Abstract
Human herpesvirus 8 (HHV-8), also known as Kaposi's sarcoma-associated herpesvirus (KSHV), discovered in 1994, is a human rhadinovirus (gamma-2 herpesvirus). Unlike other human herpesviruses (herpes simplex virus, Epstein-Barr virus, varicella-zoster virus, cytomegalovirus, HHV-6, and HHV-7), it is not widespread in the general population and has many unique proteins. HHV-8 is strongly associated with all subtypes of Kaposi's sarcoma (KS), multicentric Castleman's disease, and a rare form of B-cell lymphoma, primary effusion lymphoma. In addition, HHV-8 DNA sequences have been found in association with other diseases, but the role of the virus in these diseases is largely unconfirmed and remains controversial. The seroprevalence of HHV-8, based on detection of latent and lytic proteins, is 2 to 5% in healthy donors except in certain geographic areas where the virus is endemic, 80 to 95% in classic KS patients, and 40 to 50% in HIV-1 patients without KS. This virus can be transmitted both sexually and through body fluids (e.g., saliva and blood). HHV-8 is a transforming virus, as evidenced by its presence in human malignancies, by the in vitro transforming properties of several of its viral genes, and by its ability to transform some primary cells in culture. It is not, however, sufficient for transformation, and other cofactors such as immunosuppressive cytokines are involved in the development of HHV-8-associated malignancies. In this article, we review the biology, molecular virology, epidemiology, transmission, detection methods, pathogenesis, and antiviral therapy of this newly discovered human herpesvirus.
INTRODUCTION
Most human herpesviruses are ubiquitous in most populations. They usually persist as long-term latent infections, and asymptomatic shedding of infectious virus is common. This shedding is responsible for horizontal primary transmission, usually from mother to child, so that initial infection occurs very early in life. Because they are so common, it has been very difficult to prove their role in the pathogenesis of malignant or nonmalignant diseases.
An important exception to this rule, because of its limited and uneven distribution, is human herpesvirus 8 (HHV-8), also called Kaposi's sarcoma-associated herpesvirus (KSHV). In sub-Saharan Africa, antibodies to HHV-8 can be found in upwards of 30% of the general population (55, 134, 258, 263). From 10 to 25% of people from the Mediterranean area are seropositive for the virus. Geographic pockets in this area with higher or lower prevalences can be found. In the rest of the world, the seroprevalency is low, 2 to 5% (58).
HHV-8 was first detected by Chang et al. (56) in Kaposi's sarcoma (KS) tissues from a patient with AIDS by representational difference analysis. Since its initial discovery, HHV-8 has been found in all forms of KS: classical, endemic, and AIDS-associated iatrogenically acquired KS (265). In situ hybridization techniques have pinpointed the location of HHV-8 in the vascular endothelial cells and perivascular spindle-shaped cells in KS lesions (31,172). This association has been supported both by molecular analysis (33, 50, 212, 262) and by seroepidemiological studies (11, 51, 134, 258, 263). The pathogenic role of HHV-8 in other malignancies, such as multicentric Castleman's disease and primary effusion lymphoma, was again based on molecular, seroepidemiological, and cell biology studies (264).
On the basis of phylogenic analysis (205, 248), HHV-8 is the first human rhadinovirus (gamma-2 herpesvirus) identified. HHV-8 is related to the rhadinoviruses herpesvirus saimiri, found in squirrel monkeys, and herpesvirus ateles, found in spider monkeys. Both primates are native to South America. HHV-8 is also in the lineage of rhadinoviruses that infect macaques and African green monkeys (30, 70). More recent studies (5, 70, 121, 122, 261, 267) have found additional rhadinoviruses that are closely related to HHV-8 infecting monkeys and chimpanzees. PCR has detected the DNA polymerase from rhadinoviruses in rhesus monkeys and pigtail macaques suffering from retroperitoneal fibromatosis (virus strains FHVMm and RFHVMn) and also in asymptomatic African green monkeys (virus strain ChRV-1). Retroperitoneal fibromatosis is characterized by a proliferation of spindle cells that is somewhat similar to KS. HHV-8 homologues were also detected in drill, mandrill, and a hybrid of Mandrillus leucophaeus-Mandrill sphinx, nonhuman primates living in Cameroon and Gabon, central Africa (155). Gamma-2 herpesviruses of higher primates closely related to HHV-8 were isolated from chimpanzees and gorillas after finding that they expressed KHSV antigens (154).
STRUCTURE AND MORPHOLOGY
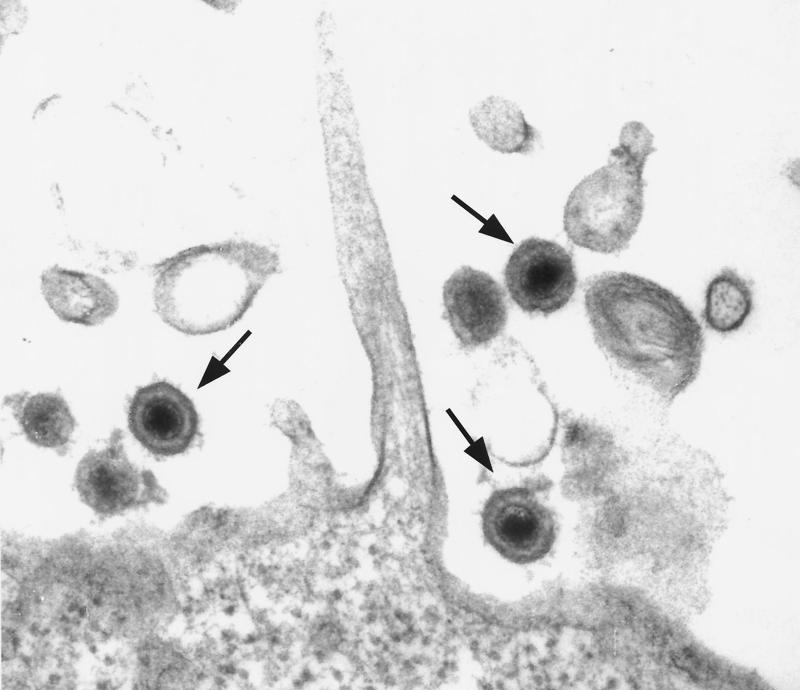
Before the discovery of HHV-8 (56), herpesvirus particles were described in a short-term tissue culture of a KS lesion (92, 115), and they were subsequently identified as cytomegalovirus (116). HHV-8 exhibits typical herpesvirus morphology: 100- to 150-nm particles with a lipid envelope and an electron-dense central core (242). Figure 1 is an electron micrograph showing the morphology of HHV-8 particles in KS-1 cells, a primary effusion body cavity B-cell lymphoma cell line which is persistently infected with HHV-8 (250). Its capsid is icosahedral, with a 110-nm diameter, and consists of 162 hexagonal capsomeres (295). Mature virions with a glycoprotein coat are 140 nm in diameter. The tegument is a protein-filled region between the capsid and the envelope. The 75-nm torus-shaped core is a complex of DNA and protein.
FIG. 1.
Morphology of KSHV (HHV-8) virions produced in PEL cell line KS-1. Arrows indicate fully mature virions.
In appearance, HHV-8 is indistinguishable from alpha-, beta-, and other gammaherpesvirus particles (134). Orenstein et al. (219) found herpesvirus particles in electron micrographs of KS lesions from three patients. Since HHV-8 has been found to productively infect spindle cells derived from microvascular endothelial cells and mononuclear cells, the particles found in the KS lesions are consistent with HHV-8. PCR analysis on the skin, lymph node, and spleen from the three patients was positive for HHV-8; however, the lymph node was positive for Epstein-Barr virus (EBV) as well.
When the envelope glycoproteins of HHV-8 bind the proteoglycans on the surface of the host cell, penetration can occur by fusion of the viral envelope with the plasma membrane of the cell (134). HHV-8 infects dividing B cells (CD45+) during mitosis, much like EBV, the human gammaherpesvirus. Following circularization of the viral genome DNA replication and capsid assembly occur in the nucleus of the host cell.
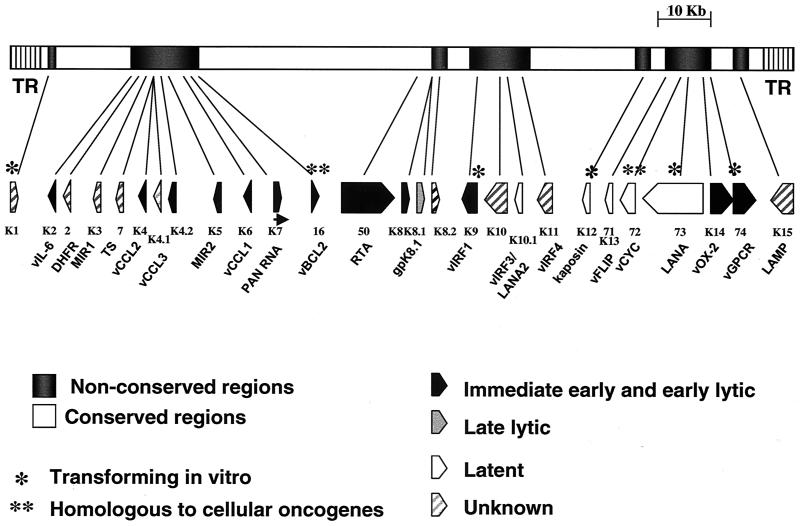
Pulsed-field gel electrophoresis of DNA extracted from purified HHV-8 virions shows that the full-length genome is 165 to 170 kb (Fig. 2). Primary characterization of the genome was done by Moore et al. (205), and others (213, 248) have done additional studies. The genome of HHV-8 is similar to that of herpesvirus saimiri in that it has a single contiguous region, 140 to 145 kb, containing all the coding regions. Some permissive and nonpermissive tumor cell lines harbor forms of HHV-8 viral DNA up to 270 kb in size (14). The genome has repeats of 803 bp in length that are 85% guanidine and cytosine. Each molecule harbors 35 to 45 such repeats, but they are not arrayed uniformly or symmetrically at each end (158). Like EBV, the latent HHV-8 genome appears to have a circular conformation, but the active DNA found during lytic replication is linear (242).
FIG. 2.
Structure of the KSHV (HHV-8) genome. The central portion of the genome is flanked by the terminal repeats, labeled TR. The KSHV genome contains close to 100 open reading frames. Many of these are conserved in most herpesviruses; these are present in the conserved blocks (white boxes) and are not indicated. Other open reading frames are unique to rhadinoviruses, gammaherpesviruses, or KSHV and are present in more divergent areas of the genome (indicated by gray boxes). ORFs that have homology with herpesvirus saimiri are assigned the corresponding numbers, and ORFs without recognizable homologues were numbered separately and given the prefix K (K1 to K15) (248). Adapted from references 212 and 248 with permission of the publishers.
Strain variation is very common among human herpesviruses, e.g., herpes simplex virus types 1 and 2 in the alphaherpesvirus group, HHV-6 variants A and B in the betaherpesvirus group, and EBV types A and B in the gammaherpesvirus group. Hayward (126) found similar variation in the gene products of KSHV ORF-K1 and ORF-K15. Both of these seem to code for membrane-signaling proteins; they contain conserved tyrosine kinase interaction motifs within their C-terminal cytoplasmic domains (165, 214). ORF-K1 and ORF-15 may play a key role in the biology and disease manifestation associated with HHV-8 (126). Based on the analysis of the ORF-K1 and ORF-15 genes, five HHV-8 variants (groups A to E) have been identified. Group B is dominant in Africa; D and E are confined to Pacific Island and Amerindian populations. In Europe and North America, groups A and C predominate (264). Geographic strain variation in gene sequences has been identified in viruses isolated from Japan, Kuwait, Europe, Russia, Australia, South America, and the United States (94, 153, 195, 196, 297). Although specific variants have not been associated with different pathologies, the high level of strain variability in HHV-8 may have important functional implications, although no serological differences have been noted using the currently available serological assays.
HHV-8 has many homologies with closely related viruses (134, 267) but also has many unique sequences (30). ORF-26 of HHV-8, which encodes the minor capsid protein, has 51% homology to VP23 of herpesvirus saimiri and is 39% homologous to the EBV open reading frame (ORF) for tegument, BD LF1 (56). Of the approximately 95 genes in the HHV-8 genome, nearly 25 encode novel proteins not found in other human herpesviruses. Many of these represent captured and diverged homologues of cellular genes that are referred to by ORF-K numbers if they do not have homologues in the herpesvirus saimiri genome. A number of genes seem to be responsible for KS pathogenesis: K1, K2, vMIPS, K4, K4.1, K5, K9, K12, ORF-6, ORF-71, ORF-73, ORF-74, and K15 (212). The products and functions of these genes in pathogenesis have been reviewed by Neipel and Fleckenstein (212).
BIOLOGY, INFECTIVITY, TRANSFORMATION, AND TUMORIGENESIS
Biology
To date, HHV-8 has been detected in tissues from KS, multicentric Castleman's disease (MCD), and primary effusion lymphoma (PEL) (Table 1). Although it is difficult to get infectious virus from culture explants of KS and MCD tissues, two studies have been performed with cells cultured from KS lesions. Virus was detected in the cultured cells (26, 93). Cell lines derived from PEL do exhibit HHV-8 particles (250), although many PEL-derived lines are also infected with EBV (e.g., BC-1 and BC-2) (14, 52). However, PEL cell lines BCBL, BCP-1, BC-3, and KS-1 (which are free of both EBV and human immunodeficiency virus [HIV] as well as other herpesviruses) have proven very valuable for studies of HHV-8. These cells are latently infected with the virus and rarely produce many virus particles, but if they are chemically induced with phorbol myristate acetate (PMA) and butyrate, the virus undergoes DNA synthesis, and infectious virus is produced. Although BC-3 and KS-1 were derived from the same patient (14, 52), the KS-1 line, which has been passed through immunodeficient mice, gives much higher yields of virus with successive passages, but the BC-3 line gave its highest yields in early passages.
TABLE 1.
Spectrum of HHV-8-associated diseasesa
| Disease type | Description |
|---|---|
| Primary infection | Infectious mononucleosis-like illness |
| Very strong HHV-8 association | KS (all types), i.e., endemic, classic, and AIDS related |
| Multicentric Castleman's disease in HIV-infected and non-HIV-infected individuals | |
| Primary effusion lymphoma, also known as body cavity B-cell lymphoma (a rare tumor, but more cases in HIV-infected individuals) | |
| Development of KS or PEL in transplant recipients; PEL of T-cell origin has also been reported | |
| Evidence of HHV-8 DNA | Non-KS skin lesion in transplant recipients |
| Reactive lymphadenopathy Bowen's disease | |
| Pemphigus vulgaris with or without HIV infection | |
| Salivary gland tumors (bilateral MALT lymphoma of the parotid gland in HHV-8-seropositive patients) | |
| HIV-infected patients with interstitial pneumonitis | |
| Persistent HHV-8 viremia with coinfection of human T-lymphotropic virus type 1 and myelofibrosis | |
| Hemophagocytic syndrome | |
| Noncleaved cell lymphoma | |
| Multiple myeloma | |
| Kikuchi's disease (histocytic necrotizing lymphadenitis) | |
| Sarcoidosis |
HHV-8 association with the listed infections was based on DNA PCR, IgG antibody responses, in situ hybridization on tumor cells, and immunohistochemistry on tissue sections with HHV-8 monoclonal antibodies.
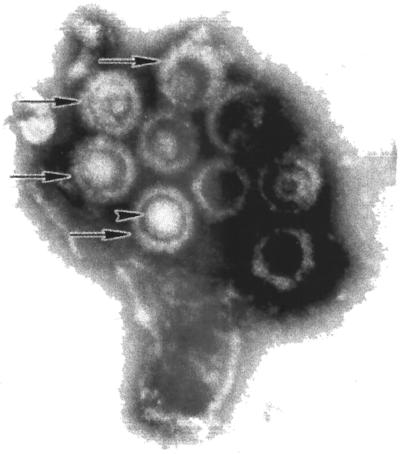
Negative staining of HHV-8 particles derived from KS-1 cells (Fig. 3) reveals that 35% of the virions are intact and the remaining 65% are defective, with viral capsids of 100 to 150 nm in diameter. Both KS-1 and BC-3 cell lines have been fully characterized (14, 250) and have been found to express CD45 but no other B- or T-cell lineage-restricted antigens, an unusual immunophenotype. They exhibit immunoglobulin gene rearrangements typical of B-cell clonal populations, and they retain the germ line configuration for T-cell genes, indicating their B-cell derivation. Immunoglobulin (Ig) chain genes, the Ia heavy-chain genes, and kappa and lambda light-chain genes retain the germ line configuration for Tβ genes.
FIG. 3.
Purified HHV-8 virions. Supernatant from PMA-butyrate-induced KS-1 cells was harvested and fractionated by sucrose gradient centrifugation. The micrograph shows a cluster of negatively stained, mature HHV-8 virions (arrows), having a nucleocapsid core, defined tegument region, and outer lipid envelope. Surface spikes are evident on some particles.
Both of these cell lines, as well as others listed above, are easily propagated in standard cell culture medium, RPMI 1640 with 10% or less fetal bovine serum. They generally grow in large clumps, and <1% of the cells express lytic viral antigen by immunofluorescence assay with monoclonal and polyclonal antibody (Advanced Biotechnologies Inc., Columbia, Md.) to ORF-59 (DNA polymerase processing gene), ORF-K8.1 A or A/B (envelope protein), rabbit anti-ORF-65 (capsid protein), and serum from a classical-KS patient. When KS-1 cells are induced with PMA and butyrate, >70% of cells express lytic antigen in approximately 72 h and become an excellent source of infectious virus or purified virus for immunological and molecular biology studies.
Infectivity of HHV-8
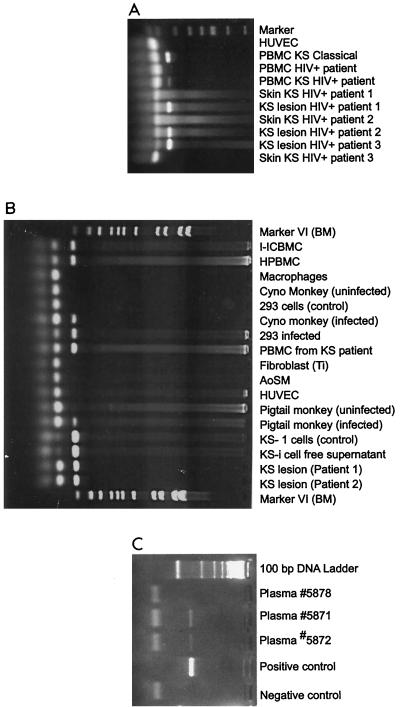
HHV-8 does not readily infect most cell types. Mesri et al. (197) and Moore et al. (205) have shown transmission of HHV-8 DNA from the BC-1 cell line into Raji (EBV-positive, nonproducing B-cell line), BJAB (EBV genome-negative B-cell line), MoLT-3 (CD4-positive mature T-cell line), and owl monkey kidney cell lines as well as into human cord blood mononuclear cells. No active infection occurred, however, and no viral DNA could be detected after several passages. HHV-8 from KS lesions has been propagated in embryonal kidney 293 cells (93); however, the transmission was very limited, and the viral DNA could be detected only by PCR amplification (Fig. 4). Renne et al. (241) were able to transmit HHV-8 from BCBL-1 cells to 293 cells by using a spliced HHV-8 late mRNA and a sensitive reverse transcriptase (RT)-PCR technique; minimal amounts of viral RNA were expressed and could not be detected by RT-PCR. Thirty-eight additional cell lines, including B cells, endothelial cells, and cells of fibroblastic origin, were used as targets for transmission, but all failed.
FIG. 4.
Detection of KSHV (HHV-8) DNA in various clinical samples from KS patients and infected cells. Genomic DNA was extracted with DNAzol reagent (Gibco-BRL, Gaithersburg, Md.), and 1 μg of total DNA was used in the PCR. For the detections of KSHV/HHV-8 expression, two primers were synthesized from the minor capsid region of the HHV-8 sequence. The sense primer was 5′-TCG AGC AGC TGT TGG TGT ACC ACA T, and the antisense primer was 5′-TCC GTG TTG TCT ACG TCC AG. These primers, used in the PCR, were designed to amplify 142 bp of HHV-8. Each PCR cycle consisted of denaturation at 94°C for 1 min, primer annealing at 60°C for 45 s, and extension at 72°C for 2 min. The samples were amplified for 30 cycles. A positive reaction for PCR of HHV-8 showed amplified product of 142 bp. Molecular weight markers (marker VI) were from Boehringer Mannheim, Indianapolis, Ind. (A) PBMCs from classic KS patients and HIV-infected KS patients, KS lesions, and skin from HIV-infected KS patients. (B) HHV-8-infected cells: PBMCs, monocytes, primary monkey peripheral blood cells, PBMCs from KS patients, the KS-1 cell line, which is persistently infected with HHV-8, and KS lesions from two patients. (C) HHV-8 DNA detected in plasma of two Israeli patients from Ethiopia.
KS-1, BC-3, and BCBL-1 cell lines produce infectious virus, but the infectivity of this virus is very low. HHV-8 from the KS-1 cell line concentrated over a sucrose gradient has a very high particle count. Preparations concentrated 1,000-fold have been found to contain ≥1.8 × 1010 virus particles/ml and a protein concentration of >1.0 mg; however, complete infectious particles constitute only 103 of these particles. The rest are either immature or noninfectious particles. Thus far, to our knowledge, no primary cell or cell line is readily permissive for HHV-8 infection and produces quantities of infectious virus.
Cultures of primary human monocytes-macrophages from patients with AIDS-associated KS can be infected with HHV-8, and treatment with cytokines seems to enhance viral production and allow the cells to maintain the virus for a longer period (21). An infection of primary cells with HHV-8 obtained from induced KS-1 cells is shown in Fig. 4.
Microvascular endothelial cells transformed with the papillomavirus type 16 E6 and E7 genes were also permissive for HHV-8 derived from the BCB-1 cell line (206). Successful transmission was demonstrated by DNA PCR, RT-PCR, immunofluorescent assay for cellular proteins, electron microscopy for morphological changes, and morphological changes in soft-agar colonies of the infected cells. The viral genome was maintained indefinitely and remained in latent form, as indicated by reaction with monoclonal antibody for ORF-73, an indicator gene of HHV-8 latency. The virus in these infected endothelial cells could be induced to go into the lytic cycle. This transition caused the cells to go from a cobblestone growth pattern to a spindle-shaped morphology, a growth pattern typical of KS lesions.
Untransformed primary fetal dermal microvascular endothelial cells, derived from large blood vessels or capillaries, have also been successfully infected with HHV-8 from PEL cell lines (JSC-1, BC-3, and BCP-1) (39, 294). In this system, the best results were obtained with virus from the JSC-1 line. Infection caused the dermal microvascular endothelial cells to change morphology from a cobblestone pattern to spindle-shaped cells, similar to the change seen in the transformed endothelial cells described above. This change is accompanied by loss of contact inhibition. Infected cells expressed latent nuclear antigen (LNA or LANA) and showed increased mitosis. Ten percent of the spindle-shaped cells spontaneously go into the lytic cycle and express K8 and other lytic cycle proteins, as demonstrated by immunofluorescence assay. The JSC-1 line is coinfected with HHV-8 and EBV, but EBV expression in dermal microvascular endothelial cells is extremely low. It is not yet clear what, if any, role EBV may play in HHV-8 infectivity in dermal microvascular endothelial cells.
Virus from PEL cell lines and virus from KS lesions passed to 293 cells were used to infect the EBV-negative Loukes B-cell line (99). After infection, viruses from both sources induced apoptotic cell death. Transient expression of the HHV-8 vBCL-2 homologue delayed apoptosis and prolonged the survival of the infected 293 cells. From this, it was concluded that HHV-8 induces apoptosis through a BCL-2-dependent pathway. The virus derived from KS lesions and grown in 293 cells has distinct characteristics compared to the virus from PEL cell lines and therefore may play different roles in the pathophysiology of KS.
Transformation
The first direct evidence of transformation caused by HHV-8 was shown by Flore et al. (92). In this study, human primary bone marrow cells were infected with purified HHV-8 from BC-3 PEL line cells. This infection led to increased long-term proliferation and survival, which were associated with the acquisition of telomerase activity and anchorage-independent growth. Only a subset of the transformed cells contained HHV-8, suggesting that paracrine factors were responsible for the survival of the cells that did not contain virus. Survival may be mediated by upregulation of a receptor for vascular endothelial growth factor. The cells could be induced to go into the lytic cycle and produce a productive infection by PMA. Eight months postinfection, 1 to 5% of the cell population expressed LNA, but the phenotype of these cells was indistinguishable from that of their uninfected normal primary endothelial cell neighbors. Kinase domain receptor downregulation is considered an important milestone in senescence and death of normal endothelial cells, and it is possible that upregulation by KSHV is involved in the long-term survival of infected cells. These findings show that HHV-8 can induce transformation of primary endothelial cells; however, the mechanism of transformation is different from that of other transforming viruses such as EBV.
Six days postinfection, cultures of primary human keratinocytes infected with HHV-8 have demonstrated transcription of viral genes by RT-PCR and protein expression by immunofluorescence assay for ORF-73 (49). This infection was tried because HHV-8 DNA can be found in endothelial keratinocytes in the basal layer of the epidermis in nodular lesions of the plaque stage of KS. HHV-8 DNA can also be found in the epithelial cells of the eccrine glands within KS lesions, in salivary glands, and rarely in prostate tissues (75, 227). Infected cultures of keratinocytes could be induced to go into the lytic cycle and transcribe lytic genes such as ORF-26. The cells continued to proliferate, and the growth pattern of the culture was changed from the pattern of uninfected cultures. They lost contact inhibition and demonstrated telomerase activity, anchorage-independent growth, and changes in cytokine production. In the study by Flore et al. (92) on primary bone marrow endothelial cells, HHV-8 could always be detected in a subset of transformed cells after culture for more than 1 year, but in the keratinocyte study, HHV-8 was undetectable after 8 weeks by nested PCR.
Viral Genes and Transformation
HHV-8-associated malignancies such as KS have been found to express a unique protein called kaposin, encoded by an abundant, latent-cycle transcript of 0.7 kb, T 0.7 or HHV-8 ORF-K12 (208, 209). The kaposin gene induces tumorigenic transformation in transfected Rat-3 cells. The fact that transformed Rat-3 cells contained mRNA for kaposin localized in the cells' cytoplasm suggests that kaposin plays a role in the maintenance of the transformed phenotype. Kaposin has also been detected in PEL cell lines which are persistently infected with HHV-8, supporting a role for kaposin in the development of HHV-8-associated malignancies, such as KS.
Most KSHV genes with oncogenic potential (Fig. 2) appear to be transcribed to some extent in PELs. Still, patterns of KSHV gene expression in de novo infection and during initial steps of lymphomagenesis are not known. KSHV carries 11 open reading frames that encode homologues to cellular proteins involved in signal transduction, cell cycle regulation, and/or inhibition of apoptosis (248) (Fig. 2). Four of these genes, K9, the viral interferon regulatory factor (vIRF) (107, 170, 299), ORF-74, the KSHV viral G protein-coupled receptor (GPCR) (15, 17), ORF-K1 (165, 166), and ORF-K12 (kaposin) (208, 209), can transform rodent cells and/or cause tumors in animal models.
The KSHV GPCR and K1 oncogenes are potentially important in PEL lymphomagenesis because they can trigger signaling cascades relevant for B- and T-cell growth. KSHV GPCR is a constitutively active G protein-coupled receptor able to trigger the mitogen-activated protein kinase signaling cascades and induce secretion of vascular endothelial growth factor (17). Mitogen-activated protein kinase cascades such as those triggered by KSHV GPCR are activated by inflammatory cytokines and mitogens. Vascular endothelial growth factor is an angiogenic and vascular permeability factor that could contribute to the effusion phenotype. K1 has an ITAM motif that can activate cytoplasmic tyrosine kinases and mimic signaling by the B-cell antigen receptor (165, 166).
KSHV also encodes homologues of cytokine and cytokine response genes: a viral interleukin 6 (vIL-6), K2, and the vIRFs (Fig. 2). vIL-6 can bind the gp130 receptor to activate IL-6-responsive genes and promote B-cell survival (201, 204, 215). vIRF1 can inhibit interferon-induced transcriptional activation (107, 170, 299) (Fig. 5). KSHV also contains ORFs homologous to cellular oncogenes involved in lymphomagenesis. These are the viral cyclin D (ORF-72), which is homologous to the BCL-1 gene, and the viral BCL-2 (ORF-16). The KSHV cyclin homologue is a functional cyclin that can associate with CDK6, induce phosphorylation of retinoblastoma (Rb) protein, and overcome Rb-mediated cell cycle arrest (169; Y. Chang, P. S. Moore, S. J. Talbot, C. Boshoff, T. Zarkowska, D. Godden-Kent, R. Weiss, and S. Mittnacht, letter to the editor, Nature 382:410, 1996). The viral cyclin D differs from the cellular cyclin D in its ability to induce degradation of the CDK inhibitor p27Kip when complexed with CDK6 (85, 182). The viral BCL-2 can block apoptosis as efficiently as cellular BCL-2, BCL-xL, or the EBV BCL-2 homologue BHRF1 (61). Interestingly, the KSHV BCL-2 cannot homodimerize or heterodimerize with other BCL-2 family members, suggesting that it may have evolved to escape any negative regulatory effects of the cellular Bax and Bak proteins. In addition to BCL-2, KSHV carries a viral FLIP (vFLIP, K13), which is an inhibitor of the proapoptotic molecule FLICE/caspase-8 (281).
FIG. 5.
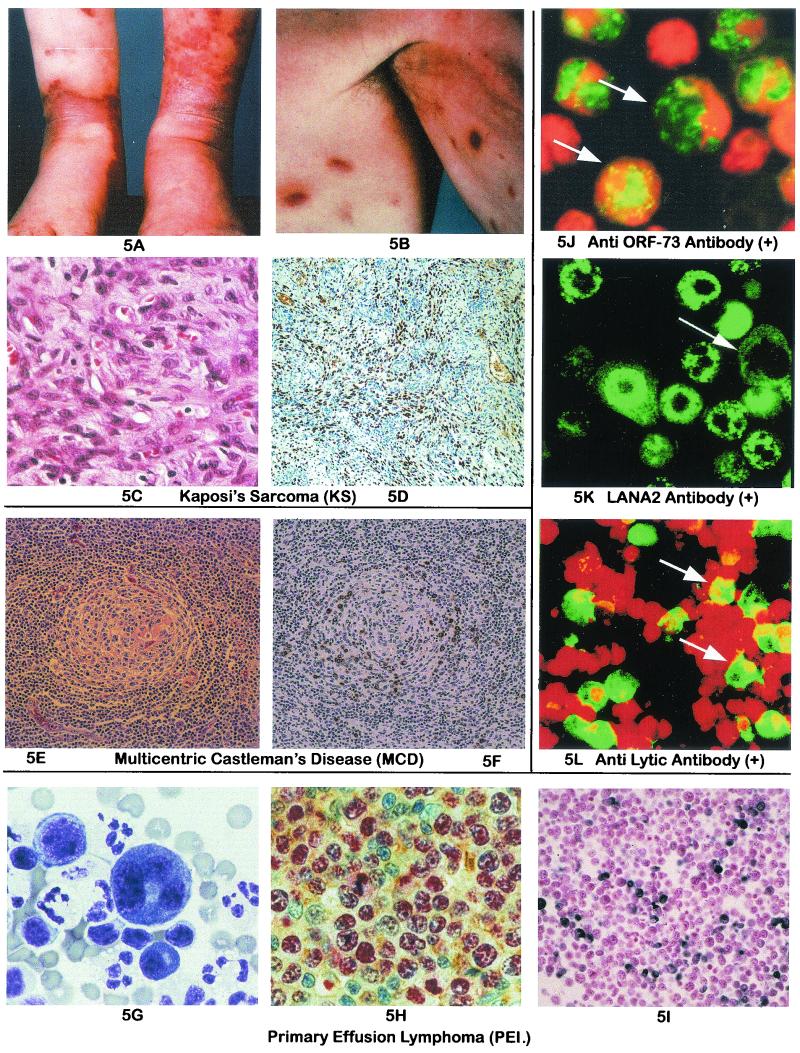
KS, PEL, and MCD: morphology and immunohistochemistry for viral antigens and detection of latent and lytic antigens to KSHV by IFA, with human sera and monoclonal antibody. (A) KS lesions in the lower extremities typical of a sporadic case. (B) Hyperpigmented KS lesions in the upper arms. (C) Histological section stained with hematoxylin and eosin of a nodular tumor stage lesion of KS. Note the spindle cell proliferation and abundant vasculature. (D) KSHV LANA (ORF-73) expression in KS. Staining with a rat monoclonal antibody revealed LANA positivity (diaminobenzidine, brown) in the nuclei of many spindle cells in a KS lesion. Positivity was also identified in endothelial cells lining the larger vascular spaces that may represent lymphatic vessels. (E) Histology of multicentric Castleman's disease. Hematoxylin- and eosin-stained section of a lymph node with HIV-associated Castleman's disease showing a single follicle with a large, concentrically arranged mantle zone surrounding a germinal center. The interfollicular area contains a network of small vessels. (F) KSHV vIL-6 expression in MCD. Immunohistochemical staining with polyclonal antiserum to vIL-6, showing cells with cytoplasmic positivity (diaminobenzidine, brown) in the mantle zone surrounding an atrophic germinal center. (G) Wright-Giemsa stain air-dried cytocentrifuge preparation of a KSHV-positive primary effusion lymphoma. The two tumor cells in this image are considerably larger than normal benign lymphocytes and neutrophils. The cells display significant polymorphism and possess moderately abundant basophilic cytoplasm. A prominent, clear perinuclear Golgi zone can be appreciated in the largest cell. The nuclei vary from large and round to highly irregular, multilobated, and pleomorphic and often contain one or more prominent nucleoli. (H) KSHV LANA (ORF-73) expression in KSHV-positive lymphomas. Staining with a rat monoclonal antibody revealed LANA positivity (alkaline phosphatase, red) in the nuclei of large, atypical lymphoma cells seen infiltrating reactive lymphoid tissue. This section was double stained with a polyclonal antiserum to kappa light chains (diaminobenzidine, brown), showing cytoplasmic positivity in a few of the surrounding cells but not the tumor cells. (I) KSHV vIL-6 expression in PELs. Immunohistochemical staining of cell block containing the BC-3 cell line was performed with a polyclonal rabbit antiserum to a vIL-6-specific peptide. Abundant expression is seen (diaminobenzidine, brown) in numerous lymphoma cells. (J) Detection by IFA of KSHV latent IgG antibody in serum from a classic KS patient. Typical nuclear speckles in the PEL cell line KS-1 are evident at a 1:50 dilution of the serum. (K) Presence of LANA-2 protein in KSHV-infected BCBL-1 cells. Diffuse finely speckled nuclear pattern of LANA-2 (green) is observed by IFA with LANA-2 monoclonal antibody. (L) Detection by IFA of KSHV lytic antibody in serum from a classic KS patient, using induced KS-1 cells. Apple green diffusely stained cells carry lytic antigen. Original magnifications: ×200 (D, E, F, and I), ×600 (C and H), and ×1,000 (G). Panels C to I copyright Amy Chadburn (Weill Medical College of Cornell University, New York, N.Y.). Panel K reproduced from reference 247 with permission.
Expression in PELs in vivo is well documented for vIL-6 protein (204) and v-cyclin mRNA (53). Additionally, vFLIP (K13), kaposin (K12), and LANA2/v-IRF3 (ORF-K10.5) are transcribed during latency in PEL cell lines (257). This observation suggests that these genes may be involved in the maintenance of the malignant status and the effusion phenotype of PELs.
LNA-1 (ORF-73), the protein most frequently identified in KS tumor cells (Fig. 5), has also been found to be tumorigenic. It transforms primary rat embryo cells (236), seems to act as a transcription cofactor, and contributes to HHV-8-induced oncogenesis by targeting the retinoblastoma protein E2F transcriptional regulatory pathway.
Receptor for HHV-8
Recently, heparan sulfate was found to be a receptor that interacts with the K8.1 envelope glycoprotein (23). Cell surface glycosaminoglycans play a crucial role in HHV-8 target cell recognition, and K8.1 is at least one of the proteins involved.
METHODS OF DETECTING VIRAL INFECTION
Serological and molecular methods have been employed independently and in concert to detect the presence of HHV-8 KSHV in cells, tissues, and body fluids.
Nucleic Acids
The majority of studies for the detection of HHV-8 DNA used PCR methods. HHV-8 DNA has been detected in 95% of HIV-associated KS, classical KS, and endemic KS (33, 134, 135, 257, 258, 262, 263). The DNA from either fresh or frozen KS samples can be detected by PCR after 30 to 35 amplifications (Fig. 4). Detection of HHV-8 DNA PCR products can be enhanced by hybridization; however, false positives are not reduced by this method. Nested PCR has been used to detect HHV-8 in paraffin-embedded tissues from KS and multicentric Castleman's disease biopsies, lymphoid tissues from PEL patients (37), semen, plasma, peripheral blood, and saliva. HHV-8 has been detected in 30 to 60% of the peripheral blood mononuclear cells (PBMCs) of KS patients by nested PCR (205, 291). HHV-8 PCR template sequences have been generated from various regions of the viral genome; these template sequences include latent genes (e.g., ORF-73) and lytic genes (e.g., ORF-65). While the use of PCR has been very useful in obtaining extensive information about the distribution of HHV-8, it has also led to the publication of multiple studies with disease associations that have not been confirmed, likely due to the high risk of contamination and false-positive results using this methodology.
In Situ Hybridization
In situ hybridization can be used to localize the specific cells that harbor HHV-8 in KS-involved tissues (31, 67, 75, 239, 274, 283). While in situ hybridization has been used effectively with several targets as probes, it is being replaced as a diagnostic procedure by immunohistochemical methods.
Immunohistochemistry
The detection of KSHV can now be easily achieved in formalin-fixed, paraffin-embedded tissues with commercially available monoclonal antibodies to several different viral antigens, both lytic and latent (Fig. 5D, F, H, and I). With this technique, KSHV has been detected in the spindle cells and some epithelial cells of KS lesions, in PEL tumor cells, in KSHV-positive lymphomas involving solid tissues, and in scattered cells in the mantle zone of multicentric Castleman's disease (51, 82). This technique has also been used to identify scattered KSHV-positive cells in reactive lymph nodes from HIV-positive, KSHV-positive individuals (A. Chadburn, personal communication). The ease of this technique makes it available to standard pathology laboratories to confirm a diagnosis of KS. It also can be used to evaluate the KSHV status in patients with multicentric Castleman's disease or non-Hodgkin's lymphomas. A polyclonal antiserum to vIL-6 has been produced that works in paraffin sections and can detect KSHV in PEL (Fig. 5I) and multicentric Castleman's disease (Fig. 5F) but only rarely in KS and therefore is not useful for KS diagnosis.
Serology
Latent and lytic antibodies to HHV-8 have been detected by immunofluorescence assay (Fig. 5J and K), Western blots, and enzyme-linked immunosorbent assay (ELISA). Recently (247), an additional latent nuclear protein, LANA-2, encoded by HHV-8 ORF-K10.5, has been identified (Fig. 5K). Like LNA-1, this protein is localized in the nucleus and has been found to be expressed in multicentric Castleman's disease tissue and PEL cells, but not in KS spindle cells. LANA-2 is thought to suppress apoptosis by inhibition of p53-induced transcription. This is a classic mechanism in tumorigenesis, and the hemopoietic cells that lead to multicentric Castleman's disease and PEL may be particularly susceptible to LANA-2.
The PEL cell lines BCP-1, KS-1, BC-3, and BCBL-1 are commonly used as substrate cells for immunofluorescence assay (IFA) for the detection of antibodies to both latent and lytic proteins (Fig. 5J and L). In order to detect latent antibody, a harsher fixation method has been used to permeate the cells so that the test sera can react with LNA (108, 268). This latent nuclear protein is 226 to 236 kDa and is localized in the cell nucleus of infected cells. Typical IFA of LNA shows dotted fluorescence in the nucleus of infected cells, found in more than 95% of PEL cells (Fig. 5J). LNA IFA detection was the primary method first used in HHV-8 serology to detect antibody prevalence in patients and healthy donors. Recently, an ELISA with recombinant LNA (ORF-73) baculovirus protein has been developed and is available commercially; this assay can detect latent antibody in more specimens from patient groups and healthy blood donors than the traditional IFA-LNA. The recombinant ORF-73 ELISA and IFA-LNA gave similar results in over 90% of testing trials (C. R. Lee, M. Roman, D. Thomas, D. Bourboulia, C. Boshoff, O. Flore, A. Friedman-Kien, P. S. Gill, R. Masood, T. Schulz, J. E. Whitman, B. Chandran, and D. V. Ablashi, HHV-8 ORF-73 latent antigen [LNA-1] ELISA: development and performance comparison with LANA IFA, Fifth International AIDS Malignancy Conference, 23-25 April 2001, National Cancer Institute, abstract 31). This ELISA to detect latent antibody is highly specific. The assay has >10% more sensitivity than IFA-LANA.
For the detection of lytic antibody to HHV-8, both IFA (Fig. 5L) and ELISA have been used. In order to test for lytic antibodies by IFA, the PEL cell lines must be chemically induced to express the lytic antigens. Positive cells range from 20 to 70% depending on the cell line used, the time course of induction, and other biological factors. Chatlynne et al. (59) described an IFA with the KS-1 cell line (253) and a lytic antibody ELISA that uses sucrose-purified whole virus from the same cell line. Both of these assays have proven to be sensitive and specific, correlate very well with other assays and disease incidence, and are commercially available (Advanced Biotechnologies Inc.). Homemade ELISAs for antibodies to small viral capsid antigen (175) and to the major capsid protein with recombinant ORF-65 have also demonstrated good results (38, 234, 268).
KS patients are 80 to 90% positive and demonstrate elevated lytic antibody titers in the whole-virus ELISA. By the same test, normal healthy donors show a prevalence of 2 to 5%, varying slightly with the population except for areas of central Africa, where the virus is endemic (1). Gao et al. (108) used ORF-73 isolated from the nuclei of BC-1 PEL cell line for immunoblots, with which 80% of AIDS-related KS sera tested positive. With a combination of Western blots and an ORF-65 ELISA to test serological samples, the following percentages were positive: 75 to 85% of AIDS-KS, 31% of homosexual men, 2% of hemophiliacs in the United Kingdom, 1.7 to 5% of healthy blood donors from the United Kingdom and United States, and 35% of control groups from Uganda (134, 175, 268). An ELISA using targeted peptides from ORF-65 and ORF-K8.1 to detect lytic IgG HHV-8 antibodies found the following were positive: 92% of KS patients, 3% of United States blood donors, and 55% of HIV-positive men (40).
Rabkin et al. (234) and later Enbom et al. (86) did comparative studies of most of the serological methods available at that time and found considerable variations in the specificity and sensitivity of the assays. They found no reliable consistency between all the assays. In Enbom's study, the samples that were positive by the K8.1 ELISA were positive in all the other assays used, but very few samples were positive by this assay. More recently, Schatz et al. (260) compared many second-generation assays plus some novel ones and found much better concordance than had been seen previously. Five panels of serum were sent to seven European laboratories and tested with 18 different assays, 9 of which were used to screen all five panels. The panels contained samples from Uganda, Germany, Switzerland, and Italy, from people both with and without KS, people both positive and negative for HIV-1, and various combinations of these states. Since no “gold standard” for determining positivity for HHV-8 antibodies is yet available, they assumed that all KS patients were infected with HHV-8 because all KS lesions are HHV-8 positive by PCR.
Using this assumption as a basis, they compared the nine assays for specificity and sensitivity by statistical methods. All nine showed excellent concordance with AIDS-KS sera, HIV-1-positive/KS-negative sera, and Italian blood donors. The combination of the lytic IFA from the Laboratory of Virology, Istituto Superiore di Sanità, in Rome and the latent nuclear antigen IFA from the Department of Medical Microbiology of the University of Liverpool was found to be the most specific, but only marginally better than the ELISA ORF-K8.1 and ELISA ORF-K12 from the Institute for Clinical and Medical Virology, University of Erlangen-Nürberg, Germany, and the Munich mix 2 peptide ELISA from the Max von Pettenkofer Institute, Gezentrum, Munich, Germany. For sensitivity in this survey, the Rome lytic IFA and the Liverpool LANA IFA also performed the best, but several other assays proved almost as sensitive. The ORF-K8.1 and ORF-K12 ELISAs, while shown to be very specific, did not prove to be as sensitive as other assays.
TRANSMISSION
PBMCs
HHV-8 can be found in the PBMCs of people carrying HHV-8 (Fig. 4B) (84, 131, 270, 286); in one case, it was even found in the PBMCs of a healthy North American blood donor (24). The likelihood of finding such infected cells varies with the seroprevalence for a particular region. In areas of low prevalence, such as the United Kingdom, France, United States, and Canada, nested PCR turned up no positive PBMCs in healthy individuals (167, 184, 291). HHV-8 was detected in PBMCs in 9% of HIV-1 patients in parts of Italy. In Uganda, a country with a high incidence of classical KS, 14% of the total population had HHV-8 detected in PBMCs (11). HHV-8 cannot be demonstrated in the PBMCs of all patients with classical or AIDS-related KS (Fig. 4) (68), but the cases where it can be found suggest that HHV-8 can replicate in the PBMCs of KS patients. Blasig et al. (25), by immunohistochemical staining and in situ hybridization, found HHV-8 replication in cells of monocytic origin from KS lesions. Evidence for the presence of HHV-8 infection of the PBMCs from other patients has been reported (27, 74, 84, 131, 157, 203, 269, 270, 287, 293).
It is probable that the viral load in the blood of HHV-8-infected subjects may vary considerably depending upon the state of infection, as demonstrated by Sitas et al. (269), who found that HHV-8 peripheral blood viral load and the IgG antibody to HHV-8 were well correlated. Ariyoshi et al. (13) also found that in Gambia, West Africa, KS is less frequent in HIV-2-positive individuals in spite of evidence of HHV-8 infection. In HIV patients with no diagnosis of KS, HHV-8 DNA has been found in 13% (18 of 135) of the PBMCs of the patients studied (198). Furthermore, HHV-8 DNA in PBMCs and lower CD4+ cell counts correlated with elevated plasma levels of HIV mRNA, giving good evidence for the relationship between immunosuppression, HIV replication, and HHV-8 expression (84, 198). After finding a high copy number (9,000 copies) of HHV-8 DNA in the PBMCs of 45% of KS patients studied, several researchers (87, 156) raised the question of why there has been no evidence of blood-borne viral transmission.
Saliva and Mucosal Shedding
Vieira et al. (285) found that HHV-8 DNA recovered from saliva was similar to HHV-8 DNA recovered from the cultured 293 cells that had been infected with saliva fluid and carried for up to 13 passages. PMA treatment of infected 293 cells increased the amount of HHV-8 DNA produced. This study suggests not only that infectious virus is carried in the saliva of men with a history of KS, but also that the virus can be transmitted via saliva.
HHV-8 DNA was detected in the saliva of 75% of HIV-1 patients tested plus in the saliva of one HIV-negative patient (150), where concentrations ranged from 102.4 to 106/ml, but no HHV-8 DNA was found in the saliva of healthy donor controls. This suggests that HHV-8 is transmitted in the saliva like other human herpesviruses (EBV, cytomegalovirus, HHV-6, HHV-7, and human herpes virus simplex type 1) (28, 47, 157, 177, 178). In a study, LaDuca and colleagues (46, 156) quantitated HHV-8 DNA (33,000 copies per μg) in the saliva of 37% of the KS patients studied. HHV-8 DNA was also found in PBMCs of 46% (9,000 copies per μg), plasma of 7% (40 copies per μg), semen of 12% (300 copies per μg), normal skin of 23%, and KS skin lesions of 92% (64,000 copies per μg) of the same KS patients. The finding of high copy numbers of HHV-8 DNA in the saliva makes it a likely route for transmission of the virus.
Mucosal Shedding
HHV-8 DNA was found in oropharyngeal cells in 30% of HHV-8-seropositive males who had sex with other men but in only 1% of their anal and genital samples (227). The DNA PCR copy number from the oral samples was also twice as high as the copy number from the other tissue samples. Pauk et al. (227) concluded that exposure to infectious saliva is a risk factor for infection with HHV-8. Although transmission of HHV-8 via saliva is possible, it is not likely to be common, because even in those infected with HHV-8, only a 15% frequency of salivary virus shedding has been demonstrated. In immunosuppressed individuals, such as those with AIDS, viral shedding via saliva is, in contrast, much more frequent. Lucchini et al. (177) came to similar conclusions about mucosal shedding of HHV-8. These data suggest that oral transmission may be possible, but with so little shedding of HHV-8, the transmission may be difficult. Biggar and Goedert (22) stated that HHV-8 transmission via the oral route may depend on the infectious virus shed and that HHV-8 levels could be low in HHV-8-infected persons who are not HIV positive. This may include homosexual men.
Semen and Prostate Glands
Corbellino et al. (64) found that prostate tissues harbored the HHV-8 genome, suggesting that the prostate is a major site of infection. Later, in situ hybridization of the prostatic glandular epithelium for the presence of a latent HHV-8 gene showed that viral gene expression in prostatic biopsy tissue is common, evident even in some men without KS (274). Furthermore, two studies reported that HHV-8 is preferentially detected in semen rather than spermatocytes, suggesting secretion into seminal fluids (129, 202). Later studies by Diamond et al. (75) measured the quantities of the virus in the seminal secretions. HHV-8 DNA was detected in the nuclei of >90% of the glandular epithelial cells. Solution-based PCRs on prostate ultrasound-guided biopsies from HIV-KS patients and HIV patients without KS were positive for HHV-8 DNA. Histological sections stained by in situ hybridization showed that the infection localized to discrete areas of the prostate. From 1 to 5% expressed mRNA transcripts associated with HHV-8 replication, and 90% expressed the T-0.7 transcript associated with HHV-8 latency. The authors of this study concluded that if these prostate cells shed virus into the semen, this may be a crucial factor in determining whether HHV-8 is transmitted through sexual activity.
HHV-8 DNA was found in 8% of 184 semen samples of HIV-infected individuals by Pellett et al. (228), indicating that semen can carry the virus, albeit in small amounts. This was confirmed by other reports (74, 129, 202), except that of Lin et al. (173), in which a high prevalence of HHV-8 was detected. In a note to Lancet, Lin et al. (174) retracted the previous report by stating that the high incidence of HHV-8 DNA reported previously was probably due to contamination.
Sexual Transmission
On the assumption that HHV-8 can be sexually transmitted, the virus has to be at a higher titer in the semen than in PBMCs. The presence of HHV-8 in the semen of healthy subjects is controversial. Almost all of the studies on semen samples reveal either a very low number of HHV-8 positive samples (0 to 33%) regardless of the KS or HIV status of the patients or the absence of HHV-8 DNA (124, 129, 202, 271, 280). A study (271) of female commercial sex workers in Honduras found that those positive for HIV had an HHV-8 seroprevalence rate four times as high as those that were HIV negative (36% versus 10%). Martin et al. (186), in a multicenter cohort study, found a correlation between HHV-8 infection and the number of male homosexual partners a man had. A study of 130 male homosexuals in Sydney, Australia (123), found that HHV-8-seropositive men reported having more casual sex partners than seronegative men and were also likely to report oroanal contact with these partners, but the numbers were not statistically significant. This study found that in 3 years, 53% of HHV-8-seropositive men developed KS (137, 185).
HHV-8 was detected in the genital tract of women who were HHV-8 seropositive (293). Sexual transmission of HHV-8 was also reported in young men who have sex with other men (76). High-risk sexual behavior and drug use were factors in the incidence of HHV-8 infection in a study of a group of 1,295 women from the United States (40). The independent association of HHV-8 infection with injection drug use suggested that HHV-8 can be transmitted via needle sharing, albeit less efficiently than EBV, hepatitis C virus, or HIV-1. HHV-8 seropositivity increased with the frequency of injection drug use (P < 0.001), e.g., in women who used drugs daily; in women who used cocaine, the HHV-8 seropositivity was three times higher.
If HHV-8 can be transmitted via needle sharing, it should also be able to be transmitted via blood transfusion and through blood derivative products. To date, no studies have been published regarding the potential risk of HHV-8 transmission or its association with disease development. The lack of detectable HHV-8 in the semen of non-KS HIV-infected individuals is highly suggestive that it may be a rare event in transmitting HHV-8 by the genital route in HIV-1 and HHV-8 antibody-positive patients (74).
Transmission during Childhood and Adolescence
There is now enough epidemiologic evidence and laboratory data to support that the high seroprevalence of HHV-8 in eastern Africa and in Egyptian children is due to a nonsexual mode of viral transmission. Furthermore, saliva and close interpersonal contact are the main routes of transmission in these populations (9, 34, 72, 113, 144, 168, 190, 298).
The pattern of familial aggregation between mother and child and among siblings in French Guiana plus the variation with age in the seroprevalence of HHV-8 suggests transmission from mother to child and between siblings in areas of Africa where HHV-8 is endemic (185, 230). Similar evidence of horizontal transmission has been found in the central African country of Cameroon (113). Additional evidence for horizontal transmission from mother to child has been reported in South Africa by Bourboulia et al. (34) and by Andreoni et al. (9) in Egyptian children. In another report, high correlations of seropositivity were found between mother and child and among siblings but not between spouses (230).
Experimental Transmission
Dittmer et al. (77) transmitted HHV-8 to SCID-hu Thy/Liv mice. In spite of the fact that no disease was evident, this model should be useful for the detailed study of HHV-8 latency and tropism and testing of antiviral drugs.
SEROEPIDEMIOLOGY
Many studies (reviewed in reference 58) have been done to measure the prevalence of antibodies to HHV-8. A variety of techniques have been used in these studies, but to date there is no gold standard against which to measure the efficacy of these techniques, so some may overestimate the number of positives, and some may underestimate the number. Nonetheless, certain trends are clear (264). Unlike most human herpesviruses, HHV-8 is not spread universally among all human populations. The highest seroprevalences in healthy populations are found in sub-Saharan Africa, where approximately 40% are positive for antibodies to HHV-8 (1, 264). In black South African blood donors and patients with cancers other than KS, the seroprevalence is 32%; in KS patients, it rises to 83% (269). In countries surrounding the Mediterranean Sea, the seroprevalence averages 10%, although population pockets where the prevalence is higher can be found (7, 10, 58, 66, 264); this may be why classic KS is most often diagnosed in men of Italian or Jewish extraction.
In northern Europe, southeast Asia, and the Caribbean, seroprevalence falls into the 2 to 4% range (1). In Japan, the seroprevalence of HHV-8 antibodies in 1,000 blood donors was measured at 0.2% and rose to 11% in HIV-1-positive patients (n = 276) (102). In northern Europe, the prevalence of HHV-8 and the incidence of KS are so low that HHV-8 antibodies can be used to track the risk for KS. In Amsterdam, HHV-8 antibodies were most often found in HIV-infected men who had recently seroconverted and could be used to predict risk for KS (119). In the French-speaking part of Switzerland, no cases of classic KS were recorded before 1983; thereafter, most KS was found in HIV patients, but the assays used were unable to predict the incidence of KS by testing for HHV-8 antibody (232). The exception for northern Europe is Sweden, where 20% of blood donors were reported to test positive for HHV-8 antibodies; however, the same report shows a wide variety of results depending on the assay used (86).
In the United States, prevalences have been measured in the 5 to 20% range. In populations infected with HIV-1, the prevalence of HHV-8 can rise from 20 to 50% above that in the local healthy population. No rise in prevalence in HIV-1 patients is seen in the Caribbean or southeast Asia, areas where no AIDS-associated KS has been reported (1).
In patients with one of the diseases associated with HHV-8, such as KS, primary effusion lymphoma, and multicentric Castleman's disease, prevalence rates can rise to 100% (58, 260). In contrast, HIV-associated non-Hodgkin's lymphoma, a disease which is not associated with HHV-8, showed no correlation with HHV-8 antibodies (110). In areas of Africa where juvenile KS is endemic, such as Uganda and Zambia, the seroprevalence of HHV-8 is the same as in the Gambia and the Ivory Coast where endemic juvenile KS does not occur (118), suggesting that other factors may be involved in disease expression.
The seroprevalence in children tends to reflect that in the adult population, but in somewhat lower percentages (3). Increase in seroprevalence with age has been found in many studies (7, 246, 269). In central African countries with high seroprevalences, such as Cameroon (112), French Guiana (230), and Uganda (190), seropositivity increased with age and usually reached adult prevalence before puberty. For example, in Cameroon, the overall prevalence for children and young adolescents studied was 27.5% and rose to 48% in those above 15, similar to the rate of 54.4% in pregnant women. Similar results were found in Italy, with seroprevalences in children reflecting those of the surrounding adult population but in somewhat lower percentages that rose with age (292). In Italy, only 2 of 57 infants studied carried antibody, but in older children, the rate rose to 4.4% (292). This clustering is strongly indicative of nonsexual horizontal transmission before puberty.
KAPOSI'S SARCOMA
In 1872, Moriz Kaposi reported several cases of a multifocal pigmented sarcoma in elderly Viennese men (140). These patients had cutaneous lesions, typically on the lower extremities (Fig. 5A). All the patients described by Kaposi eventually died of what is now known as “classic” KS, which predominantly affects older men of Mediterranean and eastern European descent. Since Kaposi's initial description, three more forms of KS have been identified. The second type, endemic-African KS, which is more aggressive than classic KS, may involve lymph nodes in addition to skin and often affects HIV-negative hosts and children (298). A third, iatrogenic form of KS occurs after solid-organ transplantation in patients on immunosuppressive medications. Like classic KS, this form is more common in those of Mediterranean ancestry (62, 97). A fourth and very aggressive type of KS was described in the early 1980s in otherwise healthy homosexual men (100) (Fig. 5B). AIDS-KS not only involves skin (Fig. 5B) and lymph nodes but often disseminates to the lung, gastrointestinal tract, liver, and spleen. Early in the epidemic, lifetime incidence of KS in gay men was around 50% (145), but this incidence has declined in the 1980s and then more precipitously with the introduction of effective HIV therapy in the late 1990s (117, 163).
The four clinical-epidemiological forms of KS have indistinguishable histologic features. KS is composed of a variable mixture of ectatic, irregularly shaped, round capillary and slit-like endothelium-lined vascular spaces and spindle-shaped cells accompanied by a variable inflammatory mononuclear cell infiltrate. Red blood cells and hemosiderin pigment are frequently present, often extravasated between the spindle cells. Small granules of intracytoplasmic or extracellular hyalin material may be identified. Sometimes the earliest patch and plaque stage lesions are difficult to distinguish from granulation tissue. The spindle cells eventually become the predominant cell population, forming fascicles that compress the vascular slits, and the lesions become progressively nodular (63, 99).
The histogenesis of the KS spindle cell has not been easy to trace. Although KS cells stain for certain endothelial cell markers such as CD34+ and factor VIII, some studies show that they express proteins similar to dendritic cells, macrophages, or smooth muscle cells (275). It is debated, therefore, whether KS cells represent a heterogeneous population of cells or, instead, arise from a pluripotent mesenchymal precursor cell. More recent cell surface marker studies suggest that spindle cells may be of lymphatic endothelial cell origin (82). It has also been questioned whether KS represents a clonal, neoplastic process or a polyclonal inflammatory lesion.
In early KS lesions, there are few spindle cells compared to the surrounding inflammatory cells. Furthermore, KS cells in culture are dependent on exogenous growth factors and, when implanted into nude mice, can induce an inflammatory and angiogenic reaction but do not induce tumors as would fully transformed cells (255). Moreover, regression of KS can happen spontaneously or when immunosuppression is corrected. Such characteristics, along with the multifocality of KS lesions, argue that the process is primarily one of dysregulated inflammation. Confusing the picture, however, X chromosome inactivation studies within single lesions as well as comparisons of multiple lesions from a single patient support a clonal origin in a subset of advanced cases (233).
More recent studies have shown varying monoclonality, oligoclonality, and polyclonality from lesions of various patients (114). Furthermore, three neoplastic cell lines have been established from KS lesions (18, 179). A likely possibility is that KS starts as a hyperplastic polyclonal lesion that later gives rise to a clonal cell population only under specific circumstances, such as immunosuppression and selective pressures. KS may be similar to posttransplantation lymphoproliferative disorders, which are EBV-driven B-cell proliferations, which may progress from a polyclonal hyperplasia to monoclonal tumors with no evident genetic abnormalities, to frank malignant lymphomas with oncogene and tumor suppressor gene alterations (148). Figure 4 shows HHV-8 sequences detected by PCR in PBMCs, KS lesion skin, and plasma from KS patients with an HHV-8 primer sequence.
PRIMARY EFFUSION LYMPHOMAS
The first reported cases of malignant lymphoma occurring as body cavity effusions were described as AIDS-associated lymphohematopoietic neoplasms displaying an indeterminate immunophenotype (149). In this report of three cases, two of which were lymphomatous effusions, a B-cell lineage and the presence of EBV were demonstrated by using DNA-based assays. Subsequent studies recognized these lymphomatous effusions as occurring relatively frequently in HIV-infected individuals (120, 288; D. Karcher, F. Dawkins, C. T. Garret, and R. S. Schulof, abstract, Lab. Investig. 66:80, 1992). However, these were thought of as unusual AIDS-related lymphomas, and it only became clear that they represent a quite distinct clinicopathological entity with the recognition of the presence of KSHV within them (50).
The initial study documenting this association revealed KSHV DNA in all AIDS-related lymphomas presenting in body cavities as lymphomatous effusion; therefore, they were initially called body cavity-based lymphomas and, subsequently, PELs. This virus was not found in other AIDS- and non-AIDS-related non-Hodgkin's lymphoma disease or lymphoid leukemia (50). These lymphomas contained very large amounts of viral DNA, ranging between 40 and 80 copies per cell; therefore, this virus was easily identifiable by Southern blot analysis, in contrast to KS tissues, which may contain less than 1 copy per cell, making it necessary to perform PCR for its detection in some cases.
The specific association between KSHV and PEL has been confirmed by multiple investigators (42, 112, 143, 220, 225; A. Carbone, U. Tirelli, A. Gloghini, C. Pastore, E. Vaccher, and G. Gaidano, letter to the editor, Eur. J. Cancer 32A:555-556, 1996). In addition, cases of PEL occurring in HIV-negative men as well as in women have been identified, and these PEL cases also contained KSHV (42, 210, 253; R. G. Nador, E. Cesarman, D. M. Knowles, and J. W. Said, letter to the editor, N. Engl. J. Med. 333:943, 1995). Lymphomas lacking KSHV can, however, involve body cavities as lymphomatous effusions, even in the absence of a tumor mass. Effusions are particularly common in Burkitt's lymphomas but can also be seen in non-Hodgkin's lymphomas; therefore, certain criteria should be used for the diagnosis of PEL (210). In our experience these criteria include (i) presentation as a lymphomatous effusion in the pleural, peritoneal and/or pericardial cavity without a contiguous tumor mass (86%), frequently remaining localized to the body cavity of origin (81%); (ii) morphology bridging large-cell immunoblastic lymphoma and anaplastic large-cell lymphoma (100%) (Fig. 5G); (iii) expression of CD45 and one or more activation-associated antigens (95%) in the frequent absence of B-cell-associated antigens (95%) and immunoglobulin expression (76%); (iv) B-cell origin as demonstrated by the presence of clonal immunoglobulin gene rearrangements (97%); (v) coinfection with Epstein-Barr virus (86%); (vi) lack of c-myc gene rearrangements (97%); and (vii) lack of bcl-2, ras, and p53 gene alterations (87%).
Small series and isolated cases reported by other investigators have shown a similar set of characteristics (220, 225; A. Carbone, U. Tirelli, A. Gloghini, C. Pastore, E. Vaccher, and G. Gaidano, letter to the editor, Eur. J. Cancer 32A:555-556, 1996; D. S. Karcher and S. Alkan, letter to the editor, N. Engl. J. Med. 333:797-798, 1995). AIDS-related lymphomas displaying several of these features should be evaluated for the presence of KSHV/HHV-8 to confirm the diagnosis of PEL. Patients with this type of lymphoma have a very poor clinical outcome, with a median survival of 5 months. PELs are extremely rare tumors, estimated to account for about 3% of AIDS-related lymphomas and 0.4% of all AIDS-unrelated diffuse large-cell non-Hodgkin's lymphomas (42).
An additional complication to the understanding of PELs is the finding that lymphomas containing KSHV can present as solid tissue masses, usually extranodally, similar to other AIDS-related non-Hodgkin's lymphomas. While some of these lymphomas subsequently develop an effusion, others apparently do not. In fact, we have seen several cases that have presented as solid extranodal lymphomas and were diagnosed as diffuse large-cell, immunoblastic, or anaplastic large-cell lymphomas, in which the presence of KSHV in practically all the lymphoma cells could be demonstrated by in situ hybridization and immunohistochemistry (Fig. 5H). In support of the notion that these lymphomas fall in the spectrum of PEL are the observations that they usually lack expression of B-cell antigens and immunoglobulin, have an immunoblastic or anaplastic morphology, and are frequently coinfected with EBV. In addition, a recent epidemiologic study found a statistically significant association between the presence of KS and immunoblastic lymphoma in patients with AIDS (88). Therefore, it appears that KSHV-associated lymphomas represent a distinct pathobiologic category which is frequently, but not exclusively, associated with a lymphomatous effusion, comprising approximately 5% or less of all AIDS-related lymphomas.
Cases of AIDS-related lymphoma which have been positive for KSHV by PCR but not by Southern blot analysis have been identified by us, as well as by others (112, 220), suggesting low viral copy numbers in the tumor tissue. In situ hybridization studies of these cases have shown that the virus is present only in scattered atypical cells and some reactive-appearing cells in these cases (R. G. Nador et al., unpublished data). The cases we identified were not reminiscent of PEL, since they exhibited a polymorphic morphology and expression of B-cell-associated antigens CD19 and CD20. While KSHV may be playing an indirect role in these lymphomas as well, cases containing low copy numbers of KSHV should not be considered part of the spectrum of PEL. Further studies are necessary to determine whether KSHV is acting as an antigenic stimulus or using paracrine mechanisms to stimulate a proliferation of B cells and therefore is involved in the pathogenesis of these lesions, or whether its presence in these lymphomas is merely the result of disseminated viremia in KSHV-positive patients.
Additional cases of lymphoma that do not have the features of PEL but containing KSHV have been identified (161). Lymphomas arising from multicentric Castleman's disease with plasmablastic morphology, lacking EBV, and expressing lambda light chain have been reported (80). In addition, a case of Burkitt's lymphoma was found to contain KSHV by PCR and to occur in a child who had IgG antibody to KSHV lytic antigens (1:640). Therefore, the spectrum of KSHV-associated lymphomas may expand, but these observations await identification of additional similar cases and confirmation by other investigators.
Closer examination of PELs has provided information about the biology of this type of disease and its place in the spectrum of non-Hodgkin's lymphomas. Most cases have been B-cell lymphomas, as determined by the presence of clonal immunoglobulin gene rearrangements. However, two biphenotypic cases expressing CD3 have been identified, both of which contained B- and T-cell antigen receptor gene rearrangements (220, 252), as well as one case with a T-cell phenotype and genotype (164). PELs usually lack expression of B-cell-associated antigens, although some of them have been reported to express monotypic κ or λ mRNA (103, 104), and others have been shown to express surface or cytoplasmic immunoglobulin (210, 220). In our experience, faint but distinct cytoplasmic staining using antisera to κ or λ can be seen in a subset of cells in some PEL cell lines (unpublished observation).
Most PELs are thought to originate from post-germinal center B cells, since they have hypermutation of the immunoglobulin genes (89, 189). In addition to an immunoblastic morphology, PELs have a set of immunophenotypic features that suggest they are at a preterminal stage of B-cell differentiation. Loss of expression of B-cell antigens occurs in plasma cells, and this is a frequent finding in multiple myeloma as well as in immunoblastic lymphomas. Furthermore, most PELs express CD138/syndecan-1, an adhesion molecule that is selectively expressed by a subset of pre-B cells and by plasma cells, including myeloma plasma cells. The expression of CD138 by PELs seems to be quite specific, as it is not expressed by other lymphomatous effusions, primary or secondary, or by most other solid lymphomas (103).
The almost invariable presence of KSHV in lymphomas having the features described above suggests that this virus is necessary for the development of PELs. However, since PELs are so uncommon, even in populations in which the seroprevalence of KSHV is relatively high, it is evident that infection by this virus represents only one of several genetic events involved in their development. One other such factor appears to be EBV infection, as the vast majority of PELs, especially in immunocompromised hosts, contain both viral genomes. The specific role of each of these viruses and their interaction is still poorly understood, but analysis of the genes expressed by both of them has shed some light on their possible roles. Both herpesviruses can be lytic or latent, expressing distinct subsets of genes. PELs in vivo as well as in culture (see below) express mostly latent genes, but there is always a small proportion of cells in which EBV and KSHV lytic gene expression occurs. However, most cells have a latent pattern of gene expression.
It is known that EBV can establish different types of latency. Latency type I (restricted latent gene expression) is seen in Burkitt's lymphomas, while latency type III (full pattern of latent gene expression) is seen in lymphoblastoid cell lines and large-cell lymphomas in immunocompromised patients, particularly those with immunoblastic features. While the EBNA1 gene, necessary for EBV replication, is expressed in all latency types, other latent genes, including the EBV transforming genes LMP1 and EBNA 2, are only expressed in latencies type II (LMP1) and type III (LMP1 and all EBNAs). It is thought that expression of these transforming genes in Burkitt's lymphomas is not “necessary,” since these carry a translocated c-myc oncogene, and the lack of expression of these immunogenic proteins further provides this type of lymphoma with an advantage to evade the immune system.
Analysis of the pattern of EBV gene expression in PELs revealed that only EBNA1 was expressed, corresponding to type I latency (128, 276). This was an unexpected finding, given the resemblance of PEL cells to immunoblastic lymphoma cells. This observation suggests that KSHV is playing a transforming role in PELs, as the major EBV oncogenes are not expressed. Also consistent with this hypothesis is the lack of structural alterations in the cellular transforming genes frequently involved in malignant lymphomas. In particular, c-myc gene rearrangements have been identified in only one case (E. Cesarman, unpublished observation); they are extremely uncommon in contrast to other lymphomatous effusions and EBV-associated lymphomas, where alterations in this gene are a frequent finding.
While mutations of the p53 gene have been identified in only one case of PEL (210), mutations in the noncoding, presumably regulatory region of the BCL-6 gene have been reported in a significant proportion of cases (41). The BCL-6 gene is frequently mutated in diffuse large B-cell lymphomas, although the functional significance of these mutations remains unclear. Cytogenetic analyses have not provided additional concrete evidence for the presence of a single specific genetic alteration. Among nine PEL cell lines examined, it appears that numerous abnormalities are present, with complex hyperdiploid karyotypes. Frequent abnormalities are trisomy 7, trisomy 12, and aberrations of chromosomal bands 1q21-q25 (79, 148). Translocations specific to other lymphoma types have not been identified.
MULTICENTRIC CASTLEMAN’S DISEASE
KSHV has also been found to be present in MCD (54, 272; A. Gessain, A. Sudaka, J. Brière, N. Fouchard, M.-A. Nicola, B. Rio, M. Arborio, X. Troussard, J. Ausouin, J. Diebold, and G. de Thé, letter to the editor, Blood 87:414-416, 1995). Multicentric Castleman's disease is a poorly understood atypical lymphoproliferative disorder, thought to be related to immune dysregulation (101). Castleman's disease is usually described as a polyclonal, nonneoplastic disorder. It presents in two distinct histopathologic subtypes with different clinical characteristics. The first and more common is the hyaline vascular type, which presents as a solitary mass that is usually curable surgically. The second is the plasma cell type, which is associated with more generalized lymphadenopathy and immunological abnormalities. This disease occurs more frequently in older individuals and is more common in men. The patients usually present with multiple lymphadenopathies, this giving rise to the name “multicentric” Castleman's disease, and a variety of constitutional symptoms. They may develop autoimmune phenomena, cytopenias, skin rashes, and intercurrent infections. Patients with MCD frequently develop malignancies, most commonly KS and non-Hodgkin's lymphoma (272).
KSHV is present in almost all cases of MCD occurring in patients with AIDS. The identification of KSHV in AIDS- and non-AIDS-associated MCD supports an even closer relationship between MCD and KS than previously hypothesized, as in both KSHV may be playing a crucial role. In fact, in HIV-positive patients, MCD has been found to be frequently associated with KS and is usually observed in men infected with HIV by sexual contact (217). Notably, MCD, also called multicentric angiofollicular hyperplasia, is characterized by vascular proliferation in the germinal centers which is reminiscent of KS. However, this virus is present in MCD in patients with AIDS whether accompanied by KS or not. Furthermore, KSHV has also been identified in approximately half of the cases of MCD occurring in HIV-negative individuals, suggesting that it is not a coincidental association occurring in HIV-positive individuals. KSHV sequences have also been detected in PBMCs of patients with MCD (272).
The role of KSHV in the pathobiology of MCD remains poorly understood, and it is not clear whether the KSHV-positive cases differ clinically from KSHV-negative ones in individuals without AIDS. It has been shown that the germinal centers of hyperplastic lymph nodes of patients with Castleman's disease produce large quantities of IL-6. This may explain the large proportion of plasma cells in these lymph nodes, since IL-6 induces B-cell differentiation. Notably, KSHV encodes a viral IL-6 homologue, which is also expressed in MCD in scattered cells surrounding the lymphoid follicles (204, 223). KSHV has been found in mantle zone large immunoblastic B cells by using an ORF-73 monoclonal antibody (82). These cells are monotypic for lambda light chain and can sometimes proliferate to give rise to monoclonal expansions and frank lymphomas, which are called plasmablastic lymphomas and are distinct from PEL (80).
EVIDENCE OF HHV-8 IN OTHER DISEASES
The following diseases have been associated with KSHV, but many of these associations have been disputed in the literature.
HHV-8 in Patients with Pemphigus
Pemphigus vulgaris and pemphigus foliaceus are autoimmune diseases of the skin characterized by separation of the dermis and epidermis; the origin is unknown, but KS is the most frequent malignancy observed in pemphigus patients (284). A group studying pemphigus patients without HIV in Texas (193, 194) found HHV-8 DNA in the lesional skin of four of six patients with pemphigus vulgaris and six of six patients with pemphigus foliaceus who were studied. The PCR DNA sequences differed among all these patients. No HHV-8 DNA was found in normal skin from 10 controls tested from the same area. Patients with pemphigus vulgaris have a more frequent incidence of KS than those with pemphigus foliaceus.
It has been suggested that HHV-8 has a tropism for pemphigus lesions. Researchers in New Mexico have found a high incidence of pemphigus in a New Mexican population, identified by self-identification and HLA typing as descendants of Sephardic Jews who came to the southwestern United States to avoid persecution during the Spanish Inquisition (29). It could well be that the patients from Texas have a similar heritage and that there is no cause and effect between HHV-8 and pemphigus, but both have a high frequency in this unique population that traces its origin to the Mediterranean basin. Other investigators have failed to identify an association between KSHV infection and pemphigus (20, 45, 81, 83).
KS in Association with Bullous Phemigoid
At least five cases have been reported in which KS has developed in association with bullous phemigoid (91, 109, 284). With the exception of one case, these were not studied for the presence of HHV-8. In the case that was investigated (109), KS developed during a 3-year period of radiotherapy for bullous phemigoid. HHV-8 DNA sequences were found in two separate KS lesions but not in control skin from the same patient. The immunosuppressive therapy that the bullous phemigoid patient received could have activated HHV-8, which led to the development of KS.
Presence of HHV-8 in Other Skin Diseases
HHV-8 has also been found in a variety of skin tumors. In a study of 69 patients who were HIV positive or who were immunosuppressed following organ transplantation, many subsequently developed skin tumors. Among those that had premalignant Bowen's disease, 71.4% had HHV-8 DNA; 50% of those who developed squamous cell carcinoma were positive for HHV-8 DNA; and 33.3% of those with actinic keratosis, a malignant epidermal disorder, were positive. A lower frequency, 16.7%, was found in those who developed extramammary Paget's disease, in both proliferative and nonproliferative lesions (133, 237). Similar associations were reported by a second group (216). These studies suggested that HHV-8 infection may increase the likelihood of proliferative skin disease. However, several additional well-controlled studies failed to confirm this association (32, 151, 162).
In a study of HIV-1 carriers in Thailand (16), HHV-8 DNA was found in 25% of those with skin diseases and only 7.4% with no skin involvement. Carriers with antibodies to lytic HHV-8 antigens also had low CD4 and CD8 counts, and specific HHV-8 polypeptides with a molecular mass of 34,000 to 40,000 Da were identified by immunoprecipitation. This adds to the body of evidence linking HHV-8 to skin disorders in the presence of immunosuppression.
An association between KSHV and some angiosarcomas has also been reported. In one study, 7 of 24 patients with angiosarcoma studied plus one of five who had hemangioma, none of whom had systemic immunosuppression, were positive for HHV-8 DNA. The association of these two diseases involving endothelial cells was the first evidence for disease associated with HHV-8 other than KS that does not require immune suppression (191). HHV-8 DNA was detected in vascular neoplasms, which are endothelial in origin. HHV-8 DNA was found only in hemangioma (1 of 20) and angiosarcoma (7 of 24). While these studies indicated a tropism of HHV-8 for endothelial cells, they did not demonstrate whether HHV-8 contributes actively to the pathology or is just a passenger virus. Although angiosarcoma and KS are different diseases, these tumors have a common histiogenesis from within the vascular compartment. Other investigators have failed to identify HHV-8 in angiosarcomas, casting doubts on the specificity of this association (12, 136, 160, 171, 187, 221, 277, 287).
HHV-8 in Salivary Gland Tumors
It is common for healthy individuals to carry a latent infection of endemic human herpesviruses in their salivary glands, and shedding of EBV, cytomegalovirus, HHV-6, and HHV-7 during reactivation is well documented. Even though HHV-8 is not as widespread as other human herpesviruses, it has been detected in the saliva of HIV-1-infected individuals (285), leading to the supposition that HHV-8 could spread via saliva as well as by sexual routes. Even though HHV-8 has been found in saliva, to date there has been only one identification of HHV-8 DNA by PCR in bilateral mucosa-associated lymphoid tissue lymphoma of the parotid gland of a female patient with Sjögren's syndrome (147); therefore, it is unlikely HHV-8 plays any etiological role in vascular or epithelial neoplasms in immunocompromised patients.
HHV-8 in Nonneoplastic Lymphadenopathies and Chronic Interstitial Pneumonitis
HHV-8 was localized in lymphoid and monocyte-macrophage cells scattered in the interfollicular regions of lymph nodes but not in the endothelial cells of two patients with reactive lymphadenopathy (283). In another patient with chronic interstitial pneumonitis characterized by florid hyperplasia, HHV-8 has been found in inflammatory cells infiltrating the intervalvular interstitium of lung tissue, in endothelial cells of the pulmonary vasculature, and rarely in pneumocytes (283). This study indicates that HHV-8 can infect nonneoplastic lymph nodes of immunocompetent individuals. HHV-8 has also been associated with interstitial pneumonitis in HIV-infected patients (207).
KS and Sarcoidosis Coexistence in Lesions of an HIV-Seronegative Patient
Sarcoidosis has been described as a systemic disorder characterized by the presence in multiple tissues of noncaseating epithelioid cell granulomas, which may spontaneously resolve or convert to hyaline connective tissue. The etiology of sarcoidosis is unknown. HHV-8 DNA was found in a wide range of sarcoid but not nonsarcoid tissues (73). The finding of a high frequency of HHV-8 DNA was attributed to PCR cross-contamination.
A diagnosis of KS coexisting in the same lesion as a sarcoidosis, a disease characterized by suppressed cell-mediated immunity, was made in an HIV-negative patient. The biopsy of the newly disrupted skin lesion revealed a mid-dermal tumor composed of irregularly shaped vascular spaces, proliferation of spindle-shaped cells, and extravasated erythrocytes typical of KS, plus multiple noncaseating tuberculoid granulomas. HHV-8 was detected in this lesion by PCR (71).
HHV-8 in Kikuchi's Disease
Kikuchi's disease (histocytic necrotizing lymphadenitis) is a common self-limited disorder of the cervical lymph nodes that occurs predominantly in young Asian females. It is characterized by fever, flu-like symptoms, elevated erythrocytes, neuropenia, an elevated sedimentation rate, and lymphocytosis. Huh et al. (130) found HHV-8 DNA by PCR followed by hybridization and Southern blot analysis in six samples of archival tissues from 26 patients with Kikuchi's disease. The authors concluded that HHV-8 may be an important factor in the pathogenesis of Kikuchi's disease. However, this result has not been confirmed by other researchers.
Multiple Myeloma and HHV-8 Infection
Initial studies evaluating the presence of KSHV in a large series of lymphoid proliferations failed to identify this virus in multiple myeloma or other plasma cell malignancies (50, 112, 220, 225). However, an association between this virus and multiple myeloma was subsequently reported (19, 244). In that study, KSHV DNA and RNA sequences were detected in bone marrow stromal cell cultures from patients with multiple myeloma as well as two of eight patients with monoclonal gammopathy of undetermined significance (MGUS). This result, which was based on PCR and RT-PCR analyses, was subsequently confirmed by the same team using in situ hybridization and PCR analysis of DNA extracted from fresh bone marrow core biopsies (244, 249). Since transcripts of KSHV vIL-6 were detected, it was hypothesized that KSHV uses a paracrine mechanism through production of this cytokine to stimulate the proliferation of the myeloma plasma cells. Multiple studies have been published since, with conflicting results. Several additional independent laboratories confirmed the specific presence of KSHV DNA sequences in multiple myeloma biopsies (4, 60, 238; P. Brousette, F. Meggetto, M. Attal, and G. Delsol, Letter, Science 278:1972, 1997; M. B. Rettig, J. W. Said, R. Sun, R. A. Vescio, and J. R. Berenson, Author's Reply, Science 278:1972-1973, 1997). However, other investigators have been unable to confirm this association when looking for KSHV DNA by PCR in bone marrow biopsies and/or dendritic cell cultures from bone marrow or peripheral blood (35, 44, 69, 188, 200, 218, 224, 229, 278, 279, 282, 296).
Serologic studies have been almost uniform in their findings, demonstrating that patients with multiple myeloma lack antibodies to KSHV (44, 188, 200, 224, 256, 290; J. MacKenzie, J. Sheldon, G. Morgan, G. Cook, T. F. Schulz, and R. F. Jarrett, letter to the editor, Lancet 350:1144-1145, 1997; A. G. Marcelin, N. Dupin, D. Bouscary, P. Bossi, P. Cacoub, P. Ravaud, and V. Calvez, letter to the editor, Lancet 350:1144, 1997). This lack of reactivity is not due to generalized immunosuppression, since antibodies to other more ubiquitous herpesviruses, such as EBV, HHV-6, and cytomegalovirus, are easily detectable in these patients by using comparable methodologies. Since MGUS patients are known to develop multiple myeloma or other lymphoproliferative diseases within 10 to 20 years (152), the serum of MGUS patients, some of whom later developed multiple myeloma, was tested for the presence of HHV-8 IgG antibodies to both lytic and latent antigens (2). Even though the majority of these sera were positive for EBV antibody, no difference was found among normal blood donors, MGUS patients, and multiple myeloma patients for HHV-8 antibody.
Our own group has also been unable to confirm the association between KSHV infection and multiple myeloma, using a variety of methods (222; E. Cesarman, unpublished observation). In one paper, very weak seroreactivity to KSHV antigens was detected by immunoblotting (106). While this may be confirmatory of the association between KSHV infection and multiple myeloma, it is possible that this low reactivity represents higher levels of nonspecific cross-reactivity than in control patients because of the gammopathy in multiple myeloma patients. Furthermore, the epidemiologic patterns of KS and multiple myeloma are distinct, suggesting different etiologies (65, 127). Three explanations can be proposed to explain this controversy. (i) KSHV is not present in multiple myeloma or MGUS, and the identification of viral sequences by a fraction of investigators is due to technical artifacts, such as PCR contamination. (ii) KSHV is present in such low copy numbers in bone marrow and dendritic cell preparations in patients with multiple myeloma that the technique used by most laboratories is not sufficiently sensitive to detect them, although in several studies sensitivity controls suggest detection of 1 to 10 copies of KSHV. In this instance, the absent or very low serologic reactivity remains to be explained; it has been hypothesized that HHV-8 localization within the bone marrow-based dendritic cells may prevent the maturation of an antigenic response due to B-cell tolerance of antigens presented in the bone marrow (251). This reasoning would not explain, however, the discrepant epidemiology of both diseases. (iii) A different virus is present in multiple myeloma, similar enough to be recognized by some PCR primers when certain protocols with low stringency are used. Antibodies to this novel virus may not recognize most KSHV antigens (2). This explanation may be supported by sequence analysis of PCR products from specimens obtained from patients with multiple myeloma, where significant interpatient variation has been noted.
There is higher reported homology in the sequences among different myeloma patients than when these sequences are compared with the KSHV sequences for KS specimens (251). One study has suggested that sequences homologous to one of the regions of KSHV are widely disseminated in a variety of tissues, including multiple myeloma (282). While this last possibility appears to be the most conciliatory explanation, much additional evidence needs to be provided to confirm the presence of a distinct KSHV-like virus and then to confirm its specific association with multiple myeloma and MGUS. In addition, a study using degenerate primers failed to detect novel herpesvirus sequences in multiple myeloma, suggesting that the presence of a novel human herpesvirus in this disease is not likely (78).
HHV-8 in Hemophagocytic Syndrome with KSHV-Related Effusion Lymphomanone
Novel association of HHV-8 with hemophagocytic syndrome (HPS) has been reported. HPS is a fulminant, fatal, systemic illness that occurs in association with infection and malignancy. HPS is characterized as an infiltration of the reticuloendothelial system by histocytes that engulf and destroy the formed elements of blood. Patients with HPS suffer from systemic illness with fever, adenopathy, hepatosplenomegaly, liver failure, cytopenia, and coagulopathy. HPS is generally associated with AIDS patients in advanced stages of the disease. Examination of the spleen of an HPS patient revealed HHV-8 DNA by PCR. No EBV DNA was detected (226). Another case of HPS was reported by Low et al. (176), in which the patient had HHV-8 infection. After he was treated with the antiviral agent foscarnet, the patient improved dramatically. This suggested that foscarnet, an antiherpesvirus agent, blocked in vivo HHV-8 replication. More cases of HPS are needed to confirm the association with HHV-8.
TRANSPLANTATION RISKS
There is a 1 to 3% risk of KS with renal transplantation (95), with the median interval to diagnosis of KS being 29 to 31 months (11, 235). In Saudi Arabia, where there is a higher prevalency of HHV-8, the transplantation risk of KS rises to 3 to 5% (11). Cattani et al. (47) assessed the risk of development of KS in pre- and postrenal transplantation. Overall, 23% of the patients who were HHV-8 positive before transplantation developed KS, whereas only 0.7% of seronegative control patients developed the disease. Posttransplantation KS has also been associated with transplantation of heart and lungs (6, 90, 223, 240). However, it is still unclear whether posttransplantation KS is due to the reactivation of HHV-8. Most KS was from reactivation of latent HHV-8 infection due to immunosuppression rather than from new infection from the transplanted organ or transfusion.
Studies of renal transplant patients in regions of high HHV-8 prevalency reveal that the immunosuppression treatment that precedes transplantation can activate latent virus and lead to iatrogenic KS (47, 96, 231). Therefore, in these areas, serological screening of the recipients is as important as screening of donors. Although most cases of transplant KS seem to be due to reactivation of virus in the recipient, cases of KS being transmitted from a donor organ have been reported (96, 240). Serological and molecular testing on renal transplant patients has demonstrated persistent reactivation of HHV-8, which can lead to KS (132).
HHV-8 infection has been cited as a cause of bone marrow transplant failure (180). A primary HHV-8 infection developed in two patients after both received kidneys from a seropositive cadaver donor. The patients developed disseminated KS after experiencing an acute syndrome characterized by fever with plasmacytosis. A case of HHV-8 viremia was reported after transplantation of autologous peripheral stem cells in an HHV-8-seropositive patient with non-Hodgkin's lymphoma, but there was no evidence of other infection. The reactivation manifested itself with a fever and marrow anaplasia with plasmacytosis. HHV-8 latent antigen and transcripts were expressed in the aplastic marrow but not in two normal marrow samples.
It seems clear there is a high risk of KS associated with transplants due to the immunosuppression of the recipient, regardless of whether the HHV-8 is from the patient, the donor, or a blood transfusion (125). HHV-8 DNA was found in kidney allografts in two of three transplant recipients prior to the development of KS. An increase in the level of HHV-8 DNA was detected in the third patient. This study suggested that direct genotypic and serologic analyses in kidney allografts in primary infection or increased viral antibody can predict the development of KS. Therefore, monitoring the transplant recipient for HHV-8 infection could be used to provide effective antiviral therapy (259).
ANTIVIRAL THERAPY
Inhibitors of herpesvirus DNA polymerase are effective in combating lytic but not latent DNA infection. Foscarnet and ganciclovir induced regression of KS lesions in a small trial of HIV-infected patients and in three large follow-up studies (48). In spite of these encouraging results, no change in the number of PBMCs infected with HHV-8 was found. HHV-8 was very sensitive to cidofovir when tested in vitro, whereas HHV-8 was only moderately sensitive to foscarnet and ganciclovir (146, 192). Therefore, low doses of cidofovir or a high dose of foscarnet or ganciclovir could suppress clinical reactivation of HHV-8. These antiviral drugs did not inhibit episomal virus DNA polymerase, suggesting that the latent form of viral DNA is replicated by host DNA polymerase (192).
Foscarnet is known to be very toxic, and therefore, clinical dosages for this drug must be worked out for each patient. Although acyclovir has been very effective in preventing EBV (gamma-1 herpesvirus) infection of oral hairy leukoplakia (243) in AIDS patients, it has shown no such efficacy against HHV-8. Since these drugs work on the level of the viral polymerase, they are effective only in combating actively replicating virus and have no effect on the latent stage of infection. While the latent virus is not likely to cause damage, people at risk for virus reactivation, such as AIDS patients, should be monitored so that effective therapy can be instituted if the virus becomes active.
The effect of antiretroviral therapy and the use of zidovudine to prevent perinatal transmission were also reported (8, 139, 159, 199). Boivin et al. (27) showed that one AIDS patient with KS had a low viral load in KS skin lesions and PBMCs while on highly active antiretroviral therapy (HAART), suggesting a strong relationship between tumor burden and HHV-8 viral load in spite of HAART's having no direct anti-HHV-8 activity (138). Antitumor activity of fractionated doses of oral etoposide in the treatment of AIDS-related KS was reported (273), with a significant reduction in KS at a manageable clinical toxicity of the drug. These investigators, however, did not measure the effect on HHV-8.
The institution of HAART triple-drug combination therapy has correlated with a decrease in the incidence of AIDS-related KS (128). Cattelan et al. (48) studied AIDS-KS patients after they had been placed on the HAART regimen. Reduction in anti-ORF-65 antibody correlated with clinical improvements, but LNA showed a variable pattern. Decrease in plasma HIV-1 RNA levels and an increase in CD4 lymphocytes due to antiviral therapy with nucleotide analogs and protease inhibitors correlated with a regression of KS lesions.
Another antiviral therapy of HIV-1 also had an ameliorative effect on AIDS-associated KS (266). Topical treatment with 10% docosanel cream inhibits a broad spectrum of enveloped viruses in vitro, including herpes simplex virus types 1 and 2, cytomegalovirus, HHV-6, and HIV-1; KS lesions were reduced by 20%, and no treated patients experienced KS disease progression. In this study, no attempt was made to measure or detect HHV-8.
CONCLUSIONS AND POINTS FOR FUTURE RESEARCH
It took more than 20 years after EBV was found for another human herpesvirus to be discovered. HHV-6 was discovered in 1986 (254), followed 4 years later by the discovery in 1990 of HHV-7 (98); and in another 4 years, 1994, Chang et al. (56) detected the sequence for KSHV/HHV-8 in KS lesions by representational difference analysis. From serological studies, we have learned that HHV-8 distinguishes itself from other human herpesviruses in that it is endemic in a very restricted geographical area—sub-Saharan Africa—as well as in pockets of Italy, other Mediterranean countries, and eastern Europe. These areas also have a high incidence of KS.
The methods of HHV-8 transmission reflect the pattern of prevalence as well. In areas where HHV-8 is endemic, horizontal transmission has been documented, but in the rest of the world, transmission is mainly by sexual routes, even though very low viral counts in the semen of infected men have been documented. No KS or other HHV-8-associated diseases have been found in the southeast Asian countries of India, Thailand, Malaysia, and Sri Lanka. Low rates of seroprevalence have been found in these countries, especially associated with the HIV-infected population; this suggests that the virus may have been newly introduced into these areas, and those who are infected may be at risk for disease development. Alternatively, there may be some factor in addition to HHV-8 that is necessary for KS to develop, and that factor may be missing in southeast Asia. Since the general population in most of the world is free of the virus and there is documented risk of disease associated with HHV-8 infection from transfusion and organ transplantation, it has been suggested that the blood supply should be screened for the presence of HHV-8, as is presently done for HIV and HTLV.
Like the other human gammaherpesvirus, EBV, HHV-8 DNA sequences have been associated with tumorigenesis. HHV-8 sequences have been found in the atypical tumor cells of KS, MCD, and PEL, making a strong case for the pathogenicity of the virus. When EBV was first discovered, it was associated only with infectious mononucleosis and endemic Burkitt's lymphoma. HHV-8 is always found in patients with HIV who develop MCD, but cases of MCD have been found in HIV-negative patients in whom no HHV-8 can be detected. This is reminiscent of cases of nonendemic Burkitt's lymphoma in which no EBV is found. Since EBV was discovered, more sensitive diagnostic techniques have been developed, and investigators have been able to link EBV to a host of additional tumorigenic diseases. We can expect similar findings to occur for many of the diseases listed above where HHV-8 has been found, but its role, if any, in these diseases has not been clarified.
Many mysteries surrounding this virus have yet to be solved. Even where it has been established that HHV-8 is necessary for disease development, its role in pathogenesis is not entirely clear. Rabkin et al. (233) found that KS is a monoclonal tumor, but this finding remains controversial because many KS lesions are known to be polyclonal (114). Also, the link between multiple myeloma and HHV-8 remains in dispute, especially since it cannot be supported by serology. The techniques used to link HHV-8 to multiple myeloma leave open the possibility that perhaps a new human herpesvirus is waiting to be found, although this possibility appears unlikely based on our current knowledge. Even in KS, where the link between HHV-8 and disease is the strongest, it has been shown that while HHV-8 is necessary, it is not sufficient in itself to cause KS.
The disease is caused by a cascade involving the tat gene and various cytokines that combine to keep the tumor state intact (105). Is HHV-8 just the trigger for KS, or is the virus necessary to maintain the cytokines that feed the tumor? To illustrate this quandary, a few examples are in order. HIV-1 tat can indirectly affect tumor cell proliferation in KS via induction of cellular growth and angiogenic factors or indirectly by the activation of transcription regulation with cytokines such as NF-κB (21). tat transgenic male mice that are CD4+-T-cell depleted show much faster tumor growth when infected with tumor cells than similar animals that lack tat. Many transformed cell lines derived from KS lesions, such as SLK cells, lack any HHV-8 DNA and appear not to be derived from KS spindle cells (289).
The KS lesion is complex in its cellular composition, origin, epidemiology, and pathogenesis. HHV-8 may promote cell growth by augmentation of cell growth-promoting cytokines. Robert Gallo (105) describes KS as starting as a hyperplasia that may evolve into malignant clones in some individuals. Patients on HAART see a regression in their KS tumors, making KS one of the few tumors that can be controlled with medication; yet HAART has little if any antiviral effect on HHV-8 itself.
The search for antiviral drugs has been hampered by the fact that no fully permissive cell line has been found so that biological assays can be conducted. HHV-8 will infect microvascular endothelial cells, but most laboratories have not found these cells convenient to work with. On the other hand, many serological assays have been developed with various degrees of sensitivity and specificity, but no assay has yet been developed that detects both lytic and latent antibodies in one assay. Furthermore, most laboratories seem to prefer to use their own in-house testing methods, so that there is, as yet, no overall consensus on which testing methods are best. Better and more comprehensive testing methods are being developed, and these may use a combination of antigens made of synthetic or recombinant proteins.
The future for HHV-8 research is very bright. In the few years since its discovery, a lot has been learned about its biology, its association with disease, and the function of some of its novel genes. These novel genes include some that are expressed during latency, such as D-type cyclin homologue, LANA-1 and LANA-2 (vIRF-3), kaposin and vFLIP. Other genes are expressed as early lytic genes, and by a variable proportion of latently infected cells, including vIL-6, other viral homologues of interferon regulatory factor (K9 and K11.1), K1, K15 (LAMP), and vGPCR. Many other functional and structural lytic genes have been characterized, like ORF-50, ORF-65, and ORF-K8.1. Important reagents, including monoclonal antibodies, have been developed for several of these virally encoded proteins (reviewed in reference 264). Only time will tell what applications will be derived from this basic research and how it will benefit patients who are at risk for developing HHV-8-associated disease.
Since the prevalence of HHV-8 in the general population is quite low but HIV-1-infected individuals are at a higher risk for developing HHV-8-associated malignancies, would a vaccine be beneficial for those at risk?
Acknowledgments
Figures 5A and 5B were kindly provided by A. Friedman-Kien, New York University Medical Center, New York, N.Y. Figures 5C to I were provided by Amy Chadburn and Elizabeth Hyjek (Weill Medical College of Cornell University, New York, N.Y.). Figures 5J and L were stained with the human KS serum kindly provided by Parkash Gill, Norris Cancer Research Center, University of Southern California, Los Angeles. Figure 4, HHV-8 DNA PCR, was provided by Rizwan Masood, University of Southern California, Los Angeles. We acknowledge the assistance of Ruth Weinroth, Phyllis Kushner, and Candy D'Adamo in the preparation of this article. The editorial assistance of Kristine Ablashi is greatly appreciated. The technical assistance from ABI of Carole Owen and Malgorzata Roman with KSHV DNA PCR and IFA photographs is acknowledged.
REFERENCES
- 1.Ablashi, D. V., L. G. Chatlynne, H. C. Cooper, D. A. Thomas, M. Yadav, A. W. Norhanom, A. K. Chandana, V. Churdboonchart, S. A. R. Kulpradist, M. Patnaik, K. Liegmann, R. Masood, M. Reitz, F. Cleghorn, A. Manns, P. H. Levine, C. S. Rabkin, R. Biggar, F. Jensen, P. S. Gill, N. Jack, J. Edwards, J. E. Whitman, and C. Boshoff. 1999. Seroprevalence of human herpesvirus 8 (HHV-8) in countries of Southeast Asia compared to the United States, the Caribbean, and Africa. Br. J. Cancer 81:893-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ablashi, D. V., L. Chatlynne, D. Thomas, D. Bourboulia, M. B. Rettig, R. A. Vescio, D. Viza, P. Gill, R. A. Kyle, J. R. Berenson, and J. E. Whitman, Jr. 2000. Lack of serologic association of human herpesvirus-8 (KSHV) in patients with monoclonal gammopathy of undetermined significance with and without progression to multiple myeloma. Blood 96:2304-2306. [PubMed] [Google Scholar]
- 3.Ablashi, D. V., L. Chatlynne, D. Thomas, C.-R. Lee, M. Roman, D. Bourboulia, S. Yoksan, C. Hall, R. Biggar, A. W. Norhanom, T. Yoshikawa, M. Margalith, and J. E. Whitman. 2000. Prevalence of HHV-8 (KSHV) latent and lytic IgG antibody in children: an international study. J. Hum. Virol. 3:261. [Google Scholar]
- 4.Agbalika, F., X. Mariette, J.-P. Marolleau, J.-P. Fermand, and J.-C. Brouet. 1998. Detection of human herpesvirus-8 DNA in bone marrow biopsies from patients with multiple myeloma and Waldenstrom's macroglobulinemia. Blood 91:4393-4394. [PubMed] [Google Scholar]
- 5.Alexander, L., L. Denekamp, A. Knapp, M. R. Auerbach, B. Damania, and R. C. Desrosiers. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 74:3388-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allain, J.-P. 2000. Emerging viral infections relevant to transfusion medicine. Blood 14:173-181. [DOI] [PubMed] [Google Scholar]
- 7.Almuneef, M., S. Nimjee, K. Khoshnood, G. Miller, and M. O. Rigsby. 2001. Prevalence of antibodies to human herpesvirus 8 (HHV-8) in Saudi Arabian patients with and without renal failure. Transplantation 71:1120-1124. [DOI] [PubMed] [Google Scholar]
- 8.Anderson, J. E., R. W. Wilson, P. Barker, L. Doll, T. S. Jones, and D. Holtgrave. 1999. Prevalence of sexual and drug-related HIV risk behaviors in the U.S. adult population: results of the 1996 National Household Survey on Drug Abuse. J. Acquir. Immune Defic. Syndr. 21:148-156. [PubMed] [Google Scholar]
- 9.Andreoni, M., G. El-Sawaf, G. Rezza, B. Ensoli, E. Nicastri, L. Ventura, L. Ercoli, L. Sarmati, and G. Rocchi. 1999. High seroprevalence of antibodies to human herpesvirus-8 in Egyptian children: evidence of nonsexual transmission. J. Natl. Cancer Inst. 91:465-469. [DOI] [PubMed] [Google Scholar]
- 10.Angeloni, A., L. Heston, S. Uccini, M. C. Sirianni, F. Cottoni, M. V. Masala, D. Cerimele, S. F. Lin, R. Sun, M. Rigsby, A. Faggioni, and G. Miller. 1998. High prevalence of antibodies to human herpesvirus 8 in relatives of patients with classic Kaposi's sarcoma from Sardinia. J. Infect. Dis. 177:1715-1718. [DOI] [PubMed] [Google Scholar]
- 11.Antman, K., and Y. Chang. 2000. Kaposi's sarcoma. N. Engl. J. Med. 342:1027-1038. [DOI] [PubMed] [Google Scholar]
- 12.Arber, D. A., J. G. Strickler, Y. Y. Chen, and L. M. Weiss. 1997. Splenic vascular tumors: a histologic, immunophenotypic, and virologic study. Am. J. Surg. Pathol. 21:827-835. [DOI] [PubMed] [Google Scholar]
- 13.Ariyoshi, K., M. Schim van der Loeff, P. Cook, D. Whitby, T. Corrah, S. Jaffar, F. Cham, S. Sabally, D. O'Donovan, R. A. Weiss, T. F. Schulz, and H. Whittle. 1998. Kaposi's sarcoma in Gambia, West Africa, is less frequent in human immunodeficiency virus type2 than in human immunodeficiency virus type 1 infection despite a high prevalence of human herpesvirus 8. J. Hum. Virol. 1:193-199. [PubMed] [Google Scholar]
- 14.Arvanitakis, L., E. A. Mesri, R. G. Nador, J. W. Said, A. S. Asch, D. M. Knowles, and E. Cesarman. 1996. Establishment and characterization of a primary effusion (body cavity based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 88:2648-2654. [PubMed] [Google Scholar]
- 15.Arvanitakis, L., E. Geras-Raaka, M. C. Gershengorn, and E. Cesarman. 1997. Human herpesvirus KSHV encodes a constitutively active G protein-coupled receptor linked to cell proliferation. Nature 385:347-350. [DOI] [PubMed] [Google Scholar]
- 16.Ayuthaya, N. P. I., R. Inagi, W. Auwanit, P. Warachit, P. Kullavanijaya, V. Wessagowit, R. Ungpakorn, N. Loaphoomphan, P. Piradhammanon, and K. Yamanishi. 1998. The association of skin diseases with human herpesvirus 8 infection in HIV carriers. Arch. Virol. 143:1881-1892. [DOI] [PubMed] [Google Scholar]
- 17.Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. Geras Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, and E. Mesri. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. [DOI] [PubMed] [Google Scholar]
- 18.Benelli, R., A. Albini, C. Parravicini, S. Carlone, L. Repetto, G. Tambussi, and A. Lazzarin. 1996. Isolation of spindle-shaped cell populations from primary cultures of Kaposi's sarcoma of different stages. Cancer Lett. 100:125-132. [DOI] [PubMed] [Google Scholar]
- 19.Berenson, J. R., and R. A. Vescio. 1999. Herpesvirus and multiple myeloma. Blood 93:3157-3159. [PubMed] [Google Scholar]
- 20.Bezold, G., C. A. Sander, M. J. Flaig, R. U. Peter, and G. Messer. 2000. Lack of detection of human herpesvirus (HHV)-8 DNA in lesional skin of German pemphigus vulgaris and pemphigus foliaceus patients. J. Investig. Dermatol. 114:739-741. [DOI] [PubMed] [Google Scholar]
- 21.Biberfeld, P., B. Ensoli, M. Stürzl, and T. F. Schulz. 1998. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8, cytokines, growth factors, and HIV in pathogenesis of Kaposi's sarcoma. Curr. Opin. Infect. Dis. 11:97-105. [DOI] [PubMed] [Google Scholar]
- 22.Biggar, R. J., and J. J. Goedert. 2001. Mucosal shedding of human herpesvirus 8. N. Engl. J. Med. 44:690. [DOI] [PubMed] [Google Scholar]
- 23.Birkmann, A., K. Mahr, A. Ensser, S. Yağuboğlu, F. Titgemeyer, B. Fleckenstein, and F. Neipel. 2001. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1. J. Virol. 75:11583-11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blackbourn, D. J., J. Ambroziak, E. Lennette, M. Adams, B. Ramachandran, and J. A. Levy. 1997. Infectious human herpesvirus 8 in a healthy North American blood donor. Lancet 349:609-611. [DOI] [PubMed] [Google Scholar]
- 25.Blasig, C., C. Zietz, B. Haar, F. Neipel, S. Esser, N. H. Brockmeyer, E. Tschachler, S. Colombini, B. Ensoli, and M. Sturzl. 1997. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. J. Virol. 71:7963-7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blauvelt, A., B. G. Herndier, and J. M. Orenstein. 1997. Propagation of a human herpesvirus from AIDS-associated Kaposi's sarcoma. N. Engl. J. Med. 336:1837-1839. [DOI] [PubMed] [Google Scholar]
- 27.Boivin, G., A. Gaudreau, and J. P. Routy. 2000. Evaluation of the human herpesvirus 8 DNA load in blood and Kaposi's sarcoma skin lesions from AIDS patients on highly active antiretroviral therapy. AIDS 14:1907-1910. [DOI] [PubMed] [Google Scholar]
- 28.Boldogh, I., P. Szaniszlo, W. A. Bresnahan, C. M. Flaitz, M. C. Nichols, and T. Albrecht. 1996. Kaposi's sarcoma herpesvirus-like DNA sequences in the saliva of individuals infected with human immunodeficiency virus. Clin. Infect. Dis. 23:406-407. [DOI] [PubMed] [Google Scholar]
- 29.Bordenave, K., J. Griffith, S. M. Hordes, T. M. Williams, and R. S. Padilla. 2001. The historical and geomedical immunogenetics of pemphigus among the descendants of Sephardic Jews in New Mexico. Arch. Dermatol. 137:825-826. [PubMed]
- 30.Bosch, M. L., K. B. Strand, and T. M. Rose. 1998. The gamma herpesviruses: sequence comparisons. J. Virol. 72:8458-8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. D. McGee, R. A. Weiss, and J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 32.Boshoff, C., S. Talbot, M. Kennedy, J. O'Leary, T. Schulz, and Y. Chang. 1996. HHV-8 and skin cancers in immunosuppressed patients. Lancet 347:338-339. [DOI] [PubMed] [Google Scholar]
- 33.Boshoff, C., and R. A. Weiss. 1998. Kaposi's sarcoma-associated herpesvirus: Adv. Cancer Res. 75:57-86. [DOI] [PubMed] [Google Scholar]
- 34.Bourboulia, D., D. Whitby, C. Boshoff, R. Newton, V. Beral, H. Carra, A. Lane, and F. Sitas. 1998. Serologic evidence for mother-to-child transmission of Kaposi's sarcoma-associated herpesvirus infection. J. Am. Med. 280:31-32. [DOI] [PubMed] [Google Scholar]
- 35.Bouscary, D., N. Dupin, S. Fichelson, M. Grandadam, M. Fontenay-Roupie, A. G. Marcelin, P. Blanche, F. Picard, J. M. Freyssinier, P. Ravaud, F. Dreyfus, and V. Calvez. 1998. Lack of evidence of an association between HHV-8 and multiple myeloma. Leukemia 12:1840-1841. [DOI] [PubMed] [Google Scholar]
- 36.Reference deleted.
- 37.Calabrò, M. L., J. Sheldon, A. Favero, G. R. Simpson, J. R. Fiore, E. Gomes, G. Angarno, L. Chieco-Bianchi, and T. F. Schulz. 1998. Kaposi's sarcoma-associated herpesvirus DNA sequences in AIDS-related lymphomatous effusions. Br. J. Haematol. 332:1186-1191. [Google Scholar]
- 38.Calabrò, M. L., J. R. Fiore, A. Favero, A. Lepera, A. Saracino, G. Angarano, T. F. Schulz, and L. Chieco-Bianchi. 1999. Detection of human herpesvirus 8 in cervicovaginal secretions and seroprevalence in human immunodeficiency virus type 1-seropositive and -seronegative women. J. Infect. Dis. 179:1534-1537. [DOI] [PubMed] [Google Scholar]
- 39.Cannon, J. S., D. Ciufo, A. L. Hawkins, C. A. Griffin, M. J. Borowitz, G. W. Hayward, and R. F. Ambinder. 2000. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi's sarcoma herpesvirus-containing supernatant. J. Virol. 74:10187-10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cannon, M. J., S. C. Dollard, D. K. Smith, R. S. Klein, P. Schuman, J. D. Rich, D. Vlahov, and P. E. Pellett. 2001. Blood-borne and sexual transmission of human herpesvirus 8 in women with or at risk for human immunodeficiency virus infection. N. Engl. J. Med. 344:637-643. [DOI] [PubMed] [Google Scholar]
- 41.Carbone, A., G. Gaidano, A. Gloghini, L. M. Larocca, D. Capello, V. Canzonieri, A. Antinori, U. Tirelli, B. Falini, and R. Dalla-Favera. 1998. Differential expression of BCL-6, CD138/syndecan-1, and Epstein-Barr virus-encoded latent membrane protein-1 identifies distinct histogenetic subsets of acquired immunodeficiency syndrome-related non-Hodgkin's lymphomas. Blood 91:747-755. [PubMed] [Google Scholar]
- 42.Carbone, A., A. Gloghini, E. Vaccher, V. Zagonel, C. Pastore, P. Dalla Palma, F. Branz, G. Saglio, R. Volpe, U. Tirelli, and G. Gaidano. 1996. Kaposi's sarcoma-associated herpesvirus DNA sequences in AIDS-related and AIDS-unrelated lymphomatous effusions. Br. J. Haematol. 94:533-543. [DOI] [PubMed] [Google Scholar]
- 43.Reference deleted.
- 44.Cathomas, G., A. Stalder, M. O. Kurrer, N. Regamey, P. Erb, and H. I. Joller-Jemelka. 1998. Multiple myeloma and HHV-8 infection. Blood 91:4391-4393. [PubMed] [Google Scholar]
- 45.Cathomas, G. A. Stalder, N. Regamey, P. Erb, and P. H. Itin. 1998. No evidence of HHV-8 infection in patients with pemphigus vulgaris/foliaceus. Arch. Dermatol. 134:1162. [DOI] [PubMed] [Google Scholar]
- 46.Cattani, P., M. Capuano, F. Cerimele, I. L. La Parola, R. Santangelo, C. Masini, D. Cerimele, and G. Fadda. 1999. Human herpesvirus 8 seroprevalence and evaluation of nonsexual transmission routes by detection of DNA in clinical specimens from human immunodeficiency virus-seronegative patients from central and southern Italy, with and without Kaposi's sarcoma. J. Clin. Microbiol. 37:1150-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cattani, P., M. Capuano, R. Graffeo, R. Ricci, F. Cerimele, D. Cerimele, G. Nanni, and G. Fadda. 2001. Kaposi's sarcoma associated with previous human herpesvirus 8 infection in kidney transplant recipients. J. Clin. Microbiol. 39:506-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cattelan, A. M., M. L. Calabro, P. Gasperini, S. M. L. Aversa, M. Zanchetta, F. Meneghetti, A. Rossi, and L. Chieco-Bianchi. 2000. Acquired immunodeficiency syndrome-related Kaposi's sarcoma regression after highly active antiviral therapy: biologic correlates of clinical outcome. J. Natl. Cancer Inst. Monogr. 28:44-49. [DOI] [PubMed] [Google Scholar]
- 49.Cerimele, F., F. Curreli, S. Ely, A. E. Friedman-Kien, E. Cesarman, and O. Flore. 2001. Kaposi's sarcoma-associated herpesvirus can productively infect primary human keratinocytes and alter their growth properties. J. Virol. 75:2435-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 51.Cesarman, E., and D. M. Knowles. 1999. The role of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Semin. Cancer Biol. 9:165-174. [DOI] [PubMed] [Google Scholar]
- 52.Cesarman, E., P. S. Moore, P. H. Rao, G. Ighirami, D. M. Knowles, and Y. Chang. 1995. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1, BC-2) containing Kaposi's sarcoma-associated herpesvirus-like DNA sequences. Blood 86:2708-2714. [PubMed] [Google Scholar]
- 53.Cesarman, E., R. G. Nador, F. Bai, R. A. Bohenzky, J. J. Russo, P. S. Moore, Y. Chang, and D. M. Knowles. 1996. Kaposi's sarcoma associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J. Virol. 70:8218-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chadburn, A., E. Cesarman, R. G. Nador, Y. F. Liu, and D. M. Knowles. 1996. Kaposi's sarcoma-associated herpesvirus sequences in non-HIV-associated benign lymphoproliferative lesions. Lab. Investig. 74:109. [Google Scholar]
- 55.Chang, Y., and P. S. Moore. 1996. Kaposi's sarcoma (KS)-associated herpesvirus and its role in KS. Infect. Agents Dis. 5:215-222. [PubMed] [Google Scholar]
- 56.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 57.Reference deleted.
- 58.Chatlynne, L. G., and D. V. Ablashi. 1999. Seroepidemiology of Kaposi's sarcoma-associated herpesvirus (KSHV). Semin. Cancer Biol. 9:175-185. [DOI] [PubMed] [Google Scholar]
- 59.Chatlynne, L. G., W. Lapps, M. Handy, Y. Q. Huang, R. Masood, A. S. Hamilton, J. W. Said, H. P. Koeffler, M. H. Kaplan, A. Friedman-Kien, P. S. Gill, J. E. Whitman, and D. V. Ablashi. 1998. Detection and titration of HHV-8-specific antibodies in sera from blood donors, acquired immunodeficiency syndrome patients, and Kaposi's sarcoma patients using a whole virus enzyme-linked immunosorbent assay. Blood 92:53-58. [PubMed] [Google Scholar]
- 60.Chaucan, D., A. Bharti, N. Raje, E. Gustafson, G. S. Pinkus, J. L. Pinkus, G. Teoh, T. Hideshima, S. P. Treon, J. D. Fingeroth, and K. C. Anderson. 1999. Detection of Kaposi's sarcoma herpesvirus DNA sequences in multiple myeloma bone marrow stromal cells. Blood 93:1482-1486. [PubMed] [Google Scholar]
- 61.Cheng, E. H. Y., J. Nicholas, D. S. Bellows, G. S. Hayward, H. G. Guo, M. S. Reitz, and J. M. Hardwick. 1997. A BCL-2 homolog encoded by Kaposi's sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc. Natl. Acad. Sci. USA 94:690-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Civati, G., G. Busnach, B. Brando, M. L. Broggi, C. Brunati, G. P. Casadei, and L. Minetti. 1988. Occurrence of Kaposi's sarcoma in renal transplant recipients treated with low doses of cyclosporine. Transplant. Proc. 20:924-928. [PubMed] [Google Scholar]
- 63.Cockerell, C. J. 1991. Histopathological features of Kaposi's sarcoma in HIV-infected individuals. Cancer Surv. 10:73-90. [PubMed] [Google Scholar]
- 64.Corbellino, M., L. Poirel, G. Bestetti, M. Pizzuto, J. T. Aubin, M. Capra, C. Bifulco, E. Berti, H. Agut, G. Rizzardini, M. Galli, and C. Parravicini. 1996. Restricted tissue distribution of extralesional Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS patients with Kaposi's sarcoma. AIDS Res. Hum. Retroviruses 12:651-657. [DOI] [PubMed] [Google Scholar]
- 65.Cottoni, F., and S. Uccini. 1997. Kaposi's sarcoma-associated herpesvirus infection and multiple myeloma. Science 278:1972. [PubMed] [Google Scholar]
- 66.Davidovici, B., I. Karakis, D. Bourboulia, S. Ariad, J.-C. Zong, D. Benharroch, N. Dupin, R. Weiss, G. Hayward, B. Sarov, and C. Boshoff. 2001. Seroepidemiology and molecular epidemiology of Kaposi's sarcoma-associated herpesvirus among Jewish population groups in Israel. J. Natl. Cancer Inst. 93:194-202. [DOI] [PubMed] [Google Scholar]
- 67.Davis, M. A., M. Stürzl, C. Blasig, A. Schreier, H. G. Guo, M. Reitz, S. R. Opalenik, and P. J. Browning. 1997. Expression of human herpesvirus 8-encoded cyclin D in Kaposi's sarcoma spindle cells. J. Natl. Cancer Inst. 89:1868-1874. [DOI] [PubMed] [Google Scholar]
- 68.Decker, L. L., P. Shankar, and G. Khan. 1996. The Kaposi's sarcoma-associated herpesvirus (KSHV) is present as an intact latent genome in KS tissue but replicates in the peripheral blood mononuclear cells of KS patients. J. Exp. Med. 184:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deichmann, M., M. Thome, M. Bock, A. Jäckel, V. Waldmann, and H. Näher. 1999. The human herpesvirus-type 8 is not involved in malignant melanoma. Br. J. Cancer 80:67-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Desrosiers, R. C., V. G. Sasseville, S. C. Czajak, X. Zhang, K. G. Mansfield, A. Kaur, R. P. Johnson, A. Lackner, and J. U. Jung. 1997. A herpesvirus of rhesus monkeys related to human Kaposi's sarcoma-associated herpesvirus. J. Virol. 71:9764-9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dessoukey, M. W., H. A. Dayem, and M. F. Omer. 1996. Kaposi's sarcoma and sarcoidosis coexisting in lesions of HIV-seronegative patient. Int. J. Dermatol. 35:824-826. [DOI] [PubMed] [Google Scholar]
- 72.de Thé, G., G. Bestetti, M. Van Beveren, and A. Gessain. 1998. The AIDS associated epidemic of Kaposi's sarcoma in East Africa occurred under a KSHV/HHV-8 prevalence unchanged since 1975. J. Natl. Cancer Inst. 91:1888-1889. [DOI] [PubMed] [Google Scholar]
- 73.Di Alberti, L., A. Piattelli, L. Artese, G. Favia, S. Patel, N. Saunders, S. R. Porter, C. M. Scully, S. L. Ngui, and C. G. Teo. 1997. Human herpesvirus 8 variants in sarcoid tissues. Lancet 350:1655-1661. [DOI] [PubMed] [Google Scholar]
- 74.Diamond, C., M. L. Huang, D. H. Kedes, C. Speck, G. W. Rankin, Jr., D. Ganem, R. W. Coombs, T. M. Rose, J. N. Krieger, and L. Corey. 1997. Absence of detectable human herpesvirus 8 in the semen of human immunodeficiency virus-infected men without Kaposi's sarcoma. J. Infect. Dis. 176:775-777. [DOI] [PubMed] [Google Scholar]
- 75.Diamond, C., S. J. Brodie, J. N. Krieger, M.-L. Huang, D. M. Koelle, K. Diem, D. Muthui, and L. Corey. 1998. Human herpesvirus 8 in the prostate glands of men with Kaposi's sarcoma. J. Virol. 72:6223-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diamond, C., H. Thiede, T. Perdue, D. MacKellar, L. A. Valleroy, and L. Corey. 2001. Seroepidemiology of human herpesvirus 8 among young men who have sex with men. Sex. Transm. Dis. 28:176-183. [DOI] [PubMed] [Google Scholar]
- 77.Dittmer, D., C. Stoddart, R. Renne, V. Linquist-Stepps, M. E. Moreno, C. Bare, J. M. McCune, and D. Ganem. 1999. Experimental transmission of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) to SCID-hu Thy/Liv mice. J. Exp. Med. 190:1857-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dominici, M., M. Luppi, D. Campioni, F. Lanza, P. Barozzi, R. Milani, S. Moretti, G. Nadali, R. Spanedda, R. Trovato, G. Torelli, and G. Castoldi. 2000. PCR with degenerate primers for highly conserved DNA polymerase gene of the herpesvirus family shows neither human herpesvirus 8 nor a related variant in bone marrow stromal cells from multiple myeloma patients. Int. J. Cancer 86:76-82. [DOI] [PubMed] [Google Scholar]
- 79.Drexler, H. G., C. C. Uphoff, G. Gaidano, and A. Carbone. 1998. Lymphoma cell lines: in vitro models for the study of HHV-8+ primary effusion lymphomas (body cavity-based lymphomas). Leukemia 12:1507-1517. [DOI] [PubMed] [Google Scholar]
- 80.Du, M. Q., H. Liu, T. C. Diss, H. Ye, R. A. Hamoudi, N. Dupin, V. Meignin, E. Oksenhendler, C. Boshoff, and P. G. Isaacson. 2001. Kaposi sarcoma-associated herpesvirus infects monotypic (IgM lambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood 97:2130-2136. [DOI] [PubMed] [Google Scholar]
- 81.Dupin, N., A. G. Marcelin, I. Gorin, P. Bossi, N. Franck, B. Weill, J. M. Huraux, J. P. Escande, and V. Calvez. 1998. Prevalence of human herpesvirus 8 infection measured by antibodies to a latent nuclear antigen in patients with various dermatologic diseases. Arch. Dermatol. 134:700-702. [DOI] [PubMed] [Google Scholar]
- 82.Dupin, N., C. Fisher, P. Kellam, S. Ariad, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dupin, N., A. G. Marcelin, V. Calvez, and C. Andr. 1999. Absence of a link between human herpesvirus 8 and pemphigus. Br. J. Dermatol. 141:159-160. [DOI] [PubMed] [Google Scholar]
- 84.Dupon, M., B. Masquelier, C. Cazorla, G. Chene, B. Dumon, J. M. Ragnaud, B. de Barbeyrac, C. Bebear, J. Y. Lacut, and H. J. Fleury. 1997. Acquired immunodeficiency syndrome-associated Kaposi's sarcoma and human herpesvirus 8 DNA detection in serial peripheral blood mononuclear cell samples. Res. Virol. 148:417-425. [DOI] [PubMed] [Google Scholar]
- 85.Ellis, M., Y. P. Chew, L. Fallis, S. Freddersdorf, C. Boshoff, R. A. Weiss, X. Lu, and S. Mittnacht. 1999. Degradation of p27(Kip) cdk inhibitor triggered by Kaposi's sarcoma virus cyclin-cdk6 complex. EMBO J. 18:644-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Enbom, M., J. Sheldon, E. Lennette, T. Schulz, D. V. Ablashi, F. Neipel, P. Biberfeld, H. Carlberg, P. Ljungman, A. Nilsson, T. Söderström, J. Wadström, and A. Linde. 2000. Antibodies to human herpesvirus 8 latent and lytic antigens in blood donors and potential high-risk groups in Sweden: variable frequencies found in a multicenter serological study. J. Med. Virol. 62:498-504. [PubMed] [Google Scholar]
- 87.Engels, E. A., H. Eastman, D. V. Ablashi, R. J. Wilks, J. Braham, and A. Manns. 1999. Risk of transfusion-associated transmission of human herpesvirus 8. J. Natl. Cancer Inst. 91:1773-1775. [DOI] [PubMed] [Google Scholar]
- 88.Engels, E. A., P. S. Rosenberg, M. Frisch, and J. J. Goedert. 2001. Cancers associated with Kaposi's sarcoma (KS) in AIDS: a link between KS herpesvirus and immunoblastic lymphoma. Br. J. Cancer 85:1298-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fais, F., G. Gaidano, D. Capello, A. Gloghini, F. Ghiotto, S. Roncella, A. Carbone, N. Chiorazzi, and M. Ferrarini. 1999. Immunoglobulin V region gene use and structure suggest antigen selection in AIDS-related primary effusion lymphomas. Leukemia 13:1093-1099. [DOI] [PubMed] [Google Scholar]
- 90.Farge, D., C. Lebbe, and Z. Marjanovic. 1999. Human herpesvirus 8 and risk factors for Kaposi's sarcoma in kidney transplant recipients. Transplantation 67:1236-1242. [DOI] [PubMed] [Google Scholar]
- 91.Feller, M. J. 1993. Drug-induced bullous pemphigoid. Clin. Dermatol. 11:515-520. [DOI] [PubMed] [Google Scholar]
- 92.Flore, O., S. Rafii, S. Ely, J. J. O'Leary, E. M. Hyjek, and E. Cesarman. 1998. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature 394:588-592. [DOI] [PubMed] [Google Scholar]
- 93.Foreman, K. E., J. Friborg, W. P. Kong, C. Woffendin, J. Polverini, B. J. Nickoloff, and G. J. Nabel. 1997. Propagation of human herpesvirus from AIDS-associated Kaposi's sarcoma. N. Engl. J. Med. 336:163-171. [DOI] [PubMed] [Google Scholar]
- 94.Fouchard, N., V. Lacoste, P. Couppie, M. Develoux, P. Mauclère, P. Michel, V. Herve, R. Pradinaud, G. Bestetti, M. Huerre, F. Tekaia, G. de Thé, and A. Gessain. 2000. Detection and genetic polymorphism of human herpesvirus type 8 in endemic or epidemic Kaposi's sarcoma from West and Central Africa, and South America. Int. J. Cancer 85:166-170. [PubMed] [Google Scholar]
- 95.Francès, C., C. Mouquet, and V. Calvez. 1999. Human herpesvirus 8 and renal transplantation. N. Engl. J. Med. 340:1045. [DOI] [PubMed] [Google Scholar]
- 96.Francès, C., C. Mouquet, A. G. Marcelin, S. Barete, R. Agher, D. Charron, H. Benalia, N. Dupin, J. C. Piette, M. O. Bitker, and V. Calvez. 2000. Outcome of kidney transplant recipients with previous human herpesvirus-8 infection. Transplantation 69:1776-1779. [DOI] [PubMed] [Google Scholar]
- 97.Franceschi, S., and M. Geddes. 1995. Epidemiology of classic Kaposi's sarcoma, with special reference to Mediterranean population. Tumori 81:308-314. [DOI] [PubMed] [Google Scholar]
- 98.Frenkel, N., E. C. Schirmer, L. S. Wyatt, G. Katsafanas, E. Roffman, R. M. Danowich, and C. H. June. 1990. Isolation of a new herpesvirus from human CD4 T-cells. Proc. Natl. Acad. Sci. USA 87:748-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fribourg, J., W.-P. Kong, C. Flowers, S. L. Flowers, Y. Sun, K. E. Foreman, B. J. Nickoloff, and G. J. Nabel. 1998. Distinct biology of Kaposi's sarcoma-associated herpesvirus from primary lesions and body cavity lymphomas. J. Virol. 12:10073-10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Friedman-Kien, A. E. 1981. Disseminated Kaposi's sarcoma syndrome in young homosexual men. J. Am. Acad. Dermatol. 5:468-471. [DOI] [PubMed] [Google Scholar]
- 101.Frizzera, G. 1992. Atypical lymphoproliferative disorders, p. 459-496. In D. M. Knowles (ed.), Neoplastic hematopathology. Williams and Wilkins, Baltimore, Md.
- 102.Fujii, T., H. Taguchi, H. Katano, S. Mori, T. Nakamura, N. Nojiri, K. Nakajima, K. Tadokoro, T. Juji, and A. Iwamoto. 1999. Seroprevalence of human herpesvirus 8 in human immunodeficiency virus 1-positive and human immunodeficiency virus 1-negative populations in Japan. J. Med. Virol. 57:159-162. [DOI] [PubMed] [Google Scholar]
- 103.Gaidano, G., A. Gloghini, V. Gattei, M. F. Rossi, A. M. Cilia, C. Godeas, M. Degan, T. Perin, V. Canzonieri, D. Aldinucci, G. Saglio, A. Carbone, and A. Pinto. 1997. Association of Kaposi's sarcoma-associated herpesvirus-positive primary effusion lymphoma with expression of the CD138/syndecan-1 antigen. Blood 90:4894-4900. [PubMed] [Google Scholar]
- 104.Gaidano, G., C. Pastore, A. Gloghini, G. Volpe, D. Capello, P. Polito, E. Vaccher, U. Tirelli, G. Saglio, and A. Carbone. 1997. Human herpesvirus type-8 (HHV-8) in haematopoietic neoplasia. Leuk. Lymphoma 24:257-266. [DOI] [PubMed] [Google Scholar]
- 105.Gallo, R. 1998. The enigmas of Kaposi's sarcoma. Science 282:1837-1839. [DOI] [PubMed] [Google Scholar]
- 106.Gao, S.-J., M. Alsina, J.-H. Deng, C. R. Harrison, E. A. Montalvo, C. T. Leach, G. D. Roodman, and H. B. Jenson. 1998. Antibodies to Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in patients with multiple myeloma. J. Infect. Dis. 178:846-849. [DOI] [PubMed] [Google Scholar]
- 107.Gao, S. J., C. Boshoff, S. Jayachandra, R. A. Weiss, Y. Chang, and P. S. Moore. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15:1979-1985. [DOI] [PubMed] [Google Scholar]
- 108.Gao, S.-J., L. Kingsley, D. R. Hoover, T. J. Spira, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, P. Parry, Y. Chang, and P. S. Moore. 1996. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N. Engl. J. Med. 335:233-241. [DOI] [PubMed] [Google Scholar]
- 109.Gaspari, A. A., S. Marchese, D. Powell, P. Rady, and S. K. Tyring. 1997. Identification of HHV-8 DNA in the skin lesion of Kaposi's sarcoma in immunosuppressed patients with bullous pemphigoid. J. Am. Acad. Dermatol. 37:843-847. [DOI] [PubMed] [Google Scholar]
- 110.Gérard, L., F. Agbalika, J. Sheldon, A. Maillard, T. F. Schulz, and E. Oksenhendler. 2001. No increased human herpesvirus 8 seroprevalence in patients with HIV-associated non-Hodgkin's lymphoma. J. Acquir. Immune Defic. Syndr. 26:182-184. [DOI] [PubMed] [Google Scholar]
- 111.Reference deleted.
- 112.Gessain, A., J. Brière, C. Angelin-Duclos, F. Valensi, H. Merle Béral, F. Davi, M.-A. Nicola, A. Sudaka, N. Fouchard, J. Gabarre, X. Troussard, E. Dulmet, J. Audouin, J. Diebold, and G. de Thé. 1997. Human herpesvirus 8 (Kaposi's sarcoma herpes virus) and malignant lymphoproliferations in France: a molecular study of 250 cases including two AIDS-associated body cavity based lymphomas. Leukemia 11:266-272. [DOI] [PubMed] [Google Scholar]
- 113.Gessain, A., P. Mauclère, M. Van Beveren, S. Plancoulaine, A. Ayouba, J.-L. Essame-Oyono, P. M. V. Martin, and G. de Thé. 1999. Human herpesvirus 8 primary infection occurs during childhood in Cameroon, Central Africa. Int. J. Cancer 81:189-192. [DOI] [PubMed] [Google Scholar]
- 114.Gill, P. S., Y. C. Tsai, A. P. Rao, C. H. Spruck 3rd, T. Zheng, W. A. Harrington, Jr., T. Cheung, B. Nathwani, and P. A. Jones. 1998. Evidence for multiclonality in multicentric Kaposi's sarcoma. Proc. Natl. Acad. Sci. USA 95:8257-8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Giraldo, G., E. Beth, and F. Haguenau. 1972. Herpes-type particles in tissue culture of Kaposi's sarcoma from different geographic regions. J. Natl. Cancer Inst. 49:1509. [DOI] [PubMed] [Google Scholar]
- 116.Giraldo, G., E. Beth, and E. S. Huang. 1980. Kaposi's sarcoma and its relationship to cytomegalovirus (CMV). III. CMV DNA and CMV early antigens in Kaposi's sarcoma. Int. J. Cancer 26:23-29. [DOI] [PubMed] [Google Scholar]
- 117.Goedert, J. J. 2000. The epidemiology of acquired immunodeficiency syndrome malignancies. Semin. Oncol. 27:390-401. [PubMed] [Google Scholar]
- 118.Gompels, U. A., and F. C. Kasolo. 1996. HHV-8 serology and Kaposi's sarcoma. Lancet 348:1587-1588. [DOI] [PubMed] [Google Scholar]
- 119.Goudsmit, J., N. Renwick, N. H. T. M. Dukers, R. A. Coutinho, S. Heisterkamp, M. Bakker, T. F. Schulz, M. Cornelissen, and G. J. Weverling. 2000. Human herpesvirus 8 infections in the Amsterdam cohort studies (1984-1997): analysis of seroconversions to ORF65 and ORF73. Proc. Natl. Acad. Sci. USA 97:4838-4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Green, I., E. Espiritu, M. Ladanyi, R. Chaponda, R. Wieczorek, L. Gallo, and H. Feiner. 1995. Primary lymphomatous effusions in AIDS: a morphological, immunophenotypic, and molecular study. Mod. Pathol. 8:39-45. [PubMed] [Google Scholar]
- 121.Greensill, J., J. A. Sheldon, M. Renwich, E. Beer, S. Norley, J. Goudsmit, and T. F. Schulz. 2000. Two distinct gamma-2 herpesviruses in African green monkeys: a second gamma-2 herpesvirus lineage among Old World primates. J. Virol. 74:1572-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Greensill, J., J. A. Sheldon, K. K. Murthy, J. S. Bessonette, B. E. Beer, and T. F. Schulz. 2000. A chimpanzee rhadinovirus sequence related to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8: increased detection after HIV-1 infection in the absence of disease. AIDS 14:129-135. [DOI] [PubMed] [Google Scholar]
- 123.Grulich, A. E., S. J. Olsen, K. Luo, O. Hendry, P. Cunningham, D. A. Cooper, S.-J. Gao, Y. Chang, P. S. Moore, and J. M. Kaldor. 1999. Kaposi's sarcoma-associated herpesvirus: sexually transmissible infection. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20:387-393. [DOI] [PubMed] [Google Scholar]
- 124.Gupta, P., M. K. Singh, C. Rinaldo, M. Ding, H. Farzadegan, A. Saah, D. Hoover, P. Moore, and L. Kingsley. 1996. Detection of Kaposi's sarcoma herpesvirus DNA in semen of homosexual men with Kaposi's sarcoma. AIDS 10:1596-1598. [DOI] [PubMed] [Google Scholar]
- 125.Hansen, J. A., T. A. Gooley, P. J. Martin, F. Appelbaum, T. R. Chauncey, R. A. Clift, E. W. Petersdorf, J. Radich, J. E. Sanders, R. F. Storb, K. M. Sullivan, and C. Anasetti. 1998. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N. Engl. J. Med. 338:962-968. [DOI] [PubMed] [Google Scholar]
- 126.Hayward, G. S. 1999. KSHV strains: the origin and global spread of the virus. Semin. Cancer Biol. 9:187-199. [DOI] [PubMed] [Google Scholar]
- 127.Hjalgrim, H., M. Frisch, and M. Melbye. 1998. Incidence rates of classical Kaposi's sarcoma and multiple myeloma do not correlate. Br. J. Cancer 78:419-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Horenstein, M. G., R. G. Nador, A. Chadburn, E. M. Hyjek, G. Inghirami, D. M. Knowles, and E. Cesarman. 1997. Epstein-Barr virus latent gene expression in primary effusion lymphomas containing Kaposi's sarcoma-associated herpesvirus human herpesvirus-8. Blood 90:1186-1191. [PubMed] [Google Scholar]
- 129.Howard, M. R., D. Whitby, G. Bahadur, F. Suggett, C. Boshoff, M. Tenant-Flowers, T. F. Schulz, S. Kirk, S. Matthews, I. V. Weller, R. S. Tedder, and R. A. Weiss. 1997. Detection of human herpesvirus 8 DNA in semen from HIV-infected individuals but not healthy semen donors. AIDS 11:F15-F19. [DOI] [PubMed] [Google Scholar]
- 130.Huh, J., G. H. Kang, G. Gong, S. S. Kim, J. Y. Ro, and C. W. Kim. 1998. Kaposi's sarcoma-associated herpesvirus in Kikuchi's disease. Hum. Pathol. 29:1091-1095. [DOI] [PubMed] [Google Scholar]
- 131.Humphrey, R. W., T. R. O'Brien, F. M. Newcomb, H. Nishihara, K. M. Wyvill, G. A. Ramos, M. W. Saville, J. J. Goedert, S. E. Straus, and R. Yarchoan. 1996. Kaposi's sarcoma (KS)-associated herpesvirus-like DNA sequences in peripheral blood mononuclear cells: association with KS and persistence in patients receiving anti-herpesvirus drugs. Blood 88:297-301. [PubMed] [Google Scholar]
- 132.Hundall, S. D., L. P. Rady, S. K. Tyring, and J. C. Fish. 1998. Serologic and molecular evidence of human herpesvirus 8 activation in renal transplant recipients. J. Infect. Dis. 178:1791-1794. [DOI] [PubMed] [Google Scholar]
- 133.Inagi, R., H. Kosuge, S. Nishimoto, K. Yoshikawa, and K. Yaminishi. 1996. Kaposi's sarcoma-associated herpesvirus (KSHV) sequences in premalignant and malignant skin tumors. Arch. Virol. 141:2217-2223. [DOI] [PubMed] [Google Scholar]
- 134.International Agency for Research on Cancer. 1997. Epstein-Barr virus and Kaposi's sarcoma herpesvirus/human herpesvirus 8, p. 375-492. IARC monographs on the evaluation of carcinogenic risks to humans, vol. 70. Epstein-Barr virus and Kaposi’s sarcoma herpesvirus/human herpesvirus 8. IARC, Lyon, France. [PMC free article] [PubMed]
- 135.Iscovich, J., P. Boffetta, S. Franceschi, E. Azizi, and R. Sarid. 1999. Classic Kaposi's sarcoma. Cancer 88:500-517. [PubMed] [Google Scholar]
- 136.Ishak, K. G., K. E. Bijwaard, H. R. Makhlouf, J. K. Taubenberger, J. H. Lichy, and Z. D. Goodman. 1998. Absence of human herpesvirus 8 DNA sequences in vascular tumors of the liver. Liver 18:124-127. [DOI] [PubMed] [Google Scholar]
- 137.Jacobson, L. P., F. J. Jenkins, G. Springer, A. Munoz, K. V. Shah, J. Phair, Z. Zhang, and H. Armenian. 2000. Interaction of human immunodeficiency virus type 1 and human herpesvirus type 8 infections on the incidence of Kaposi's sarcoma. J. Infect. Dis. 181:1940-1949. [DOI] [PubMed] [Google Scholar]
- 138.Jacobson, L. P., T. E. Yamashita, R. Detels, J. B. Margolick, J. S. Chmiel, L. A. Kingsley, S. Melnick, and A. Munoz. 1999. Impact of potent antiretroviral therapy on the incidence of Kaposi's sarcoma and non-Hodgkin's lymphomas among HIV-1-infected individuals. Multicenter AIDS Cohort Study. J. Acquir. Immune Defic. Syndr. 21(Suppl. 1):S34-41. [PubMed] [Google Scholar]
- 139.Jones, J. L., D. L. Hanson, M. S. Dworkin, J. W. Ward, and H. W. Jaffe. 1999. Effect of antiretroviral therapy on recent trends in selected cancers among HIV-infected persons. Adult/Adolescent Spectrum of HIV Disease Project Group. J. Acquir. Immune Defic. Syndr. 21(Suppl. 1):S11-17. [PubMed] [Google Scholar]
- 140.Kaposi, M. 1872. Idiopatisches multiples Pigmentsarkom der Haut. Arch. Dermatol. Syphillis 4:265-273. [Google Scholar]
- 141.Reference deleted.
- 142.Reference deleted.
- 143.Karcher, D. S., and S. Alkan. 1997. Human herpesvirus-6 associated body cavity based lymphoma in human immunodeficiency virus-infected patients: a unique B-cell neoplasm. Hum. Pathol. 28:801-808. [DOI] [PubMed] [Google Scholar]
- 144.Kasolo, F. C., E. Mpabalwani, and U. A. Gompels. 1997. Infection with AIDS-related herpesviruses in human immunodeficiency virus-negative infants and endemic childhood Kaposi's sarcoma in Africa. J. Gen. Virol. 78:847-855. [DOI] [PubMed] [Google Scholar]
- 145.Katz, M. H., N. A. Hessol, S. P. Buchbinder, A. Hirozawa, P. O'Malley, and S. D. Holmberg. 1994. Temporal trends of opportunistic infections and malignancies in homosexual men with AIDS. J. Infect. Dis. 170:198-202. [DOI] [PubMed] [Google Scholar]
- 146.Kedes, D. H., D. Ganem, N. Ameli, P. Bacchetti, and R. Greenblatt. 1997. The prevalence of serum antibody to human herpesvirus 8 Kaposi's sarcoma-associated herpesvirus among HIV seropositive and high risk seronegative women. J. Am. Med. 277:478-481. [PubMed] [Google Scholar]
- 147.Klussmann, J. P., A. Muller, M. Wagner, O. Guntinas-Lichius, M. Jungehuelsing, T. Sloots, D. V. Ablashi, and G. R. F. Krueger. 2000. Human herpesvirus type 8 in salivary gland tumors. J. Clin. Virol. 16:239-246. [DOI] [PubMed] [Google Scholar]
- 148.Knowles, D. M., E. Cesarman, A. Chadburn, G. Frizzera, J. Chen, E. A. Rose, and R. E. Michler. 1995. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood 85:552-565. [PubMed] [Google Scholar]
- 149.Knowles, D. M., G. Inghirami, A. Ubriaco, and R. Dalla-Favera. 1989. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood 73:792-799. [PubMed] [Google Scholar]
- 150.Koelle, D. M., M.-L. Huang, B. Chandran, J. Vieira, M. Piepkorn, and L. Corey. 1997. Frequent detection of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in saliva of human immunodeficiency virus-infected men: clinical and immunologic correlates. J. Infect. 176:94-102. [DOI] [PubMed] [Google Scholar]
- 151.Kohler, S., O. W. Kamel, P. P. Chang, and B. R. Smoller. 1997. Absence of human herpesvirus 8 and Epstein-Barr virus genome sequences in cutaneous epithelial neoplasms arising in immunosuppressed organ-transplant patients. J. Cutan. Pathol. 24:559-563. [DOI] [PubMed] [Google Scholar]
- 152.Kyle, R. A. 1994. Monoclonal gammopathy of undetermined significance. Blood Rev. 8:135-141. [DOI] [PubMed] [Google Scholar]
- 153.Lacoste, V., E. Kadyrova, J. Chistiakova, V. Gurtsevitch, J. G. Judde, and A. Gessain. 2000. Molecular characterization of Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 strains from Russia. J. Gen. Virol. 81:1217-1222. [DOI] [PubMed] [Google Scholar]
- 154.Lacoste, V., P. Mauclère, G. Dubreuil, J. Lewis, M.-C. Georges-Courbot, and A. Gessain. 2000. KSHV-like herpesviruses in chimps and gorillas. Nature 407:151-152. [DOI] [PubMed] [Google Scholar]
- 155.Lacoste, V., P. Mauclère, G. Dubreuil, J. Lewis, M.-C. Georges-Courbot, J. Rigoulet, T. Petit, and A. Gessain. 2000. Simian homologues of human gamma-2 and betaherpesviruses in mandrill and drill monkeys. J. Virol. 74:11993-11999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.LaDuca, J. R., J. L. Love, L. Z. Abbott, S. Dube, A. Friedman-Kien, and B. Poiesz. 1998. Detection of human herpesvirus 8 DNA in tissues and bodily fluids. J. Infect. Dis. 178:1610-1615. [DOI] [PubMed] [Google Scholar]
- 157.Lampinen, T. M., S. Kulasingam, J. Min, M. Borok, L. Gwanzura, J. Lamb, K. Mahomed, G. B. Woelk, K. B. Strand, M. L. Bosch, D. C. Edelman, N. T. Constantine, D. Katzenstein, and M. A. Williams. 2000. Detection of Kaposi's sarcoma-associated herpesvirus in oral and genital secretions of Zimbabwean women. J. Infect. Dis. 181:1785-1790. [DOI] [PubMed] [Google Scholar]
- 158.Langunoff, M., and D. Ganem. 1997. The structure and coding organization of the genomic termini of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8). Virology 236:147-154. [DOI] [PubMed] [Google Scholar]
- 159.Lansky, A., J. L. Jones, S. Burkham, K. Reynolds, B. Bohannon, and J. Bertolli. 1999. Adequacy of prenatal care and prescription of zidovudine to prevent perinatal HIV transmission. J. Acquir. Immune Defic. Syndr. 21:223-227. [DOI] [PubMed] [Google Scholar]
- 160.Lasota, J., and M. Miettinen. 1999. Absence of Kaposi's sarcoma-associated virus (human herpesvirus-8) sequences in angiosarcoma. Virchows Arch. 434:51-56. [DOI] [PubMed] [Google Scholar]
- 161.Leach, C. T., C. Frantz, D. R. Head, S. J. Gao, K. L. McClain, M. Cohen, A. B. Campbell, B. H. Pollock, S. B. Murphy, and H. B. Jenson. 1999. Human herpesvirus-8 (HHV-8) associated with small noncleaved cell lymphoma in a child with AIDS. Am. J. Hematol. 60:215-221. [DOI] [PubMed] [Google Scholar]
- 162.Lebbe, C., R. Tatoud, P. Morel, F. Calvo, S. Euvrard, J. Kanitakis, M. Faure, and A. Claudy. 1997. Human herpesvirus 8 sequences are not detected in epithelial tumors from patients receiving transplants. Arch. Dermatol. 133:111. [PubMed] [Google Scholar]
- 163.Lebbe, C., L. Blum, C. Pellet, G. Blanchard, O. Verola, P. Morel, O. Danne, and F. Calvo. 1998. Clinical and biological impact of antiretroviral therapy with protease inhibitors on HIV-related Kaposi's sarcoma. AIDS 12:F45-F49. [DOI] [PubMed] [Google Scholar]
- 164.Lechapt-Zalcman, E., D. Challine, M. H. Delfau-Larue, C. Haioun, D. Desvaux, and P. Gaulard. 2001. Association of primary pleural effusion lymphoma of T-cell origin and human herpesvirus 8 in a human immunodeficiency virus-seronegative man. Arch. Pathol. Lab. Med. 125:1246-1248. [DOI] [PubMed] [Google Scholar]
- 165.Lee, H., J. Guo, M. Li, J. K. Choi, M. DeMaria, M. Rosenzweig, and J. U. Jung. 1998. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi's sarcoma-associated herpesvirus. Mol. Cell. Biol. 18:5219-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee, H., R. Veazey, K. Williams, M. Li, J. Guo, F. Neipel, B. Fleckenstein, A. Lackner, R. C. Desrosiers, and J. U. Jung. 1998. Deregulation of cell growth by the K1 gene of Kaposi's sarcoma-associated herpesvirus. Nat. Med. 4:435-440. [DOI] [PubMed] [Google Scholar]
- 167.Lefrere, J. J., M. C. Meyohas, M. Mariott, J. L. Meynard, M. Thauvin, and J. Frottier. 1996. Detection of human herpesvirus 8 DNA sequences before the appearance of Kaposi's sarcoma in human immunodeficiency virus (HIV) positive subjects with a known date of HIV seroconversion. J. Infect. Dis. 174:283-287. [DOI] [PubMed] [Google Scholar]
- 168.Lennette, E. T., D. J. Blackbourn, and J. A. Levy. 1996. Antibodies to human herpesvirus type 8 in the general population and in Kaposi's sarcoma patients. Lancet 348:858-861. [DOI] [PubMed] [Google Scholar]
- 169.Li, M., H. Lee, J. Guo, F. Neipel, B. Fleckenstein, K. Ozato, and J. U. Jung. 1998. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor. J. Virol. 72:5433-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li, M., H. Lee, D.-W. Yoon, J.-C. Albrecht, B. Fleckenstein, and J. Jung. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional cyclin. J. Virol. 71:1984-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li, N., W. K. Anderson, and J. Bhawan. 1998. Further confirmation of the association of human herpesvirus 8 with Kaposi's sarcoma. J. Cutan. Pathol. 25:413-419. [DOI] [PubMed] [Google Scholar]
- 172.Li, S. J., Y. Q. Huang, C. J. Cockerell, and A. E. Friedman-Kien. 1996. Localization of human herpesvirus type 8 in vascular endothelial and perivascular spindle-shaped cells of Kaposi's sarcoma lesions by in situ hybridization. Am. J. Pathol. 148:1714-1748. [PMC free article] [PubMed] [Google Scholar]
- 173.Lin, J. C., S. C. Lin, E. C. Mar, P. E. Pellett, F. R. Stamey, J. A. Stewart, and T. J. Spira. 1995. Is Kaposi's sarcoma-associated herpesvirus detectable in semen of HIV-infected homosexual men? Lancet 346:1601-1602. [DOI] [PubMed] [Google Scholar]
- 174.Lin, J. C., S. C. Lin, E. C. Mar, P. E. Pellett, F. R. Stamey, J. A. Stewart, and T. J. Spira. 1998. Retraction: is Kaposi's sarcoma-associated herpesvirus in semen of HIV-infected homosexual men? Lancet 351:1365. [DOI] [PubMed] [Google Scholar]
- 175.Lin, S. F., R. Sun, L. Heston, L. Granville, D. Shodd, K. Haglaund, M. Rigsby, and G. Miller. 1997. Identification, expression, and immunogenicity of Kaposi's sarcoma-associated herpesvirus-encoded small viral capsid antigen. J. Virol. 71:3069-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Low, P., F. Neipel, A. Rascu, H. Steininger, B. Manger, B. Fleckenstein, J. R. Kalden, and T. Harrer. 1998. Suppression of HHV-8 viremia by foscarnet in an HIV-infected patient with Kaposi's sarcoma and HHV-8 associated hemophagocytic syndrome. Eur. J. Med. Res. 3:461-464. [PubMed] [Google Scholar]
- 177.Lucchini, A., I. Dal Conte, and G. Di Perri. 2001. Mucosal shedding of human herpesvirus 8. N. Engl. J. Med. 344:691-692. [PubMed] [Google Scholar]
- 178.Lucht, E., M. Brytting, L. Bjerregaard, I. Julander, and A. Linde. 1998. Shedding of cytomegalovirus and herpesviruses 6, 7, and 8 in saliva of human immunodeficiency virus type 1-infected patients and healthy controls. Clin. Infect. Dis. 27:137-141. [DOI] [PubMed] [Google Scholar]
- 179.Lunardi-Iskandar, Y., P. Gill, V. H. Lam, R. A. Zeman, F. Michaels, D. L. Mann, M. S. Reitz, M. Kaplan, Z. N. Berneman, D. Carter, J. L. Bryant, and R. C. Gallo. 1995. Isolation and characterization of an immortal neoplastic cell line (KS Y-1) from AIDS-associated Kaposi's sarcoma. J. Natl. Cancer Inst. 87:974-981. [DOI] [PubMed] [Google Scholar]
- 180.Luppi, M., P. Barozzi, T. F. Schulz, G. Setti, K. Staskus, R. Trovato, F. Narni, A. Donelli, A. Maiorana, R. Marasca, S. Sandrini, and G. Torelli. 2000. Bone marrow failure associated with human herpesvirus 8 infection after transplantation. N. Engl. J. Med. 343:1378-1385. [DOI] [PubMed] [Google Scholar]
- 181.Reference deleted.
- 182.Mann, D. J., E. S. Child, C. Swanton, H. Laman, and N. Jones. 1999. Modulation of p27Kip1 levels by the cyclin encoded by Kaposi's sarcoma-associated herpesvirus. EMBO J. 18:654-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Reference deleted.
- 184.Marchioli, C. C., J. L. Love, L. Z. Abbott, L. Z. Huang, Y. Q. Remick, S. C. Surtento-Reodica, N. Hutchison, E. Maldvan, A. Friedman-Kien, and A. E. Poiesz. 1996. Prevalence of human herpesvirus 8 DNA sequences in several patient populations. J. Clin. Microbiol. 34:2635-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Martin, J. N., J. L. Gerberding, and D. H. Osmond. 1996. Testing for antibodies in Kaposi’s sarcoma. N. Engl. J. Med. 335:819. [DOI] [PubMed] [Google Scholar]
- 186.Martin, J. N., D. E. Ganem, D. H. Osmond, K. A. Page-Shafer, D. Macrae, and D. H. Kedes. 1998. Sexual transmission and the natural history of human herpesvirus 8 infection. N. Engl. J. Med. 338:948-954. [DOI] [PubMed] [Google Scholar]
- 187.Martinez-Escribano, J. A., M. del Pino Gil-Mateo, J. Miquel, E. Ledesma, and A. Aliaga. 1998. Human herpesvirus 8 is not detectable by polymerase chain reaction in angiosarcoma. Br. J. Dermatol. 138:546-547. [DOI] [PubMed] [Google Scholar]
- 188.Masood, R., T. Zheng, A. Tulpule, N. Arora, L. Chatlynne, M. Handy, J. Whitman, Jr., M. Kaplan, M. Dosik, D. V. Ablashi, and P. S. Gill. 1997. Kaposi's sarcoma-associated herpesvirus infection in multiple myeloma. Science 278:1970-1971. [PubMed] [Google Scholar]
- 189.Matolcsy, A., R. G. Nador, E. Cesarman, and D. M. Knowles. 1998. Immunoglobulin VH gene mutational analysis suggests that primary effusion lymphomas derive from different stages of B cell maturation. Am. J. Pathol. 153:1609-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mayama, S., L. E. Cuevas, J. Sheldon, O. H. Omar, D. H. Smith, P. Okong, B. Silvel, C. A. Hart, and T. F. Schulz. 1998. Prevalence and transmission of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in Ugandan children and adolescents. Int. J. Cancer 77:817-820. [DOI] [PubMed] [Google Scholar]
- 191.McDonagh, D. P., J. Liu, M. J. Gaffey, L. J. Layfield, N. Azumi, and S. T. Traweek. 1996. Detection of Kaposi's sarcoma-associated herpesvirus-like DNA sequences in angiosarcoma. Am. J. Pathol. 49:1363-1368. [PMC free article] [PubMed] [Google Scholar]
- 192.Medveczky, M. M., E. Horvath, T. Lund, and P. G. Medveczky. 1997. In vitro antiviral drug sensitivity of the Kaposi's sarcoma-associated herpesvirus. AIDS 11:1327-1332. [DOI] [PubMed] [Google Scholar]
- 193.Memar, O. M., P. L. Rady, R. M. Goldblum, and S. K. Tyring. 1997. Human herpesvirus-8 DNA sequences in a patient with pemphigus vulgaris, but without HIV infection or Kaposi's sarcoma. J. Investig. Dermatol. 108:118-119. [DOI] [PubMed] [Google Scholar]
- 194.Memar, O. M., P. L. Rady, R. M. Goldblum, A. Yen, and S. K. Tyring. 1997. Human herpesvirus 8 DNA sequences in blistering skin from patients with pemphigus. Arch. Dermatol. 133:1247-1251. [PubMed] [Google Scholar]
- 195.Meng, Y.-X., T. J. Spira, G. J. Bhat, C. J. Birch, J. D. Druce, B. R. Edlin, R. Edwards, C. Gunthel, R. Newton, F. R. Stamey, C. Wood, and P. E. Pellett. 1999. Individuals from North America, Australia, and Africa are infected with four different genetypes of human herpesvirus 8. Virology 261:106-119. [DOI] [PubMed] [Google Scholar]
- 196.Meng, Y.-X., T. Sata, F. R. Stamey, A. Voevodin, H. Katano, H. Koizumi, M. Deleon, M. A. Decristofano, and R. Galimberti. 2001. Molecular characterization of strains of human herpesvirus 8 from Japan, Argentina, and Kuwait. J. Gen. Virol. 82:499-506. [DOI] [PubMed] [Google Scholar]
- 197.Mesri, E. A., E. Cesarman, L. Arvanitakis, S. Rafü, M. A. S. Moore, D. N. Posnett, D. M. Knowles, and A. S. Asch. 1996. Human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus is a new transmissible virus that infects B-cells. J. Exp. Med. 183:2385-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Min, J., and D. A. Katzenstein. 1999. Detection of Kaposi's sarcoma-associated herpesvirus in peripheral blood cells in human immunodeficiency virus infection with Kaposi's sarcoma CD4 cell count and HIV RNA levels. AIDS Res. Hum. Retrovir. 15:51-53. [DOI] [PubMed] [Google Scholar]
- 199.Miro, O., M. Gomez, E. Pedrol, F. Cardellach, V. Nunes, and J. Casademont. 2000. Respiratory chain dysfunction associated with multiple mitochondrial DNA deletions in antiretroviral therapy-related lipodystrophy. AIDS 14:1855-1857. [DOI] [PubMed] [Google Scholar]
- 200.Mitterer, M., W. Mair, D. Gatti, J. Sheldon, M. Vachula, P. Coser, and T. F. Schultz. 1998. Dendritic cells derived from bone marrow and CD34+ selected blood progenitor cells of myeloma patients, cultured in serum-free media, do not contain the Kaposi's sarcoma herpesvirus genome. Br. J. Haematol. 102:1338-1340. [DOI] [PubMed] [Google Scholar]
- 201.Molden, J., Y. Chang, Y. You, P. S. Moore, and M. A. Goldsmith. 1997. A Kaposi's sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J. Biol. Chem. 272:19625-19631. [DOI] [PubMed] [Google Scholar]
- 202.Monini, P., L. de Lellis, M. Fabris, F. Rigolin, and E. Cassai. 1996. Kaposi's sarcoma-associated herpesvirus DNA sequences in prostate tissue and human semen. N. Engl. J. Med. 334:1168-1172. [DOI] [PubMed] [Google Scholar]
- 203.Monini, P., F. Carlini, M. Sturzl, P. Rimessi, F. Superti, M. Franco, G. Melucci-Vigo, A. Cafaro, D. Goletti, C. Sgadari, S. Butto, P. Leone, C. Chiozzini, C. Barresi, A. Tinari, A. Bonaccorsi, M. R. Capobianchi, M. Giuliani, A. di Carlo, M. Andreoni, G. Rezza, and B. Ensoli. 1999. Alpha interferon inhibits human herpesvirus 8 (HHV-8) reactivation in primary effusion lymphoma cells and reduces HHV-8 load in cultured peripheral blood mononuclear cells. J. Virol. 73:4029-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Moore, P. S., C. Boshoff, R. A. Weiss, and Y. Chang. 1996. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274:1739-1744. [DOI] [PubMed] [Google Scholar]
- 205.Moore, P. S., J. S. Gao, G. Dominguez, E. Cesarman, O. Lungu, M. Knowles, R. Garber, D. J. McGeoch, P. Pellett, and Y. Chang. 1996. Primary characterization of a herpesvirus-like agent associated with Kaposi's sarcoma. J. Virol. 70:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Müller, A. C. Franzen, P. Klussmann, M. Wagner, V. Diehl, G. Fätkenheuer, B. Salzberger, D. V. Ablashi, and G. R. F. Krueger. 2000. Human herpesvirus type 8 in HIV-infected patients with interstitial pneumonitis. J. Infect. 40:1-6. [DOI] [PubMed] [Google Scholar]
- 208.Muralidhar, S., A. M. Pumfrery, M. Hassani, M. R. Sadaie, N. Azumi, M. Kishishita, J. N. Brady, J. Doniger, P. Medveczky, and L. J. Rosenthal. 1998. Identification of kaposin (open reading frame K12) as a human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus) transforming gene. J. Virol. 72:4980-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Muralidhar, S., A. Pumfrery, M. Hassani, M. R. Sadaie, N. Azumi, J. N. Brady, P. Medveszky, and L. J. Rosenthal. 1998. Identification of kaposin (ORF K12) as a human herpesvirus 8 (Kaposi's sarcoma associated herpesvirus) oncogene. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:A27. [Google Scholar]
- 210.Nador, R. G., E. Cesarman, A. Chadburn, D. B. Dawson, M. Q. Ansari, J. Said, and D. M. Knowles. 1996. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpesvirus. Blood 88:645-656. [PubMed] [Google Scholar]
- 211.Reference deleted.
- 212.Neipel, F., and B. Fleckenstein. 1999. The role of HHV-8 in Kaposi's sarcoma. Semin. Cancer Biol. 9:151-164. [DOI] [PubMed] [Google Scholar]
- 213.Neipel, F., J. C. Albrecht, A. Ensser, Y. Q. Huang, I. J. Lee, A. E. Friedman-Kien, and B. Fleckenstein. 1997. Cell homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity. J. Virol. 71:4187-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nicholas, J., J. C. Zong, X. Wan, H. G. Guo, M. S. Reitz, and G. S. Hayward. 1998. Novel organizational features captured cellular genes and strain variability within the genome of KSHV/HHV-8. J. Natl. Cancer Inst. Monogr. 23:79-88. [DOI] [PubMed] [Google Scholar]
- 215.Nicholas, J., V. R. Ruvolo, W. H. Burns, G. Sandford, X. Y. Wan, D. Ciufo, S. B. Hendrickson, H. G. Guo, G. S. Hayward, and M. S. Reitz. 1997. Kaposi's sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat. Med. 3:287-292. [DOI] [PubMed] [Google Scholar]
- 216.Nishimoto, S., R. Inagi, K. Yamanishi, K. Hosokawa, M. Kakibuchi, and K. Yoshikawa. 1997. Prevalence of human herpesvirus-8 in skin lesions. Br. J. Dermatol. 137:179-184. [DOI] [PubMed] [Google Scholar]
- 217.Oksenhendler, E., M. Duarte, J. Soulier, P. Cacoub, Y. Welker, J. Cadranel, D. Cazals-Hatem, B. Autran, J.-P. Clauvel, and M. Raphael. 1996. Multicentric Castleman's disease in HIV infection: a clinical and pathological study of 20 patients. AIDS 10:61-67. [PubMed] [Google Scholar]
- 218.Olsen, S. J., K. Tarte, W. Sherman, E. E. Hale, M. T. Weisse, A. Orazi, B. Klein, and Y. Chang. 1998. Evidence against KSHV infection in the pathogenesis of multiple myeloma. Virus Res. 57:197-202. [DOI] [PubMed] [Google Scholar]
- 219.Orenstein, J. M., S. Alkan, A. Bauvelt, K. T. Jeang, M. D. Weinstein, D. Ganem, and B. Herndier. 1997. Visualization of human herpesvirus 8 in Kaposi's sarcoma by light and transmission electron microscopy. AIDS 11:F35-F45. [DOI] [PubMed] [Google Scholar]
- 220.Otsuki, T., S. Kumar, B. Ensoli, D. W. Kingma, T. Yano, T., M. Stetler-Stevenson, E. S. Jaffe, and M. Raffeld. 1996. Detection of HHV-8/KSHV DNA sequences in AIDS-associated extranodal lymphoid malignancies. Leukemia 10:1358-1362. [PubMed] [Google Scholar]
- 221.Palacios, I., I. Umbert, and A. Celada. 1999. Absence of human herpesvirus-8 DNA in angiosarcoma. Br. J. Dermatol. 140:170-171. [DOI] [PubMed] [Google Scholar]
- 222.Pan, L., L. Milligan, J. Michaeli, E. Cesarman, and D. M. Knowles. 2001. Polymerase chain reaction detection of Kaposi's sarcoma-associated herpesvirus-optimized protocols and their application to myeloma. J. Mol. Diagn. 3:32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Parravicini, C., M. Corbellino, M. Paulli, U. Magrini, M. Lazzarino, P. S. Moore, and Y. Chang. 1997. Expression of a virus-derived cytokine, KSHV vIL-6, in HIV-seronegative Castleman's disease. Am. J. Pathol. 151:1517-1522. [PMC free article] [PubMed] [Google Scholar]
- 224.Parravicini, C., E. Lauri, L. Baldini, A. Neri, F. Poli, G. Sirchia, M. Moroni, M. Galli, and M. Corbellino. 1997. Kaposi's sarcoma-associated herpesvirus infection and multiple myeloma. Science 278:1969-1970. [DOI] [PubMed] [Google Scholar]
- 225.Pastore, C., A. Gloghini, G. Volpe, J. Nomdedeu, E. Leonardo, U. Mazza, G. Saglio, A. Carbone, and G. Gaidano. 1995. Distribution of Kaposi's sarcoma herpesvirus sequences among lymphoid malignancies in Italy and Spain. Br. J. Haematol 91:918-920. [DOI] [PubMed] [Google Scholar]
- 226.Pastore, R. D., A. Chadburn, C. Kripas, and E. J. Schattner. 2000. Novel association of haemophagocytic syndrome with Kaposi's sarcoma-associated herpesvirus-related primary effusion lymphoma. Br. J. Haematol. 111:1112-1115. [DOI] [PubMed] [Google Scholar]
- 227.Pauk, J., M. L. Huang, S. J. Brodie, A. Wald, D. M. Koelle, T. Schacker, C. Celum, S. Selke, and L. Corey. 2000. Mucosal shedding of human herpesvirus 8 in men. N. Engl. J. Med. 343:1369-1377. [DOI] [PubMed] [Google Scholar]
- 228.Pellett, P. E., T. J. Spira, O. Bagasra, C. Boshoff, L. Corey, L. DeLellis, M. L. Huang, J. C. Lin, S. Matthews, P. Nonini, P. Rimessi, C. Sosa, C. Wood, and J. A. Stewart. 1999. Multicenter comparison of PCR assays for the detection of human herpesvirus 8 DNA in semen. J. Clin. Microbiol. 37:1298-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Perna, A. M., E. Viviano, E. Iannitto, R. Marceno, and N. Romano. 1998. No association between human herpesvirus type 8 infection and multiple myeloma. J. Natl. Cancer Inst. 90:1013-1014. [DOI] [PubMed] [Google Scholar]
- 230.Plancoulaine, S., L. Abel, M. van Beveren, D.-A. Trégouët, M. Joubert, P. Tortevoye, G. de Thé, and A. Gessain. 2000. Human herpesvirus 8 transmission from mother to child and between siblings in an endemic population. Lancet 356:1062-1065. [DOI] [PubMed] [Google Scholar]
- 231.Quinbi, W., O. Al-Furayh, K. Almeshan, S. F. Lin, R. Sun, L. Heston, D. Ross, M. Rigsby, and G. Miller. 1998. Serologic association of human herpesvirus-8 with posttransplant Kaposi's sarcoma in Saudi Arabia. Transplantation 65:583-585. [DOI] [PubMed] [Google Scholar]
- 232.Quinlivan, E. B., R. X. Wang, P. W. Stewart, C. Kolmoltri, N. Regamey, P. Erb, P. L. Vernazza, and the Swiss HIV Cohort Study. 2001. Longitudinal seroreactivity to human herpesvirus 8 (KSHV) in the Swiss HIV cohort 4.7 years before KS. J. Med. Virol. 64:157-166. [DOI] [PubMed] [Google Scholar]
- 233.Rabkin, C. S., S. Janz, A. Lash, A. E. Coleman, E. Musaba, L. Liotta, R. J. Biggar, and Z. P. Zhuang. 1997. Monoclonal origin of multicentric Kaposi's sarcoma lesions. N. Engl. J. Med. 336:988-993. [DOI] [PubMed] [Google Scholar]
- 234.Rabkin, C. S., T. F. Schulz, D. Whitby, E. T. Lennette, L. I. Magpantay, L. G. Chatlynne, and R. J. Biggar. 1998. Intra-assay correlation of human herpesvirus 8 serological tests. J. Infect. Dis. 178:304-309. [DOI] [PubMed] [Google Scholar]
- 235.Rabkin, C. S., F. A. Shepherd, and J. A. Wade. 1999. Human herpesvirus 8 and renal transplantation. N. Engl. J. Med. 340:1045-1046. [PubMed] [Google Scholar]
- 236.Radkov, S. A., P. Kellam, and C. Boshoff. 2000. The latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene H-ras transforms primary rat cells. Nat. Med. 6:1121-1127. [DOI] [PubMed] [Google Scholar]
- 237.Rady, P. L., A. Yen, J. L. Rollefson, I. Orengo, S. Bruce, T. K. Hughes, and S. K. Tyring. 1995. Herpesvirus-like DNA sequences in non-Kaposi's sarcoma skin lesions of transplant patients. Lancet 345:1339-1340. [DOI] [PubMed] [Google Scholar]
- 238.Raji, N., J. Gong, D. Chauhan, G. Teoh, D. Avigan, Z. Wu, D. Chen, S. P. Treon, I. J. Webb, D. W. Kufe, and K. C. Anderson. 1999. Bone marrow and peripheral blood dendritic cells from patients with multiple myeloma are phenotypically and functionally normal despite the detection of Kaposi's sarcoma herpesvirus gene sequences. Blood 93:1487-1495. [PubMed] [Google Scholar]
- 239.Reed, J. A., R. G. Nador, D. Spaulding, Y. Tani, E. Cesarman, and D. M. Knowles. 1998. Demonstration of Kaposi's sarcoma-associated herpesvirus cyclin D homologue in cutaneous Kaposi's sarcoma by colorimetric in situ hybridization using a catalyzed signal amplification system. Blood 91:3825-3832. [PubMed] [Google Scholar]
- 240.Regamey, N., M. Tamm, M. Wernli, A. Witschi, G. Thiel, G. Cathomas, and P. Erb. 1998. Transmission of human herpesvirus 8 infection from renal transplant donors to recipients. N. Engl. J. Med. 339:1358-1363. [DOI] [PubMed] [Google Scholar]
- 241.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Renne, R., M. Langunoff, W. Zhong, and D. Ganem. 1996. The size and conformation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J. Virol. 70:8151-8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Resnick, L., J. S. Herbst, D. V. Ablashi, S. Atherton, B. Frank, L. Rosen, and S. N. Horwitz. 1988. Regression of oral hairy leukoplakia after oral administered acyclovir therapy. J. Am. Med. 259:384-388. [PubMed] [Google Scholar]
- 244.Rettig, M. B., H. J. Ma, R. A. Vescio, M. Põld, G. Schiller, D. Belson, A. Savage, C. Nishikubo, C. Wu, J. Fraser, J. W. Said, and J. R. Berenson. 1997. Kaposi's sarcoma-associated herpesvirus infection of bone marrow dendritic cells from multiple myeloma patients. Science 276:1851-1854. [DOI] [PubMed] [Google Scholar]
- 245.Reference deleted.
- 246.Rezza, G., E. T. Lennette, M. Giuliani, P. Pezzotti, F. Caprilli, P. Monini, S. Butto, G. Lodi, A. Di Carlo, J. A. Levy, and B. Ensoli. 1998. Prevalence and determinants of anti-lytic and anti-latent antibodies to human herpesvirus-8 among Italian individuals at risk of sexually and parenterally transmitted infections. Int. J. Cancer 77:361-366. [DOI] [PubMed] [Google Scholar]
- 247.Rivas, C., A. E. Thlick, C. Parravicini, P. S. Moore, and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus LANA-2 is a B-cell specific latent viral protein that inhibits P53. J. Virol. 75:429-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Russo, J. J., R. A. Bohenzky, M.-C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Perry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi's sarcoma-associated herpesvirus (HHV-8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Said, J. W., M. R. Rettig, K. Heppner, R. A. Vescio, G. Schiller, H. J. Ma, D. Belson, A. Savage, I. P. Shintaku, H. P. Koeffler, H. Asou, G. Pinkus, J. Pinkus, M. Schrage, E. Green, and J. R. Berenson. 1997. Localization of Kaposi's sarcoma-associated herpesvirus in bone marrow biopsy samples from patients with multiple myeloma. Blood 90:4278-4282. [PubMed] [Google Scholar]
- 250.Said, J. W., K. Chien, S. Takeuchi, T. Tasaka, H. Asou, S. K. Cho, S. de Vos, E. Cesarman, M. Knowles, and P. Koeffler. 1996. Kaposi's sarcoma-associated herpesvirus (KSHV or HHV-8) in primary effusion lymphoma: ultrastructural demonstration of herpesvirus in lymphoma cells. Blood 87:4937-4943. [PubMed] [Google Scholar]
- 251.Said, J. W., K. Heppner, I. P. Shintaku, M. Schrage, E. Green, M. R. Rettig, R. A. Vescio, G. Schiller, H. J. Ma, D. Belson, A. Savage, J. R. Berenson, H. P. Koeffler, H. Asou, G. Pinkus, and J. Pinkus. 1998. Multiple myeloma and HHV8 infection—response. Blood 91:4391-4393.9596692 [Google Scholar]
- 252.Said, J. W., I. P. Shintaku, H. Asou, S. de Vos, J. Baker, G. Hanson, E. Cesarman, R. Nador, and H. P. Koeffler. 1999. Herpesvirus 8 inclusions in primary effusion lymphoma. Report of a unique case with T-cell phenotype. Arch. Pathol. Lab. Med. 123:257-260. [DOI] [PubMed]
- 253.Said, J. W., T. Tasaka, S. Takeuchi, H. Asou, S. de Vos, E. Cesarman, D. M. Knowles, and H. P. Koeffler. 1996. Primary effusion lymphoma in women: report of two cases of Kaposi's sarcoma-herpes virus-associated effusion-based lymphoma in human immunodeficiency virus-negative women. Blood 88:3124-3128. [PubMed] [Google Scholar]
- 254.Salahuddin, S. Z., D. V. Ablashi, P. D. Markham, S. F. Josephs, S. Sturzenegger, M. Kaplan, G. Halligan, P. Biberfeld, F. Wong-Staal, B. Kramarsky, and R. C. Gallo. 1986. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 234:596-601. [DOI] [PubMed] [Google Scholar]
- 255.Salahuddin, S. Z., S. Nakamura, P. Biberfeld, M. H. Kaplan, P. D. Markham, L. Larsson, and R. C. Gallo. 1988. Angiogenic properties of Kaposi's sarcoma-derived cells after long-term culture in vitro. Science 242:430-433. [DOI] [PubMed] [Google Scholar]
- 256.Santarelli, R., A. Angeloni, A. Farina, R. Gonnella, G. Gentile, P. Martino, M. T. Petrucci, F. Mandelli, L. Frati, and A. Faggioni. 1998. Lack of serologic association between human herpesvirus-8 infection and multiple myeloma and monoclonal gammopathies of undetermined significance. J. Natl. Cancer Inst. 90:781-782. [DOI] [PubMed] [Google Scholar]
- 257.Sarid, R., J. S. Wiezorek, P. S. Moore, and Y. Chang. 1999. Characterization and cell cycle regulation of the major Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) latent genes and their promoter. J. Virol. 73:1438-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sarid, R., S. J. Olsen, and P. S. Moore. 1999. Kaposi's sarcoma-associated herpesvirus: epidemiology, virology, and molecular biology. Adv. Virus Res. 52:139-231. [DOI] [PubMed] [Google Scholar]
- 259.Sarid, R., G. Pizov, D. Rubinger, R. Backenroth, M. M. Friedlaender, F. Schwartz, and D. G. Wolf. 2001. Detection of human herpesvirus-8 DNA in kidney allografts prior to the development of Kaposi's sarcoma. Clin. Infect. Dis. 32:1502-1505. [DOI] [PubMed] [Google Scholar]
- 260.Schatz, O., P. Monini, R. Bugarini, F. Neipel, T. F. Schulz, M. Andreoni, P. Erb, M. Eggers, J. Haas, S. Butto, M. Lukwiya, J. R. Bogner, S. Yaguboglu, J. Sheldon, L. Sarmati, F. D. Goebel, R. Hintermaier, G. Enders, N. Regamey, M. Wernli, M. Stürzl, G. Rezza, and B. Ensoli. 2001. Kaposi's sarcoma-associated herpesvirus serology in Europe and Uganda: multicentric study with multiple and novel assays. J. Med. Virol. 65:123-132. [PubMed] [Google Scholar]
- 261.Schultz, E. R., G. W. Rankin, M. P. Blanc, B. W. Raden, C. C. Tsai, and T. M. Rose. 2000. Characterization of two divergent lineages of macaque rhadinoviruses related to Kaposi's sarcoma-associated herpesvirus. J. Virol. 74:4919-4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Schulz, T. F. 1998. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8). J. Gen. Virol. 79:1573-1591. [DOI] [PubMed] [Google Scholar]
- 263.Schulz, T. F., Y. Chang, and P. S. Moore. 1998. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8), p. 87-134. In D. J. McCance (ed.), Human tumor viruses. ASM Press, Washington, D.C.
- 264.Schulz, T. F. 2000. KSHV (HHV8) infection. J. Infect. 41:125-129. [DOI] [PubMed] [Google Scholar]
- 265.Schulz, T. F. 2001. KSHV/HHV8-associated lymphoproliferations in the AIDS setting. Eur. J. Cancer 37:1217-1226. [DOI] [PubMed] [Google Scholar]
- 266.Scolaro, M. J., L. B. Gunnill, L. E. Pope, M. H. Khalil, D. H. Katz, and J. E. Berg. 2001. The antiviral drug docosanol as a treatment for Kaposi's sarcoma lesions in HIV type 1-infected patients: a pilot clinical study. AIDS Res. Hum. Retrovirol. 17:35-43. [DOI] [PubMed] [Google Scholar]
- 267.Searles, R. P., E. P. Bergquam, M. K. Axlhelm, and S. W. Wong. 1999. Sequence and genomic analysis of a rhesus macaque rhadinovirus with similarity to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 73:3040-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Simpson, G. R., T. F. Schulz, D. Whitby, P. M. Cook, C. Boshoff, L. Rainbow, M. R. Howard, S.-J. Gao, R. A. Bohenzky, P. Simmons, C. Lee, A. de Ruiter, A. Hatzakis, R. S. Tedder, I. V. D. Weller, R. A. Weiss, and P. S. Moore. 1996. Prevalence of Kaposi's sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet 348:1133-1138. [DOI] [PubMed] [Google Scholar]
- 269.Sitas, F., H. Carrara, V. Beral, R. Newton, G. Reeves, D. Bull, U. Jentsch, R. Pacella-Norman, D. Bourboulia, D. Whitby, C. Boshoff, and R. Weiss. 1999. Antibodies against human herpesvirus 8 in black South African patients with cancer. N. Engl. J. Med. 340:1863-1871. [DOI] [PubMed] [Google Scholar]
- 270.Smith, M. S., C. Bloomer, R. Horvat, E. Goldstein, J. M. Casparian, and B. Chandran. 1997. Detection of human herpesvirus 8 DNA in Kaposi's sarcoma lesions and peripheral blood of human immunodeficiency virus-positive patients and correlation with serologic measurements. J. Infect. Dis. 176:84-93. [DOI] [PubMed] [Google Scholar]
- 271.Sosa, C., W. Klaskala, B. Chandran, R. Soto, L. Sieczkowski, M. H. Wu, M. Baum, and C. Wood. 1998. Human herpesvirus 8 as a potential sexually transmitted agent in Honduras. J. Infect. Dis. 178:547-551. [DOI] [PubMed] [Google Scholar]
- 272.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M.-F. d'Agay, J.-P. Clauvel, M. Raphael, L. Degos, and F. Sigaux. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1275-1280. [PubMed] [Google Scholar]
- 273.Sprinz, E., A. P. Caldas, D. R. Mans, A. Cancela, L. DiLeone, T. Dalla Costa, and G. Schwartsmann. 2001. Fractionated doses of oral etoposide in the treatment of patients with AIDS-related Kaposi sarcoma: a clinical and pharmacologic study to improve therapeutic index. Am. J. Clin. Oncol. 24:177-184. [DOI] [PubMed] [Google Scholar]
- 274.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sturzl, M., H. Brandstetter, and W. K. Roth. 1992. Kaposi's sarcoma: a review of gene expression and ultrastructure of KS spindle cells in vivo. AIDS Res. Hum. Retrovir. 8:1753-1763. [DOI] [PubMed] [Google Scholar]
- 276.Szekely, L., F. Chen, N. Teramoto, B. Ehlin-Henriksson, K. Pokrovskaja, A. Szeles, A. Manneborg-Sandlund, M. Lowbeer, E. T. Lennette, and G. Klein. 1998. Restricted expression of Epstein-Barr virus (EBV)-encoded, growth transformation-associated antigens in an EBV- and human herpesvirus type 8-carrying body cavity lymphoma line. J. Gen. Virol. 79:1445-1452. [DOI] [PubMed] [Google Scholar]
- 277.Takata, M., N. Hatta, K. Takehara, and H. Fujiwara. 1997. Absence of human herpesvirus-8 DNA in angiosarcomas and other skin tumours in immunocompetent patients, and in graft-versus-host disease in the immunosuppressed recipients of bone marrow transplants. Br. J. Dermatol. 137:156-157. [DOI] [PubMed] [Google Scholar]
- 278.Tarte, K., S. J. Olsen, J. F. Rossi, E. Legouffe, Z. Y. Lu, M. Jourdan, Y. Chang, and B. Klein. 1998. Kaposi's sarcoma-associated herpesvirus is not detected with immunosuppression in multiple myeloma. Blood 92:2186-2188. [PubMed] [Google Scholar]
- 279.Tarte, K., S. J. Olsen, Z. Y. Lu, E. Legouffe, J. F. Rossi, Y. Chang, and B. Klein. 1998. Clinical-grade functional dendritic cells from patients with multiple myeloma are not infected with Kaposi's sarcoma-associated herpesvirus. Blood 91:1852-1857. [PubMed] [Google Scholar]
- 280.Tasaka, T., J. W. Said, and H. P. Koeffler. 1996. Absence of HHV-8 in prostate and semen. N. Engl. J. Med. 335:1237-1238. [PubMed] [Google Scholar]
- 281.Thome, M., P. Schneider, K. Hofmann, H. Fickenscher, E. Meinl, F. Neipel, C. Mattmann, K. Burns, J. L. Bodmer, M. Schroter, C. Scaffidi, P. H. Krammer, M. E. Peter, and J. Tschopp. 1997. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517-521. [DOI] [PubMed] [Google Scholar]
- 282.Tisdale, J. F., A. K. Stewart, B. Dickstein, R. F. Little, I. Dubé, D. Cappe, C. E. Dunbar, and K. E. Brown. 1998. Molecular and serological examination of the relationship of human herpesvirus 8 in multiple myeloma: ORF-26 sequences in bone marrow stroma are not restricted to myeloma patients and other regions of the genome are not detected. Blood 92:2681-2687. [PubMed] [Google Scholar]
- 283.Trovato, R., M. Luppi, P. Barozzi, L. Da Prato, A. Maiorana, S. Lico, R. Marasca, P. Torricelli, G. Torelli, and L. Ceccherini-Nelli. 1999. Cellular localization of human herpesvirus 8 in nonneoplastic lymphadenopathies and chronic interstitial pneumonitis by in situ polymerase chain reaction studies. J. Hum. Virol. 2:38-44. [PubMed] [Google Scholar]
- 284.Tye, M. J. 1970. Bullous pemphigoid and Kaposi's sarcoma. Arch. Dermatol. 101:690-691. [DOI] [PubMed] [Google Scholar]
- 285.Vieira, J., M.-L. Huang, D. M. Koelle, and L. Corey. 1997. Transmissible Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in saliva of men with a history of Kaposi's sarcoma. J. Virol. 71:7083-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Viviano, E., F. Vitale, F. Ajello, A. M. Perna, M. R. Villafrate, F. Bonura, M. Arico, G. Mazzola, and N. Romano. 1997. Human herpesvirus type 8 DNA sequences in biological samples of HIV-positive and negative individuals in Sicily. AIDS 11:607-612. [DOI] [PubMed] [Google Scholar]
- 287.Viviano, E., N. Romano, M. Sorce, E. Castelli, and L. Marasa. 1997. Absence of human herpesvirus 8 DNA in benign and malignant endothelial lesions. J. Clin. Microbiol. 35:3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Walts, A. E., I. P. Shintaku, and J. W. Said. 1990. Diagnosis of malignant lymphoma in effusions from patients with AIDS by gene rearrangement. Am. J. Clin. Pathol. 94:170-175. [DOI] [PubMed] [Google Scholar]
- 289.Weiss, R., and C. Boshoff. 2000. Addressing controversies over Kaposi's sarcoma. J. Natl. Cancer Inst. 92:677-679. [DOI] [PubMed] [Google Scholar]
- 290.Whitby, D., C. Boshoff, M. Luppi, and G. Torelli. 1997. Kaposi's sarcoma-associated herpesvirus infection and multiple myeloma. Science 278:1971-1972. [PubMed] [Google Scholar]
- 291.Whitby, D., M. R. Howard, M. Tenant-Flowers, N. S. Brink, A. Copas, C. Boshoff, T. Hatzioannou, F. E. A. Suggett, D. M. Aldam, A. S. Denton, R. F. Miller, I. V. D. Weller, R. A. Weiss, R. S. Tedder, and T. F. Schulz. 1995. Detection of Kaposi sarcoma-associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet 346:799-800. [DOI] [PubMed] [Google Scholar]
- 292.Whitby, D., M. Luppi, C. Sabin, P. Barozzi, A. R. Di Biase, F. Balli, F. Cucci, R. A. Weiss, C. Boshoff, and G. Torelli. 2000. Detection of antibodies to human herpesvirus 8 in Italian children: evidence for horizontal transmission. Br. J. Cancer 82:702-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Whitby, D., N. A. Smith, S. Matthews, S. O'Shea, C. A. Sabin, R. Kulasegaram, C. Boshoff, R. A. Weiss, A. de Ruiter, and J. M. Best. 1999. Human herpesvirus 8: seroepidemiology among women and detection in the genital tract of seropositive women. J. Infect. Dis. 179:234-236. [DOI] [PubMed] [Google Scholar]
- 294.Wu, F. Y., J.-H. Ahn, D. J. Alcendor, W.-J. Jang, J. Xiao, D. Hayward, and G. Hayward. 2001. Origin-independent assembly of Kaposi's sarcoma-associated herpesvirus DNA replication compartments in transient cotransfection assays and association with the ORF-K8 protein and cellular PML. J. Virol. 75:1487-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wu, L., P. Lo, X. Yu, J. K. Stoops, B. Forghani, and Z. H. Zhou. 2000. Three-dimensional structure of the human herpesvirus 8 capsid. J. Virol. 74:9646-9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Yi, Q., M. Ekman, D. Anton, S. Bergenbrant, A. Osterborg, P. Georgii-Hemming, G. Holm, K. Nilsson, and P. Biberfeld. 1998. Blood dendritic cells from myeloma patients are not infected with Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8). Blood 92:402-404. [PubMed] [Google Scholar]
- 297.Zhang, Y. J., T. L. Davis, X. P. Wang, J. H. Deng, J. Baillargeon, I. T. Yeh, H. B. Jenson, and S. J. Gao. 2001. Distinct distribution of rare US genetypes of Kaposi's sarcoma-associated herpesvirus (KSHV) in south Texas for KSHV epidemiology. J. Infect. Dis. 183:125-129. [DOI] [PubMed] [Google Scholar]
- 298.Ziegler, J. L., and E. Katongole-Mbidde. 1996. Kaposi's sarcoma in childhood: an analysis of 100 cases from Uganda and relationship to HIV infection. Int. J. Cancer 65:200-203. [DOI] [PubMed] [Google Scholar]
- 299.Zimring, J. C., S. Goodbourn, and M. K. Offermann. 1998. Human herpesvirus 8 encodes an interferon regulatory factor (IRF) homolog that represses IRF-1-mediated transcription. J. Virol. 72:701-707. [DOI] [PMC free article] [PubMed] [Google Scholar]