assumed that, in these experimental models, the antitumor activity of GHRH antagonists could be partly due to a decrease of the tumoral bFGF levels.
In conclusion, this study demonstrates that GHRH antagonists can inhibit the growth of RL and HT human NHL in vitro and in vivo. The inhibition of growth was probably mediated by a direct effect of GHRH antagonists on tumoral GHRH receptors. Our findings suggest that GHRH antagonists might provide a promising new treatment modality for advanced NHL.
Acknowledgments
This work was supported by the Medical Research Service of the Veterans Affairs Department and a grant from Zentaris Gmbh (Frankfurt on Main, Germany) to Tulane University (to A.V.S). G.K. was supported by a fellowship within the postdoctorate program of the German Academic Exchange Service.
Abbreviations: NHL, non-Hodgkin's lymphoma; GH, growth hormone; GHRH, GH-releasing hormone; SV, splice variant; IGF-IR, IGF-I receptor.
References
- 1.Jemal, A., Murray, T., Ward, E., Samuels, A., Tiwari, R. C., Ghafoor, A., Feuer, E. J. & Thun, M. J. (2005) CA Cancer J. Clin. 55, 10-30. [DOI] [PubMed] [Google Scholar]
- 2.Freedman, A. S. (2004) in Cancer Medicine, eds. Kufe, D. W., Weichselbaum, R. R., Bast, R. C., Jr., Gansler, T. S., Holland, J. F. & Frei, E., III (B.C. Decker, Hamilton, Canada), pp. 2189-2210.
- 3.The International Non-Hodgkin's Lymphoma Prognostic Factors Project (1993) N. Engl. J. Med. 329, 987-994. [DOI] [PubMed] [Google Scholar]
- 4.Hinoda, Y., Sasaki, S., Ishida, T. & Imai, K. (2004) Cancer Sci. 95, 621-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schally, A. V. & Comaru-Schally, A. (2003) in Cancer Medicine, eds. Kufe, D. W., Pollock, R. E., Weichselbaum, R. R., Bast, R. C., Jr., Gansler, T. S., Holland, J. F. & Frei, E., III (B.C. Decker, Hamilton, Canada), pp. 911-926.
- 6.Thorpe, P. E. (2004) Clin. Cancer Res. 10, 415-427. [DOI] [PubMed] [Google Scholar]
- 7.Mendelsohn, J. & Baselga, J. (2003) J. Clin. Oncol. 21, 2787-2799. [DOI] [PubMed] [Google Scholar]
- 8.Gschwind, A., Fischer, O. M. & Ullrich, A. (2004) Nat. Rev. Cancer 4, 361-370. [DOI] [PubMed] [Google Scholar]
- 9.Roskoski, R., Jr. (2004) Biochem. Biophys. Res. Commun. 319, 1-11. [DOI] [PubMed] [Google Scholar]
- 10.Schally, A. V., Comaru-Schally, A. M., Nagy, A., Kovacs, M., Szepeshazi, K., Plonowski, A., Varga, J. L. & Halmos, G. (2001) Front. Neuroendocrinol. 22, 248-291. [DOI] [PubMed] [Google Scholar]
- 11.Schally, A. V. & Varga, J. L. (1999) Trends Endocrinol. Metab. 10, 383-391. [DOI] [PubMed] [Google Scholar]
- 12.Varga, J. L., Schally, A. V., Csernus, V. J., Zarandi, M., Halmos, G. & Rekasi, Z. (1999) Proc. Natl. Acad. Sci. USA 96, 692-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varga, J. L., Schally, A. V., Horvath, J. E., Kovacs, M., Halmos, G., Groot, K, Toller, G. L., Rekasi, Z. & Zarandi, M. (2004) Proc. Natl. Acad. Sci. USA 101, 1708-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahan, Z., Varga, J. L., Schally, A. V., Rekasi, Z., Armatis, P., Chatzistamou, L., Czompoly, T. & Halmos, G. (2000) Breast Cancer Res. Treat. 60, 71-79. [DOI] [PubMed] [Google Scholar]
- 15.Letsch, M., Schally, A. V., Busto, R., Bajo, A. M. & Varga, J. L. (2003) Proc. Natl. Acad. Sci. USA 100, 1250-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szepeshazi, K., Schally, A. V., Groot, K., Armatis, P., Halmos, G., Hebert, F., Szende, B., Varga, J. L. & Zarandi, M. (2000) Br. J. Cancer 82, 1724-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szepeshazi, K., Schally, A. V., Groot, K., Armatis, P., Hebert, F. & Halmos, G. (2000) Eur. J. Cancer 36, 128-136. [DOI] [PubMed] [Google Scholar]
- 18.Pinski, J., Schally, A. V., Groot, K., Halmos, G., Szepeshazi, K., Zarandi, M. & Armatis, P. (1995) J. Natl. Cancer Inst. 87, 1787-1794. [DOI] [PubMed] [Google Scholar]
- 19.Braczkowski, R., Schally, A. V., Plonowski, A., Varga, J. L., Groot, K., Krupa, M. & Armatis, P. (2002) Cancer 95, 1735-1745. [DOI] [PubMed] [Google Scholar]
- 20.Szereday, Z., Schally, A. V., Varga, J. L., Kanashiro, C., Hebert, F., Armatis, P., Groot, K., Szepeshazi, K., Halmos, G. & Busto, R. (2003) Cancer Res. 63, 7913-7919. [PubMed] [Google Scholar]
- 21.Kiaris, H., Schally, A. V., Varga, J. L., Groot, K. & Armatis, P. (1999) Proc. Natl. Acad. Sci. USA 96, 14894-14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khandwala, H. M., McCutcheon, I. E., Flyvbjerg, A. & Friend, K. E. (2000) Endocr. Rev. 21, 215-244. [DOI] [PubMed] [Google Scholar]
- 23.Chatzistamou, I., Schally, A. V., Varga, J., Groot, K., Armatis, P. & Bajo, A. M. (2001) J. Cancer Res. Clin. Oncol. 127, 645-652. [DOI] [PubMed] [Google Scholar]
- 24.Chatzistamou, I., Schally, A. V., Varga, J. L., Groot, K., Armatis, P., Busto, R. & Halmos, G. (2001) J. Clin. Endocrinol. Metab. 86, 2144-2152. [DOI] [PubMed] [Google Scholar]
- 25.Chatzistamou, I., Schally, A. V., Varga, J. L., Groot, K., Busto, R., Armatis, P. & Halmos, G. (2001) Anticancer Drugs 12, 761-768. [DOI] [PubMed] [Google Scholar]
- 26.Plonowski, A., Schally, A. V., Krupa, A., Hebert, F., Groot, K. & Varga, J. L. (2002) Prostate 52, 173-182. [DOI] [PubMed] [Google Scholar]
- 27.Szepeshazi, K., Schally, A. V., Armatis, P., Groot, K., Hebert, F., Feil, A., Varga, J. L. & Halmos, G. (2001) Endocrinology 142, 4371-4378. [DOI] [PubMed] [Google Scholar]
- 28.Csernus, V. J., Schally, A. V., Kiaris, H. & Armatis, P. (1999) Proc. Natl. Acad. Sci. USA 96, 3098-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rekasi, Z., Varga, J. L., Schally, A. V., Halmos, G., Armatis, P., Groot, K. & Czompoly, T. (2000) Endocrinology 141, 2120-2128. [DOI] [PubMed] [Google Scholar]
- 30.Busto, R., Schally, A. V., Varga, J. L., Garcia-Fernandez, M. O., Groot, K., Armatis, P. & Szepeshazi, K. (2002) Proc. Natl. Acad. Sci. USA 99, 11866-11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busto, R., Schally, A. V., Braczkowski, R., Plonowski, A., Krupa, M., Groot, K, Armatis, P. & Varga, J. L. (2002) Regul. Pept. 108, 47-53. [DOI] [PubMed] [Google Scholar]
- 32.Frohman, L. A. & Szabo, M. (1981) Prog. Clin. Biol. Res. 74, 259-271. [PubMed] [Google Scholar]
- 33.Garcia-Fernandez, M. O., Schally, A. V., Varga, J. L., Groot, K. & Busto, R. (2003) Breast Cancer Res. Treat. 77, 15-26. [DOI] [PubMed] [Google Scholar]
- 34.Halmos, G., Schally, A. V., Czompoly, T., Krupa, M., Varga, J. L. & Rekasi, Z. (2002) J. Clin. Endocrinol. Metab. 87, 4707-4714. [DOI] [PubMed] [Google Scholar]
- 35.Kahan, Z., Arencibia, J., Csernus, V., Groot, K., Kineman, R., Robinson, W. R. & Schally, A. V. (1999) J. Clin. Endocrinol. Metab. 84, 582-589. [DOI] [PubMed] [Google Scholar]
- 36.Benlot, C., Levy, L., Fontanaud, P., Roche, A., Rouannet, P. & Joubert, D. (1997) J. Clin. Endocrinol. Metab. 82, 690-696. [DOI] [PubMed] [Google Scholar]
- 37.Rekasi, Z., Czompoly, T., Schally, A. V. & Halmos, G. (2000) Proc. Natl. Acad. Sci. USA 97, 10561-10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halmos, G., Schally, A. V., Varga, J. L., Plonowski, A., Rekasi, Z. & Czompoly, T. (2000) Proc. Natl. Acad. Sci. USA 97, 10555-10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chopin, L. K. & Herington, A. C. (2001) Prostate 49, 116-121. [DOI] [PubMed] [Google Scholar]
- 40.Plonowski, A., Schally, A. V., Busto, R., Krupa, M., Varga, J. L. & Halmos, G. (2002) Peptides 23, 1127-1133. [DOI] [PubMed] [Google Scholar]
- 41.Kineman, R. D. (2000) Proc. Natl. Acad. Sci. USA 97, 532-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiaris, H., Schally, A. V., Busto, R., Halmos, G., Artavanis-Tsakonas, S. & Varga, J. L. (2002) Proc. Natl. Acad. Sci. USA 99, 196-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stephanou, A., Knight, R. A. & Lightman, S. L. (1991) Neuroendocrinology 53, 628-633. [PubMed] [Google Scholar]
- 44.Khorram, O., Garthwaite, M. & Golos, T. (2001) J. Clin. Endocrinol. Metab. 86, 3157-3161. [DOI] [PubMed] [Google Scholar]
- 45.Blazar, B. R., Brennan, C. A., Broxmeyer, H. E., Shultz, L. D. & Vallera, D. A. (1995) Exp. Hematol. 23, 1397-1406. [PubMed] [Google Scholar]
- 46.Zarandi, M., Kovacs, M., Horvath, J., Toth, K., Halmos, G., Groot, K., Nagy, A., Kele, Z. & Schally, A. V. (1997) Peptides 18, 423-430. [DOI] [PubMed] [Google Scholar]
- 47.Kovacs, M., Kineman, R. D., Schally, A. V., Zarandi, M., Groot, K. & Frohman, L. A. (1997) Endocrinology 138, 4536-4542. [DOI] [PubMed] [Google Scholar]
- 48.Munson, P. J. & Rodbard, D. (1980) Anal. Biochem. 107, 220-239. [DOI] [PubMed] [Google Scholar]
- 49.Pfaffl, M. W. (2001) Nucleic Acids Res. 29, 2003-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breier, B. H., Gallaher, B. W. & Gluckman, P. D. (1991) J. Endocrinol. 128, 347-357. [DOI] [PubMed] [Google Scholar]
- 51.Plonowski, A., Schally, A. V., Varga, J. L., Rekasi, Z., Hebert, F., Halmos, G. & Groot, K. (2000) Prostate 44, 172-180. [DOI] [PubMed] [Google Scholar]
- 52.Toller, G. L., Horvath, J. E., Schally, A. V., Halmos, G., Varga, J. L., Groot, K., Chism, D. & Zarandi, M. (2004) Proc. Natl. Acad. Sci. USA 101, 15160-15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pazgal, I., Zimra, Y., Tzabar, C., Okon, E., Rabizadeh, E., Shaklai, M. & Bairey, O. (2002) Br. J. Cancer 86, 1770-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salven, P., Teerenhovi, L. & Joensuu, H. (1999) Blood 94, 3334-3339. [PubMed] [Google Scholar]
- 55.Bertolini, F., Paolucci, M., Peccatori, F., Cinieri, S., Agazzi, A., Ferrucci, P. F., Cocorocchio, E., Goldhirsch, A. & Martinelli, G. (1999) Br. J. Haematol. 106, 504-509. [DOI] [PubMed] [Google Scholar]
- 56.Salven, P., Teerenhovi, L. & Joensuu, H. (1997) Blood 90, 3167-3172. [PubMed] [Google Scholar]
- 57.Siejka, A., Lawnicka, H., Komorowski, J., Schally, A. V., Stepien, T., Krupinski, R. & Stepien, H. (2003) Life Sci. 72, 2473-2479. [DOI] [PubMed] [Google Scholar]
- 58.Stangelberger, A., Schally, A. V., Varga, J. L., Hammann, B. D., Groot, K., Halmos, G., Cai, R. Z. & Zarandi, M. (2005) Prostate 64, 305-315. [DOI] [PubMed] [Google Scholar]