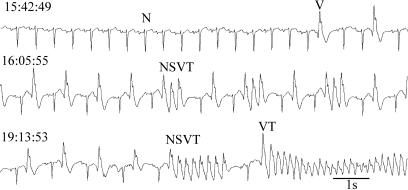
One thing is certain. Between now and some time in the future, let's say within the next 60 years for most of you, your heart will undergo a transition from its current behavior, which is pumping adequate amounts of blood to your brain, to a different behavior that leads to inadequate pumping of blood and subsequent death. The terminal cardiac rhythm of the heart is usually ventricular fibrillation, a rhythm in which the main pumping chambers of the heart, the ventricles, do not exhibit the coordinated contractions needed to pump blood effectively. Several hundred thousand people each year in the U.S. experience unexpected “sudden cardiac death” (1). Fig. 1 is an example in an 82-year-old woman with heart failure who was wearing a portable (Holter) heart monitor recording her electrocardiogram at the time of death. Fig. 1 shows a variety of different rhythms, including normal sinus beats (N), abnormal premature ventricular ectopic beats (V), nonsustained ventricular tachycardia (NSVT) consisting of short sequences of abnormally rapid ventricular beats, and rapid sustained ventricular tachycardia (VT). Shortly after the end of this recording, there is a second transition to ventricular fibrillation, leading to the death of the individual. These data are recorded from the surface of the body, and it is not possible to know the spatiotemporal pattern of cardiac activity during these arrhythmias. The ventricular beats may arise from an abnormal localized focus, they may be associated with excitation traveling in a reentrant path, or they may arise from a combination of both mechanisms (2, 3). Recordings of cardiac activity using voltage-sensitive dyes in mammalian hearts have documented circulating reentrant spiral waves. These provide a possible physiological basis for ventricular fibrillation and for some types of ventricular tachycardia (4-6). In a recent issue of PNAS, Hwang et al. (7) carried out recordings of cardiac cells in tissue culture that reveal spontaneous transitions in spatiotemporal organization of reentrant spiral wave activity. Because they use a motion artifact to record the data, the recordings can be carried out continuously over many hours or even days. These data demonstrate spontaneous transitions in the organization of cardiac activity, the presence of multiple stable spatiotemporal patterns, and the role of heterogeneities in shaping and organizing cardiac dynamics. This raises important questions for both basic science and medicine.
Fig. 1.
Sudden cardiac death recorded in an 82-year-old woman with heart failure who was wearing a portable monitor (Holter tape) that measures the continuous electrocardiogram. At 15:42:49, the rhythm consists of beats originating from the normal sinus node pacemaker (N), with two isolated premature ventricular ectopic beats arising from an abnormal site in the ventricles (V). The trace at 16:05:55 shows an alternation of normal sinus beats with ventricular ectopic beats and three sequences of three consecutive ventricular ectopic beats, nonsustained ventricular tachycardia (NSVT). At 19:13:53, the record is initially similar to the record at 16:05:55, but there is a more prolonged nonsustained ventricular tachycardia, followed by a sinus beat, and then a sustained ventricular tachycardia (VT) that leads to sudden death. The trace (recording from patient no. 52) is taken from the Sudden Cardiac Death Holter Database of the National Institutes of Health Research Resource for Complex Physiologic Signals (www.physionet.org). Figure prepared by C. Lerma, McGill University.
Spiral Wave Dynamics
Excitable media, such as neurons, cardiac tissue, or the Belousov-Zhabotinsky chemical reaction, support waves that, upon collision, do not pass through each
Recordings of cardiac cells reveal spontaneous transitions in spatiotemporal organization of reentrant spiral wave activity.
other but annihilate (8). A preparation of an excitable medium in two dimensions displays multistability. Depending on the initial condition, the medium may be quiescent or it might support a circular wave spreading from a point source or a rotating spiral wave. Further, there is a remarkable richness of spiral wave dynamics: spiral waves can rotate stably (4) or appear as bursts of activity (9), they can exist as spirals with multiple arms (10, 11), they can undergo a complex meandering pattern (8, 12), and they can break up so that one spiral can lead to many (13, 14). The work by Hwang et al. (7) describes previously unknown behaviors in which the excitation circulates in reentrant loops in well organized, repetitive patterns with delicate and elegant spatial and temporal structure. It is likely that the details of the patterns are determined by the underlying heterogeneity of the spatial structure and the physiology. Thus, the work sets challenges for physical and biological scientists to identify and manipulate heterogeneities and to analyze their role in shaping dynamics.
Cardiac Arrhythmia
In addition to the basic science context, the work is also relevant for the study of cardiac arrhythmias in people (2, 3). In healthy individuals, the sinus node acts as a pacemaker that responds to a wide variety of physiologic perturbations by varying its frequency. From a perspective of mathematics, the sinus rhythm can be thought of as an attractor sitting in a deep basin. Although in normal hearts there is a second attractor corresponding to ventricular fibrillation, in most people, the normal healthy heart rhythm persists for billions of beats before it encounters a condition that will lead to this fatal attractor. However, in rare circumstances such as might be encountered in a person struck by lightning, or a patient whose heart rhythm is severely disrupted by physicians during heart operations or specialized diagnostic medical procedures, a major perturbation to the heart leads to a rhythm in the fibrillatory attractor. A large shock to the heart by a defibrillator can generally convert the arrhythmia back to the normal sinus rhythm if delivered sufficiently soon after fibrillation starts, once again demonstrating bistability in the heart.
In abnormal hearts, the basin of attraction of the sinus rhythm is not as deep. We can imagine erosion of a portion of the walls of the basin so that a transition to a fibrillatory attractor becomes easier or perhaps inevitable. A large number of different mechanisms can lead to changes in the anatomy and physiology of the heart and hence affect the stability of the sinus rhythm (2, 3). Among these are gradual changes secondary to high blood pressure, sudden changes due to a blocked artery that leads to localized cell death, or even changes induced by medications prescribed to treat cardiac or other conditions. Patients identified as having a high risk of fibrillation are eligible for an implantable cardiac defibrillator, which is programmed to automatically detect fibrillation if it occurs unexpectedly and convert it to a sinus rhythm. However, because many individuals who die of sudden cardiac death do not have identifiable abnormalities that place them in a high-risk category using current criteria, physicians face a significant challenge to predict a high risk of sudden cardiac death in individuals (15).
Cardiac tissue-culture preparations enable long-term observation of spontaneous activity.
The essence of the problem is to understand the physiology of cardiac arrhythmias and of the spontaneous transitions that occur between cardiac rhythms. Cardiac tissue-culture preparations enable long-term observation of spontaneous activity using a variety of experimental techniques, including varying the geometry of growing cells and using pharmacologic agents to modify physiological cellular properties leading to novel dynamic behaviors (14, 16-18). Consequently, studies of dynamics in cardiac tissue culture should lead to a better understanding of the origin of spontaneous transitions in dynamics observed clinically.
Cardiac Ballet
In 1969, Smirk and Ng (19) observed that electrocardiographic recordings often displayed complex repetitive sequences of ventricular beats associated with a high risk for sudden cardiac death and proposed the term “cardiac ballet” to describe these rhythms. The repetitive pirouettes of the spiral waves in cardiac tissue observed by Hwang et al. (7) evoke the same image. Thus, there is a strong parallel between the dynamics of hearts displaying complex arrhythmia and the repetitive dynamic sequences observed in tissue culture. The details of morphology and dynamics may differ from patient to patient or from one tissue culture to the next, but the underlying principles will be the same.
From the initial theoretical predictions of rotating spiral waves in hearts (20) to the current experimental observation of unexpected spontaneous transitions in spatiotemporal excitation patterns, the study of dynamics in excitable media has been marked by a game of leapfrog between experiment and theory. In the current article (7), experiment is leading the way, posing a problem for theoreticians. But the work also poses a challenge to clinicians who treat cardiac patients: Will it be possible to identify and understand the origins of complex arrhythmias to better predict, before they occur, the spontaneous transitions in spatiotemporal cardiac activity in people that lead to sudden cardiac death?
See companion article on page 10363 in issue 29 of volume 102.
References
- 1.Zheng, Z. J., Croft, J. B., Giles, W. H. & Mensah, G. A. (2001) Circulation 104, 2158-2163. [DOI] [PubMed] [Google Scholar]
- 2.Jalife, J., Delmar, M., Davidenko, J. & Anumonwo, J. M. B. (1999) Basic Cardiac Electrophysiology for the Clinician (Futura, Armonk, NY).
- 3.Kléber, A. G. & Rudy, Y. (2004) Physiol. Rev. 84, 431-488. [DOI] [PubMed] [Google Scholar]
- 4.Davidenko, J. M., Pertsov, A. V., Salomonsz, R., Baxter, W. & Jalife, J. (1992) Nature 355, 349-351. [DOI] [PubMed] [Google Scholar]
- 5.Garfinkel, A., Kim, Y.H., Voroshilovsky, O., Qu, Z., Kil, J. R., Lee, M.-H., Karagueuzian, H. S., Weiss, J. N. & Chen, P.-S. (2000) Proc. Natl. Acad. Sci. USA 97, 6061-6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray, R. A. & Chattipakorn, N. (2005) Proc. Natl. Acad. Sci. USA 102, 4672-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang, S.-m., Kim, T. Y. & Lee, K. J. (2005) Proc. Natl. Acad. Sci. USA 102, 10363-10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winfree, A. T. (2002) The Geometry of Biological Time (Springer, New York).
- 9.Bub, G., Glass, L., Publicover, N. G. & Shrier, A. (1998) Proc. Natl. Acad. Sci. USA 95, 10283-10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agladze, K. I. & Krinsky, V. I. (1982) Nature 296, 424-426. [Google Scholar]
- 11.Bursac, N., Aguel, F. & Tung, L. (2005) Proc. Natl. Acad. Sci. USA 101, 15530-15534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, G., Ouyang, Q., Petrov, V. & Swinney, H. L. (1996) Phys. Rev. Lett. 77, 2105-2108. [DOI] [PubMed] [Google Scholar]
- 13.Fenton, F. H., Cherry, E. M., Hastings, H. M. & Evans, S. J. (2002) Chaos 12, 852-892. [DOI] [PubMed] [Google Scholar]
- 14.Bub, G., Shrier, A. & Glass, L. (2005) Phys. Rev. Lett. 94, 028105. [DOI] [PubMed] [Google Scholar]
- 15.Josephson, M. & Wellens, H. J. J. (2004) Circulation 109, 2685-2691. [DOI] [PubMed] [Google Scholar]
- 16.Fast, V. G. & Kleber, A. G. (1995) Cardiovasc. Res. 5, 697-707. [PubMed] [Google Scholar]
- 17.Nagai, Y., González, H., Shrier, A. & Glass, L. (2000) Phys. Rev. Lett. 84, 4248-4251. [DOI] [PubMed] [Google Scholar]
- 18.Arutunyan, A., Swift, L. M. & Sarvazyan, N. (2002) Am. J. Physiol. 283, H741-H749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smirk, F. H. & Ng, J. (1969) Br. Heart J. 31, 426-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiener, N. & Rosenblueth, A. (1946) Arch. Inst. Cardiol. Mex. 16, 205-265. [PubMed] [Google Scholar]